Abstract
The ability of Pseudomonas aeruginosa to form antibiotic-resistant biofilms is thought to account for the inability of current therapies to resolve bacterial infections in the lungs of patients with cystic fibrosis (CF). We recently described a system in which highly antibiotic-resistant P. aeruginosa biofilms grow on human CF airway epithelial cells, and using this system we showed that enhanced iron release from CF cells facilitates the development of such highly antibiotic-resistant biofilms. Given the positive role for iron in biofilm development, we investigated whether the FDA-approved iron chelators deferoxamine and deferasirox would enhance the ability of tobramycin, the primary antibiotic used to treat CF lung infections, to eliminate P. aeruginosa biofilms. The combination of tobramycin with deferoxamine or deferasirox reduced established biofilm biomass by approximately 90% and reduced viable bacteria by 7-log units. Neither tobramycin nor deferoxamine nor deferasirox alone had such a marked effect. The combination of tobramycin and FDA-approved iron chelators also prevented the formation of biofilms on CF airway cells. These data suggest that the combined use of tobramycin and FDA-approved iron chelators may be an effective therapy to treat patients with CF and other lung disease characterized by antibiotic-resistant P. aeruginosa biofilms.
Keywords: antibiotic resistance, biofilms, deferoxamine, deferasirox, cystic fibrosis model
CLINICAL RELEVANCE
This study shows that FDA-approved iron chelators can potentiate the tobramycin-mediated killing of Pseudomonas aeruginosa biofilms grown in a cystic fibrosis co-culture model. This is the first report of an effective therapeutic approach to kill antibiotic-resistant bacterial biofilms established on live cystic fibrosis airway epithelial cells.
Chronic airway infections are one of the most debilitating manifestations of cystic fibrosis (CF) (1). Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) lead to the accumulation of a thick and dehydrated mucus, reduced airway surface liquid, and compromised mucociliary clearance (2, 3). This static mucus layer becomes rapidly colonized by microorganisms and evolves into a nidus for persistent and recurrent bacterial infections, causing progressive bronchiectasis and ultimately the premature death of patients with CF (4, 5). Pseudomonas aeruginosa, a gram-negative bacterium, thrives in the CF lung environment and is the leading pathogen associated with pulmonary disease in these patients (5). Approximately 80% of the adult population with CF is permanently colonized by P. aeruginosa despite the development of new antibiotics for aerosol therapy, including tobramycin (6, 7). There is some evidence that early aggressive antibiotic therapy can delay the onset of chronic P. aeruginosa infection in infants or children with CF (8). However, reoccurrence or re-infection eventually leads to the development of chronic P. aeruginosa infections by teenagers and adults (8). These established P. aeruginosa infections in the CF airways are, to date, not resolvable. The ability of P. aeruginosa to form biofilms has been linked to this increased resistance toward antibiotics and is thought to account for the lack of efficacy of current therapies (8–14).
Iron is crucial to many important physiologic functions in microbial pathogens, including energy production, oxygen transport, and the regulation of gene expression. Iron also promotes biofilm formation on abiotic surfaces (e.g., plastic), in part by regulating surface motility and by stabilizing the biofilm polysaccharide matrix (15–19). In a recent study, we showed that enhanced iron release from human CF airway epithelial cells promoted robust P. aeruginosa biofilm formation on mucus-producing and non–mucus-producing CF airway epithelial cells (20). Incredibly, the minimum bactericidal concentration for tobramycin was over 10,000 μg/ml for P. aeruginosa biofilms grown on airway cells, as opposed to 400 μg/ml for P. aeruginosa biofilms grown on an abiotic surface (20). This high-level resistance is significant because the mean concentration of tobramycin in the bronchoaveolar lavage fluid (BALF) of patients with CF after inhalation is approximately 1,000 μg/ml (21).
Because biofilm formation is strongly dependent on iron availability, chelating iron may be a promising new therapy to eliminate P. aeruginosa biofilm formation on CF airway epithelial cells. Several compounds have been tested for their ability to prevent biofilm formation or disrupt established biofilms on abiotic surfaces. For example, lactoferrin, an iron-binding protein present in airway secretions (22, 23), inhibits the formation of biofilms by preventing P. aeruginosa from adhering to a surface (16). EDTA, a metal chelator, disperses and kills P. aeruginosa biofilms growing on polycarbonate chips (24), on catheters (25–27), and on stainless steel discs (28). We recently demonstrated that chelation of iron with conalbumin reduced P. aeruginosa biofilm formation on CF airway epithelial cells (20). However, conalbumin is highly immunogenic and cannot be used in humans. Finally, disrupting P. aeruginosa iron metabolism by gallium, a transition metal chemically similar to iron, also leads to destruction of abiotic biofilms (29–31).
The goal of this study was to investigate the ability of FDA-approved iron chelators to both prevent and disrupt P. aeruginosa biofilms grown on CF-derived airway cells using a co-culture model system we recently described (20). The data reveal that chelating iron with deferasirox (DSX) or deferoxamine (DFO), both FDA-approved drugs, enhances tobramycin-mediated killing of mature biofilms established on airway cells, reduces the virulence of P. aeruginosa, and prevents the formation of biofilms on CF airway cells. To our knowledge, this is the first study reporting an effective therapeutic approach to kill established P. aeruginosa biofilms growing on CF airway epithelial cells.
MATERIALS AND METHODS
Materials
The antibiotics and iron chelators used in this study are listed in Table 1 and were obtained from Sigma (St. Louis, MO), with the exception of deferasirox, which was a generous gift from Novartis (Basel, Switzerland). Stock solutions of compounds were prepared in minimum essential medium (MEM) and made fresh immediately before use. Unless otherwise stated, conalbumin, DFO, and DSX were used at 20 μg/ml, 400 μg/ml, and 1 μM respectively. These concentrations are able to chelate the approximately 0.1 μM iron present in the medium. In addition, the concentrations of DFO and DSX used in this study were lower than or equal to the daily doses used in clinical practice to treat chronic iron overload. For information, the recommended daily dose for DFO is 20 to 40 mg/kg/day (equivalent to 280–560 μg/ml or 426–852 μM for an average 70-kg young adult) and 20 mg/kg/day for DSX (equivalent to 280 μg/ml or 750 μM for an average 70-kg young adult). Tobramycin was used at 1,000 μg/ml, the peak concentration measured in the BAL isolated from the lungs of patients with CF (21).
TABLE 1.
DRUGS AND COMPOUNDS USED IN THIS STUDY
| Compound | Brand Name | Abbreviation | Properties | Status | Administration |
|---|---|---|---|---|---|
| Conalbumin | ConA | Iron chelator | |||
| Desferasirox | Exjade | DSX | Iron chelator | FDA approved for the treatment of chronic iron overload | Oral |
| Desferoxamine mesylate | Desferal | DFO | Iron chelator | FDA approved for the treatment of acute iron intoxication and chronic iron overload | Parenteral injection |
| Tobramycin sulfate | Tobi | Tb | Antibiotic | FDA approved, used in CF clinic | Inhalation |
Definition of abbreviations: CF, cystic fibrosis; FDA, United States Food and Drug Administration.
Cell Lines and Cell Culture
Human bronchial epithelial cells (CFBE41o−) homozygous for the ΔF508-CFTR mutation and stably overexpressing ΔF508-CFTR (hereafter called CFBE cells) (32, 33) were maintained in MEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 2 μg/ml puromycin, 5 μg/ml plasmocin, 50 U/ml penicillin, and 50 μg/ml streptomycin in a 5% CO2–95% air incubator at 37°C. We have previously reported that these growth culture conditions lead to an extracellular iron concentration of approximately 0.1 μM (20). Antibiotics were removed immediately before experiments. CFBE cells were seeded on 40-mm diameter glass coverslips (Bioptechs, Butler, PA) at 2 × 106 cells/coverslip and grown at 37°C for 8 to 10 days to establish confluent monolayers. In some experiments, CFBE cells were seeded at 1 × 106 cells on 24-mm permeable filter inserts (Snapwell; Corning Costar, Kennebunk, ME) and grown in air–liquid interface culture at 37°C for 8 to 10 days to establish confluent monolayers. In a previous study we found that P. aeruginosa biofilm formation was similar on parental CFBE41o− cells, on the CFBE cells stably overexpressing ΔF508-CFTR, and on human airway epithelial cells that produce mucus (20).
Bacterial Strains
P. aeruginosa strain PAO1 carrying the pSMC21 plasmid was grown in lysogeny broth (LB) overnight. This plasmid constitutively expresses green fluorescent protein (GFP), and is stable in the absence of antibiotic selection (34, 35). As described in detail previously (20), for co-culture studies with CFBE cells, overnight cultures of P. aeruginosa were washed, resuspended in microscopy medium (see below), and used at a multiplicity of infection (M.O.I.) of 25.
Flow Chamber Imaging Experiments
GFP-labeled P. aeruginosa biofilms were grown on the apical side of live, polarized CFBE cells in a FCS2 flow chamber (Bioptechs) as described previously (20). Briefly, polarized and confluent CFBE cells grown on glass coverslips were placed in the flow chamber and perfused with a modified cell growth medium (MEM without phenol red, 2 mM L-glutamine), hereafter referred to as “microscopy medium.” Bacteria were injected on the apical side of the cells and allowed to attach, without flow, for 2 hours. The flow was subsequently started and maintained throughout the experiment at a rate of 20 ml/hour. Microscopic observations were performed on a Nikon LiveScan Swept Field confocal microscope (Tokyo, Japan) or on an Olympus IX-70 inverted microscope (Tokyo, Japan). Digital images were acquired with the Nikon Software's Suite or with the OpenLab 4.0.3 software package (Improvision, Inc., Lexington, MA). Volumes were deconvolved by iterative restoration using the Volocity 3.5.1 software (Improvision). Quantitative analysis of three-dimensional biofilm structures was performed with the COMSTAT image analysis software package (36, 37). Experiments were performed a minimum of three times. Data are expressed as mean ± SEM. A P value < 0.05 was considered significant.
Static Co-Culture Biofilm Assay
To assess the viability of bacteria after drug treatment, biofilms were grown on polarized and confluent CFBE cells under static conditions as previously described (38). Briefly, P. aeruginosa was inoculated on the apical side of epithelial cells grown on filters. After 1 hour of incubation at 37°C, unattached bacteria were removed by gently removal of the supernatant and replacing it with microscopy medium supplemented with 0.4% arginine. Filters containing the airway cells and the attached bacteria were returned to 37°C and 5% CO2 for the duration of each experiment. Arginine was added to the medium to prolong the viability of airway cells incubated with P. aeruginosa under static conditions, as described previously (38). At the end of each drug treatment, biofilms remaining at the apical side of airway cells were washed once with microscopy medium, and then 0.1% Triton X-100 was added to the medium for 15 minutes to lyse the epithelial cells and dissociate biofilms. The lysate was vortexed for 3 minutes and serial dilutions were spot titered onto LB plates to determine the colony-forming units (CFU)/well.
Abiotic Biofilm Assay
Biofilm formation on 96-well microtiter dishes was performed as described previously (39). Briefly, microtiter wells were inoculated with overnight PAO1 culture diluted 1:100 in microscopy medium and were grown at 37°C for 24 hours. Antibiotic and/or iron chelator treatments were added to 24-hour-old biofilms and maintained for 6 hours. At the end of each treatment, wells were washed four times with microscopy medium to remove any planktonic cells. Samples were sonicated as described previously (40) and viable bacteria were quantified by dilution plating and enumeration of CFU/well.
Cytotoxicity Assays
Biofilms were grown on the apical side of confluent and polarized CFBE cells grown on filters, as described above for the static co-culture biofilm assay. At the end of each drug treatment, lactate dehydrogenase (LDH) levels were measured in the medium and inside cells. Apical (AP) and basolateral (BL) fluids were collected, pooled, and centrifuged briefly to pellet bacteria and cell debris. Subsequently, 500 μl of microscopy medium was added to the apical side of cells and the cells were lysed by a freeze-thaw cycle. LDH levels were measured using the Cyto Tox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI) according to the manufacturer's instructions. Cytotoxicity was expressed as [LDHAP+BL/(LDHAP+BL+LDHcells)] × 100. Assays were performed in triplicate and experiments were performed three times.
RESULTS
Tobramycin and the Iron Chelator Conalbumin Reduce Established P. aeruginosa Biofilms
Previous studies demonstrated that the viability of P. aeruginosa biofilms grown on abiotic surfaces is reduced by iron chelators, including EDTA and lactoferrin (15, 16, 18, 24, 27, 30). However, the effect of iron chelators on P. aeruginosa biofilms grown on polarized CF airway cells has not been reported. Thus, studies were conducted to determine if an iron chelator, alone or in combination with the clinically relevant antibiotic, tobramycin, reduced the viability of established P. aeruginosa biofilms grown on CF airway cells. Accordingly, P. aeruginosa biofilms were grown on airway cells for 6 hours, followed by a 30-minute exposure to either tobramycin or conalbumin, or to a combination of both (Figure 1). Biofilm biomass, a direct and quantitative measure of biofilm formation on CFBE cells, was determined for each treatment condition as described in Materials and Methods.
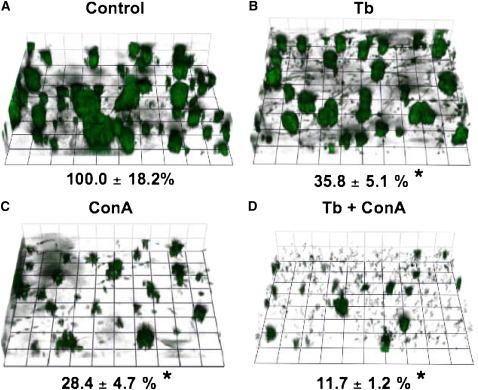
Figure 1.
Tobramycin and conalbumin disrupt established biofilms. Green fluorescent protein (GFP)-labeled Pseudomonas aeruginosa biofilms were grown on cystic fibrosis (CF) human airway epithelial cells in a flow chamber for 6 h before drug treatment. Z-series image stacks were acquired with a 1-μm step, (A) immediately before injection of compounds (Control), 30 minutes after injection of either (B) tobramycin (Tb, 1,000 μg/ml) or (C) conalbumin (ConA, 20 μg/ml), or (D) the combination of tobramycin and conalbumin (Tb + ConA) at the concentrations indicated above. The biofilm biomass remaining after treatment is indicated under each panel as a percent of control (*P < 0.05 compared with control). The three-dimensional images are presented at a slight angle to facilitate viewing. Experiments were performed in triplicate and representative fields are shown. Grid unit, 14.5 μm.
P. aeruginosa rapidly forms biofilms on CF airway cells, consistent with a previous publication by our group, which provided five lines of evidence, including enhanced antibiotic resistance, to support the view that the clusters of P. aeruginosa observed on these airways cells are indeed biofilms (Figure 1A) (please consult Ref. 20 for details). Tobramycin or conalbumin alone significantly reduced P. aeruginosa biofilm biomass by 64% and 72%, respectively, compared with the control, untreated condition (Figure 1A versus Figures 1B and 1C). The effect of tobramycin and conalbumin were not significantly different from each other. Although tobramycin and conalbumin added together reduced P. aeruginosa biofilm biomass (88%), the reduction was not significantly different from conalbumin or tobramycin alone. Thus, whereas tobramycin and conalbumin individually reduced the biomass of established biofilms, these compounds did not have either an additive or a synergistic effect on P. aeruginosa biofilms when combined.
FDA-Approved Iron Chelators Prevent P. aeruginosa Biofilm Formation
Because of the high immunogenicity of conalbumin, and thus its inability to serve as a therapeutic agent for humans, we investigated the ability of FDA-approved iron chelators to prevent and/or disrupt biofilms. We focused our efforts on two iron chelators, deferoxamine (DFO) and deferasirox (DSX), both approved for the treatment of acute iron poisoning and chronic iron overload in humans (Table 1).
We first investigated the ability of DFO and DSX to prevent biofilm formation. To this end P. aeruginosa was inoculated in the flow chamber and allowed to grow on CFBE cells for 2 hours in the absence of flow. The flow was then initiated to eliminate unattached planktonic bacteria, and either tobramycin, DFO, or DSX was added to the medium. DFO and DSX were used at clinically relevant concentrations (400 μg/ml and 1 μM, respectively) that also chelate the approximately 0.1 μM Fe in the medium (20). After 4 hours of treatment (total co-culture time of 6 h), microscopic observation of the airway monolayer and of the biofilms was performed and P. aeruginosa biomass was measured as described above.
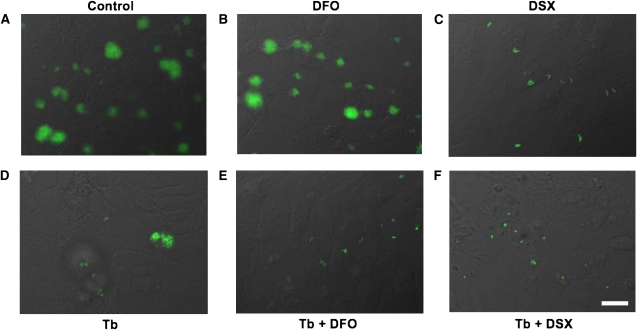
In the absence of drug treatment, P. aeruginosa formed the typical cluster biofilms described above and in our previous study (20) (Figure 2A). DFO reduced the formation of P. aeruginosa biofilms by 42% (Figures 2B and 3), and DSX reduced P. aeruginosa biofilm formation by 99% (Figures 2C and 3). Tobramycin also reduced P. aeruginosa biofilm formation by 97% (Figures 2D and 3). DSX was significantly more effective at reducing biofilm formation than tobramycin alone, although this difference was modest (Figure 3). Thus, chelation of iron with FDA-approved drugs, especially DSX, prevents the development of P. aeruginosa biofilms on CF airway epithelial cells.
Figure 2.
Prevention of P. aeruginosa biofilm formation by tobramycin and FDA-approved iron chelators. P. aeruginosa was grown on a confluent monolayer of airway cells for 2 hours in the absence of flow. The flow was then initiated and the indicated compounds were added to the input medium. Biofilms were allowed to develop for another 4 hours, after which images were captured. En face view of GFP-labeled P. aeruginosa PAO1 grown on human bronchial epithelial cells (CFBE41o−) homozygous for the ΔF508-CFTR mutation and stably overexpressing ΔF508-CFTR (CFBE cells) (A) in the absence of treatment (Control), after a 4-hour treatment with (B) deferoxamine (DFO) (400 μg/ml), (C) deferasirox (DSX) (1 μM), (D) tobramycin (1,000 μg/ml), or combined treatment with (E) tobramycin and DFO or (F) tobramycin and DSX at the concentrations indicated above. Experiments were performed in triplicate and representative images are shown. Bar, 20 μm.
Figure 3.
Quantitative analysis of biofilms and viable bacteria. P. aeruginosa biomass on CFBE cells was quantified with the COMSTAT program, as described in Materials and Methods. For each treatment (for which representative images are shown in Figure 2), six randomly chosen z-series image stacks were analyzed 6 hours after inoculation (i.e., 4 h after starting the drug treatment), and experiments were repeated at least three times. *P < 0.05 versus the untreated control; **P < 0.05 as indicated.
The next series of studies was conducted to examine the potential additive or synergistic effect of tobramycin and FDA-approved iron chelators on biofilm formation. Tobramycin and DFO together did not reduce biofilm formation to a greater extent than tobramycin alone (Figures 2E and 3). However, tobramycin and DSX together significantly decreased the ability of P. aeruginosa to form biofilms compared with tobramycin alone. Taken together, these data show that treatment with either tobramycin or an iron chelator alone inhibits biofilm formation on human CF airway epithelial cells and that combining tobramycin and DSX had an additive effect on preventing biofilm formation, compared with tobramycin alone.
Finally, to assess the potential toxicity of these chelators on the airway cells, CFBE cell monolayers were incubated with either DFO or DSX in the absence of bacteria. No evidence of cytotoxicity was observed as determined by the Cyto Tox 96 nonradioactive cytotoxicity assay (data not shown).
Combined Treatment with Tobramycin and FDA-Approved Iron Chelators Disrupt Established P. aeruginosa Biofilms
We next investigated whether the combination of tobramycin and an iron chelator would disrupt established biofilms on confluent human CF airway epithelial cells. For these studies, P. aeruginosa was grown on CFBE cells in a flow chamber for 6 hours in the absence of drugs to allow the development of mature, antibiotic resistant biofilms (20). Iron chelators and/or tobramycin were subsequently added to the medium. After 16 hours of drug treatment, biofilm biomass was determined and compared with the biomass at T = 6 hours (immediately before addition of drugs).
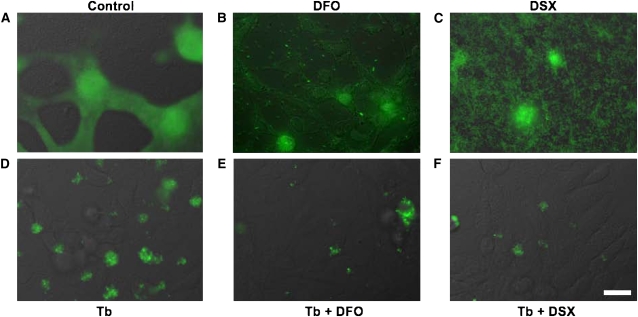
In the absence of any added drugs, P. aeruginosa caused extensive damage to CFBE cells and most airway cells detached from the glass coverslip (Figure 4A). Bacteria subsequently colonized those empty regions on the glass substratum and formed a flat biofilm on this abiotic substratum (Figure 4A). Neither DFO nor DSX alone prevented P. aeruginosa from damaging CFBE cells (Figures 4B and 4C). However, in the presence of DFO or DSX, P. aeruginosa did not form multicellular, biofilm-like aggregates, but rather they grew primarily as individual bacteria attached to the glass substratum (Figure 4B, DFO) or as a lawn of individual bacteria (Figure 4C, DSX). The inability of P. aeruginosa to form biofilm-like aggregates in the presence of iron chelators is consistent with previous studies by Singh and colleagues (16).
Figure 4.
Combined treatment with tobramycin and iron chelators disrupt established P. aeruginosa biofilms. P. aeruginosa was grown on a confluent monolayer of airway cells for 6 hours. Established biofilms were then incubated for another 16 hours (A) in the absence of drug (Control) or were treated with (B) DFO (400 μg/ml), (C) DSX (1 μM), (D) tobramycin (1,000 μg/ml), or to both (E) tobramycin and DFO or (F) tobramycin and DSX at the concentrations indicated above. Images were acquired 22 hours after inoculation (i.e., 16 h after treatment). Differential interference contrast (DIC) images and the corresponding fluorescent images, pseudo-colored green, were merged. In A, B, and C, it is evident that the airway cells were damaged by exposure to the bacteria. However, in D, E, and F the airway monolayer was intact. Experiments were performed in triplicate and representative images are shown. Bar, 20 μm.
As opposed to the iron chelators alone, treatment of established biofilms with tobramycin protected the CFBE cells from being destroyed by P. aeruginosa (compare Figures 4A–4C with Figure 4D). This finding is consistent with our previous report showing that tobramycin reduces the virulence of biofilm bacteria both by reducing bacterial numbers and by altering the expression of virulence factors (38). However, despite the fact that the CFBE monolayer remained intact, P. aeruginosa biomass after a 16-hour exposure to tobramycin increased to approximately 140% of its pre-treatment size (Figure 5A). This finding is consistent with our previous work showing that P. aeruginosa biofilms grown on airway cells are highly resistant to tobramycin (20).
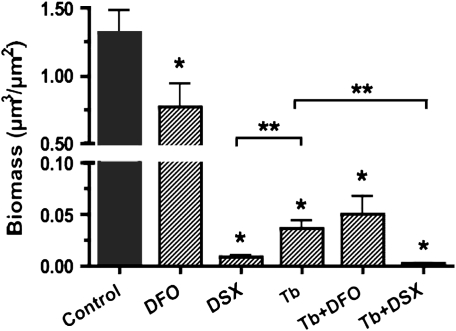
Figure 5.
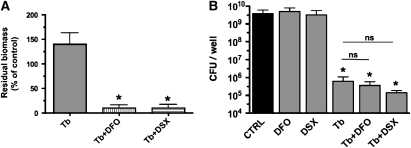
Quantitative analysis of biofilm disruption by tobramycin and iron chelators. (A) Summary of P. aeruginosa biomass after treatments described in Figure 4. Image stacks were captured 22 hours after inoculation (i.e., 16 h after starting the treatment), and experiments were repeated at least three times. Data are expressed as percent of the biomass present at 6 hours after inoculation (i.e., before starting the treatment). Because P. aeruginosa damaged untreated airway cell monolayers and monolayers exposed to DFO or DSX only, biomass data for these conditions could not be determined and thus are not reported. The concentrations of DFO and DSX used in these experiments (400 μg/ml and 1 μM, respectively) chelate the approximately 0.1 μM Fe present in the growth medium. *P < 0.05 versus the tobramycin treatment. (B) Biofilms were grown on plastic microtiter dishes for 24 hours before being treated with tobramycin (1,000 μg/ml), DFO (400 μg/ml), DSX (1 μM), or to both tobramycin and DFO or tobramycin and DSX, at the concentrations indicated above. Treatment was maintained for 6 hours before remaining attached viable bacteria were dislodged from the surface via sonication, and the number of colony-forming units (CFU)/well was determined. *P < 0.05 versus the untreated control; ns, not significantly different.
In contrast to tobramycin or iron chelators alone, the combination of tobramycin and either DFO or DSX significantly reduced the biomass of established biofilms by 90% of the pre-treatment value (Figures 4E, 4F, and 5A). Thus, a combination of tobramycin and an iron chelator disrupted established, highly drug-resistant biofilms on human airway epithelial cells and, furthermore, prevented P. aeruginosa from damaging the monolayer of CFBE cells, even after exposure to bacteria for 24 hours.
We also investigated the effect of tobramycin and iron chelators on established biofilms grown on an abiotic plastic surface, rather than on airway cells. In these studies, biofilms were formed for 24 hours, then treated with tobramycin and iron chelators, alone or in combination. After 6 hours of treatment, neither DFO nor DSX reduced the number of viable bacteria compared with untreated abiotic biofilms (Figure 5B). Tobramycin reduced the viable bacteria in these biofilms by approximately 104 CFU/well. No further decrease in CFU count was observed when abiotic biofilms were treated with a combination of tobramycin and iron chelators (compare Figures 5A and 5B). Therefore, whereas iron chelators enhanced the ability of Tb to disrupt established biofims grown on human airway cells, neither DFO nor DSX facilitated the ability of Tb to disrupt established biofilms growing on an abiotic, plastic surface.
DSX Disrupts Established Biofilms by Chelating Iron
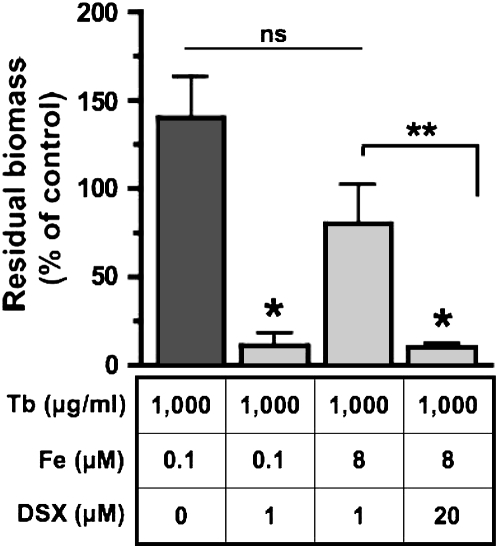
Our studies presented above suggest that the enhanced killing of biofilms by tobramycin when combined with iron chelators may be due, at least in part, to the ability of DXO and DSX to chelate iron (see also Figure 6). Since DFO and DSX had similar effects in disrupting biofilms, and because DFO was not as efficient as DSX in preventing biofilm formation, we chose to focus on DSX, the most promising compound, for the rest of this study. To determine if the ability of DSX to reduce established biofilms was linked to its ability to chelate iron, we repeated the tobramycin and DSX treatment in the presence of excess iron (8 μM of FeCl3). This concentration of iron was chosen because it is typically found in the BALF isolated from patients with CF (41–43) and exceeds the iron chelation capability of DSX (1 μM) used in our experiments by 16-fold. The increase in [Fe], from approximately 0.1 to 8 μM in the presence of Tb and DSX (1 μM) reduced the ability of tobramycin and DSX to decrease P. aeruginosa biofilm biomass (Figure 6). By contrast, increasing the DSX concentration from 1 to 20 μM (which has an Fe chelation capacity of 10 μM iron) in the presence of 8 μM iron and tobramycin reduced biomass by 90% (Figure 6). Together, these data show that the ability of DSX to reduce P. aeruginosa biomass was related, at least in part, to its ability to chelate iron.
Figure 6.
DSX disrupts established biofilms by chelating iron. Experiments were conducted to determine if DSX reduced P. aeruginosa biomass by chelating iron. Increasing DSX concentration from 0 to 1 μM at constant [Fe, 0.1 μM] reduced biomass (compare first versus second bar). Increasing [Fe] from ∼ 0.1 to 8 μM at constant [DSX, 1 μM] increased biofilm formation (compare second versus third bar). Finally, increasing [DSX] from 1 to 20 μM at constant [Fe, 8 μM] once again decreased biofilm formation (compare third versus fourth bar). In all four experiments Tb was added to the medium at 1,000 μg/ml. Where indicated, ferric chloride (Fe) was added to the medium at a final concentration of 8 μM. Unsupplemented medium contains approximately 0.1 μM Fe (20). DSX was added to the medium at a final concentration of either 0, 1, or 20 μM. Biofilm biomass was quantified 22 hours after inoculation (i.e., 16 h after starting the treatment) using the COMSTAT program, and was compared with the biomass measured at 6 hours after inoculation. *P < 0.05 versus the tobramycin treatment, **P < 0.05; ns, not significantly different.
Apical and Basolateral Application of DSX Promotes Tobramycin-Mediated Biofilm Killing
In the studies presented above assessing the ability of tobramycin and DSX to reduce established biofilms, both tobramycin and DSX were added to the apical side of CFBE cells. Clinically, while tobramycin is delivered to the lung by nebulization and is thus presented to the apical side of airway cells, DSX is provided systemically, and airway cells would then be exposed to this compound via their basolateral side. Therefore, studies were conducted to compare apical versus basolateral treatment with DSX. For these assays, CFBE cells were grown on filters at an air–liquid interface to allow addition of DSX to either the apical (i.e., airways) or basolateral (i.e., blood side) compartment. P. aeruginosa biofilms were grown in a static coculture system on the apical side of the airway cells, as previously described (38), and the efficacy of treatment was assessed by determining the bacterial CFU attached to the epithelial cells at the end of the treatment period. Bacteria were measured via CFU rather than microscopically because the filters used to grow airway cells are not optically clear, and therefore this method allowed a more efficient and accurate assessment of bacterial counts under these experimental conditions. In these studies, drugs were applied to 6-hour-established biofilms and maintained for an additional 16 hours, followed by the counting of viable bacteria. The viability of the airway cells was monitored at the end of each treatment by measuring LDH levels.
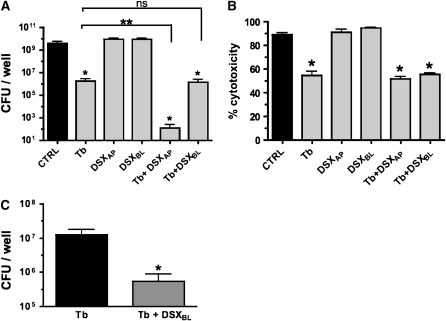
In the absence of drugs, the bacteria reached a density of greater than 109 CFU/well (Figure 7A), and the cytotoxicity was approximately 90% (Figure 7B). These data are consistent with the confocal microscopic observations reported above (Figure 4); that is, after 22 hours in the absence of treatment, P. aeruginosa biofilms kill a majority of CFBE cells (Figure 4A). Tobramycin alone added to the apical side of CFBE cells decreased the CFU count by 3-log units, to approximately 106 CFU/well (Figure 7A), and reduced the cytotoxicity from 90% to 55% (Figure 7B). DSX alone, applied to either the apical or basolateral side of CFBE cells, had no effect on the CFU count or on the cytotoxicity of P. aeruginosa (Figures 7A and 7B), observations consistent with the microscopy data (Figure 4C). However, DSX and tobramycin added together to the apical side of the airway cells reduced the CFU by 4-log units versus tobramycin alone (Figure 7A). Thus, DSX combined with tobramycin at the apical side of the airway cells reduced the viable bacteria count from 4 × 109 CFU/well in the untreated control to approximately 100 CFU/well. Together, these data show that combined treatment with tobramycin and DSX, an FDA-approved iron chelator, reduces P. aeruginosa CFU by approximately 7-log units (Figure 7A) and significantly reduces the cytotoxic effects of P. aeruginosa on polarized monolayers of CFBE cells (Figure 7B).
Figure 7.
Apical and basolateral application of DSX promotes tobramycin-mediated biofilm killing. (A) CFBE cells were grown on filters at an air–liquid interface and P. aeruginosa was added to the apical side of cells. Drugs were applied to 6-hour-old biofilms and biofilms were exposed to drugs for an additional 16 hours. Tobramycin was added to the apical compartment in all experiments, while DSX (1 μM) was added to the apical (DSXAP) or to the basolateral (DSXBL) compartment of airway cells. Bacterial CFU/well in the apical compartment were determined at the end of each treatment (22 h after inoculation). *P < 0.05 versus untreated control, **P < 0.05; ns, not significantly different. (B) The cytotoxic effects of P. aeruginosa on CFBE cells and the effect of tobramycin and DSX on P. aeruginosa–induced cytotoxicity were evaluated by measuring LDH release by airway cells, as described in Materials and Methods. Experiments were performed in triplicate. *P < 0.05 versus control (CTRL). (C) Effect of an extended basolateral application of DSX on P. aeruginosa CFU growing on CFBE cells. Seventy-two hours before inoculation with P. aeruginosa, polarized human airway cells were switched to serum-free medium or serum-free medium supplemented with DSX (800 μM) added to the basolateral side. P. aeruginosa was then added to the apical compartment and biofilms were allowed to develop for 6 hours. Subsequently, tobramycin was added to 6-hour-old biofilms. Biofilms were exposed to tobramycin for a total of 16 hours. Viable bacteria remaining in the apical side of airway cells were determined at the end of each treatment (CFU/well). Experiments were performed in triplicate. *P < 0.05 (tobramycin versus tobramycin plus DSXBL).
In comparison with apical treatment, no additive effect of DSX and tobramycin was observed when DSX was applied to the basolateral compartment of polarized CFBE monolayers. Addition of DSX to the basolateral side of the airway cells combined with tobramycin at the apical side reduced the CFU by 3-log units versus the untreated control, an effect that was not significantly different from tobramycin (apical) alone (Figure 7A).
We predicted that it may take a higher concentration or more time for DSX to reduce P. aeruginosa CFU when applied to the basolateral side of polarized CFBE cells. Thus, DSX (800 μM) was added to the basolateral compartment for 72 hours before inoculation with P. aeruginosa on the apical side of CFBE cells. The concentration of DSX used in this experiment is comparable to the recommended initial daily dose of 20 mg/kg body weight to reduce iron levels in patients using this therapy over the long term (44). This combination of tobramycin (apical) and DSX (basolateral) reduced the CFU counts by 1.5 log units compared with tobramycin alone, from 107 CFU/well to 5 × 105 CFU/well (Figure 7C). However, this extended basolateral application of DSX was still not as effective as the addition of DSX at the apical side of the cells (Figure 7A).
DISCUSSION
Bacterial biofilms are notoriously resistant to antibiotics compared with their free-living planktonic counterparts (10, 45, 46). In a previous study we showed that P. aeruginosa biofilms grown on CF-derived airway epithelial cells were 25-fold more resistant to the killing action of tobramycin than biofilms grown on plastic surfaces, and that the concentration of tobramycin achieved clinically in the lungs of patients with CF (∼ 1,000 μg/ml) does not eliminate established biofilms in our in vitro co-culture system (20). The major new observations in this study are that the combination of tobramycin with the FDA-approved iron chelators deferoxamine or deferasirox reduces established P. aeruginosa biofilm biomass on polarized CF airway cells by approximately 90%, reduces viable bacteria in these biofilms by 7-log units, and also prevents the formation of P. aeruginosa biofilms on these airway cells. The mechanism of this effect is mediated, at least in part, by the iron-chelating ability of DSX. Moreover, the combination of Tb and DSX also reduced the cytotoxic effects of P. aeruginosa on CF airway epithelial cells. Importantly, tobramycin alone did not reduce established P. aeruginosa biofilms to the extent observed for the combination treatment. Taken together, these data suggest that the combined use of tobramycin and FDA-approved iron chelators may be an effective therapy to treat CF and other lung diseases characterized by antibiotic-resistant P. aeruginosa biofilms. By contrast, our data also demonstrate that the combined use of Tb and iron chelators, compared with the use of Tb alone, is not more effective in eliminating established biofilms that form on abiotic surfaces, providing additional evidence that biofilms that form on abiotic surfaces are phenotypically different from biofilms that form on human airway epithelial cells.
Previously, we demonstrated that the formation of biofilms on airway cells is stimulated by iron (20), a finding consistent with previous studies investigating biofilm formation on abiotic surfaces (15–19). A role for iron in stimulation of biofilm formation on epithelial cells is a clinically relevant finding because the ASL in the CF lung has an iron concentration 400-fold higher than in a non-CF lung (41–43). Indeed, it appears that P. aeruginosa biofilms seem to have even greater need for iron than planktonic bacteria (17). Here, we report that the combination of tobramycin with FDA-approved iron chelators DSX or DFO efficiently prevents biofilm formation, indicating that we can exploit this increased need of biofilm bacteria for iron to develop new anti-biofilm therapeutic approaches. Although DSX reduces P. aeruginosa CFU when added to either the apical or basolateral side of CFBE cells (in combination with tobramycin), DSX is more effective when added to the apical side of airway cells. Interestingly, DFO has recently been shown to be a good candidate for nebulization, as it distributed efficiently in a model of the human lung (47). Thus, clinically, nebulization of tobramyin with DFO might be an effective way to eliminate established P. aerginosa biofilms in patients with CF.
Tobramycin, as well as the iron chelators DFO and DSX, are clinically approved for adults and children (age 6 yr and above). In addition, the concentration of DFO and DSX used in this study, and shown to be efficient in preventing or disrupting biofilms, was similar to or lower than the recommended daily doses used in the clinic to treat chronic iron overload. These data are of interest, because it may be possible to delay colonization of the CF lung, or decrease the bacterial burden, by using a treatment regimen combining antimicrobial agents and iron chelators. Clinical studies suggest that any delay in the colonization of the CF lung results in a slower decline in lung function (8, 48–52). However, it may not be possible to use chelators as a prophylactic treatment due to the possibility of depleting iron in patients with CF, who may already be somewhat anemic (53–55). Because of the rapid killing of the bacterial biofilm by the combination treatment, we propose that iron chelators would not need to be administered daily. Instead, they could be used, in conjunction with antibiotic treatment, during the acute exacerbations that typically plague patients with CF. These episodic co-treatments would hopefully eliminate the infections in patients who no longer respond to antibiotic-only therapy. Such an administration protocol presents the advantage of adding very little burden to the regimen of patients with CF already undergoing tobramycin nebulization. In support of the strategy proposed here, recent work with chelator–gallium complexes, which disrupt bacterial iron metabolism, have been shown to be effective versus two different models of P. aeruginosa infection (29, 30).
Are there possible detrimental side-effects of using iron chelators in the context of the CF lung? For example, P. aeruginosa can use iron-loaded DFO as an iron source (18, 56, 57), which may enhance bacteria growth. The ability of P. aeruginosa to accumulate iron-loaded DFO may explain in part why DFO is less effective than DSX in disrupting biofilms, since Fe-loaded DSX cannot be used by P. aeruginosa as an iron source and thus may be more effective in restricting Fe uptake by P. aeruginosa. Interestingly, studies performed in animal models of septicemia present conflicting results regarding the effect of DFO on pathogenesis, with reports ranging from a lack of prevention of lung damage (58) to a significant attenuation of lung injury (59). Thus, it is unclear what effect this chelator might have in the context of the CF lung. Alternatively, because iron deficiency is known to induce the expression of several virulence factors in P. aeruginosa (60–62), it is possible that the use of iron chelators in patients could lead to increased lung damage. Although we did not examine the effect of DFO and DSX on the expression of P. aeruginosa virulence factors per se, we did report that, in accordance with our previous study (38), tobramycin, alone or in combination with DSX, decreased the cytotoxic effect of P. aeruginosa on airway cells. Therefore, at least in our co-culture model, possible increased growth of bacteria due to DFO-mediated increased iron availability or expression of virulence genes known to be associated with iron deficiency do not appear to be a concern.
In addition to our studies of biofilms on airway cells, we examined the effects of tobramycin and chelator treatment, alone or in combination, on biofilms grown on an abiotic surface, specifically plastic. While tobramycin at the levels used was effective in reducing biofilms grown on plastic surfaces, the addition of iron chelators did not enhance tobramycin-mediated killing of these biofilms. These data indicate that biofilms grown on airway cells versus on an abiotic substratum are phenotypically different. Such a conclusion is consistent with our previous findings, including the marked increase in antibiotic resistance and differential gene expression profiles upon tobramycin treatment observed for biofilms grown on plastic versus their counterparts grown on plastic (20, 38). These findings further validate the necessity of studying biofilms in the context of host cell epithelium.
Currently, we do not fully understand the mechanism underlying the improved killing of biofilms when combining tobramycin and DSX. However, the findings presented here support the conclusion that it is the iron-binding activity of DSX that contributes, at least in part, to enhance the ability of tobramycin to prevent and disrupt biofilm formation. Ongoing work by our group is exploring the basis of the effects of tobramycin and DSX on biofilms grown on airway cells. A better understanding of these mechanisms may allow us to further improve the efficacy of such combination therapies.
Acknowledgments
The authors thank Novartis for providing deferasirox. They also thank Drs. J. P. Clancy for the CFBE cells, H. W. Parker and A. H. Gifford for critical reading of the manuscript, and G.G. Anderson for technical assistance.
This work was supported by Cystic Fibrosis Research Development Program (STANTO97RO) and the National Institutes of Health (HL074175) to B.A.S.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0299OC on January 23, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Tümmler B, Kiewitz C. Cystic fibrosis: an inherited susceptibility to bacterial respiratory infections. Mol Med Today 1999;5:351–358. [DOI] [PubMed] [Google Scholar]
- 2.Deneuville E, Perrot-Minot C, Pennaforte F, Roussey M, Zahm JM, Clavel C, Puchelle E, de Bentzmann S. Revisited physicochemical and transport properties of respiratory mucus in genotyped cystic fibrosis patients. Am J Respir Crit Care Med 1997;156:166–172. [DOI] [PubMed] [Google Scholar]
- 3.Tomkiewicz R, App E, Zayas J, Ramirez O, Church N, Boucher R, Knowles M, King M. Amiloride inhalation therapy in cystic fibrosis: influence on ion content, hydration, and rheology of sputum. Am Rev Respir Dis 1993;148:1002–1007. [DOI] [PubMed] [Google Scholar]
- 4.Gomez MI, Prince A. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr Opin Pharmacol 2007;7:244–251. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med 1996;335:179–188. [DOI] [PubMed] [Google Scholar]
- 6.Heijerman H. Infection and inflammation in cystic fibrosis: a short review. J Cyst Fibros 2005;4:3–5. [DOI] [PubMed] [Google Scholar]
- 7.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 2002;15:194–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros 2005;4:49–54. [DOI] [PubMed] [Google Scholar]
- 9.Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002;416:740–743. [DOI] [PubMed] [Google Scholar]
- 10.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–1322. [DOI] [PubMed] [Google Scholar]
- 11.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 2001;9:34–39. [DOI] [PubMed] [Google Scholar]
- 13.Chernish R, Aaron S. Approach to resistant gram-negative bacterial pulmonary infections in patients with cystic fibrosis. Curr Opin Pulm Med 2003;9:509–515. [DOI] [PubMed] [Google Scholar]
- 14.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918–951. [DOI] [PubMed] [Google Scholar]
- 15.Singh P. Iron sequestration by human lactoferrin stimulates P. aeruginosa surface motility and blocks biofilm formation. Biometals 2004;17:267–270. [DOI] [PubMed] [Google Scholar]
- 16.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature 2002;417:552–555. [DOI] [PubMed] [Google Scholar]
- 17.Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K. Influence of quorum-sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J Bacteriol 2008;190:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banin E, Vasil ML, Greenberg EP. Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA 2005;102:11076–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berlutti F, Morea C, Battistoni A, Sarli S, Cipriani P, Superti F, Ammendolia M, Valenti P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int J Immunopathol Pharmacol 2005;18:661–670. [DOI] [PubMed] [Google Scholar]
- 20.Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O'Toole GA, Stanton BA. The ΔF508-CFTR mutation results in increased biofilm formation by P. aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol 2008;295:L25–L37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest 2002;122:219–226. [DOI] [PubMed] [Google Scholar]
- 22.Harbitz O, Jenssen A, Smidsrød O. Lysozyme and lactoferrin in sputum from patients with chronic obstructive lung disease. Eur J Respir Dis 1984;65:512–520. [PubMed] [Google Scholar]
- 23.Thompson A, Bohling T, Payvandi F, Rennard S. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med 1990;115:148–158. [PubMed] [Google Scholar]
- 24.Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol 2006;72:2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakandawala N, Gawande PV, LoVetri K, Madhyastha S. Effect of ovotransferrin, protamine sulfate and EDTA combination on biofilm formation by catheter-associated bacteria. J Appl Microbiol 2007;102:722–727. [DOI] [PubMed] [Google Scholar]
- 26.Kite P, Eastwood K, Sugden S, Percival SL. Use of in vivo-generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J Clin Microbiol 2004;42:3073–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raad I, Chatzinikolaou I, Chaiban G, Hanna H, Hachem R, Dvorak T, Cook G, Costerton W. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob Agents Chemother 2003;47:3580–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayres HM, Payne DN, Furr JR, Russell AD. Effect of permeabilizing agents on antibacterial activity against a simple Pseudomonas aeruginosa biofilm. Lett Appl Microbiol 1998;27:79–82. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 2007;117:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, Greenberg EP, Banin E. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc Natl Acad Sci USA 2008;105:16761–16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halwani M, Yebio B, Suntres Z, Alipour M, Azghani A, Omri A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J Antimicrob Chemother 2008;62:1291–1297. [DOI] [PubMed] [Google Scholar]
- 32.Bruscia E, Sangiuolo F, Sinibaldi P, Goncz K, Novelli G, Gruenert D. Isolation of CF cell lines corrected at DeltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther 2002;9:683–685. [DOI] [PubMed] [Google Scholar]
- 33.Cozens A, Yezzi M, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner W, Widdicombe J, Gruenert D. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 1994;10:38–47. [DOI] [PubMed] [Google Scholar]
- 34.Bloemberg G, O'Toole G, Lugtenberg B, Kolter R. Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol 1997;63:4543–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuchma SL, Connolly JP, O'Toole GA. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J Bacteriol 2005;187:1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heydorn A, Ersboll BK, Hentzer M, Parsek MR, Givskov M, Molin S. Experimental reproducibility in flow-chamber biofilms. Microbiology 2000;146:2409–2415. [DOI] [PubMed] [Google Scholar]
- 37.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000;146:2395–2407. [DOI] [PubMed] [Google Scholar]
- 38.Anderson GG, Moreau-Marquis S, Stanton BA, O'Toole GA. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun 2008;76:1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 1998;30:295–304. [DOI] [PubMed] [Google Scholar]
- 40.Kadouri D, O'Toole G. Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl Environ Microbiol 2005;71:4044–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stites S, Walters B, O'Brien-Ladner A, Bailey K, Wesselius L. Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest 1998;114:814–819. [DOI] [PubMed] [Google Scholar]
- 42.Stites SW, Plautz MW, Bailey K, O'Brien-Ladner AR, Wesselius LJ. Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am J Respir Crit Care Med 1999;160:796–801. [DOI] [PubMed] [Google Scholar]
- 43.Alexis N, Richards J, Carter JD, Ghio AJ. Iron-binding and storage proteins in sputum. Inhal Toxicol 2002;14:387–400. [DOI] [PubMed] [Google Scholar]
- 44.Nick H, Acklin P, Lattmann R, Buehlmayer P, Hauffe S, Schupp J, Alberti D. Development of tridentate iron chelators: from desferrithiocin to ICL670. Curr Med Chem 2003;10:1065–1076. [DOI] [PubMed] [Google Scholar]
- 45.Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O'Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003;426:306–310. [DOI] [PubMed] [Google Scholar]
- 46.Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 1985;27:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musk DJ, Hergenrother PJ. Chelated iron sources are inhibitors of Pseudomonas aeruginosa biofilms and distribute efficiently in an in vitro model of drug delivery to the human lung. J Appl Microbiol 2008;105:380–388. [DOI] [PubMed] [Google Scholar]
- 48.Kozlowska WJ, Bush A, Wade A, Aurora P, Carr SB, Castle RA, Hoo A-F, Lum S, Price J, Ranganathan S, et al. Lung function from infancy to the preschool years after clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med 2008;178:42–49. [DOI] [PubMed] [Google Scholar]
- 49.Javadpour S, Jones A, Brownlee K. Longitudinal analysis of FEV1 changes related to antibiotic therapy in children with cystic fibrosis. Ir Med J 2007;100:529–532. [PubMed] [Google Scholar]
- 50.Konstan M, Morgan W, Butler S, Pasta D, Craib M, Silva S, Stokes D, Wohl M, Wagener J, Regelmann W, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 2007;151:134–139. [DOI] [PubMed] [Google Scholar]
- 51.Courtney J, Bradley J, Mccaughan J, O'Connor T, Shortt C, Bredin C, Bradbury I, Elborn J. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol 2007;42:525–532. [DOI] [PubMed] [Google Scholar]
- 52.Ren C, Morgan W, Konstan M, Schechter M, Wagener J, Fisher K, Regelmann W; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol 2007;42:513–518. [DOI] [PubMed] [Google Scholar]
- 53.Fischer R, Simmerlein R, Huber RM, Schiffl H, Lang SM. Lung disease severity, chronic inflammation, iron deficiency, and erythropoietin response in adults with cystic fibrosis. Pediatr Pulmonol 2007;42:1193–1197. [DOI] [PubMed] [Google Scholar]
- 54.Reid DW, Withers NJ, Francis L, Wilson JW, Kotsimbos TC. Iron deficiency in cystic fibrosis: relationship to lung disease severity and chronic Pseudomonas aeruginosa Infection. Chest 2002;121:48–54. [DOI] [PubMed] [Google Scholar]
- 55.Ater J, Herbst J, Landaw S, O'Brien R. Relative anemia and iron deficiency in cystic fibrosis. Pediatrics 1983;71:810–814. [PubMed] [Google Scholar]
- 56.Llamas MA, Sparrius M, Kloet R, Jimenez CR, Vandenbroucke-Grauls C, Bitter W. The heterologous siderophores ferrioxamine B and ferrichrome activate signaling pathways in Pseudomonas aeruginosa. J Bacteriol 2006;188:1882–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuiv PO, Keogh D, Clarke P, O'Connell M. FoxB of Pseudomonas aeruginosa functions in the utilization of the xenosiderophores ferrichrome, ferrioxamine B, and schizokinen: evidence for transport redundancy at the inner membrane. J Bacteriol 2007;189:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mustard R, Bohnen J, Mullen J, Schouten B, Swanson H. Deferoxamine induces hypotension in experimental gram-negative septicemia. Shock 1994;1:221–227. [DOI] [PubMed] [Google Scholar]
- 59.Moch D, Schröppel B, Schoenberg M, Schulz H, Thorab F, Marzinzag M, Hedlund B, Brückner U. Protective effects of hydroxyethyl starch-deferoxamine in early sepsis. Shock 1995;4:425–432. [PubMed] [Google Scholar]
- 60.Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 2002;99:7072–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasil ML, Ochsner UA. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol Microbiol 1999;34:399–413. [DOI] [PubMed] [Google Scholar]
- 62.Wilderman PJ, Vasil AI, Johnson Z, Wilson MJ, Cunliffe HE, Lamont IL, Vasil ML. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect Immun 2001;69:5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]