Abstract
OBJECTIVE
The objective of this article was to systematically review all published studies that investigated the association between bone density and fractures in children.
DESIGN
Potentially relevant articles were identified by searching electronic databases. Duplicates were removed, abstracts were inspected, and relevant articles were obtained. Studies were included in the systematic review if participants were <16.0 years old, were healthy, had extractable data on bone mass, and had fractures as the outcome.
RESULTS
Ten case-control studies were identified. No prospective studies were found. There was no evidence of heterogeneity between studies or of funnel-plot asymmetry. Eight of the studies were included in the meta-analysis, because they presented results as means and standard deviations of bone density in cases and controls. The pooled standardized mean difference for bone mass in children with and without fractures, from a fixed-effects model, was −0.32 (95% confidence interval: −0.43 to −0.21).
CONCLUSIONS
Evidence for an association between bone density and fractures in children is limited. The results from this meta-analysis suggest that there is an association between low bone density and fractures in children. Although there was no evidence of heterogeneity or publication bias, this meta-analysis is based on case-control studies that are prone to bias. Large, well-conducted prospective cohort studies are required to confirm the association between bone density and fractures in children.
Keywords: bone density, children, fractures, meta-analytic methods, systematic reviews
Fractures in children are common; the reported incidence of fractures in the United Kingdom in children ranges from 1.6% per year1 to 3.6% per year.2 There is also evidence that the incidence of fractures in childhood is increasing over time.3 It is well recognized that bone mass in adults influences fracture risk, but the evidence for an association between bone mass and fractures in children is limited. Indirect evidence that bone mass may influence fracture risk in children can be found in several randomized, double-blind, intervention trials that examined the effects of calcium intake in children and adolescents.4-6 These studies demonstrated improvements in bone mass, and further study found that children who avoid drinking cow’s milk are at an increased risk for prepubertal bone fractures.7
Bone densitometry is commonly used to measure bone mass in adults, particularly in postmenopausal women. The most commonly used technique is dualenergy x-ray absorptiometry (DXA), and it is being used increasingly in children.8 DXA machines produce values for bone mineral content (BMC) and bone area (BA) and then calculate “areal” bone mineral density (BMD) by dividing BMC by BA. This is not a true density but a 2-dimensional measurement that can be affected by the subject’s size. Although the problem of size in bone densitometry is well appreciated, there is no consensus on the most appropriate way to correct results for size.9 Other techniques for measuring bone density in children include peripheral quantitative computed tomography (QCT), quantitative ultrasound (QUS), and metacarpal morphometry.
The incidence of fractures increases with age, and fractures in later life are associated with osteoporosis (lower bone mass).10 Fractures in children are generally thought to reflect the fact that falls and other injuries are common in childhood,11 but there is emerging evidence that fractures in childhood are related to underlying skeletal fragility. The purpose of this systematic review is to quantify this relationship. There have been no previous systematic reviews of this association
METHODS
All observational epidemiologic studies that examined the relationship between bone mass and fractures in children were included. “Children” were defined as those who were ≤16.0 years of age. Children were excluded if they had a chronic illness that is likely to affect bone mass. All studies were required to have extractable data on bone mass measured by any method. The primary outcome measure was all fractures.
A systematic strategy was used to search electronic databases of published articles using both Medical Subject Headings and text-words. The databases searched were Medline (1966-2005), Embase (1988-2005), Web of Science (1965-2005), the Cochrane Musculoskeletal Injuries Group, the Cochrane Controlled Trials Register, and Sigle for “gray” literature. Articles about bone mass were obtained by using the words “bone density,” “bone mineral density,” “bone mineral content,” “bone mass,” “bone mineral apparent density,” or “calcification” and their abbreviations. Articles about fractures were obtained by using the words “fracture” or “fractures.” Articles on children were obtained by either using Medical Subject Headings of “infant,” “child,” or “adolescent” or limiting the search. Reference lists of articles obtained were also searched.
We assessed the methodologic quality of the studies. If the article did not contain sufficient information on the methodology, the authors were contacted. The key components of study quality that were assessed were comparability of fracture and control group at entry; selection of control group; definition of inclusion and exclusion criteria; clearly defined outcome measure; the measure and control for potential confounders in either the recruitment or analysis stage; and use of multiple comparisons or subgroup analyses.
The methods and results of all studies that reported the association between bone density and fracture risk in children were tabulated. Data from the studies that reported means and SDs were combined. A test of heterogeneity was performed and a funnel plot was drawn to look for publication bias and heterogeneity.12 Analysis was performed by using Stata 8.0 (Stata Corp, College Station, TX) using the “metan” and “funnel” commands. The standardized mean difference (SMD) was calculated by the difference in means divided by the pooled SD of participants’ outcomes across the whole trial.13
RESULTS
Using our search strategy, 257 articles were identified; 234 were rejected after reading the title and abstract, because they included children older than 16 years, included children with chronic illnesses, were not relevant, were case reports, had no measure of association, were letters without original research, were duplicate references, or the children had no fractures. Twenty-three full articles were retrieved. Of these, 13 were rejected (Table 1). Ten case-control studies were found and included in this systematic review.14-23 No population-based cohort studies were identified. The methods of the 10 case-control studies that investigated the association between bone density and fracture risk in children are shown in Table 2. The number in each study ranged from 16 fractures23 to 321 fractures.21 Three studies recruited only females,17,18,20 but the rest recruited both genders. DXA alone was used to assess bone mass in 7 studies; 1 study used QCT18; 1 study used QUS22; and 1 study used all 3 methods.20 Six studies showed an association between low bone mass and fractures in children,14,15,17,20-22 and 4 studies showed no association.16,18,19,23 One study was rejected because of the inclusion of children older than 16 years,24 and this showed an association between low bone mass and fractures.
TABLE 1. Rejected Articles for This Systematic Review.
| Article | Reason for Rejection |
|---|---|
| Elsasser U, Wilkins B, Hesp R, Thurnham DI, Reeve J, Ansell BM. Bone rarefaction and crush fractures in JCA. Arch Dis Child. 1982;57:377-380 | Unknown age of children; unable to contact authors |
| Koo WWK, Sherman R, Succop P, et al. Sequential BMC in small preterm infants with and without fractures and rickets. J Bone Miner Res. 1988;3:193-197 | Unable to obtain data for children with and without fractures |
| Hagino H, Yamamoto K, Teshima R, Kishimoto H, Nakamura T. Fracture incidence and bone mineral density of the distal radius in Japanese children. Arch Orthop Trauma Surg. 1990;109:262-264 | Included children >16 y of age |
| Blimkie CJ, Lefevre J, Beunen GP, Renson R, Dequeker J, Van Damme P. Fractures, physical activity and growth velocity in adolescent Belgium boys. Med Sci Sports Exerc. 1992;25:801-808 | No bone density measurement |
| Greenfield D. Risk Factors for Fracture [PhD thesis]. Sheffield, United Kingdom: Sheffield University; 1998 | Adults |
| Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4 year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011-2018 | Identical study children as in ref 17 |
| Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509-515 | Included children >16 y of age |
| Jones IE, Williams SM, Dow N, Goulding A. How many children remain fracture-free during growth? A longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study. Osteoporos Int. 2002;13:990-995 | No bone density measurement |
| Jones IE, Taylor RW, Williams SM, Manning PJ, Goulding A. Four year gain in bone mass in girls with and without past forearm fractures. J Bone Miner Res. 2002;17:1065-1072 | Identical study children as in ref 17 |
| Davidson P, Goulding A, Chalmers D. Biomechanical analysis of arm fracture in obese boys. J Paediatr Child Health. 2003;39:657-664 | Included children >16 y of age |
| Goulding A, Jones IE, Taylor RW, Piggot JM, Taylor D. Dynamic and static tests of balance and postural sway in boys: effects of previous wrist bone fractures and high adiposity. Gait Posture. 2003;17:136-141 | Included children >16 y of age |
| Ma DQ, Jones G. TV, computer and video viewing; physical activity and upper limb fracture risk in children. J Bone Miner Res. 2003;19:1970-1977 | Values of bone mass in fractures and controls not presented |
| Ma DQ, Jones G. Soft drink, milk consumption, physical activity, bone mass and upper limb fractures in children. Calcif Tissue Int. 2004;75:286-291 | Identical study children as in ref 21 |
TABLE 2. Case-Control Studies That Reported Measures of Association Between Fractures and Bone Density in Children.
| Study | Geographical Area Covereda; Latitude | Age, y; Gender | Cases (No. of Children With Fractures) | Controls | Exclusions for Cases and Controls | Bone Density Measure | Time Between Fracture and Bone Density Measurement |
|---|---|---|---|---|---|---|---|
| Landin and Nilsson14 (1982) |
Malmo, Sweden; 59°N | 4-16; M and F |
90 | 131 controls from same population as cases | Hand, finger, skull, tooth, and rib fractures, metabolic bone disease, malnutrition, growth impairment | DXA of radius | 40 d (±25) |
| Chan et al15(1984) | Salt Lake City, UT; 40°N | 2-12; M and F |
17 | 17, unknown from where they were drawn | Existing chronic illness, malnutrition, underlying bone abnormalities | DXA nondominant or nonfractured radius | 16 mo |
| Cook et al16(1987) | Louisiana; 30°N | 3-14; M and F |
17 | 17, unknown from where they were drawn | Metabolic bone disease, malnutrition, growth impairment, fractures of fingers, skull, teeth, or ribs | DXA lumbar spine, left femoral neck | Within 4 wk |
| Goulding et al17 (1998) | Dunedin, New Zealand; 46°S | 3-15; F |
100 | 100 controls (friends of cases with same age) | Nonwhite | DXA lumbar spine, left femur, total body, radius | Within 6 wk |
| Skaggs and Loro18 (2001) | Los Angeles, CA; 34°N | 6 to 15; F |
50 | 50 matched community controls | Medium/high-energy trauma fractures, chronic illness, ill for >2 wk in last 6 mo, previous hospitalization, medications, vitamins, calcium supplements | Computed tomography radius |
Within 1 mo |
| Ma and Jones19 (2002) | Southern Tasmania; 42°S | 8; M and F |
32 | 292 controls | Not at risk of sudden infant death syndrome | DXA of total body, lumbar spine, and femur | Unknown |
| Suuriniemi et al20 (2003) | Jyvaskyla, Finland; 60°N | 11; F |
37 | 212 controls from same population as cases | History of serious medical conditions, medications known to affect bone, fracture <1 y ago, serious trauma | DXA of total body, femur, lumbar spine, pQCT of left distal radius, and broadband attenuation left calcaneus by QUS | >1 y |
| Ma and Jones21 (2003) | Southern Tasmania; 42°S | 9-16; M and F |
321 | 321 from same school class as a case | Diseases that may prevent them from completing the study, moved out of area, not enrolled in school, previous upper limb fractures since age of 9 | DXA total body, lumbar spine, and right femoral neck | Average: 6 wk; max: 3 mo |
| Schalamon et al22 (2004) | Graz, Austria; 48°N | 9-12; M and F |
50 | 154 recruited from a school within same period | High-impact trauma, illnesses except allergies | Speed of sound of proximal phalanges of dominant hand measured by QUS | Within 12 h |
| Goulding et al23 (2004) | Dunedin, New Zealand; 46°S | 0-13; M and F |
16 | 34 controls | No history of cow’s milk avoidance | DXA total body, lumbar spine, and forearm | Unknown |
A summary of study quality is shown in Table 3. The method of control selection was not clearly described in 2 studies.15,16 Neighborhood controls were used in 5 studies.14,17,18,21,22 The cases and controls in refs 19, 20, and 23 were drawn from previously recruited cohorts. The comparability of cases and controls at baseline was not described for 2 studies14,22 but was clearly stated for all the other studies. Two studies15,17 showed a difference in weight between children with fractures and controls at baseline. In all the studies, inclusion and exclusion criteria were stated clearly and applied to both cases and controls.
TABLE 3. Summary of Study Quality of Articles Included in This Systematic Review.
| Study | Control Selection | Comparability of Cases and Controls at Baseline | Verification of Fractures | Control for the Potential Confounding Effects of Body Size | Temporality Ensured (ie, Bone Density Measured Before Fracture Occurred) | Multiple Comparisons and Subgroup Analyses Carried out |
|---|---|---|---|---|---|---|
| Landin and Nilsson14 (1982) |
Neighborhood controls | Unknown | Unknown | Attempted | No | No |
| Chan et al15(1984) | Unknown | Difference in weight between cases and controls | Radiograph review | No difference found in height | No | No |
| Cook et al16(1987) | Unknown | Good | Unknown | No difference found in height or weight | No | No |
| Goulding et al17 (1998) | Neighborhood controls | Difference in weight between cases and controls | Radiograph review | No difference found in height | No | Yes |
| Skaggs and Loro18 (2001) | Neighborhood controls | Good | Unknown | Attempted | No | No |
| Ma and Jones19 (2002) | From previously recruited cohorts | Good | Radiograph review | Attempted | No | No |
| Suuriniemi et al20 (2003) | From previously recruited cohorts | Good | Radiograph review | Attempted | No | Yes |
| Ma and Jones21 (2003) | Neighborhood controls | Good | Radiograph review | Attempted | No | Yes |
| Schalamon et al22 (2004) | Neighborhood controls | Unknown | Unknown | No difference found in height or weight | No | No |
| Goulding et al23 (2004) | From previously recruited cohorts |
Good | Radiograph review | Attempted | No | No |
It was unclear how fractures were verified in the studies by Skaggs and Loro18 and Schalamon et al.22 In 5 studies,17,19-21,23 fractures were verified by chart or radiograph review where possible, but it is unknown how many were verified in each study. The other studies14,16 may have confirmed all fractures, but it is not made clear in the article.
The studies by Schalamon et al22 and Goulding et al23 did not control for potential confounders during either recruitment or analysis, but Goulding et al23 showed no difference between fractures and controls in terms of age and body size at baseline. All other studies controlled for the potential confounding effects of age; 4 studies controlled for the potential confounding effects of body size (either weight14,21 or both height and weight18,20). Puberty was assessed by Tanner stage in 4 studies: 1 study20 limited entry to participants who were prepubertal or in early puberty (Tanner stage I or II); 2 studies21,23 noted that there was no difference in Tanner stage between children with fractures and those without; and 1 study18 matched cases and controls for Tanner stage. Two studies17,19 presented data on children who were assumed to be prepubertal because of their ages, but no formal testing of pubertal stage had been undertaken.
In all studies, the measure of bone density was taken after the fracture with the time delay ranging from 12 hours22 to >1 year.20 Multiple comparisons and subgroup analyses were conducted in 3 studies.17,20,21
Eight of the studies presented results as means and SDs of bone density in cases and controls.15-22 Landin and Nilsson14 presented bone density of cases as percentage difference (cases minus controls). The study by Goulding et al23 presented results as the percentage of children with volumetric bone density below 1 SD of the study population. Using these 8 studies,15-22 a funnel plot was drawn to assess publication bias and heterogeneity and showed no evidence of asymmetry. Formal testing of heterogeneity was conducted by using the χ2 test, which showed no evidence of heterogeneity (χ2 = 13.03, with 9 degrees of freedom; P = .161). These 8 studies were combined by using a fixed-effects meta-analysis. Because many of the studies presented multiple comparisons, estimates were chosen that included a measure of body size, which used BMC and a peripheral measure of bone mass (forearm or femur) where possible. One study17 presented data for 3 age groups of children, and results for these groups were included separately in the analysis.
The combined SMD in mean bone mass between children with fractures and controls was −0.32 (95% confidence interval [CI]: −0.43 to −0.21; P < .001). A forest plot is shown in Fig 1. The fixed-effects meta-analysis was repeated after excluding the largest study,21 and the results still showed an overall lower bone mass in children with fractures compared with controls (SMD: −0.26; 95% CI: −0.40 to −0.11; P < .001). Additional analysis was performed on the 3 studies that presented results for children with wrist and forearm fractures.17,18,21 This subgroup analysis showed a similar association to that observed in the main analysis with an SMD of −0.25 (95% CI: −0.40 to −0.10). When latitude of the study centers was assessed, the studies that were based further away from the equator were more likely to show an association between low bone mass and fractures in children.
FIGURE 1.
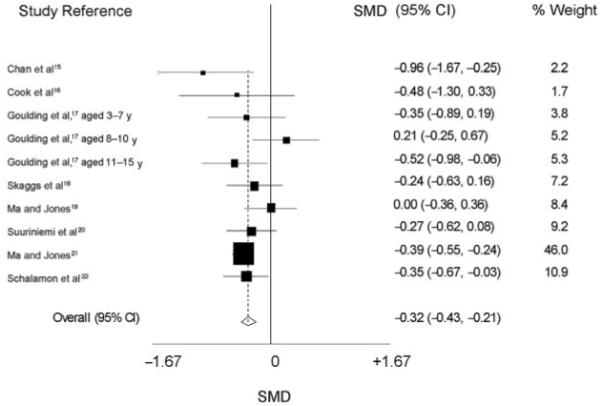
Forest plot for fixed-effect meta-analysis of the association between bone mass and fractures in children.
DISCUSSION
Ten case-control studies, with a total of 730 fractures and 1328 control children, met the criteria for this review. After combination of 8 case-control studies, our results show evidence of an association between low bone mass and fractures in children, with an SMD of −0.32 (95% CI: −0.43 to −0.21; P < .001).
All the studies were case-control studies and therefore are prone to bias. In these studies, unclear verification of fractures may introduce bias because some “cases” may not have had a fracture. This is possible in 2 studies18,22 and would tend to move the observed association closer to the null. Thus, our observed difference in mean bone mass between children with fractures and controls of −0.32 may be an underestimate. Lack of representativeness of the control selection may lead to a biased estimate of the effect of bone mass on fracture risk. However, most of the studies included in this review used accepted methods of control selection.
Confounding, both measured and unmeasured, is a problem in case-control studies. In bone-mass estimates made by using DXA, adjusting for body size is important but difficult. If adjustment is not complete, it may lead to an inaccurate estimate of the effect of bone mass on fracture risk. There is no ideal technique, but all studies used at least 1 method to account for differences in body size, such as adjusting for height, weight, or both, during either the recruitment or analysis stage. Some studies noted that there was no difference in either height or weight between the children with fractures and the control group.15-17,22 Two studies19,23 used BMAD, which is BMD corrected for area and is less influenced by body size than either BMC or BMD. Other potential confounders that were considered by most studies were age and gender. Schalamon et al22 did not seem to adjust for gender, but direct communication with the lead author confirmed that there was no difference in gender between children with fractures and those in the control group.
All the studies measured bone mass in the children after the bone fracture had occurred, which means that a reduction in bone mass resulting from the previous fractures cannot be excluded. However, repeat bonedensity measurements were taken on the children used in the study by Goulding et al17 4 years after the original fracture.25 This showed a sustained lower bone mass in the children with fractures compared with those without. It is possible that behavior is modified permanently by a fracture and results in a persistent low bone mass. However, the sustained low bone mass shown in the study by Goulding et al25 is more likely to represent long-term bone mass and reduces the likelihood of reverse causality.
Multiple comparisons and subgroup analyses such as those conducted by Goulding et al,17 Suuriniemi et al,20 and Ma and Jones21 increase the likelihood that a “significant” result will be seen by chance. Because the biggest weight in the fixed-effects meta-analysis was given to the study by Ma and Jones,21 this may mean that our results are biased. However, repeating the fixed-effects meta-analysis without this study showed a similar difference in bone mass in children with fractures compared with controls. No asymmetry was shown by the funnel plot, so publication bias is less likely; however, the studies were small and 6 of the 10 studies had positive results, so we cannot exclude publication bias as a possible explanation.
CONCLUSIONS
The methodologic quality of the studies included in this review were variable, with potential for bias and confounding. Although our combined estimate should be interpreted with caution, our results suggest that bone mass may contribute to fracture risk in childhood. In adults, each SD decrease in BMD approximately doubles fracture risk.26 Because most of the studies reported differences in mean values rather than differences in risk, it is difficult to speculate how the results of this meta-analysis can be used to predict fracture risk in children, and additional work is required. Our study did not investigate the underlying causes for the association between bone mass and fractures in children, but geography may be important; our results suggested that latitude influenced the results, perhaps via cutaneous vitamin D synthesis. To investigate the association between bone mass and fractures in children further, large prospective cohort studies are required.
ACKNOWLEDGMENT
Emma Clark is funded by a Wellcome Clinical Research Training Fellowship.
Abbreviations
- DXA
dual-energy x-ray absorptiometry
- BMC
bone mineral content
- BA
bone area
- BMD
bone mineral density
- QCT
quantitative computed tomography
- QUS
quantitative ultrasound
- SMD
standardized mean difference
- CI
confidence interval
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Stark AD, Bennet GC, Stone DH, Chishti P. Association between childhood fractures and poverty: population based study. BMJ. 2002;324:457. doi: 10.1136/bmj.324.7335.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons RA, Delahunty AM, Heaven M, McCabe M, Allen H, Nash P. Incidence of childhood fractures in affluent and deprived areas: population based study. BMJ. 2000;320:149. doi: 10.1136/bmj.320.7228.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years. JAMA. 2003;290:1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 4.Bonjour JP, Carrie AL, Ferrari S, et al. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized double-blind, placebo-controlled trial. J Clin Invest. 1997;99:1287–1294. doi: 10.1172/JCI119287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston CC, Jr, Miller JZ, Slemenda CW, et al. Calcium supplementation and increases in bone mineral density in children. N Engl J Med. 1992;327:82–87. doi: 10.1056/NEJM199207093270204. [DOI] [PubMed] [Google Scholar]
- 6.Lee WT, Leung SS, Wang SH, et al. Double-blind, controlled calcium supplementation and bone mineral accretion in children accustomed to a low-calcium diet. Am J Clin Nutr. 1994;60:744–750. doi: 10.1093/ajcn/60.5.744. [DOI] [PubMed] [Google Scholar]
- 7.Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr. 2002;76:675–680. doi: 10.1093/ajcn/76.3.675. [DOI] [PubMed] [Google Scholar]
- 8.Fewtrell MS, British Paediatric and Adolescent Bone Group Bone densitometry in children assessed by dual x ray absorptiometry: uses and pitfalls. Arch Dis Child. 2003;88:795–798. doi: 10.1136/adc.88.9.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachrach LK. Dual energy X-ray absorptiometry (DEXA) measurements of bone density and body composition: promise and pitfalls. J Pediatr Endocrinol Metab. 2000;13(suppl 2):983–988. [PubMed] [Google Scholar]
- 10.Walker-Bone K, Dennison E, Cooper C. Epidemiology of osteoporosis. Rheum Dis Clin North Am. 2001;27:1–18. doi: 10.1016/s0889-857x(05)70185-x. [DOI] [PubMed] [Google Scholar]
- 11.Dowd MD, Keenan HT, Bratton SL. Epidemiology and prevention of childhood injuries. Crit Care Med. 2002;30(11 suppl):S385–S392. doi: 10.1097/00003246-200211001-00002. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Cochrane Collaboration Additional module 1: meta-analysis of continuous data. Available at: www.cochrane-net.org/openlearning/HTML/modA1.htm. Accessed November 8, 2005.
- 14.Landin LA, Nilsson BE. BMC in children with fractures. Clin Orthop Relat Res. 1983;178:292–296. [PubMed] [Google Scholar]
- 15.Chan GM, Hess M, Hollis J, Book LS. Bone mineral status in childhood accidental fractures. Am J Dis Child. 1984;138:569–570. doi: 10.1001/archpedi.1984.02140440053013. [DOI] [PubMed] [Google Scholar]
- 16.Cook SD, Harding AF, Morgan EL, et al. Association of BMD and pediatric fractures. J Pediatr Orthop. 1987;7:424–427. doi: 10.1097/01241398-198707000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. BMD in girls with forearm fractures. J Bone Miner Res. 1998;13:143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Skaggs DL, Loro ML, Pitukcheewanont P, Tolo V, Gilsanz V. Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J Bone Miner Res. 2001;16:1337–1342. doi: 10.1359/jbmr.2001.16.7.1337. [DOI] [PubMed] [Google Scholar]
- 19.Ma DQ, Jones G. Clinical risk factors but not bone density are associated with prevalent fractures in prepubertal children. J Paediatr Child Health. 2002;38:497–500. doi: 10.1046/j.1440-1754.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- 20.Suuriniemi M, Mahonen A, Kovanen V, Alen M, Cheng S. Relation of PuvII site polymorphism in the COL1A2 gene to the risk of fractures in prepubertal Finnish girls. Physiol Genomics. 2003;14:217–224. doi: 10.1152/physiolgenomics.00070.2003. [DOI] [PubMed] [Google Scholar]
- 21.Ma DQ, Jones G. The association between BMD, metacarpal morphometry and upper limb fractures in children: a population-based case-control study. J Clin Endocrinol Metab. 2003;88:1486–1491. doi: 10.1210/jc.2002-021682. [DOI] [PubMed] [Google Scholar]
- 22.Schalamon J, Singer G, Schwantzer G, et al. Quantitative ultrasound assessment in children with fractures. J Bone Miner Res. 2004;19:1276–1279. doi: 10.1359/JBMR.040401. [DOI] [PubMed] [Google Scholar]
- 23.Goulding A, Rockell JE, Black RE, Grant AM, Jones IE, Williams SM. Children who avoid drinking cow’s milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc. 2004;104:250–253. doi: 10.1016/j.jada.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. BMD and body composition in boys with distal forearm fractures: a DEXA study. J Pediatr. 2001;139:509–515. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 25.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 26.Genant HK, Cooper C, Poor G, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10:259–264. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]


