Summary
This study examined the metabolic response of Drosophila melanogaster exposed to O2 concentrations ranging from 0 to 21% and at 100%. The metabolic rate of flies exposed to graded hypoxia remained nearly constant as O2 tensions were reduced from normoxia to ∼3 kPa. There was a rapid, approximately linear reduction in fly metabolic rate at PO2s between 3 and 0.5 kPa. The reduction in metabolic rate was especially pronounced at PO2 levels <0.5 kPa, and at a PO2 of 0.1 kPa fly metabolic rate was reduced ∼10-fold relative to normoxic levels. The metabolic rate of flies exposed to anoxia and then returned to normoxia recovered to pre-anoxic levels within 30 min with no apparent payment of a hypoxia-induced oxygen debt. Flies tolerated exposure to hypoxia and/or anoxia for 40 min with nearly 100% survival. Fly mortality increased rapidly after 2 h of anoxia and >16 h exposure was uniformly lethal. Flies exposed to pure O2 for 24 h showed no apparent alteration of metabolic rate, even though such O2 tensions should damage respiratory enzymes critical to mitochondria function. Within a few hours the metabolic rate of flies recovering from exposure to repeated short bouts of anoxia was the same as flies exposed to a single anoxia exposure.
Keywords: metabolic rate, reperfusion injury, critical oxygen tension, hypoxic response, hyperoxia
INTRODUCTION
Understanding how organisms respond to, and tolerate low O2 tensions has become an area of intense scientific study. Much of this interest is due to the recent identification of a widely conserved set of genes, termed hypoxia inducible factors (HIF), that sense and respond to hypoxia (Kaelin, 2005; Semenza, 2004). In addition to providing basic insights into how organisms cope with reduced levels of O2, understanding the hypoxic response has important medical ramifications in the treatment of ischemic injuries induced by events such as stroke or cardiac failure, and in the treatment of cancer (Dayan et al., 2006; Hochachka et al., 2002; Murphy and Steenbergen, 2008; Pouyssegur et al., 2006; Yun et al., 2002). Studies using model organisms such as the nematode Caenorhabditis elegans and Drosophila melanogaster, have provided valuable insights into the underlying molecular and physiological responses to hypoxia (Bishop et al., 2004; Centanin et al., 2005; Chen et al., 2004; Epstein et al., 2001).
Oxygen is required for all known metazoans to complete their life cycle (Fenchel and Finlay, 1995; Jensen, 1995). Under aerobic conditions, animals utilize O2 in the mitochondrial electron transport chain for oxidative phosphorylation and the synthesis of adenosine triphosphate (ATP) (Saraste, 1999). Metazoans have evolved a variety of mechanisms to either supply O2 to cells, or to compensate for reduced O2 levels when faced with fluctuations in O2 levels. Factors that expose organisms to low O2 tensions include environmental events, such as decreased O2 partial pressures with increased altitude, or chemical reactions that remove or displace O2 in aquatic and terrestrial environments (Baumgartl et al., 1994; Drew, 1992; Hoback and Stanley, 2001). Hypoxia can also occur at the cellular level during heavy exercise, and during physiological insults, such as strokes or heart attack, which limit perfusion (Hochachka, 1998). Although the requirement for O2 is absolute, animals do have the ability to tolerate varying degrees and periods of hypoxia (Hochachka, 1980).
Organisms respond to reduced O2 levels with adaptations that include increasing rates of glycolytic flux, reducing metabolic rate and modulating the levels of hypoxia inducible factors (HIFs) that regulate hypoxia-sensitive genes (Baumgartl et al., 1994; Brooks and Storey, 1997; Chandel and Schumacker, 2000; Clegg, 1997; Johansson et al., 1995). Even with such modifications O2 levels can still become limiting to cellular function, forcing the organism to withstand the harmful effects of hypoxia or anoxia (Hand, 1998). At the cellular level hypoxia can rapidly induce a wide range of potentially detrimental physiological effects that include alterations in levels of nicotinamide adenine dinucleotide hydride (NADH), adenosine diphosphate (ADP), adenosine monophosphate (AMP), inorganic phosphate, ATP and pH (Foe and Alberts, 1985).
The deleterious effects of hypoxia are thought to be mediated mainly by reducing the rate of ATP production. This results in the rapid failure of ATP-driven ion pumps, which allows the unopposed flow of ions into the cell causing rapid membrane depolarization. The resulting unregulated Ca2+ influx causes a wide array of cellular disruptions including spontaneous stimulation of phospholipases, proteases and nucleases (Choi, 2005; Lutz and Nilsson, 1997; Murphy and Steenbergen, 2008; Storey and Storey, 1990; Thompson et al., 2006). Toxic effects of hypoxia also include neuron excitotoxicity, free radical damage, inflammation and immune system over-activation (Bickler et al., 2004).
These hypoxia-induced changes at the cellular level result in alterations in physiological function of the organism. At the level of the whole organism two well-studied indicators of the effects of hypoxia are the O2 tension at which metabolic rate begins to be reduced, and how well an organism can survive a complete lack of O2. The point at which a reduction in metabolic rate occurs is commonly referred to as the critical oxygen tension (Pc). This value can vary from normoxic O2 tensions of 21 kPa in frog muscle to 1 kPa or less in some invertebrates (Boutilier and St Pierre, 2002; Holter and Spangenberg, 1997; Taylor and Moore, 1995). Whereas exposures to low O2 levels are eventually lethal to all metazoans, the length of time that organisms can tolerate anoxic and/or hypoxic conditions varies enormously. A few minutes without O2 can be lethal to most vertebrates (Arthur et al., 1997; Hermes-Lima and Zenteno-Savin, 2002), whereas copepod eggs buried in anoxic lake sediments remained viable for up to 332 years (Hairston et al., 1995; Marcus et al., 1994). Although some animals can survive long periods of anoxia (Hand, 1998; Hand and Hardewig, 1996; Jackson, 2000; Lutz and Nilsson, 1997), the great majority of free-living, eukaryotic organisms cannot withstand more than 24 h of anoxia (Clegg, 1997).
Elevated O2 levels can also be toxic to organisms. Hyperoxia toxicity is thought to be caused by an increased production of free radicals and hydrogen peroxide generation (Joenje, 1989; Paget et al., 1987). Among other effects, hyperoxia can disrupt metabolic pathways and reduce respiration rates (Gille and Joenje, 1992; Jamieson, 1989; Schoonen et al., 1990). Humans exposed to pure O2 for more than 24 h begin to show compromised lung function and exposure to several days of pure O2 is lethal to many mammals and insects (Mockett et al., 2001; Smith and Gottlieb, 1975).
Insects have long been used to study respiration physiology and the response of organisms to varied O2 tensions (Chadwick and Gilmour, 1940; Ellenby, 1953; Fenn et al., 1967; Harrison et al., 2006; Hetz and Bradley, 2005; Hoback and Stanley, 2001; Jarecki et al., 1999; Lighton and Schilman, 2007). A major advantage in the use of insects to study physiological responses to varying O2 levels is that the tissues in insects are typically directly exposed to the ambient O2 tensions. This contrasts with many animals (e.g. mammals) that rely on respiratory pigments and a circulatory system to meet gas exchange requirements. This means the O2 tensions that cells are exposed to can be quite different from ambient levels, particularly at increased O2 tensions (Massabuau, 2003).
The well-characterized development and genetics of Drosophila make it particularly useful in studies of hypoxia tolerance (Douglas et al., 2003; Haddad, 2006). As in most insects, gas exchange in Drosophila occurs via pairs of spiracles on the segments of the thorax and abdomen from where branching tracheae penetrate the tissues terminating in blind-ended tracheoles that deliver O2 directly from the atmosphere to the organs and tissues. Ambient gases enter into Drosophila through spiracles located laterally along the thorax and abdomen. Small muscles control valves that regulate the flow of air in response to factors such as humidity and metabolic demand (Heymann and Lehmann, 2006; Lehmann et al., 2000; Wilson et al., 2005). This study examined the metabolic response of D. melanogaster to a wide range of O2 concentrations. As well as better characterizing the response of Drosophila to hypoxia, such studies allow changes in the physiology of the whole organism to be compared with studies on the effects of varied O2 tensions on gene expression patterns.
MATERIALS AND METHODS
Exposure to graded hypoxia
The metabolic response of a common laboratory wild-type strain of Drosophila melanogaster Meigen (strain w1118) to varying O2 tensions was assayed in groups of approximately 100 mixed-sex, 4- to 8-day-old post-emergent flies. Previous studies have shown that male and female flies appear to respond equally to hypoxia in physiological and behavioral assays (Krishnan et al., 1997). Fly stocks were maintained on a standard yeast-cornmeal-agar medium at 24°C in 50 ml glass vials. The day before the flow-through respirometry measurements were made the flies were sedated with light CO2 anesthesia and their sex determined. Flies recovered from this CO2 exposure for 24 h before the start of metabolic measurements.
On the day of the metabolic measurement flies were immobilized with a humidified stream of N2 and placed in two 25 ml glass metabolic chambers at a density of around 100 flies per chamber. Exposing flies to this short period of anoxia does not significantly affect their subsequent metabolic rate (Van Voorhies et al., 2004). The chambers were then flushed with CO2-free, H2O-saturated air at 20 ml min–1 (STPD). CO2 was removed from the inflowing gas stream with an Ascarite gas scrubbing column. Gas flow into the chambers was regulated with a mass flow controller (Sierra Instruments, Monterrey, CA, USA) and a rotameter (Gilmont Instruments, Barrington, IL, USA) directly calibrated against a mass flow controller before each experiment. To prevent the flies from being stressed by low humidity conditions, the gas stream was rehydrated before entering the metabolic chamber by passing it through a series of glass syringes filled with sterile H2O and cotton wool. After flowing through the metabolic chamber, water was removed from the air stream with magnesium perchlorate filters before entering the CO2 and O2 analyzers. The water vapor content of the air stream entering the metabolic chamber was at essentially 100% RH (18.7 mg H2O l–1) and contained 1–2 p.p.m. CO2 as assayed with a Li-Cor 6262 CO2/H2O analyzer (Lincoln, NE, USA).
The small amount of CO2 produced by outgassing from the water-filled syringes was subtracted from the final metabolic reading. Metabolic chambers were maintained at room temperature (23±1°C) as monitored by a Hobo data logger (Bourne, MA, USA). At the end of the collection of metabolic data flies were frozen at –80°C and weighed on a Sartorius M2P microbalance (Sartorius AG, Göettingen, Germany). The weight of flies stored at –80°C is stable for >1 year (Van Voorhies et al., 2004).
Oxygen and carbon dioxide concentrations were analyzed at a 2 s sampling interval beginning 10 min after the flies were sealed in the chambers. Data were recorded using Sable Systems DATACAN data acquisition hardware and software (Sable Systems, Las Vegas, NV, USA). A group of ∼100 flies produced around a 0.02% CO2 enrichment of the air stream under normoxic conditions. To normalize for the slight differences in the initial metabolic rates between the different groups of flies, the pre-hypoxic metabolic rate of each group was given a relative value of 1.00. The metabolic rates of two experimental groups of flies were measured simultaneously using a Li-Cor 6251, and 6262 analyzer operated independently of each other. The gas stream then fed into a dual channel Oxzilla fuel-cell O2 analyzer (Sable Systems) to measure O2 concentration. Data from the CO2 and O2 analyzers were lag corrected to compensate for the time required for the gas sample to flow from the metabolic chambers to the analyzers. The O2 concentration of the inflowing airstream would have been reduced by the metabolic consumption of O2 by the flies. However, even at their maximum metabolic rate a group of flies depleted the inflowing air by ∼0.02 kPa so this would have a minor effect on the O2 level. The CO2 gas analysis system was set to zero daily against CO2-free air, and calibrated regularly with a 51 p.p.m. certified gas standard (Air Products, Long Beach, CA, USA). The O2 analyzer was calibrated daily with well-mixed atmospheric air scrubbed of H2O with a column of magnesium perchlorate.
After allowing the flies to equilibrate in the chambers for ∼1 h, a stream of N2 gas (>99.9% purity) was mixed into a 2 l glass mixing flask leading into the metabolic chamber. Over the course of ∼2 h the O2 level in the air stream leading to the experimental group was reduced from 19 to 2 kPa and after 4 h reached <0.1 kPa. Flies were then exposed to 45 min of anoxia by passing pure N2 directly into the metabolic chambers. At the end of this period atmospheric air was introduced into the mixing flask. Data were collected from six independent groups with two groups measured on three separate occasions (N=6).
Each experiment also included two to four control groups of 15–20 flies that were exposed to normoxic conditions. Because the gas analyzers were in continuous use in monitoring the hypoxic groups, the CO2 production of the control groups could only be assayed immediately after the control flies were placed in the metabolic chambers and after the completion of the hypoxia recovery measurements. Since these flies had only a short period to equilibrate in the chambers before the first metabolic measurement was taken the initial metabolic rate data for this group probably overestimates the actual initial metabolic rate (Van Voorhies et al., 2004).
Recovery from anoxia
After being exposed to approximately 5 h of graded hypoxic conditions flies were reintroduced to normoxic conditions by adding room air into the mixing flask. The flies were then directly exposed to an increase in O2 concentration. This increased the PO2 of the airstream to 2 kPa within 1 min and to 16 kPa within 3 min. The metabolic rate (calculated as the amount of CO2 produced) of the flies was then recorded for an additional 4 h period. These data were used to plot the metabolic recovery of flies exposed to hypoxia.
Respiratory quotient measurements
Respiratory quotients (RQ) were determined for flies while exposed to hypoxia, during recovery from hypoxia, and in hyperoxia, using a Sable Systems PA-1 paramagnetic O2 analyzer and a Li-Cor 6262 gas analyzer. For the RQ experiments flies were sealed in chambers with a defined predetermined O2 concentration. The chambers were filled and resampled from cylinders containing pressurized mixes of air of defined O2 concentrations and scrubbed of CO2. At the end of the sampling interval the chamber was flushed at a known rate with the same gas mixture that was used to fill the chamber. The bolus of CO2 enriched/O2 depleted air was flushed into the CO2 and O2 analyzers located downstream. Gas flow into the chamber was regulated with a mass flow controller located upstream of the chamber. The accuracy of the system was assessed through injection of known volumes of a calibration gas standard with defined amounts of CO2 and N2 and by calculating the RQ generated from the combustion of pure ethanol. Based on these methods the CO2 and O2 analyzers gave readings within 2% of the predicted values.
To determine if metabolic substrate utilization was altered in hypoxia, O2 consumption and CO2 production were measured in flies progressively exposed to O2 tensions of 1.2, 0.8, 0.4, 0.2 kPa. O2 consumption and CO2 production were initially sampled at 1 h intervals. Because the metabolic rate of the hypoxic flies was greatly reduced, the sampling intervals were increased to 1.5 h during extreme hypoxia. For these measurements an average of approximately 75, 5- to 6-day-old post-emergent male and female flies were placed in 25 ml glass metabolic chambers. Five experimental groups were exposed to hypoxia, and three control groups exposed only to normoxic conditions. The amount of CO2 produced and O2 consumed was calculated using DATACAN software.
To determine if exposure to hypoxia induced a large O2 debt which was repaid during hypoxic recovery, RQ values were determined for flies after a 4 h exposure to 0.2 kPa O2. RQs were calculated on data from five groups of flies both during the hypoxic exposure and after the flies were in normoxic conditions for 0.5 and 1.5 h. The RQs of two groups of control flies that remained in normoxic conditions were also calculated.
RQ values of flies exposed to 100% O2 for 24 h were calculated for six groups of flies each with equal numbers of males and females flies. RQs were calculated for these groups after 4, 7, 11 and 26 h.
Survival of flies in 0–19 kPa O2
To determine if survival varied between flies exposed to different hypoxic conditions groups of flies were subjected to O2 levels of 0, 0.1, 0.2, 1.0 and 19 kPa (normal atmospheric O2 concentration at the site of the experiments) for periods ranging from 2–16 h. Five separate groups of ∼20 mixed-sex, flies were used for each time point and O2 tension. The gas stream leading into the fly chambers were hydrated with glass syringes filled with water-saturated cotton wool that were preflushed with 99.999% N2 for 48 h to purge O2. Gas mixtures were made by mixing N2 with room air in a 20 l pressurized gas cylinder and assaying the final O2 contents with a PA-1 O2 analyzer. Gas mixes of different O2 tensions entered the chambers at 20 ml min–1 (STPD).
Control flies had a small amount of Drosophila instant food placed in the chamber with them. The other groups of flies were essentially immobilized during exposures and were incapable of feeding. For this reason no food was placed in these chambers. Previous experiments with this same strain of flies have shown that no mortality occurs in flies starved for 24 h. Chambers were kept at room temperature, which varied between 23 and 25°C as monitored with a Hobo data logger. Survival was assayed after allowing the hypoxia-exposed flies to recover in normoxia for up to 24 h.
Exposure to multiple hypoxic events
To determine if multiple, rapid exposure to hypoxia altered metabolic function, groups of flies were immobilized with a stream of humidified N2 at a flow of 1 l min–1 (STPD) until there was no detectable fly movement. This occurred within 15–20 s of exposure to the stream of N2. The chamber was then flushed with normoxic air at 100% RH until >90% of the flies were actively walking in the vial (typically ∼2 min) and then immediately exposed to another anoxic event. Five groups of 12–15 flies were exposed to five such anoxic events while five groups of flies were exposed to 10 bouts of anoxia and reperfusion.
For each experiment there were three groups of ∼15 control flies that were exposed to a single anoxic exposure equal in duration to the total time the cyclic groups were exposed. The time that the control groups were exposed to pure N2 was 90 s for the groups exposed to five anoxic bouts and 180 s for groups exposed to 10 bouts. Flies exposed to cyclic anoxia were in a flowing gas stream for 11.5 to 23 min. This time included both the interval of the flushing with N2 (1.5 or 3 min) plus the time that the chambers were flushed with normoxic air during the recovery period (10 or 20 min). To control for the potential effect of exposing flies to a moving air stream the flies in the control group were exposed to a normoxic flow of air of 1 l min–1 for a period equal to that of the experimental group. Thus, both the experiment and control flies had equal time exposures to a flow of N2 or a flow of air. At the end of the set of anoxic exposures the anoxic and control groups were sealed in 50 ml glass metabolic chambers with a small amount of food and placed in incubators at 24°C. The flies were allowed to recover in the chamber for 1 h before collecting CO2 and O2 data for the next 22 h at a 1 h sampling interval for the first 6 h and every 2 h thereafter. After 22 h, CO2 production from the food was measured and subtracted from the output.
Exposure to hyperoxia
Hyperoxia measurements were conducted under conditions similar to the hypoxic experiment except that flies were supplied with a small amount of sterile food. To avoid the potential confounding effect of the metabolic activity of eggs and larvae laid by female flies only male flies were used. For these measurements flies were immobilized with N2 and placed in the metabolic chamber. After allowing the flies to equilibrate for 1 h in the chamber the metabolic rate of the flies in normoxia was recorded for 1.5 h to obtain a control metabolic rate. Gaseous 100% O2 was mixed into a 2-l flask and then into 25 ml glass metabolic chambers containing an average of 20, 5-day-old male flies. The same flow rates, sampling intervals and humidification methods were used in the hyperoxia conditions as used in the hypoxia experiments.
The metabolic rate of the fly groups in 100% O2 was recorded for a 24 h period. The flies were then exposed to normoxic conditions and their metabolic rate was recorded for another 2 h. The CO2 output from the food was measured at the end of each experiment and subtracted from the final output to determine the final metabolic reading. The CO2 contribution from the food was always a minor portion (<2%) of the total CO2 output. The CO2 production of the control flies continually maintained under normoxic conditions was measured several times during each experiment. Data were collected from measurements done on five separate occasions. Fly mortality was assayed several times during each experiment.
Effect of CO2 sedation on metabolic function
To determine if the use of CO2 to immobilize flies had a measurable effect on metabolic rate, groups of 20 flies were placed in 12 separate metabolic chambers divided between three groups: control flies, flies to be knocked out with CO2, and flies to be knocked out with N2. A baseline reading was taken after the flies were in the chambers for 20 min and then the chambers of the experimental groups were flushed with 100% humidified CO2 or N2 for 5 min at a flow of 200 ml min–1 and then the chambers were sealed for 25 min. After being exposed to pure N2 or CO2 for ∼30 min the chambers were flushed with normoxic air for 5 min. Three additional metabolic rate measurements were then taken of each group at 30 min intervals. To determine if high CO2 levels would immobilize flies in the presence of normoxic O2 levels a group of mixed-sex flies was exposed to a mixture of 20 kPa O2 and 80 kPa CO2.
Statistics
An ANOVA analysis (Statview 5.1) was used to determine at what O2 level the metabolic rate was significantly reduced relative to normoxic levels. As O2 tensions dropped below 0.7 kPa the rate of reduction in metabolic rate relative to the reduction in O2 tension appeared to increase. To quantify this effect, the slopes were compared between the change in metabolic rate and O2 tension, for O2 tensions between 0.1–0.6 kPa and 0.7–3.1 kPa with a Student's t-test (Statview 5.1). Student's t-tests were also used to analyze for differences in RQ between flies in hypoxic or normoxic conditions and to compare the early metabolic recovery data from flies exposed to repeated hypoxic and reperfusion events. The data were analyzed for differences in survival with ANOVA. To determine if exposure to cyclic anoxia influenced the subsequent metabolic rate, data were analyzed for differences in metabolic rate using repeated-measures ANOVA (Statview 5.1).
RESULTS
Exposure to graded hypoxia
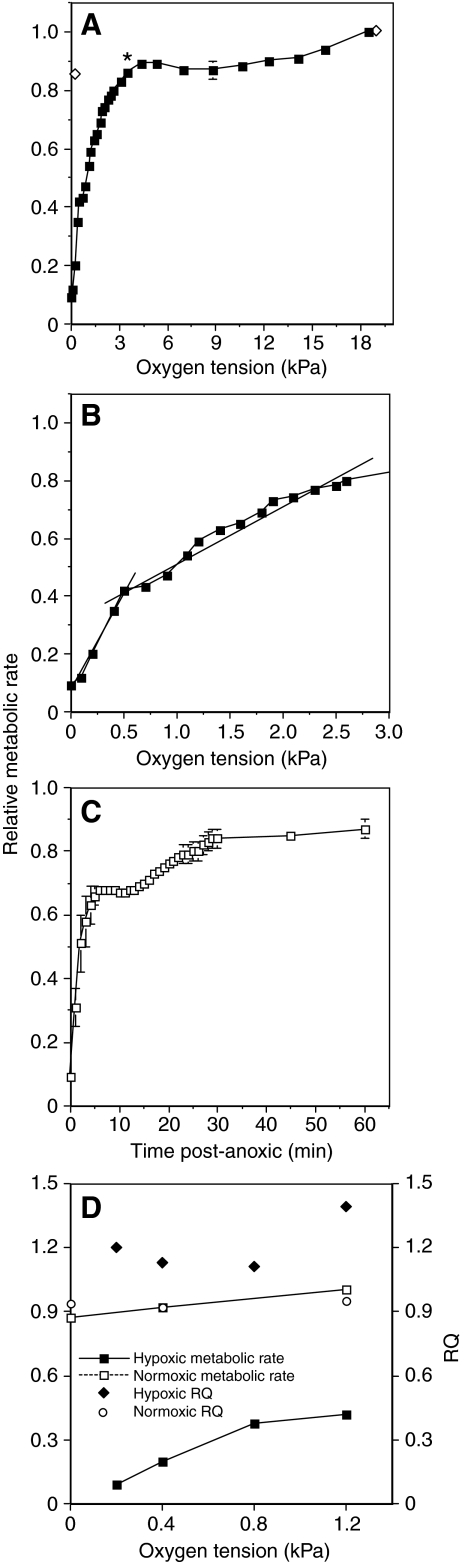
The metabolic rate of flies exposed to graded hypoxia remained relatively
unchanged until an O2 tension of ∼3.1 kPa (P=0.015,
F=2.6, d.f.=9), whereupon it decreased in a approximately linear
manner until the O2 partial pressure reached 0.6 kPa
(Fig. 1A). The rate of
metabolic depression relative to the decrease in O2 tension was
more pronounced as O2 tensions were reduced from 0.6 kPa to anoxia
(Fig. 1B). The average slope of
the line between these O2 tensions increased more than fourfold
relative to the slope of the line between 0.7 and 3.1 kPa, a highly
significant difference
(P 0.001,
t=11.1, d.f.=10). The metabolic rates of the flies in extreme hypoxia
are at least an order of magnitude lower than under normoxic conditions. The
O2 tension at which fly metabolic rate was reduced to 50% of
normoxic levels was 1.0 kPa.
0.001,
t=11.1, d.f.=10). The metabolic rates of the flies in extreme hypoxia
are at least an order of magnitude lower than under normoxic conditions. The
O2 tension at which fly metabolic rate was reduced to 50% of
normoxic levels was 1.0 kPa.
Fig. 1.
Effect of reduced O2 levels on D. melanogaster metabolic rate (measured as CO2 production). The metabolic response to hypoxia data are from metabolic rates determined at 28 different O2 tensions measured in six independent groups of flies. Data are means ± s.e.m. (A) metabolic rates at O2 tensions ranging from normoxia to 0 kPa. The asterisk indicates when metabolic rate is first significantly reduced compared with normoxia, and the open symbols are the metabolic rate of control groups not exposed to hypoxia. (B) The lower range of the same data plotted at higher resolution. The two lines are the average change in metabolic rate for O2 tensions between 0.1 and 0.6 kPa, and 0.7 and 3.1 kPa. The equation describing the relationship between relative metabolic rate and O2 tension in extreme hypoxia is: MR=0.75x+0.04, r2=0.94; and for less extreme hypoxia MR=0.16x+0.4, r2=0.83, with x as the PO2. (C) Recovery of fly metabolic rate after exposure to anoxia. (D) The RQ (respiratory quotient) and relative metabolic rate of flies progressively exposed to O2 tensions of 1.2, 0.8, 0.4, 0.2 kPa. Open symbols are the relative metabolic rate and RQ of flies maintained in normoxic conditions. Data are from five groups of flies exposed to hypoxia and three control groups.
Flies exposed to hypoxia followed a characteristic set of behaviors. They initially rested quietly on the sides or ends of the chamber. At an O2 tension of approximately 3 kPa the flies became more agitated and began actively walking around the chambers. As the O2 tension dropped below around 2 kPa the flies moved to the bottom of the chamber where they stood with greatly reduced movement. Fly movement decreased even more as the O2 tension dropped to around 1.4 kPa but the flies still maintained an upright position. At O2 tensions below ∼0.8 kPa all of the flies were completely immobile and were lying on their sides. All flies survived exposure to several hours of hypoxic conditions and >99% of the flies were alive 15 h after the end of the exposure to hypoxia.
Recovery from anoxia
The metabolic rate of flies exposed to 5 h of graded hypoxia, including 45 min of anoxia, quickly returned to near pre-hypoxic levels after reintroduction of normoxic conditions (Fig. 1C). Within 5 min of being exposed to normoxia the metabolic rate of the flies increased almost 10-fold compared with their lowest hypoxic value. Despite the rapid recovery in metabolic rate, it typically took at least 30 min of normoxic flow before the first flies began to move and close to an hour for all the flies in a group to recover. During this recovery period the metabolic rate of the flies was around 50% higher than flies in 1.4 kPa O2, an O2 tension at which flies are still capable of activity.
Respiratory quotient
The RQ of flies in normoxia was near 0.95, a value consistent with other measurements of Drosophila RQ (Van Voorhies et al., 2004). The RQ of flies exposed to O2 tensions between 1.2 and 0.2 kPa increased to between 1.11 and 1.39 (Fig. 1D). Such increases in RQ are probably caused by a hypoxia-induced increase in anaerobic metabolism. The RQ of flies in 0.2 kPa O2 was significantly higher than flies in normoxia (P=0.003, t=4.15, d.f.=8). Because the RQ values were higher in more extreme hypoxia, the use of CO2 to measure metabolic rate is a conservative estimation of the metabolic depression that occurs in very hypoxic conditions.
The post-hypoxic RQ of flies during the first 30 min of the recovery period averaged 0.91 [±0.01 (s.e.m.) N=5]. The RQ increased slightly, but significantly (P=0.004, t=4.0, d.f.=8), during the 60–90 min recovery period to 0.98 (±0.02, N=5). The RQ of the two control groups was 0.87 and 0.90 for these two time periods. The RQ of flies in 100% O2 for 24 h was constant [0.96±0.01 (mean ± s.e.m.) N=24] over the four sample intervals. The maintenance of this high RQ value over this period indicates that the flies were feeding during the entire time they were exposed to 100% O2. There was no difference in RQ between the male and female flies.
Survival in 0–19 kPa O2
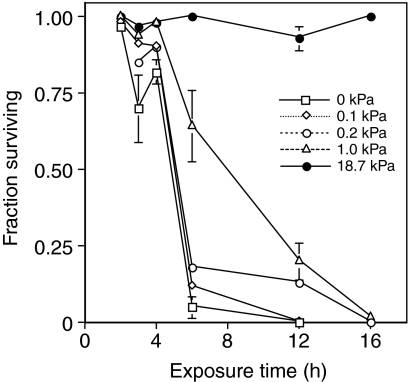
All flies were dead after a 16 h exposure to anoxia (Fig. 2). Flies survived slightly better at 1 kPa but still did far worse than normoxic control flies. There was generally a direct correlation between the O2 tension and survival. Survival of flies remained high (>75%) in hypoxia, up to 4 h exposure, but dropped rapidly with longer exposures. Around 10% of the flies in 1 kPa O2 group showed slight movement (e.g. twitching), whereas all the other groups of flies were immobile within a few minutes of exposure to the lowered O2 tensions. Flies were much slower to recover from hypoxia of longer than 2 h and often were not moving after 1 h in normoxia.
Fig. 2.
The effect of time of exposure to reduced oxygen tensions on D. melanogaster survival. Values are means ± s.e.m., N=5 for each time point.
Multiple exposure to hypoxia
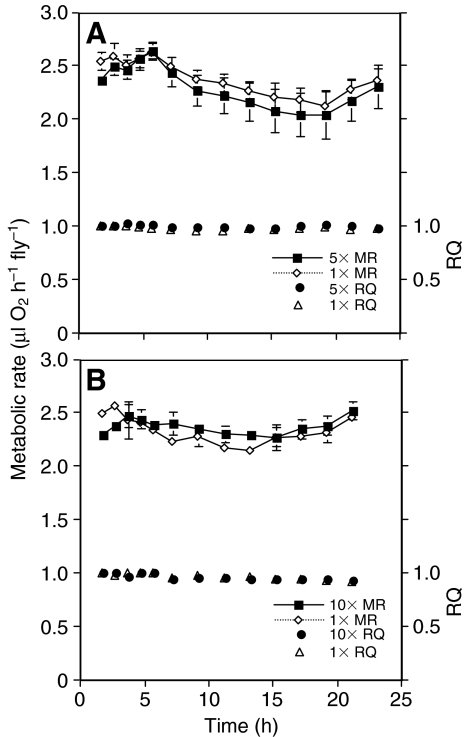
Exposure to multiple hypoxic and anoxic events did not have a significant long-term effect on metabolic rate or respiratory quotients, although it did cause a short-term depression in metabolic rate (Fig. 3A,B). Metabolic rate varied significantly over the sampling interval for groups exposed to five and 10 anoxic bouts (P<0.001 for the 5× anoxic group and 10× hypoxic groups). With the exception of the first sample, there were no significant differences between the metabolic rates of the control groups and the cyclic anoxic groups for each time point (P=0.99 for the 5× anoxic group and P=0.13 for the 10× hypoxic groups). The initial metabolic rate of the group exposed to 10 cyclic anoxic events was reduced significantly compared with the control groups for the first reading (P=0.05, t=2.41, d.f.=6). There were no deaths in either the control or cyclic groups over 24 h, and feeding state of the groups recovering from the cyclic hypoxia treatment did not appear altered as the RQ remained near 1.00.
Fig. 3.
The effect of multiple hypoxic–reperfusion events on the subsequent metabolic rate (MR) and respiratory quotient (RQ) in D. melanogaster. (A) Recovery MR and RQ of groups of flies exposed to either a single period of hypoxia or five periods of hypoxia and normoxia. (B) Recovery MR and RQ of groups of flies exposed to either a single period of hypoxia or 10 periods of hypoxia and normoxia. Values are means ± s.e.m.
Hyperoxia results
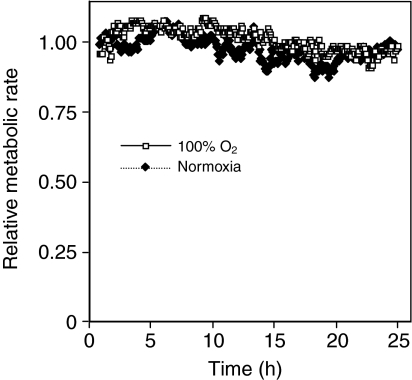
There were essentially no differences in metabolic rate between the normoxic group and groups exposed to 100% O2 (Fig. 4). All flies in both the hyperoxia and control groups were alive at the end of 8 h and after 24 h 97% of the flies in hyperoxia and control groups were alive. Two of the 100% O2 groups had ∼15% mortality after 24 h. To avoid the potentially complicating problem of this high mortality rate these groups were excluded from the analysis.
Fig. 4.
The metabolic response of D. melanogaster to hyperoxia. Data are plotted at a 6 min resolution from five groups of flies exposed to 100% O2 for 24 h and five groups of control flies exposed to normoxia. Values are means ± s.e.m.
Recovery of metabolic rate in CO2- and N2-immobilized flies
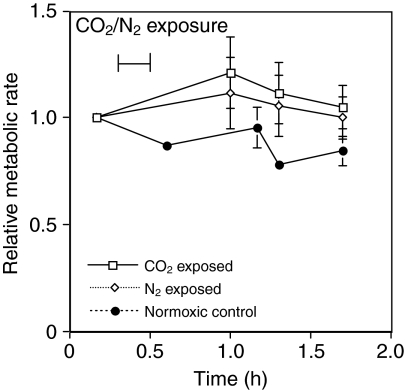
At the end of the sample period the flies in all of the groups were alive. After 24 h, 99% of the control group was alive, and 90% of the N2 and CO2 groups were alive. The metabolic rate of flies exposed to pure N2 remained constant over the entire sample period relative to the starting metabolic rate. Flies exposed to pure CO2 for 30 min did not show a subsequent reduction in metabolic rate over a 2 h period (Fig. 5), and if anything the metabolic rate of this group was elevated, but not significantly, compared with the control group (P=0.06, t=3.1, d.f.=3). Flies exposed to an atmosphere of 20% O2 and 80% CO2 were completely immobilized within 1 min of exposure to the gas mixture.
Fig. 5.
The effect on relative metabolic rate of a 30 min exposure to 100% N2 or CO2. Values are means ± s.e.m. with four groups of flies in each treatment group.
DISCUSSION
Graded hypoxia of 19–0 kPa O2
D. melanogaster maintains a near constant metabolic rate as O2 tensions were reduced from normoxic levels to approximately 3 kPa – an O2 tension equivalent to being at an altitude twice that of the summit of Mount Everest (Denny, 1993). Maintaining normal metabolic function at this low O2 tension is remarkable by mammalian standards. However, several factors, including having low resting metabolic rates relative to maximal metabolic rates and the presence of highly efficient gas exchange systems, pre-adapt insects to low critical O2 tensions (Greenlee and Harrison, 1998).
The critical O2 tensions (Pc) in resting D. melanogaster would be expected to be low since the metabolic rate of flies at rest or walking is a small fraction of flight metabolic rate. Flight increases metabolic rate approximately tenfold in D. melanogaster (Lehmann et al., 2000) and can increase it by >50-fold in other insects (Harrison et al., 2001). As such, the high apparent safety margin for O2 delivery in resting insects may be greatly reduced or nonexistent during flight (Harrison et al., 2001; Harrison et al., 2006; Heymann and Lehmann, 2006). Consistent with this prediction, D. repleta does not fly at O2 tensions below 6 kPa and flight activity is reduced in D. melanogaster placed in an altitudinal equivalent of 7 kPa O2 (Chadwick and Gilmour, 1940; Dillon and Frazier, 2006).
The critical O2 tension of an organism provides a useful estimate of the point at which its metabolic function may be limited by rates of gas exchange, and to predict the hypoxia tolerance of an organism (Herreid, 1980; Holter and Spangenberg, 1997; Loudon, 1989). Pc for insects typically range between 1–5 kPa. Higher Pc values are more common in larger insects and are probably a result of increased diffusional distances (Loudon, 1988). Previous studies have reported Pcs for Drosophila ranging from 1.6 to 2.8 kPa (Greenlee and Harrison, 1998; Loudon, 1988). Many invertebrates respond to hypoxia by reducing metabolic rates, but in mammals a reduction in metabolic rate in response to hypoxia only occurs in smaller species such as rats (Korducki et al., 1994).
Although measures of an organism's Pc are valuable, this value does not have a precise set-point (Frazier et al., 2001). Factors such as experimental and growth temperature, activity level and developmental stage all affect Pc values (Herreid, 1980; Lighton, 2007; Morris and Taylor, 1985). It also is apparent that insects reared at reduced O2 tensions well above levels that reduce metabolic rate can still show physiological alterations compared with those reared in normoxia. Placing D. melanogaster embryos at O2 tensions of 5 kPa increases the expression level of hypoxia inducible factors (Teodoro and O'Farrell, 2003). Although the Pc for adult D. melanogaster is around 3 kPa, flies reared at 10 kPa had reduced growth rates, smaller adult body size, and higher mortality than normoxic counterparts (Frazier et al., 2001). Similarly, beetle larvae (Tebebrio molitor) reared at 11 kPa O2, an O2 tension above its Pc, grew more slowly, had increased mortality and an altered sex ratio than larvae reared at 15 or 21 kPa O2 (Loudon, 1988).
As O2 tensions decreased below 3 kPa the metabolic rate of D. melanogaster showed an approximately linear rate of decrease until ∼0.7 kPa. Below this point there was a sharp decrease in the metabolic rate relative to the reduction in O2 tension. If the only factor limiting respiration in hypoxia was the rate of gas diffusion, the rate of decrease in metabolic rate should remain linear as O2 levels decrease. This suggests that the more rapid decrease in metabolic rate observed in very hypoxic conditions may be due to flies actively reducing metabolic rate in response to hypoxia. Hypoxia causes numerous changes in mitochondrial function that include opening of mitochondrial ion channels, and a reduction in mitochondrial complex IV cytochrome oxidase activity. Factors such as these may be responsible for the increased depression of metabolic rate at very low O2 levels (Lahiri et al., 2005). Also there are proteins in D. melanogaster that appear to reduce metabolism under conditions of environmental stress (Teleman et al., 2005), and it is possible that such proteins could become more active under extreme hypoxia.
It also appears that the hypoxic response in D. melanogaster differs from that caused by the loss of cellular energy reserves caused by metabolic inhibitors such as cyanide (Teodoro and O'Farrell, 2003). This implies that the physiological response to hypoxia is not a simple passive consequence of the collapse of cellular energy reserves due to insufficient levels of O2 to carry out oxidative phosphorylation. Regardless of the reasons, such a downregulation in metabolic rate should be adaptive for surviving hypoxia since suppression of energy turnover provides the greatest protection against hypoxia, and is critical to long-term anoxic survival (Arthur et al., 1997; Brooks and Storey, 1997; Hand, 1998; Hochachka et al., 1996).
Respiratory quotient during exposure to hypoxia
The RQ of flies exposed to O2 tensions of ≤1.2 kPa increased to between 1.11 and 1.39, and is consistent with results from other animals exposed to hypoxia. For example, the RQ of rats (Rattus norvegicus) in hypoxia increased from 0.75 to 1.15 (Frappell et al., 1995) and the RQ of the leafroller moth (Platynota stultana) increased from around 0.75 in normoxia, to 1.3 in 1–2 kPa O2 (Zhou et al., 2001). The most probable cause for this increase in RQ is an increased use of anaerobic metabolism in hypoxic conditions, but other factors, such as changes in acid–base balance, could also be responsible for these increased RQ values (Greenlee and Harrison, 1998). Although the rate of anaerobic metabolism may increase in flies exposed to hypoxia, this presumed increase in the rate of anaerobic metabolism was insufficient for the flies to maintain movement.
Metabolic recovery from hypoxia
In general, D. melanogaster flies tolerated hours of exposure to hypoxic and/or anoxic conditions with no apparent adverse effects. Unlike mammals, insects have the ability to spontaneously recover from extended periods of anoxia (Kolsch et al., 2002). The metabolic rate of hypoxia-exposed flies recovered to near pre-hypoxic levels within ∼1 h of being returned to normoxic conditions, even though exposure to hypoxic conditions reduced metabolic rates by more than an order of magnitude compared with normoxic levels. D. melanogaster can rapidly replenish ATP levels depleted by hypoxia. The ATP levels of D. melanogaster embryos exposed to 30 min of extreme hypoxia (<0.1 kPa O2) declined to 38% of control levels, but recovered to 86% of pre-hypoxic treatment within 5 min of being placed in normoxia (DiGregorio et al., 2001). Other investigators have found that D. melanogaster recovers from anoxia as a non-linear function of exposure time, with >2 h of anoxia causing a disproportionate increase in recovery time (Krishnan et al., 1997).
There was no indication of either a large metabolic overshoot or a major alteration in RQ in flies recovering from hypoxia. The metabolic response of D. melanogaster during hypoxia recovery is consistent with that of many invertebrates that do not show an apparent O2 debt during recovery from hypoxia. The lack of an alteration in the RQ of D. melanogaster is also consistent with the general observation that insects make minimal use of anaerobic metabolism when exposed to hypoxia (Kolsch et al., 2002; Wegener and Moratzky, 1995). The increase in O2 consumption seen in some animals after exposure to hypoxia or anoxic is primarily due to increased energy demands for the removal of anaerobic end products and recharging of the phosphagen and ATP pools (Ellington, 1983).
Hypoxic and anoxic survival
The factors that allow D. melanogaster to survive several hours of exposure to hypoxic and/or anoxic conditions are not clear. Insects possess very efficient respiratory systems and appear to seldom function anaerobically (Wegener, 1996). An argument could be made that since insects are rarely exposed to hypoxic conditions at the cellular level, they would be poorly adapted to survive severe hypoxia. This pattern is seen in organisms such as birds and mammals that rely on aerobic metabolism for the bulk of their metabolic demands. These animals can suffer irreparable damage after more than a few minutes of anoxia (Chapman et al., 2002; Hermes-Lima and Zenteno-Savin, 2002).
A general requirement for surviving hypoxia is having both a low initial metabolic rate and the ability to greatly reduce metabolic rate in hypoxia. Turtles, Chrysemys picta, are an example of this. Anoxic survival is temperature dependent with turtles surviving for 12 h at 20°C, but 90 days at 3°C. This difference in survival is thought to be mediated by a temperature-induced reduction in metabolic rate at lower temperature (Jackson, 2000). In contrast to the low metabolic rate of turtles, the mass-specific metabolic rate of non-flying D. melanogaster is well above the maximal metabolic rate recorded in human elite athletes (Coyle, 2005), leading to the prediction that they would have a limited ability to survive hypoxia.
The response of Drosophila to anoxia is similar to mammals, in that both show a rapid loss of muscle control and are quickly immobilized. The difference is that Drosophila can survive in this state for hours whereas mammals suffer irreversible damage after a few minutes of anoxia (Gu and Haddad, 1999). Among the factors potentially responsible for the survival of Drosophila in hypoxia is the ability of Drosophila neurons (unlike mammals) to become hyperpolarized in hypoxia. This reduces neuron excitability and may save energy (Gu and Haddad, 1999).
Although D. melanogaster is very tolerant of short-term hypoxia, exposure to hypoxic conditions for periods longer than a few hours rapidly increased mortality. There was typically a linear relationship between O2 tension and survival. A similar result has been reported for D. melanogaster embryos, with embryos dying more quickly in 1 kPa than 2 kPa O2 (DiGregorio et al., 2001). Anoxic conditions were the most stressful, and survival of this group was reduced compared with the hypoxic groups for all but the 2 h exposures. This contrasts with survival of hypoxic conditions in the nematode Caenorhabditis elegans in which embryos exposed to anoxia survive longer than embryos exposed to hypoxia (Nystul and Roth, 2004). The ability to withstand hypoxic and/or anoxic conditions also varies with the developmental state. Adult flies exposed to anoxia do not survive >12 h, but embryos can survive at least 36 h of anoxia with nearly 100% survival (DiGregorio et al., 2001; Foe and Alberts, 1985).
The survival of flies in hypoxia decreased rapidly after >4 h exposure, a finding reported by other investigators (Chen et al., 2002). This increase in mortality may be caused by large increases in protein aggregation which increased over 4-fold after 4 h of anoxia (Chen et al., 2002). The survival of D. melanogaster in hypoxia is comparable with that of other insects. Hypoxia and anoxia tolerance in insects varied widely from an LT50 of 1.5 h for the yellow-fever mosquito (Adedes ageypti) to 36 h for a flour beetle (Tribolium confusum) (Knipling et al., 1961). Locusts (Locusta migratoria) survive up to 4 h of anoxia, and hawk moths (Manduca sexta) up to 24 h (Wegener and Moratzky, 1995).
Multiple exposures of flies to hypoxia
With the exception of the first metabolic reading, no significant differences were apparent between the recovery metabolic rate or RQ of flies exposed to multiple bouts of anoxia, and exposure to a single anoxic episode. This result is unexpected because it has been well demonstrated that fluctuating hypoxia can increase production of mitochondrial reactive oxygen species (ROS) by up to 10-fold (Chandel and Schumacker, 2000; Drew et al., 2002; Guzy et al., 2005; Mansfield et al., 2005; Michiels et al., 2002). These ROS bursts produce DNA damage that can lead to mitochondrial apoptosis (Wyllie, 1997). Additionally in mammals, hypoxic-reperfusion events increase levels of neuronal nitric oxide synthase and generation of nitric oxide, which causes mitochondrial dysfunction (Vannucci and Hagberg, 2004). Because ROS formation can only occur in hypoxic, not anoxic conditions, once a cell is anoxic formation of ROS should be minimal, and cycling in and out of anoxia should be far more damaging than a single anoxic–reperfusion event.
Although there was no evidence from this study that anoxic–reperfusion events had a long-term effect on metabolic function, a study by Lighton and Schilman (Lighton and Schilman, 2007) found that O2 reperfusion events did cause a significant reduction in recovery metabolic rates in D. melanogaster. The factor that appears responsible for the difference between these two studies is the amount of time that the flies were exposed to anoxia. In this study the flies were in anoxic conditions for between 1.5 and 3 min whereas in the study by Lighton and Schilman flies were exposed to anoxia for 7.5 to 120 min before reperfusion occurred. The shorter anoxic exposure time used this study was probably below the level that would induce mitochondrial damage and cause a reduction in metabolic rate. It is also worth noting that in this study the group of flies exposed to 10 cycles of anoxic reperfusion did have a significantly reduced metabolic rate compared with the control group for the first recovery metabolic rate.
Effects of 100% O2 on metabolic rate
Flies exposed to an environment of 100% O2 for 24 h showed no apparent alteration in metabolic rate or RQ relative to flies in normoxic conditions. This result is unexpected. Studies in mammalian cells in culture have found that hyperoxia can inactivate many enzymes crucial to normal metabolic function (Gardner et al., 1994; Joenje, 1989). Functional consequences of such changes include protein misfolding, catalytic inactivation, the loss of protein functions and a reduction in the function of glycolysis and the tricarboxylic (TCA) acid cycle (Das et al., 2001; Schoonen et al., 1990; Yan et al., 1997; Yan and Sohal, 1998). Hyperoxia can also increase rates of oxidation of pyridine nucleotides (NAD and NADH), significantly increasing ROS production from mitochondria, as well as damage to mitochondria (Arthur et al., 1997; Gille and Joenje, 1992; Wispe et al., 1992). Many of these deleterious effects occur within a short time of exposure to hyperoxia (Das et al., 2001). Studies in insects have found that hyperoxia can cause a profound depression in metabolic rate. For example, the metabolic rate of army worm pupae (Prodenia eridania) exposed to hyperbaric hyperoxia was reduced 20- to 50-fold relative to normoxic controls (Clark and Cristofalo, 1960). Mammalian cell cultures exposed to hyperoxia also show large (approximately threefold) decreases in metabolic rate compared with normoxic cells (Schoonen et al., 1990).
It has generally been assumed that insects use tracheae and tracheoles to distribute gasses directly to cells. As a result, O2 tensions at the cell surface should closely mirror ambient O2 tensions (Miquel et al., 1975; Mockett et al., 1999). However, one method by which flies could withstand a hyperoxia insult would be to limit spiracle opening to reduce gas flow (Hetz and Bradley, 2005). Several lines of evidence argue that this is not occurring in adult Drosophila exposed to hyperoxia. Many studies have used both direct microscopic examination and biochemical assays to demonstrate damage to many different internal cells types in Drosophila exposed to elevated O2 tensions (Miquel et al., 1975; Philpott et al., 1974; Yan et al., 1997; Yan and Sohal, 1998). The deleterious effects of high O2 levels can be seen after a few hours of exposure, and the mortality rate of Drosophila increases significantly after a 24 h exposure to hyperoxia (Das et al., 2001). Additionally, the high metabolic rate of D. melanogaster, even at rest, predicts that it should continually keep it spiracles at least partially opened to avoid a rapid build-up of CO2 (Heymann and Lehmann, 2006; Lighton and Schilman, 2007). These factors do not support the hypothesis that flies are able to maintain low internal O2 levels even in the face of high external O2 tensions.
The RQ of flies exposed to 100% O2 remained near 0.95 over the 24 h period of the measurements, which is essentially the same as normoxic controls. Although there are examples of hyperoxia inhibiting carbohydrate metabolism (Haugaard, 1968), the maintenance of this high RQ indicates that the flies were actively feeding and using carbohydrates as their main metabolic substrate. It is not clear how D. melanogaster sustains the damage that is expected to be caused by hyperoxia and still maintains a normal metabolic rate. It is possible that reduction in metabolic rate is not seen because the standard metabolic rate of the flies is much less than flight metabolic rate.
Recovery of metabolic rate in CO2 or N2 immobilized flies
Exposing D. melanogaster for 30 min to either pure N2 or CO2 had relatively minor effects on the metabolic rate of the flies during recovery. The limited effect of N2 exposure on the subsequent metabolic rate of flies is consistent with data showing that the metabolic rate of flies exposed to graded hypoxia quickly recovers to pre-exposure levels when placed in normoxic conditions (Van Voorhies et al., 2004) (and this study).
It is more surprising that a 30 min exposure to pure CO2 did not cause a subsequent reduction in metabolic rate. Unlike N2, the anesthetic effect of CO2 is caused by factors other than CO2 simply displacing O2 and producing anoxic conditions (Badre et al., 2005). D. melanogaster exposed to a mixture of 20% O2 and 80% CO2 were quickly immobilized, even though O2 tensions were far higher than required for normal movement. Additionally, exposing Drosophila to elevated CO2 levels has many effects including influencing gene expression levels, reducing longevity and fecundity, and affecting mating behavior (Barron, 2000; Leenders and Beckers, 1972; Perron et al., 1972).
Studies in other insects have shown that even low levels of CO2 can also have large effects on insect respiration. Honeybees (Apis mellifera) exposed to CO2 levels of a few percent showed a significant inhibition of succinic dehydrogenase, a critical enzyme of the Krebs TCA cycle (Seeley, 1960). The metabolic rate of lepidopteran pupae (Platynota stultana) placed in an atmosphere containing >20% CO2 was reduced up to 80% compared with CO2-free air, even when 21% O2 was present (Zhou et al., 2001). In view of the potent effects of CO2 on the physiology of flies it could be reasonably predicted that exposure to pure CO2 would have some effect on the metabolic rate of flies recovering from this exposure. However, at least in the short term, no such effect was detected.
Conclusion
A notable feature of this study is the quantification of the remarkable ability of D. melanogaster to maintain or recovery normal metabolic function in the face of numerous environmental insults such as exposure over many hours to anoxic, hypoxic, pure O2 or CO2 environments and multiple episodes of hypoxia–normoxic reperfusion. Exposing most mammals, including humans, to such conditions would result in death or greatly compromise metabolic function.
Discussions with M. Bernstein, J. Graham and J. Williams and two anonymous reviewers helped greatly with this manuscript. A. Khazaeli and J. Curtsinger provided valuable advice on Drosophila rearing techniques. This research was supported by a grant from the US National Cancer Institute (MSI CCP NCI U56 CA96286) and by the US NIH. Deposited in PMC for release after 12 months.
References
- Arthur, P. G., Franklin, C. E., Cousins, K. L., Thorarensen, H., Hochachka, P. W. and Farrell, A. P. (1997). Energy turnover in the normoxic and anoxic turtle heart. Comp. Biochem. Physiol. 117A, 121-126. [DOI] [PubMed] [Google Scholar]
- Badre, N. H., Martin, M. E. and Cooper, R. L. (2005). The physiological and behavioral effects of carbon dioxide on Drosophila melanogaster larvae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 140, 363-376. [DOI] [PubMed] [Google Scholar]
- Barron, A. B. (2000). Anaesthetising Drosophila for behavioural studies. J. Insect Physiol. 46, 439-442. [DOI] [PubMed] [Google Scholar]
- Baumgartl, H., Kritzler, K., Zimelka, W. and Zinkler, D. (1994). Local PO2 measurements in the environment of submerged soil microarthropods. Acta Oecol. 15, 781-789. [Google Scholar]
- Bickler, P. E., Fahlman, C. S. and Ferriero, D. M. (2004). Hypoxia increases calcium flux through cortical neuron glutamate receptors via protein kinase C. J. Neurochem. 88, 878-884. [DOI] [PubMed] [Google Scholar]
- Bishop, T., Lau, K. W., Epstein, A. C., Kim, S. K., Jiang, M., O'Rourke, D., Pugh, C. W., Gleadle, J. M., Taylor, M. S., Hodgkin, J. et al. (2004). Genetic analysis of pathways regulated by the von Hippel-Lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2, E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier, R. G. and St Pierre, J. (2002). Adaptive plasticity of skeletal muscle energetics in hibernating frogs: mitochondrial proton leak during metabolic depression. J. Exp. Biol. 205, 2287-2296. [DOI] [PubMed] [Google Scholar]
- Brooks, S. P. and Storey, K. B. (1997). Glycolytic controls in estivation and anoxia: a comparison of metabolic arrest in land and marine molluscs. Comp. Biochem. Physiol. 118A, 1103-1114. [DOI] [PubMed] [Google Scholar]
- Centanin, L., Ratcliffe, P. J. and Wappner, P. (2005). Reversion of lethality and growth defects in Fatiga oxygen-sensor mutant flies by loss of hypoxia-inducible factor-alpha/Sima. EMBO Rep. 6, 1070-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick, L. and Gilmour, D. (1940). Respiration during flight in Drosophila repleta Wollaston: the oxygen consumption considered in relation to the wing-rate. Physiol. Zool. 13, 398-410. [Google Scholar]
- Chandel, N. S. and Schumacker, P. T. (2000). Cellular oxygen sensing by mitochondria: old questions, new insight. J. Appl. Physiol. 88, 1880-1889. [DOI] [PubMed] [Google Scholar]
- Chapman, L. J., Chapman, C. A., Nordlie, F. G. and Rosenberger, A. E. (2002). Physiological refugia: swamps, hypoxia tolerance and maintenance of fish diversity in the Lake Victoria region. Comp. Biochem. Physiol. 133A, 421-437. [DOI] [PubMed] [Google Scholar]
- Chen, L., Rio, D. C., Haddad, G. G. and Ma, E. (2004). Regulatory role of dADAR in ROS metabolism in Drosophila CNS. Brain Res. Mol. Brain Res. 131, 93-100. [DOI] [PubMed] [Google Scholar]
- Chen, Q., Ma, E., Behar, K. L., Xu, T. and Haddad, G. G. (2002). Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster. J. Biol. Chem. 277, 3274-3279. [DOI] [PubMed] [Google Scholar]
- Choi, D. W. (2005). Neurodegeneration: cellular defences destroyed. Nature 433, 696-698. [DOI] [PubMed] [Google Scholar]
- Clark, A. M. and Cristofalo, V. J. (1960). Oxygen sensitivity in the insect Prodenia eridania. Am. J. Physiol. 198, 441-444. [DOI] [PubMed] [Google Scholar]
- Clegg, J. S. (1997). Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J. Exp. Biol. 200, 467-475. [DOI] [PubMed] [Google Scholar]
- Coyle, E. F. (2005). Improved muscular efficiency displayed as Tour de France champion matures. J. Appl. Physiol. 98, 2191-2196. [DOI] [PubMed] [Google Scholar]
- Das, N., Levine, R. L., Orr, W. C. and Sohal, R. S. (2001). Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem. J. 360, 209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan, F., Roux, D., Brahimi-Horn, M. C., Pouyssegur, J. and Mazure, N. M. (2006). The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1α. Cancer Res. 66, 3688-3698. [DOI] [PubMed] [Google Scholar]
- Denny, M. (1993). Air And Water: The Biology and Physics of Life's Media. Princeton, NJ: Princeton University Press.
- DiGregorio, P. J., Ubersax, J. A. and O'Farrell, P. H. (2001). Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J. Biol. Chem. 276, 1930-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon, M. E. and Frazier, M. R. (2006). Drosophila melanogaster locomotion in cold thin air. J. Exp. Biol. 209, 364-371. [DOI] [PubMed] [Google Scholar]
- Douglas, R. M., Xue, J., Chen, J. Y., Haddad, C. G., Alper, S. L. and Haddad, G. G. (2003). Chronic intermittent hypoxia decreases the expression of Na/H exchangers and HCO3-dependent transporters in mouse CNS. J. Appl. Physiol. 95, 292-299. [DOI] [PubMed] [Google Scholar]
- Drew, K. L., Toien, O., Rivera, P. M., Smith, M. A., Perry, G. and Rice, M. E. (2002). Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp. Biochem. Physiol. 133C, 483-492. [DOI] [PubMed] [Google Scholar]
- Drew, M. C. (1992). Soil aeration and plant root metabolism. Soil Sci. 154, 259-268. [Google Scholar]
- Ellenby, C. (1953). Oxygen consumption and cell size: a comparison of the rate of oxygen consumption of diploid and triploid prepupae of Drosophila melanogaster Meigen. J. Exp. Biol. 30, 475-491. [Google Scholar]
- Ellington, W. R. (1983). The recovery from anaerobic metabolism in invertebrates. J. Exp. Zool. 228, 431-444. [Google Scholar]
- Epstein, A. C., Gleadle, J. M., McNeill, L. A., Hewitson, K. S., O'Rourke, J., Mole, D. R., Mukherji, M., Metzen, E., Wilson, M. I., Dhanda, A. et al. (2001). C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43-54. [DOI] [PubMed] [Google Scholar]
- Fenchel, T. and Finlay, B. J. (1995). Ecology and Evolution in Anoxic Worlds. Oxford: Oxford University Press.
- Fenn, W. O., Henning, M. and Philpott, M. (1967). Oxygen poisoning in Drosophila. J. Gen. Physiol. 50, 1693-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe, V. E. and Alberts, B. M. (1985). Reversible chromosome condensation induced in Drosophila embryos by anoxia: visualization of interphase nuclear organization. J. Cell Biol. 100, 1623-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappell, P., Westwood, K. and Maskrey, M. (1995). Ventilatory and metabolic responses to hypoxia during moderate hypothermia in anesthetized rats. J. Appl. Physiol. 79, 256-260. [DOI] [PubMed] [Google Scholar]
- Frazier, M. R., Woods, H. A. and Harrison, J. F. (2001). Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol. Biochem. Zool. 74, 641-650. [DOI] [PubMed] [Google Scholar]
- Gardner, P. R., Nguyen, D. D. and White, C. W. (1994). Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc. Natl. Acad. Sci. USA 91, 12248-12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille, J. J. P. and Joenje, H. (1992). Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat. Res. 275, 405-414. [DOI] [PubMed] [Google Scholar]
- Greenlee, K. J. and Harrison, J. F. (1998). Acid–base and respiratory responses to hypoxia in the grasshopper Schistocerca americana. J. Exp. Biol. 201, 2843-2855. [DOI] [PubMed] [Google Scholar]
- Gu, X. Q. and Haddad, G. G. (1999). Drosophila neurons respond differently to hypoxia and cyanide than rat neurons. Brain Res. Mol. Brain Res. 845, 6-13. [DOI] [PubMed] [Google Scholar]
- Guzy, R. D., Hoyos, B., Robin, E., Chen, H., Liu, L., Mansfield, K. D., Simon, M. C., Hammerling, U. and Schumacker, P. T. (2005). Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1, 401-408. [DOI] [PubMed] [Google Scholar]
- Haddad, G. G. (2006). Tolerance to low O2: lessons from invertebrate genetic models. Exp. Physiol. 91, 277-282. [DOI] [PubMed] [Google Scholar]
- Hairston, N. G., Van Brunt, R. A. and Kearns, C. M. (1995). Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76, 1706-1711. [Google Scholar]
- Hand, S. C. (1998). Quiescence in Artemia franciscana embryos: reversible arrest of metabolism and gene expression at low oxygen levels. J. Exp. Biol. 201, 1233-1242. [DOI] [PubMed] [Google Scholar]
- Hand, S. C. and Hardewig, I. (1996). Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu. Rev. Physiol. 58, 539-563. [DOI] [PubMed] [Google Scholar]
- Harrison, J., Frazier, M. R., Henry, J. R., Kaiser, A., Klok, C. J. and Rascon, B. (2006). Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 154, 4-17. [DOI] [PubMed] [Google Scholar]
- Harrison, J. F., Camazine, S., Marden, J. H., Kirkton, S. D., Rozo, A. and Yang, X. (2001). Mite not make it home: tracheal mites reduce the safety margin for oxygen delivery of flying honeybees. J. Exp. Biol. 204, 805-814. [DOI] [PubMed] [Google Scholar]
- Haugaard, N. (1968). Cellular mechanisms of oxygen toxicity. Physiol. Rev. 48, 311-371. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima, M. and Zenteno-Savin, T. (2002). Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. 133C, 537-556. [DOI] [PubMed] [Google Scholar]
- Herreid, C. F. (1980). Hypoxia in invertebrates. Comp. Biochem. Physiol. 67A, 311-320. [Google Scholar]
- Hetz, S. K. and Bradley, T. J. (2005). Insects breathe discontinuously to avoid oxygen toxicity. Nature 433, 516-519. [DOI] [PubMed] [Google Scholar]
- Heymann, N. and Lehmann, F. O. (2006). The significance of spiracle conductance and spatial arrangement for flight muscle function and aerodynamic performance in flying Drosophila. J. Exp. Biol. 209, 1662-1677. [DOI] [PubMed] [Google Scholar]
- Hoback, W. W. and Stanley, D. W. (2001). Insects in hypoxia. J. Insect Physiol. 47, 533-542. [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W. (1980). Living Without Oxygen. Cambridge, MA: Harvard University Press.
- Hochachka, P. W. (1998). Mechanism and evolution of hypoxia tolerance in humans. J. Exp. Biol. 201, 1243-1254. [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W., Buck, L. T., Doll, C. J. and Land, S. C. (1996). Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanism for surviving oxygen lack. Proc. Natl. Acad. Sci. USA 93, 9493-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka, P. W., Rupert, J. L., Goldenberg, L., Gleave, M. and Kozlowski, P. (2002). Going malignant: the hypoxia-cancer connection in the prostate. BioEssays 24, 749-757. [DOI] [PubMed] [Google Scholar]
- Holter, P. and Spangenberg, A. (1997). Oxygen uptake in coprophilous beetles (Aphodius, Geotrupes, Sphaeridium) at low oxygen and high carbon dioxide concentrations. Physiol. Entomol. 22, 339-343. [Google Scholar]
- Jackson, D. C. (2000). Living without oxygen: lessons from the freshwater turtle. Comp. Biochem. Physiol. 125A, 299-315. [DOI] [PubMed] [Google Scholar]
- Jamieson, D. (1989). Oxygen toxicity and reactive oxygen metabolites in mammals. Free Radic. Biol. Med. 7, 87-108. [DOI] [PubMed] [Google Scholar]
- Jarecki, J., Johnson, E. and Krasnow, M. A. (1999). Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99, 211-220. [DOI] [PubMed] [Google Scholar]
- Jensen, P. (1995). Life history of the nematode Theristus anoxybioticus from sublittoral muddy sediment at methane seepages in the northern Kattegat, Denmark. Mar. Biol. 123, 131-136. [PMC free article] [PubMed] [Google Scholar]
- Joenje, H. (1989). Genetic toxicology of oxygen. Mutat. Res. 219, 193-208. [DOI] [PubMed] [Google Scholar]
- Johansson, D., Nilsson, G., Ouml. and Rnblom, E. (1995). Effects of anoxia on energy metabolism in crucian carp brain slices studied with microcalorimetry. J. Exp. Biol. 198, 853-859. [DOI] [PubMed] [Google Scholar]
- Kaelin, W. G., Jr (2005). ROS: really involved in oxygen sensing. Cell Metab. 1, 357-358. [DOI] [PubMed] [Google Scholar]
- Knipling, G. D., Sullivan, W. N. and Fulton, R. A. (1961). The survival of several species of insects in a nitrogen atmosphere. J. Econ. Entomol. 54, 1054-1055. [Google Scholar]
- Kolsch, G., Jakobi, K., Wegener, G. and Braune, H. J. (2002). Energy metabolism and metabolic rate of the alder leaf beetle Agelastica alni (L.) (Coleoptera, Chrysomelidae) under aerobic and anaerobic conditions: a microcalorimetric study. J. Insect. Physiol. 48, 143-151. [DOI] [PubMed] [Google Scholar]
- Korducki, M. J., Forster, H. V., Lowry, T. F. and Forster, M. M. (1994). Effect of hypoxia on metabolic rate in awake ponies. J. Appl. Physiol. 76, 2380-2385. [DOI] [PubMed] [Google Scholar]
- Krishnan, S. N., Haddad, G. G., Wyman, R. J., Mohsenin, A. and Sun, Y. (1997). Behavioral and electrophysiologic responses of Drosophila melanogaster to prolonged periods of anoxia. J. Insect Physiol. 43, 203-210. [DOI] [PubMed] [Google Scholar]
- Lahiri, S., Roy, A., Baby, S. M., Hoshi, T., Semenza, G. L. and Prabhakar, N. R. (2005). Oxygen sensing in the body. Prog. Biophys. Mol. Biol. 91, 249-286. [DOI] [PubMed] [Google Scholar]
- Leenders, H. J. and Beckers, P. J. (1972). The effect of changes in the respiratory metabolism upon genome activity. A correlation between induced gene activity and an increase in activity of a respiratory enzyme. J. Cell Biol. 55, 257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, F., Dickinson, M. H. and Staunton, J. (2000). The scaling of carbon dioxide release and respiratory water loss in flying fruit flies (Drosophila spp.). J. Exp. Biol. 203, 1613-1624. [DOI] [PubMed] [Google Scholar]
- Lighton, J. R. and Schilman, P. E. (2007). Oxygen reperfusion damage in an insect. PLoS ONE 2, e1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighton, J. R. B. (2007). Hot hypoxic flies: whole-organism interactions between hypoxic and thermal stressors in Drosophila melanogaster. J. Therm. Biol. 32, 134-143. [Google Scholar]
- Loudon, C. (1988). Development of Tenebrio molitor in low oxygen levels. J. Insect Physiol. 34, 97-103. [Google Scholar]
- Loudon, C. (1989). Tracheal hypertrophy in mealworms: design and plasticity in oxygen supply systems. J. Exp. Biol. 147, 217-235. [Google Scholar]
- Lutz, P. L. and Nilsson, G. E. (1997). Contrasting strategies for anoxic brain survival - glycolysis up or down. J. Exp. Biol. 200, 411-419. [DOI] [PubMed] [Google Scholar]
- Mansfield, K. D., Guzy, R. D., Pan, Y., Young, R. M., Cash, T. P., Schumacker, P. T. and Simon, M. C. (2005). Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 1, 393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, N. H., Lutz, R., Burnett, W. and Cable, P. (1994). Age, viability, and vertical distribution of zooplankton resting eggs from an anoxic basin: evidence of an egg bank. Limnol. Oceanogr. 39, 154-158. [Google Scholar]
- Massabuau, J. C. (2003). Primitive, and protective, our cellular oxygenation status? Mech. Ageing Dev. 124, 857-863. [DOI] [PubMed] [Google Scholar]
- Michiels, C., Minet, E., Mottet, D. and Raes, M. (2002). Regulation of gene expression by oxygen: NF-κB and HIF-1, two extremes. Free Radic. Biol. Med. 33, 1231-1242. [DOI] [PubMed] [Google Scholar]
- Miquel, J., Lundgren, P. R. and Bensch, K. G. (1975). Effects of oxygen–nitrogen (1:1) at 760 Torr on the life span and fine structure of Drosophila melanogaster. Mech. Ageing Dev. 4, 41-57. [DOI] [PubMed] [Google Scholar]
- Mockett, R. J., Orr, W. C., Rahmander, J., Benes, J. J., Radyuk, S. N., Klochko, V. I. and Sohal, R. (1999). Overexpression of Mn-containing superoxidase in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 371, 260-269. [DOI] [PubMed] [Google Scholar]
- Mockett, R. J., Orr, W. C., Rahmandar, J. J., Sohal, B. H. and Sohal, R. S. (2001). Antioxidant status and stress resistance in long- and short-lived lines of Drosophila melanogaster. Exp. Gerontol. 36, 441-463. [DOI] [PubMed] [Google Scholar]
- Morris, S. and Taylor, A. C. (1985). Oxygen consumption by Palaemon elegans (Rathke) in response to temperature change: a determinant of distribution. Exp. Biol. 44, 255-268. [PubMed] [Google Scholar]
- Murphy, E. and Steenbergen, C. (2008). Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 88, 581-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul, T. G. and Roth, M. B. (2004). Carbon monoxide-induced suspended animation protects against hypoxic damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 101, 9133-9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget, T. A., Fry, M. and Lloyd, D. (1987). Effects of inhibitors on the oxygen kinetics of Nippostrongylus brasiliensis. Mol. Biochem. Parasitology 22, 125-134. [DOI] [PubMed] [Google Scholar]
- Perron, J. M., Huot, L., Corrivault, C. W. and Chawla, S. S. (1972). Effects of carbon dioxide anaesthesia on Drosophila melanogaster. J. Insect Physiol. 18, 1869-1874. [DOI] [PubMed] [Google Scholar]
- Philpott, D. E., Bensch, K. G. and Miquel, J. (1974). Life span and fine structural changes in oxygen-poisoned Drosophila melanogaster. Aerosp. Med. 45, 283-289. [PubMed] [Google Scholar]
- Pouyssegur, J., Dayan, F. and Mazure, N. M. (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437-443. [DOI] [PubMed] [Google Scholar]
- Saraste, M. (1999). Oxidative phosphorylation at the fin de siecle. Science 283, 1488-1493. [DOI] [PubMed] [Google Scholar]
- Schoonen, W. G. E. J., Wanamarta, A. H., vander Klei-vanMoorsel, J. M., Jakobs, C. and Joenje, H. (1990). Hyperoxia-induced clonogenic killing of HeLa cells associated with respiratory failure and selective inactivation of Krebs cycle enzymes. Mutat. Res. 237, 173-181. [DOI] [PubMed] [Google Scholar]
- Seeley, T. (1960). Atmospheric carbon dioxide regulation in honey-bee (Apis mellifera) colonies. J. Insect. Physiol. 20, 2301-2305. [DOI] [PubMed] [Google Scholar]
- Semenza, G. L. (2004). Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 19, 176-182. [DOI] [PubMed] [Google Scholar]
- Smith, S. L. and Gottlieb, S. F. (1975). Effects of increased partial pressures of oxygen on the embryonic and post-embryonic development of Drosophila melanogaster. Aviat. Space Environ. Med. 46, 161-169. [PubMed] [Google Scholar]
- Storey, K. B. and Storey, J. M. (1990). Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q. Rev. Biol. 65, 145-174. [DOI] [PubMed] [Google Scholar]
- Taylor, A. C. and Moore, P. G. (1995). The burrows and physiological adaptations to a burrowing lifestyle of Natatolana borealis (Isopoda: Cirolanidae). Mar. Biol. 123, 805-814. [Google Scholar]
- Teleman, A. A., Chen, Y. W. and Cohen, S. M. (2005). 4E-BP functions as a metabolic brake used under stress conditions but not during normal growth. Genes Dev. 19, 1844-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro, R. O. and O'Farrell, P. H. (2003). Nitric oxide-induced suspended animation promotes survival during hypoxia. EMBO J. 22, 580-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, R. J., Zhou, N. and MacVicar, B. A. (2006). Ischemia opens neuronal gap junction hemichannels. Science 312, 924-927. [DOI] [PubMed] [Google Scholar]
- Van Voorhies, W. A., Khazaeli, A. A. and Curtsinger, J. W. (2004). Testing the “Rate of Living” model: further evidence that longevity and metabolic rate are not inversely correlated in Drosophila melanogaster. J. Appl. Physiol. 97, 1915-1922. [DOI] [PubMed] [Google Scholar]
- Vannucci, S. J. and Hagberg, H. (2004). Hypoxia-ischemia in the immature brain. J. Exp. Biol. 207, 3149-3154. [DOI] [PubMed] [Google Scholar]
- Wegener, G. (1996). Flying insects: model systems in exercise physiology. Experientia 52, 404-412. [DOI] [PubMed] [Google Scholar]
- Wegener, G. and Moratzky, T. (1995). Hypoxia and anoxia in insects: microcalorimetric studies on two species (Locusta migratoria and Manduca sexta) showing different degrees of anoxia tolerance. Thermochimica Acta 251, 209-218. [Google Scholar]
- Wilson, M., Widdicombe, J. H., Gohil, K., Burtis, K. C., Reznick, A. Z., Cross, C. E. and Eiserich, J. P. (2005). Are Drosophila a useful model for understanding the toxicity of inhaled oxidative pollutants: a review. Inhal. Toxicol. 17, 765-774. [DOI] [PubMed] [Google Scholar]
- Wispe, J. R., Warner, B. B., Clark, J. C., Dey, C. R., Neuman, J., Glasser, S. W., Crapo, J. D., Chang, L. Y. and Whitsett, J. A. (1992). Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J. Biol. Chem. 267, 23937-23941. [PubMed] [Google Scholar]
- Wyllie, A. (1997). Clues in the p53 murder mystery. Nature 389, 237-238. [DOI] [PubMed] [Google Scholar]
- Yan, L., Levine, R. L. and Sohal, R. S. (1997). Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. USA 94, 11168-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. J. and Sohal, R. S. (1998). Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. USA 95, 12896-12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, Z., Maecker, H. L., Johnson, R. S. and Giaccia, A. J. (2002). Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2, 331-341. [DOI] [PubMed] [Google Scholar]
- Zhou, S., Criddle, R. S. and Mitcham, E. J. (2001). Metabolic response of Platynota stultana pupae during and after extended exposure to elevated CO2 and reduced O2 atmospheres. J. Insect Physiol. 47, 401-409. [DOI] [PubMed] [Google Scholar]