Abstract
Background
Neuronal ceroid lipofuscinoses (NCLs) are a group of autosomal recessive neurodegenerative diseases characterized by lysosomal accumulation of autofluorescent material in neurons and other cell types. The infantile subtype (INCL) is rare (1 in >100,000 births), the most devastating of childhood subtypes, and is caused by mutations in the gene CLN1 which encodes palmitoyl-protein thioesterase-1.
Methods
To investigate the incidence of hypothermia and bradycardia during general anesthesia in INCL patients, we conducted a case-control study to examine the perianesthetic course of INCL patients and of controls receiving anesthesia for diagnostic studies.
Results
Eight INCL children [mean age 25 months (range 10 to 32) at first anesthetic] and 25 controls (mean age 44, range 18 to 92 months) underwent 62 anesthetics for nonsurgical procedures. INCL patients had neurologic deficits including developmental delay, myoclonus, and visual impairment. INCL patients had lower baseline temperature (36.4±0.1 vs. 36.8±0.1, INCL vs. controls, p<0.007) and during anesthesia, despite active warming techniques, had significantly more hypothermia (18 vs. 0 episodes, p<0.001) and sinus bradycardia (10 vs. 1, p<0.001) compared to controls. INCL diagnosis was significantly associated with temperature decreases during anesthesia (p<0.001), whereas age, sex, and duration of anesthesia were not (p=NS).
Conclusions
We report that INCL patients have lower baseline body temperature and during general anesthesia, despite rewarming interventions, are at increased risk for hypothermia and bradycardia. This suggests a previously unknown INCL phenotype, impaired thermoregulation. Therefore, when anesthetizing these children, careful monitoring and routine use of warming interventions are warranted.
Introduction
Neuronal ceroid lipofuscinoses (NCLs), collectively referred to as Batten's disease, are a group of predominantly autosomal recessive neurodegenerative storage diseases characterized by lysosomal accumulation of autofluorescent material in neurons and other cell types (1-3). There are 4 major subtypes of NCLs: infantile (INCL, also known as CLN 1 or Haltia-Santavuori disease), late-infantile (LINCL, also known as CLN 2 or Jansky-Bielschowsky disease), juvenile (JNCL, also known as CLN 3 or Spielmeyer-Sjögren disease), and adult (also known as CLN 4 or Kuf's disease) (1-3). These NCL subtypes do differ on age of onset and composition of the storage material. The infantile subtype (INCL) is rare (1 in >100,000 births) (4), is the most devastating disease, and has the earliest age of onset. INCL is caused by mutations within the gene CLN1 on chromosome 1p32, which encodes palmitoyl-protein thioesterase-1(PPT1) (5,6). PPT1 cleaves thioester linkages in S-acylated (palmitoylated) proteins facilitating the degradation of fatty-acylated proteins by lysosomal hydrolases (5). Thus, deficiency of PPT1 leads to abnormal lysosomal accumulation of palmitoylated proteins which, in turn, leads to INCL pathogenesis (7).
Children with INCL appear normal at birth and seem to acquire developmental skills until the age of 6-11 months. These children then begin to demonstrate hyperirritability, hypotonia, myoclonic jerks, and seizures (1-3). By the age of 2 years, severe visual deterioration and slow or absent pupillary reaction are noted, by 3 years significant loss of cortical function ensues, and by 4 years, children with INCL usually manifest an isoelectric electroencephalogram attesting to lack of brain function. These children then live in a vegetative state until they are 8-12 years-of-age, at which time death occurs (1, 8-10). INCL is uniformly fatal and, although many manifestations of the disease can be ameliorated with sedatives, centrally acting skeletal muscle relaxants (such as baclofen), and anticonvulsants, effective treatment is nonexistent.
Because of the profound neurological abnormalities in children with INCL, the anesthetic management of these patients requires an understanding of the natural history of the disease. We report our experience with the anesthetic management of 8 children with INCL and have found that hypothermia and bradycardia are frequent occurrences during anesthesia.
Methods
Study Design and Patients
We conducted a case-control study of children with INCL and control children without the disease. Children in both case (INCL) and control groups were anesthetized for diagnostic procedures between 2001 and 2007 by the same anesthesiologists. Attempts were made to have controls comparable for age, duration of anesthetic, and types of procedures. Children in the INCL group were enrolled in an investigational therapeutic protocol approved by the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) since 2001. Children from the control group did not have INCL and were enrolled in unrelated investigational protocols approved by the IRBs of the NICHD and National Cancer Institute. Data analyzed included patient demographics, primary diagnoses, anesthetic technique, duration of anesthesia, and changes in temperature and heart rate. IRB review for this report was waived because it analyzed data previously collected for quality assurance and data were devoid of identifiers.
Procedures
Table 1 outlines locations of procedures and transportation between locations during the anesthetics. All INCL patients received general anesthesia for electroretinograms (ERG), brain magnetic resonance imaging (MRI), and spectroscopy. For the 2-hour ERG, tropicamide 1% and phenylephrine 2.5% were applied to both eyes and a contact lens was placed on one eye as previously described (11). Children in the control group received general anesthesia for MRI, spectroscopy, and auditory brain response and/or lumbar punctures and dental examinations. The ambient temperature in the operating rooms and radiology suite was maintained at 22 °C.
Table 1.
Procedure locations and transportation routes for anesthetics in INCL and control patients undergoing diagnostic procedures*
| Number of Anesthetics | ||
|---|---|---|
| Procedures and Location | INCL | CONTROLS |
| Operating Room (OR) | ||
| Electroretinogram | 31 | 0 |
| Auditory Brain Response | 0 | 16 |
| Lumbar Puncture | 0 | 18 |
| Dental Exam | 0 | 5 |
| Skin Biopsy | 0 | 6 |
| Radiology | ||
| Magnetic Resonance Imaging | 31 | 31 |
| Spectroscopy | 31 | 12 |
| Transportation Routes | ||
| Radiology → PACU | 0 | 5 |
| Radiology → OR → PACU | 7 | 21 |
| OR → Radiology → PACU | 24 | 5 |
INCL indicates Infantile Neuronal Ceroid Lipofuscinosis, PACU indicates postanesthesia care unit, OR=operating room. Some patients had multiple procedures per anesthetic.
Anesthetics
Anesthetics were administered by staff anesthesiologists and routine monitoring devices (ECG, noninvasive arterial blood pressure, pulse oximetry, and capnography) were used during procedures and transportation. Temperature was measured with a tympanic thermometer when patients were in the preoperative holding area (baseline temperature) and postanesthesia care unit (PACU) and with an esophageal probe when patients were in the operating room. For all patients, full body air blankets (Bair Hugger, Arizant Healthcare, Inc, Eden Prairie, MN) set at high temperature were used during portions of anesthetics conducted in the operating room. While in the MRI scanner, temperature was not monitored and patients were fully covered with blankets.
For this study, hypothermia was arbitrarily defined as temperatures below 35.6°C and bradycardia as heart rate below the 5th percentile for age. Therefore, heart rates below 85 bpm for ages 13-36 months, below 75 for ages 37-72 months, and below 74 for ages 73-108 months were considered bradycardia.
Statistical Methods
Analyses were done with Statistical Analysis System Version 9.1 software (SAS Institute, Inc, Cary, NC) and StatXact Version 6 software (Cytel Software Corporation, Cambridge, MA). Because the number of anesthetics per patient differed significantly between the 2 comparison groups (INCL patients versus controls), we formulated analyses to ensure valid comparisons. We first compared only the first anesthetic for each patient in INCL and control groups (Table 2 upper section). When analyzing data from the first anesthetic for each patient, interval data are presented as means and standard deviations, t-tests were used to compare the 2 groups and Fisher's exact test were used to compare categorical variables.
Table 2.
Comparison of patients with infantile neuronal lipofuscinosis (INCL) versus controls undergoing anesthesia for diagnostic procedures
| Variable | INCL | Controls | P value |
|---|---|---|---|
| Data from first anesthetic for each patient | |||
| Number of patients | 8 | 25 | |
| Male (%) | 4 (50) | 11 (44) | 1.00 |
| Female (%) | 4 (50) | 14 (56) | |
| Age (months) * | 25±6.6 | 44±19.8 | <0.001 |
| Range | 10.5-32 | 18-92 | |
| Baseline temperature (°C) * | 36.2±0.4 | 36.8±0.4 | <0.001 |
| Data from all anesthetics in all patients | |||
| Number of anesthetics | 31 | 31 | |
| Age (months) † | 39±5.6 | 46±3.7 | 0.34 |
| Duration of anesthesia (minutes) †‡ | 280±10.3 | 231±8.2 | 0.002 |
| Baseline temperature (°C) † | 36.4±0.1 | 36.8±0.1 | 0.007 |
| Number of anesthetics when hypothermia was observed (%) § | 18 (29%) | 0 (0%) | <0.001 |
| Lowest temperature (°C) * ‖ | 34.3±1.4 | 36.6±0.6 | |
| Range | 32-36.7 | 35.9-38.0 | |
| Change in temperature from baseline (°C) † | -1.2±0.2 | -0.2±0.1 | <0.001 |
| Baseline heart rate † | 100±4 | 108±3 | 0.134 |
| Number of anesthetics when bradycardia was recorded § | 10 (16%) | 1 (2%) | <0.001 |
| Slowest heart rate * ‖ | 73±13.3 | 103±19.1 | |
| Range | 60-100 | 73-160 | |
| Time to first occurrence of lowest temperature (minutes) † | 192±17.2 | 149±16.2 | 0.08 |
| Time in PACU (minutes) † | 67±6.9 | 56±6.7 | 0.25 |
Variables are shown as Mean± SD (standard deviation).
Least square means and associated standard error, from repeated measures analyses.
Duration of anesthesia reflects time from induction of to emergence from general anesthesia.
Exact procedure for binomial clustered data.
Lowest temperatures and heart rates were not compared because there were large differences between groups in the sample sizes. PACU= postanesthesia care unit
We then compared all anesthetics for each patient in INCL and control groups. For analyses comparing the 2 groups with repeating data from all anesthetics for each patient (Table 2 bottom section), we used specialized methods appropriate for the type of outcome (dichotomous or interval): exact tests (using StatXact) for binomial clustered data for dichotomous outcomes (e.g., occurrence/non-occurrence of hypothermia or bradycardia) or SAS's Proc Mixed (for mixed models) for interval data. These methods are necessary since, when patients have repeating outcomes (dependent variables) and associated predictor variables (e.g., comparison group, pre-procedure temperature, duration of anesthesia, time to first occurrence of lowest temperature, time in PACU, etc.), the repeating outcomes for a given group are no longer statistically independent. In these data there is only one repeating factor: the possibly multiple anesthesia events a patient had. For dichotomous outcomes these repeats per patient constitute the cluster (StatXact). In specifying analyses using SAS's Proc Mixed, the predictor variables of interest were modeled as fixed effects; patient was a random effect (i.e., a “random intercept” model). The error terms in the mixed effects model were assumed to all be statistically independent, which induced a compound symmetric covariance matrix for the repeating outcomes on a single patient. Because of small sample sizes, we used the Kenward and Roger method for computing the degrees of freedom for tests of fixed effects. Lastly, the variances of the random effects were assumed to be equal in the 2 comparison groups. The primary outcome of interest was the change in temperature from pre-procedure to the lower of the 2 temperatures recorded for each perianesthetic period. All “time” variables (duration of anesthesia, time to first occurrence of lowest temperature, time in PACU) were not repeated within an anesthetic event, but a single measure for each anesthetic event. Results from Proc Mixed repeated measures are presented as the value of the estimator (“least square means” in SAS terminology) ± associated standard error of the estimator and p-values. For all analyses, 2-sided P < 0.05 was considered statistically significant. For 2 comparisons for data from all anesthetics, we did not use repeated measures nor did we do statistical comparisons. We determined the lowest temperature and slowest heart rate, for each patient from all anesthetic events, and then for each comparison group we obtained an arithmetic mean, standard deviation and range of these lowest values. Because of the discrepancies between comparison groups in the number of anesthetics per patient, we simply report the descriptive statistics without an associated p-value.
Results
Patients
We identified 8 children with INCL who cumulatively underwent 31 consecutive anesthetics between 2001 and 2007. Demographic data and clinical findings for INCL and control patients are listed in Tables 2 and 3. All INCL patients had confirmed lethal mutations of the PPT1 gene. In INCL patients, the experimental protocol called for ERG and brain MRI at baseline and at approximately every 6 months after enrollment. With exception of patient 8, who was admitted at the age of 10 months and had no overt neurological deficit, all other INCL patients showed typical developmental delay and neurological findings at time of enrollment.
Table 3.
Demographics of patients with infantile neuronal ceroid lipofuscinosis*
| Patient | Age at presentation (months) | Age at first anesthetic (months) | Sex | Weight, kg (percentile) | Height, cm (percentile) | Number of Anesthetics |
|---|---|---|---|---|---|---|
| 1 | 15 | 22 | F | 10.0(45) | 81.6(15) | 5 |
| 2 | 18 | 25 | F | 12.4(50) | 80(3) | 6 |
| 3 | 22 | 27 | F | 11.8(25) | 86.2(25) | 5 |
| 4 | 9 | 27 | M | 14.7 (75) | 91(50) | 5 |
| 5 | 18 | 32 | M | 14.0 (6) | 96.5 (50) | 3 |
| 6 | 20 | 29 | M | 11.3 (<10) | 85.1(10) | 1 |
| 7 | 21 | 29 | M | 11.0 (2) | 92(56) | 4 |
| 8 | 18 | 10.5 | F | 8.8(<50) | 72.6(<50) | 2 |
Patient 8 was diagnosed by genetic testing at the age of 5 months and at the time of anesthesia had not yet developed neurologic deficits.
The control group comprised 25 children (11 boys and 14 girls) with primary diagnoses of Niemann-Pick disease (N=12), Smith-Lemli-Optiz syndrome (N=5), Gaucher disease (N=3), brain tumors (N=2), neurofibromatosis type 1 (N=1), rheumatoid arthritis (N=1), and chronic granulomatous disease (N=1).
Anesthesia management
Details of the 31 anesthetics for INCL and control groups are shown in Table 4. During ERGs, in order to avoid interference in the response to optical stimulation, no volatile anesthetic was used and anesthesia was maintained with nitrous oxide and propofol. In addition, in INCL patients, muscle relaxants were used to avoid eye movement during ERGs as necessary. During other diagnostic procedures, anesthesia was maintained with a volatile anesthetic or propofol at the discretion of anesthesiologists. Considering all procedures, the mean duration of anesthesia was longer in INCL patients [280±10.3 min (mean±SE)], compared with controls [231±8.2 min (mean±SE)], P=0.002 (Table 2).
Table 4.
Anesthetic technique used for children with infantile neuronal lipofuscinosis (INCL) and controls undergoing imaging and invasive diagnostic procedures
| INCL | Controls | |
|---|---|---|
| Anesthetic technique | ||
| General | 31 | 31 |
| Monitoring | ||
| Standard | 31 | 31 |
| Induction agent | ||
| Sevoflurane and nitrous oxide | 27 | 13 |
| Propofol | 4 | 18 |
| Maintenance anesthetic* | ||
| Propofol and/or inhalation agent | 31 | 31 |
| Airway management | ||
| Endotracheal tube | 31 | 8 |
| Nasal canula | 0 | 23 |
| Other intraoperative drugs | ||
| Muscle relaxant | 21 | 2 |
| Midazolam | 7 | 12 |
| Glycopyrrolate | 6 | 1 |
| Atropine | 3 | 0 |
| Ondansetron | 5 | 0 |
Inhaled anesthetics were not used during electroretinogram
After all anesthetics, patients were routinely admitted to the PACU and subsequently transferred to patient care units, except for 2 INCL patients who had hypothermia and after brief PACU stay (patients 2 and 7) were transferred to the intensive care unit for overnight observation (see below).
Perianesthetic Events: Hypothermia and bradycardia
In order to ensure valid comparisons between INCL and control groups because some patients had repeated studies, we conducted 2 analyzes: one comparing only the first anesthetic from each patient and another comparing all anesthetics for patients in the 2 groups (Table 2). Although INCL patients were younger at the time of their first anesthetic (Table 2, P<0.001), when all anesthetics were considered, there was no significant age difference comparing INCL versus controls (Table 2, P=0.34). With regards to hypothermia, INCL patients had a significantly more frequent occurrence of hypothermia than did controls: 18 vs. 0, respectively (P<0.001). In INCL patients, in these 18 anesthetics when hypothermia ensued (Table 5), the baseline temperature was 36.3±0.1 °C (mean ±SE), onset of hypothermia (temperature below 35.6 °C) was observed relatively early in the course of anesthesia (by 59±12.3 min, mean ±SE), and the average temperature decrease was -1.6±0.2 °C (mean ±SE). Furthermore, in these 18 anesthetics, hypothermia was observed during the first procedure, ERG in 12 and brain MRI in 6 patients. The presence of hypothermia was not associated with time spent in the PACU (P=0.98). Figure 1 shows the times during anesthetics when the lowest temperature was recorded in controls and INCL patients.
Table 5.
Significant events observed during anesthetics in patients with infantile neuronal lipofuscinosis
| Patient | Number of anesthetics | Hypothermia | Bradycardia | Stridor/ oxygen desaturation | Significant events during anesthetics |
|---|---|---|---|---|---|
| 1 | 5 | Yes | No | Hypothermia on 1st, 2nd, 3rd and 5th anesthetics | |
| 2 | 6 | Yes | No | Yes | Hypothermia on 1stand 2nd and stridor after extubation on 2nd and 5th anesthetics |
| 3 | 5 | Yes | Yes | Hypothermia on 3rd and 4th anesthetics and bradycardia on 4th anesthetic | |
| 4 | 5 | Yes | Yes | Hypothermia on all and bradycardia on 3rd and 4th anesthetics | |
| 5 | 3 | Yes | Yes | Hypothermia on 2nd and 3rd and bradycardia on 2nd and 3rd anesthetics | |
| 6 | 1 | Yes | Yes | Hypothermia and bradycardia on 1st anesthetic | |
| 7 | 4 | Yes | Yes | Yes | Hypothermia on 1st and 2nd, bradycardia on all four, and apnea and desaturation after 3rd anesthetics |
| 8 | 2 | No | No | None |
Figure 1.
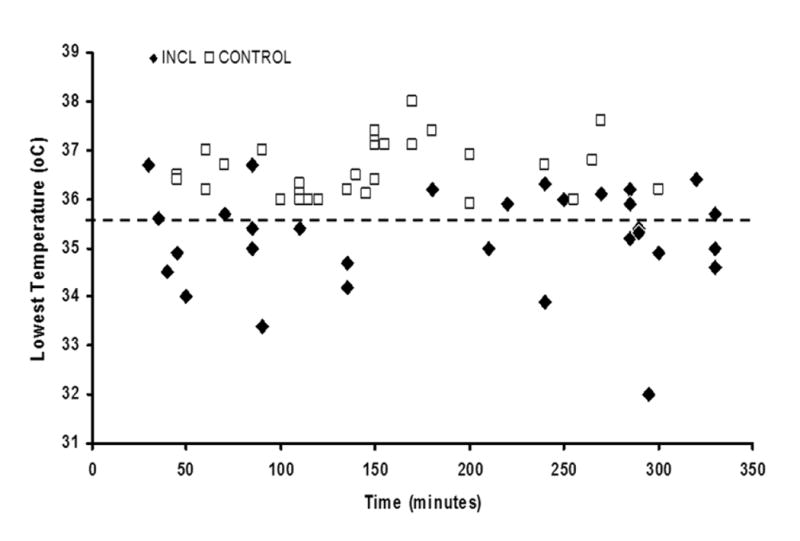
Time of lowest temperature recorded during anesthesia for diagnostic procedures in infantile neuronal ceroid lipofuscinosis and control patients. Hypothermia was defined as temperature below 35.6°C (indicated by the dashed line).
INCL patients had a significantly more frequent occurrence of sinus bradycardia than did controls, 10 versus 1, respectively (Table 2, P<0.001). As shown in Table 5, in the INCL group, 5 of 8 patients (patients 3, 4, 5, 6 and 7) displayed 10 episodes, whereas in the control group only one of 25 patients displayed one episode of sinus bradycardia. In the INCL group, in 8 of 10 episodes, bradycardia was associated with hypothermia, whereas in the remaining 2 episodes, bradycardia was observed in the absence of hypothermia. All episodes of sinus bradycardia were successfully treated with atropine or glycopyrrolate.
We evaluated possible associations of preanesthetic factors and duration of anesthesia with changes in temperature during anesthetics in INCL and control groups. Disease [INCL vs. No-INCL (controls)] was significantly associated with decreases in temperature (p<0.001), whereas age (p=0.12), baseline temperature (p=0.09), duration of anesthesia (p=0.84), and transportation route (p=0.33) were not. When we evaluated factors associated with the lowest temperature during anesthetics, results were similar except that baseline temperature was significantly associated with lowest temperature observed during anesthetics (p = 0.002). In addition, considering the INCL group alone, duration of anesthesia (p=0.30), age (p=0.35), sex (p=0.62), baseline temperature (p = 0.09), and transportation route (p=0.33) were not associated with decreases in temperature during anesthesia.
Other perianesthetic occurrences
Other perianesthetic events included stridor in INCL patient 2 who was intubated with an appropriate size endotracheal tube and yet had stridor after extubation with 2 different anesthetics. Over the 31 anesthetic events, there were no significant changes in arterial blood pressure (data not shown).
Patient 2, because of changes in brain MRI compared to that obtained 6 months earlier, and patient 7, because of episodes of transient apnea coupled with decreases in oxygen saturation, were admitted to the intensive care unit for overnight observation. In those 2 occasions, no deterioration of the baseline neurological or respiratory examinations was noted and no interventions were necessary. Both patients were discharged without sequelae the following morning.
Discussion
This case-control study showed that children with INCL have lower body temperature at baseline and that, during general anesthesia, they are at increased risk of hypothermia and bradycardia. These findings suggest that children with INCL might have impaired thermoregulation and abnormalities in the cardiac conduction system. Disturbances in temperature regulation is a previously unreported phenotype of INCL and further illustrates the critical nature of this disease. Considering these potential thermoregulatory defects in patients with INCL and the known deleterious effects of anesthesia in thermoregulation (12), in order to avoid the complications associated with profound hypothermia, careful planning for anesthesia in this patient population is imperative.
Our data suggest that, in patients with INCL, hypothermia is a common occurrence during general anesthesia. In our series, 7 of 8 patients developed hypothermia (defined as temperature <35.6 °C) despite the use of active rewarming techniques, careful measures to avoid heat loss, and in the absence of fluid shifts. We found 4 published reports describing the anesthetic courses of patients with NCLs. One report describes anesthesia for patients with LINCL (13), a second for 2 patients with JNCL (14), a third for an infant with NCL (15), and a fourth for one patient with adult NCL (16). To our knowledge, this is the first report to describe anesthetic considerations in infants and children with PPT1 mutation-confirmed INCL. Our findings are in concert with 2 other reports suggesting that mild hypothermia can occur in older patients with LINCL and JNCL (13,14). In our INCL patients, we recorded temperatures lower than 34°C despite the use of active warming techniques during anesthesia. These episodes of hypothermia occurred in environments (operating rooms and MRI) with controlled ambient temperature (22°C). In some patients hypothermia was associated with bradycardia and appeared to prolong patients' stay in the PACU. However, PACU time was not statistically different when patients with and without hypothermia were compared. It is important to note that 2 INCL patients who had hypothermia were transferred to an intensive care unit after a short stay in the PACU. Nevertheless, our findings together with those of others strongly suggest that, when anesthetizing a child with INCL, careful attention to core body temperature as well as cardiac monitoring, and active prevention of heat loss are warranted.
Although general anesthesia alters temperature thresholds for thermoregulation (12) and exposure to cold environments (such as MRI suites) can lead to decreases in body temperature, increases in core temperature and even hyperthermia have been reported in children undergoing MRIs (17-19). Our findings of hypothermia in INCL patients, which in some was observed during MRI, are in contrast to those reports (17-19). Those studies showed that children (2-77 months) sedated for MRI studies with various drugs had increases, rather than decreases, in core temperature (17,19). Furthermore, hyperthermia, rather than hypothermia, has been reported in children receiving volatile anesthetics during anesthesia for MRI (18). One could postulate that our anesthetic technique or its length could have contributed to hypothermia in INCL patients. However, we believe these possibilities to be less likely given lack of hypothermia in control patients receiving similar anesthetic techniques during the same time period. Therefore, our findings suggest that children with INCL have increased risk of hypothermia and measures to avoid heat loss are warranted during anesthetics in these patients.
Why might INCL patients have lower baseline temperature and when subjected to general anesthesia be susceptible to frequent episodes of hypothermia? One possibility is that children with INCL might have impairment of thermoregulation. In support of this hypothesis are studies of circadian temperature recordings in NCL patients showing significant disturbances in body temperature rhythms. Researchers showed that patients with INCL have maximal temperature in the morning, whereas controls had higher temperatures in the afternoon (20). It is also noteworthy that the only INCL patient in our series who did not develop hypothermia was the youngest patient (Patient 8) who had not yet developed neurological deficits at the time of first anesthesia. This may suggest that impairment in thermoregulation follows the development of neurologic deficit in INCL. Although the mechanism for hypothermia is incompletely understood, our findings suggest that during anesthesia, patients with INCL are prone to develop significant hypothermia that can reach potentially harmful levels and warrant interventions. In addition, our findings of possible impairment of thermoregulation in patients with INCL, lends further support to a body of literature suggesting that some neurodegenerative diseases (21) may involve and result from abnormalities in metabolic processes associated with energy production and thermoregulation.
Another common occurrence during anesthesia in our patients with INCL was bradycardia. Some of these episodes of bradycardia were associated with hypothermia but others occurred in the absence of hypothermia. Importantly, no episode of bradycardia was coupled with overt hemodynamic compromise and all episodes were successfully treated with anticholinergics. Although we observed no arrhythmia other than sinus bradycardia during anesthesia, others have reported the occurrence of bradycardia, sinus arrest, and severe supraventricular tachycardia during anesthesia in patients with JNCL (22). In that autopsy study, researchers showed that NCLs are associated with degeneration and infiltration of the cardiac conduction system with granular material, left ventricular hypertrophy, ventricular dilation, degenerative myocardial changes, interstitial fibrosis, and fatty replacement (22). Therefore, during the anesthetic of children with NCL, sinus bradycardia can occur with or without hypothermia and there is the possibility of other arrhythmias given possible involvement of the cardiac conduction system known to occur in this group of neurodegenerative diseases. Therefore, consideration should be given to obtaining preoperative electrocardiograms in some INCL patients to determine the presence of conduction abnormalities.
Although some may advocate avoiding the use of neuromuscular blocking drugs in patients with NCLs (14), we chose to use a nondepolarizing muscle relaxant in some of our anesthetics and found that its use was not associated with prolonged recovery or prolonged muscle relaxation. With regard to volatile anesthetics, we chose to use sevoflurane for induction of anesthesia in our patients and did not observe worsening of myoclonic activity or episodes of seizures. Although there are theoretical concerns about the use of sevoflurane in young children because of its epileptogenic potential (23,24), we found little evidence to indicate that its use is unsafe in patients with INCL (24).
In summary, we examined temperature regulation in INCL patients undergoing anesthesia and found that in these patients basal body temperature is lower than in controls and hypothermia and sinus bradycardia are common occurrences during general anesthesia. Further, the degree of hypothermia can be significant and warrants the use of active warming techniques. In our institution, besides using warming techniques, we have taken measures to minimize anesthesia time (decreasing waiting time during anesthetics, rigorously coordinating the schedule of all operators involved in the multiple procedures) and measures to decrease heat loss by turning off air circulation in the bore of the magnet during MRIs.
Our findings suggest a previously unreported phenotype of INCLUDE: that of increased risk for hypothermia and bradycardia. Although the mechanisms of disturbances in temperature control are incompletely understood and were not explored in this study, this new phenotype has implications for future investigations of the pathophysiology and therapy of INCL.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Clinical Center. The opinions or assertions contained herein are the private views of the authors (SWL) and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense
Footnotes
Conflict of Interest: None
References
- 1.Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain pathology (Zurich, Switzerland) 2004;14:61–9. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haltia M. The neuronal ceroid-lipofuscinoses: from past to present. Biochimica et biophysica acta. 2006;1762:850–6. doi: 10.1016/j.bbadis.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6:107–26. doi: 10.1007/s10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- 4.Santavuori P, Vanhanen SL, Sainio K, Nieminen M, Wallden T, Launes J, Raininko R. Infantile neuronal ceroid-lipofuscinosis (INCL): diagnostic criteria. J Inherit Metab Dis. 1993;16:227–9. doi: 10.1007/BF00710250. [DOI] [PubMed] [Google Scholar]
- 5.Camp LA, Verkruyse LA, Afendis SJ, Slaughter CA, Hofmann SL. Molecular cloning and expression of palmitoyl-protein thioesterase. The Journal of biological chemistry. 1994;269:23212–9. [PubMed] [Google Scholar]
- 6.Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–7. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann SL, Peltonen L. The neuronal ceroid lipofuscinosis. In: Scriver CR, Sly WS, Childs B, editors. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3877–94. [Google Scholar]
- 8.Santavuori P, Lauronen L, Kirveskari E, Aberg L, Sainio K, Autti T. Neuronal ceroid lipofuscinoses in childhood. Neurol Sci. 2000;21:S35–41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner RM. Genetic analysis of Batten disease. J Inherit Metab Dis. 1993;16:787–90. doi: 10.1007/BF00711910. [DOI] [PubMed] [Google Scholar]
- 10.Haltia M. The neuronal ceroid-lipofuscinoses. J Neuropathol Exp Neurol. 2003;62:1–13. doi: 10.1093/jnen/62.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Marmor MF, Zrenner E. Standard for clinical electroretinography (1999 update). International Society for Clinical Electrophysiology of Vision. Doc Ophthalmol. 1998;97:143–56. doi: 10.1023/a:1002016531591. [DOI] [PubMed] [Google Scholar]
- 12.Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–38. doi: 10.1097/ALN.0b013e31817f6d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada Y, Doi K, Sakura S, Saito Y. Anesthetic management for a patient with Jansky-Bielschowsky disease. Can J Anaesth. 2002;49:81–3. doi: 10.1007/BF03020423. [DOI] [PubMed] [Google Scholar]
- 14.Pereira D, Pereira M, Caldas F. Anesthesia management in neuronal ceroid lipofuscinoses. Paediatric anaesthesia. 2006;16:356–8. doi: 10.1111/j.1460-9592.2005.01796.x. [DOI] [PubMed] [Google Scholar]
- 15.Gopalakrishnan S, Sidduiqui S, Mayhew JF. Anesthesia in a child with Batten disease. Paediatric anaesthesia. 2004;14:890–1. doi: 10.1111/j.1460-9592.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- 16.Defalque RJ. Anesthesia for a patient with Kufs' disease. Anesthesiology. 1990;73:1041–2. doi: 10.1097/00000542-199011000-00036. [DOI] [PubMed] [Google Scholar]
- 17.Bryan YF, Templeton TW, Nick TG, Szafran M, Tung A. Brain magnetic resonance imaging increases core body temperature in sedated children. Anesth Analg. 2006;102:1674–9. doi: 10.1213/01.ane.0000216292.82271.bc. [DOI] [PubMed] [Google Scholar]
- 18.Kussman BD, Mulkern RV, Holzman RS. Iatrogenic hyperthermia during cardiac magnetic resonance imaging. Anesth Analg. 2004;99:1053–5. doi: 10.1213/01.ANE.0000133911.79161.AF. [DOI] [PubMed] [Google Scholar]
- 19.Machata AM, Willschke H, Kabon B, Prayer D, Marhofer P. Effect of brain magnetic resonance imaging on body core temperature in sedated infants and children. Br J Anaesth. 2009:aen388. doi: 10.1093/bja/aen388. [DOI] [PubMed] [Google Scholar]
- 20.Heikkila E, Hatonen TH, Telakivi T, Laakso ML, Heiskala H, Salmi T, Alila A, Santavuori P. Circadian rhythm studies in neuronal ceroid-lipofuscinosis (NCL) Am J Med Genet. 1995;57:229–34. doi: 10.1002/ajmg.1320570223. [DOI] [PubMed] [Google Scholar]
- 21.Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, Cui L, Beyer RP, Easley CN, Smith AC, Krainc D, Luquet S, Sweet IR, Schwartz MW, La Spada AR. Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell metabolism. 2006;4:349–62. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Hofman IL, van der Wal AC, Dingemans KP, Becker AE. Cardiac pathology in neuronal ceroid lipofuscinoses--a clinicopathologic correlation in three patients. Eur J Paediatr Neurol. 2001;5 A:213–7. doi: 10.1053/ejpn.2000.0465. [DOI] [PubMed] [Google Scholar]
- 23.Akeson J, Didriksson I. Convulsions on anaesthetic induction with sevoflurane in young children. Acta anaesthesiologica Scandinavica. 2004;48:405–7. doi: 10.1111/j.1399-6576.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 24.Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatric anaesthesia. 2005;15:266–74. doi: 10.1111/j.1460-9592.2004.01538.x. [DOI] [PubMed] [Google Scholar]


