Abstract
Background:
N-methyl-D-aspartic acid (NMDA) receptors play an important role in the development of hypersensitivity to visceral and somatic stimuli following inflammation or tissue injury. Our objective was to investigate the role of NMDA NR1 receptors in the spinal cord (T10-L1; L4-S1) of a subset of rats that remain hypersensitive following histological resolution of TNBS-induced colitis compared to saline treated rats and rats that had recovered both behaviorally and histologically. We hypothesized that NMDA NR1 subunit expression mediates hypersensitivity following transient TNBS colitis.
Methods:
Male Sprague-Dawley rats (150g-250g) received 20mg/rat intracolonic trinitrobenzene sulfonic acid (TNBS) in 50% ethanol or saline. Animals underwent nociceptive visceral/somatic pain testing 16 weeks after resolution of TNBS colitis. Animals were sacrificed and their spinal cord (T10-L1; L4-S1) was retrieved and 2-dimensional polyacrylamide gel electrophoresis and immunohistocytochemistry techniques were used to investigate spinal-NMDA receptor expression.
Results:
NR1001 was the only NMDA NR1 receptor subunit that was expressed in recovered and control rats, whereas hypersensitive animals expressed NR1011 and NR1111 as well as NR1001 subunits. Immunohistochemistry analysis demonstrated increased expression of NMDA NR1-N1, C1, and C2-plus expression in lamina I & II of the spinal cord (T10-L1; L4-S1) in hypersensitive rats but not in recovered/control rats.
Conclusions:
Selective increases in the expression of the NMDA NR1 splice variants occur in hypersensitive rats following resolution of TNBS colitis. This suggests that the NMDA NR1 receptor play an important role in the development of neuronal plasticity and central sensitization. The recombination of NR1 splice variants may serve as a key functional protein that maintains hypersensitivity following resolution of TNBS colitis.
Keywords: NMDA receptor, spinal cord, visceral pain, somatic hypersensitivity, central sensitization, Irritable bowl syndrome (IBS)
1. Introduction
Chronic abdominal pain is a common gastrointestinal symptom that affects large numbers of patients in the US. Even though the pathophysiology of visceral pain or functional bowel disorders is unclear, visceral hypersensitivity is a common biological marker present in some patients with functional bowel disorders such as the irritable bowel syndrome (IBS) (Naliboff et al 1997; Verne et al 2001). Although the mechanisms of IBS are still not well understood, research into visceral hypersensitivity has demonstrated that impulse frequencies of visceral primary afferent neurons increase following injury to the viscera and may lead to central sensitization (Al Chaer et al 2000; Cervero 1985; Cross 1994; Lu et al 1997; Mayer & Gebhart 1994; Mayer et al 1999; Qin et al 2002; Urban & Gebhart 1999b). Central sensitization also contributes to chronic visceral pain. A number of receptors, neurotransmitters, cytokines and second messenger systems in primary afferent and second order neurons have been implicated in the enhancement of visceral nociception (Cervero & Laird 2004; Delvaux 2002; Holzer et al 2001; Mach 2004; Mertz 2003), including serotonin, substance P, calcitonin gene-related peptide (CGRP), as well as glutamatergic NMDA receptors.
We have previously shown that a subset of IBS patients with visceral hypersensitivity have thermal hyperalgesia of the hand and foot (Verne et al 2001), consistent with their frequent complaints of pain in body regions somatotopically distinct from the gut. These patterns of hyperalgesia suggest that central sensitization mechanisms may occur in IBS patients. Other studies have also shown that IBS patients demonstrate hypersensitivity to controlled nociceptive stimuli applied to somatic tissues (Bouin et al 2002; Dunphy et al 2003; Verne et al 2003). Results from all of these studies suggest that visceral and somatic nociceptive processing overlap (viscerosomatic convergence), particularly in the lumbosacral distribution. Thus, tonic input from the gut may sensitize spinal cord neurons in which visceral and somatic nociceptive information converges.
In a previous study, we found long-term visceral and somatic hypersensitivity in a subset of rats (18 of 75 rats, 24%) that had been given intracolonic trinitrobenzene sulfonic acid (TNBS) (Zhou et al 2008a). Hypersensitivity to visceral and somatic stimuli was observed in these animals 16 weeks following resolution of colitis. This subset of hypersensitive rats had visceral and somatic hypersensitivity in response to nociceptive colonic distension and somatic stimulation similar to that seen in a subset of IBS patients (Bouin et al 2002; Dunphy et al 2003; Verne et al 2001; Verne et al 2003). Some IBS patients report greater pain in response to rectal distension and thermal stimulation of the extremities in comparison to controls. We have hypothesized that this somatic hypersensitivity, both in IBS patients and hypersensitive rats, is most pronounced in somatic areas associated with convergence of colonic and somatic afferents onto common spinal neurons (Price et al 2006).
NMDA receptors contribute to colonic inflammation-evoked hyperalgesia and dorsal horn neuron hyperexcitability (Zhou & Verne 2008). NMDA receptors integrate the activity of groups of neurons and provide a mechanism to amplify nociceptive signals leading to central sensitization. Central sensitization is characterized by enlarged neuronal receptive fields, allodynia and hyperalgesia (Baranauskas & Nistri 1998; Dubner & Ruda1992 Ma & Woolf 1995; McRoberts et al 2001; petrenko et al 2003; Ren & Dubner 1999; Urban & Gebhart 1999a; Willert et al 2004; Woolf & Thompson 1991). Further support for NMDA receptor-mediated central sensitization and chronic visceral pain is evidenced by attenuation of central sensitization with NMDA receptor antagonists (Castroman & Ness 2002; Dubner & Ruda1992 ; Klatt et al 1999; Sun et al 1998; Traub et al 2002).
The various splice variants of NR1 have distinct properties that significantly influence the function of the fully formed receptor. In this study, we examined the expression of the NR1 splice variants in the spinal cord of rats following resolution of TNBS colitis to determine if NMDA receptor expression was altered by a transient inflammatory injury. We previously found increased expression of NR1 splice variants in the colon of rats with TNBS-induced colitis and somatic/visceral hypersensitivity (Zhou et al 2006). In subsequent studies, we found subsets of rats that retained visceral and somatic hypersensitivity even after histological resolution of TNBS-induced colitis (Zhou et al 2008a; Zhou et al 2008b). Therefore, we hypothesized that NMDA NR1 subunit expression mediates hypersensitivity following transient TNBS colitis.
2. Results
Histological Examination of Colons
All saline and TNBS treated rats (recovered and hypersensitive rats) had no evidence of colitis at 16 weeks following administration of saline or TNBS respectively (data not shown). There was no evidence of neutrophils in the lamina propria or interstitial edema.
Behavioral Pain Testing
Shown in Table 1 are the results of the visceral and somatic pain testing. A total of 6 out of 27 (22%) of TNBS treated rats exhibited both somatic and visceral hypersensitivity as previously defined; 21 out of 27 (78%) of TNBS treated rats recovered from transient TNBS colitis, there is no difference between saline treated rats (n=10) and recovered rats. These results are similar to the previous studies we have reported (Zhou et al 2008a,b). There was no observed order effect based on which pain stimuli were applied first.
Table 1.
Results of behavioral visceral and somatic hypersensitivity testing. All values are means ± standard deviation (SD). One Way AVOVA indicated p< 0.0001 in all 3 modality tests. Colonic distention test F=23.99 and DF=2; Mechanical stimuli test F=21.23 and DF=2; Thermal stimuli test F=36.28 and DF=2.
| Behavior Tests | Saline Control Rats (n=10) |
Hypersensitive Rats (n=6 of 27) |
Recovered Rats (n=21 of 27) |
One Way AVOA -Tukey post test |
|---|---|---|---|---|
|
Colonic Distention (mmHg) |
53.00 ± 11.03 | 17.88 ± 5.01 | 52.98 ± 10.32 | p < 0.001 |
|
Mechanical Stimuli (force / g) |
31.95 ± 7.76 | 9.89 ± 5.54 | 32.24 ± 8.00 | p < 0.001 |
|
Thermal Stimuli (latency / second) |
16.00 ± 4.12 | 5.13 ± 1.77 | 16.29 ± 4.03 | p < 0.001 |
All values represent mean ± standard deviation (SD)
Interestingly, a total of 22% of rats maintained visceral and somatic hypersensitivity after TNBS colitis was healed. For our current study, we focused on changes in the central nerve system-spinal cord that might lead to central sensitization in hypersensitive rats. We used the NMDA NR1 subunit receptors as a marker to investigate central sensitization as it has been previously shown to play an important role in initiating and maintaining other chronic pain states. Thus, in our current study, we are specifically evaluating the unique role that the NMDA NR1 receptor might play in visceral and somatic hypersensitivity in functional bowel disorders such as IBS.
NMDA NR1 Receptor Expression in Two-Dimensional (2-D) Gel Analysis
The 8 splice variants of NR1 are predicted to have different isoelectric points and slightly different molecular weights. We have adopted 2-D polyacryamide gel electrophoresis to detect the 8 splice variants of the NR1 subunit in the spinal cord of T10-L1 and L4-S1 in 3 conditions: hypersensitive rats, recovered rats, and saline control rats.
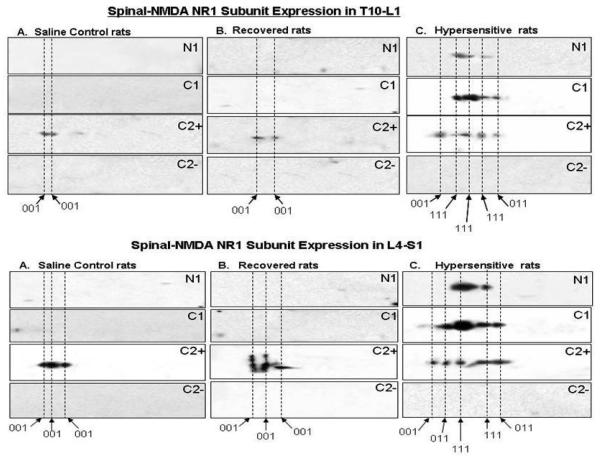
In each of the saline control (n=3) and recovered (n=3) rats, only one subunit of the NR1 receptor was detected, NR1001 (Figure 1 upper panel A & B) in the T10-L1 and L4-S1 (Figure 1 lower panel A & B) spinal cord. In contrast, there were changes in the protein profiles of NR1 subunits in each of the hypersensitive (n=3) rats. Among the eight potential splice variants of NR1, two additional subunits were expressed in the hypersensitive rats which were NR1111 and NR1011 (Figure1 upper panel C; and Figure 1 lower panel C). Thus, we found NR1001 expression in the control and recovered rat groups, and NR1111 and NR1011 expression in the hypersensitive rat group following resolution of TNBS colitis.
Figure 1.
Two-Dimensional Gel western analyses of NR1 splice variants in rat's T10-L1 (upper panel) and L4-S1 (lower panel) spinal cord following resolution of TNBS colitis. To identify proteins with antibodies to the NR1-N1, NR1 C1, NR1-C2plus and NR1-C2minus in the spinal cord, three separate membranes for each panel were used for all 4 antibodies. The membranes were stripped of primary antibody between experiments. Figure 1 A & B upper panel indicate NR1001 receptor expression in saline control and recovered rats; figure 1C upper panel indicates not only NR1001 expression, but also NR1011 and NR1111 in hypersensitive rats. Figure 2A and figure 2B lower panel indicate NR1001 receptor expression in saline control and recovered rats; figure 2C lower panel indicates not only NR1001 expression, but also NR1011 and NR1111 in hypersensitive rats.
Immunohistochemistry of NMDA NR1 Receptor Expression
Immunohistochemistry pictures of NMDA NR1 subunit in hypersensitive rats
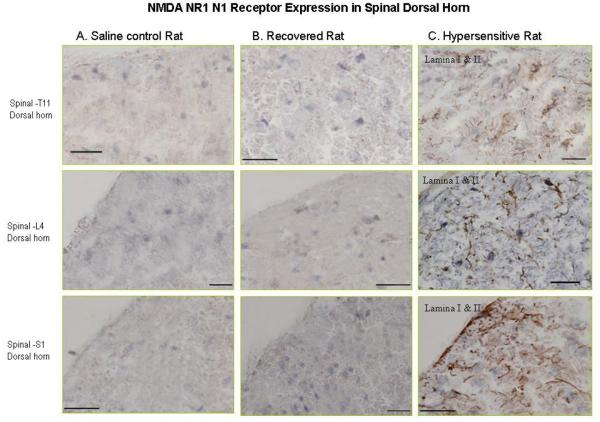
Immunohistochemistry pictures demonstrate the NR1-N1 positive cell expression. Figure 2 (panel C) are representative spinal cord section at T11, L4 and S1 that show NR1-N1 positive cell expression mainly located in lamina I & II in hypersensitive rat; however, Figure 2 (panel A & B) indicated negative NR1-N1 expression in lamina I & II in same level of spinal cord in saline control and recovered rat.
Figure 2.
Photomicrographs illustrate NMDA NR1-N1 receptor expression in the spinal cord in hypersensitive rats. Figure 2 (panel C) are representative spinal cord section at T11, L4 and S1 that show NR1-N1 positive cell (brown) expression mainly located in lamina I & II and in hypersensitive rats; however figure 2 (panel A & B) indicate negative NR1-N1 expression in lamina I & II in the same level of spinal cord in saline control and recovered rats.
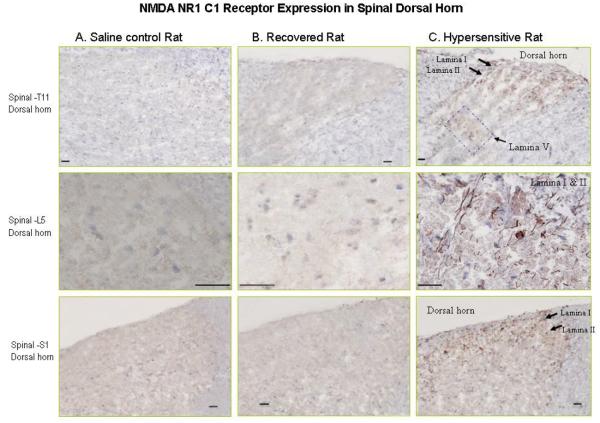
Immunohistochemistry pictures demonstrate the NR1-C1 positive cell expression. Figure 3 (panel C) demonstrated NR1-C1 protein expression mainly in lamina I & II of the T11 spinal cord in hypersensitive rat; and moderate magnitude of positive cell expression in lamina V. In figure 3 (column C) also demonstrated NR1-C1 expiration in lamina I & II of L5 and S1. There was no positive NR1-C1 detected in lamina I & II of T11, L5 and S1 of spinal cord (Figure 3, panel A & B).
Figure 3.
Photomicrographs illustrate NMDA NR1-C1 receptor expression in the spinal cord in hypersensitive rats. Figure 3 panel C: demonstrate NR1-C1 protein expression (brown) mainly in lamina I & II of the T11 spinal cord in hypersensitive rats; and moderate magnitude of positive cell expression in lamina V. Figure 3 panel C: also demonstrate NR1-C1 expiration in lamina I & II of L5 and S1. Figure 3 panel A & B: no NR1-C1 positive cell expression was detected in lamina I & II of T11, L5 and S1 of the spinal cord in saline control and recovered rat.
Quantitative NMDA NR1-N1, NR1-C1, NR1-C2 plus and NR1-C2 minus positive cell expression
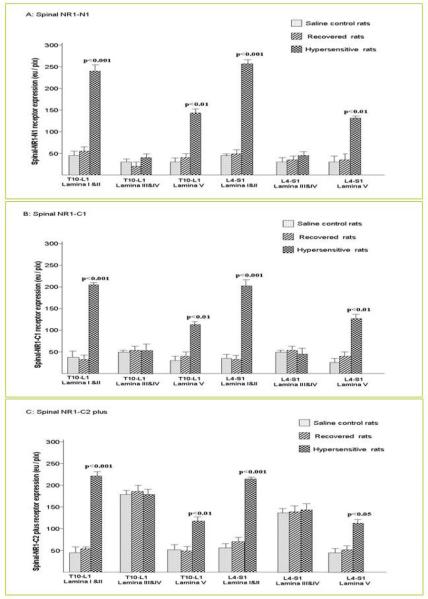
Compared to each of the control (n=3) and recovered rats (n=3), all 3 hypersensitive rats (n=3) demonstrated NR1-N1 positive cell expression predominantly located in lamina I & II in T10 -L1 and L4-S1; and moderate expression were demonstrated in Lamia V of T10-L1 and L4-S1 of the spinal cord. Two way ANOVA indicated p< 0.0001 (F=211.7; FD=5). Two way ANOVA follow by Bonferroni Post-tests indicated NR1-N1 receptor expression in Lamina I & II of T10-L1 and L4-S1 of the spinal cord (p< 0.001); NR1-N1 expression in lamina V in T10-L1 and L4-S1 of the spinal cord (p<0.01) (Figure 4A). No NR1-N1 positive cell was found in Lamina III & IV in saline control rats, recovered rats and hypersensitive rats.
Figure 4.
Quantitative NMDA NR1-N1, NR1-C1 and NR1-C2 plus positive cell expression (eu/pix) in the spinal cord from T10 to L1 and from L4 to S1. Figure 4 panel A: NR1-N1 positive cell expression was predominantly located in lamina I & II in T10-L1 and L4-S1 of the spinal cord, moderate NR1-N1 expression was present in lamina V in T10-L1 and L4-S1 of the spinal cord in hypersensitive rats compared with recovered and control rats. The data are shown as the means ± SD. Two way ANOVA indicated p< 0.0001 (F=211.7; FD=5). Two way ANOVA follow by Bonferroni Post-tests indicated NR1-N1 receptor expression in Lamina I & II of T10-L1 and L4-S1 of the spinal cord (p< 0.001), NR1-N1 expression in lamina V in T10-L1 and L4-S1 of the spinal cord (p<0.01). Figure 4 panel B: NR1-C1 expression was found predominantly expressed in lamina I, II from T10 to L1 and from L4 to S1 and moderate NR1-C1 positive cell expression show in lamina V of the T10-L1 and L4-S1 spinal cord in hypersensitive rats compared with recovered rats and control rats. Two way ANOVA indicated p< 0.0001 (F=228.1; FD=5). Two way ANOVA follow by Bonferroni Post-tests indicated NR1-C1 receptor expression in Lamina I & II of T10-L1 ( p< 0.001) and L4-S1 of the spinal cord (p< 0.001), NR1-N1 expression in lamina V in T10-L1 (p< 0.01) and L4-S1 of the spinal cord (p<0.01). Figure 4 panel C: NR1-C2 plus expression was present not only in lamina I, II and V of the T10-L1 and L4-S1 spinal cord in hypersensitive rats, but also in lamina III, IV in saline control rats, recovered rats and hypersensitive rats. Two way ANOVA indicated p<0.0001 (F=158.2; FD=5). Two way ANOVA follow by Bonferroni Post-tests indicated NR1-C2 plus receptor expression in Lamina I & II of T10-L1 ( p< 0.001) and L4-S1 of the spinal cord (p< 0.001), NR1-C2 plus expression in lamina V in T10-L1 (p< 0.01) and L4-S1 of the spinal cord (p<0.05).
Similar to NR1-N1, the NR1-C1 expression were found predominantly expressed in lamina I, II from T10 to L1 and from L4 to S1 and moderate NR1-C1 positive cell expression was shown in lamina V of the T10-L1 and L4-S1 spinal cord in hypersensitive rats compared with recovered rats and control rats. Two way ANOVA indicated p< 0.0001 (F=228.1; FD=5). Two way ANOVA follow by Bonferroni Post-tests indicted NR1-C1 receptor expression in Lamina I & II of T10-L1 ( p< 0.001) and L4-S1 of the spinal cord (p< 0.001), NR1-N1 expression in lamina V in T10-L1 (p< 0.01) and L4-S1 of the spinal cord (p<0.01) (Figure 4, panel B).
We did not detect NR1-N1 and NR1-C1 positive cell expression in the T10-L1 and L4-S1 of ventral area spinal cord either the control, recovered group of rats or hypersensitive rats.
In contrast to NR1-N1 and NR1-C1 above, NR1-C2 plus expression was present not only in lamina I, II and V of the T10-L1 and L4-S1 spinal cord in hypersensitive rats, but also in lamina III, IV in saline control rats, recovered rats and hypersensitive rats. There was a significant increase of NR1-C2 plus expression in lamina I & II and moderate expression in lamina V of the spinal cord from T10 to L1 and from L4 to S1. Two way ANOVA indicated p<0.0001 (F=158.2; FD=5). Two way ANOVA follow by Bonferroni Post-tests indicated NR1-C2 plus receptor expression in Lamina I & II of T10-L1 ( p< 0.001) and L4-S1 of the spinal cord (p< 0.001), NR1-C2 plus expression in lamina V in T10-L1 (p< 0.01) and L4-S1 of the spinal cord (p<0.05) (Figure 4 panel C). In all groups of rats (control, recovered, and hypersensitive) the NR1-C2 plus positive cell expression was also demonstrated in the ventral horn areas (data not shown). We did not detect NR1-C2 minus positive cell expression in the T10-L1 and L4-S1 spinal cord of any of the groups of rats.
Increased NMDA NR1 receptor expression, such as NR1-N1, NR1-C1, and NR1-C2 plus was positively correlated with hypersensitivity on all 3 pain behavioral tests (colonic distension, mechanical stimuli, and thermal stimuli) in hypersensitive rats in lamina I&II and lamina V of spinal cord.
3. Discussion
This study examined enduring changes in NR1 expression in the spinal cord in response to a transient inflammatory injury to the colon in rats. These enduring changes occurred in 22% of rats that retained visceral and somatic hypersensitivity after histological resolution of colitis. This percentage is similar to that of our previous studies (24% in (Zhou et al 2008a) and 20% in (Zhou et al 2008b) and to the approximate 25% incidence of IBS following infectious diarrhea (Spiller 2003). The first unique finding of our current study indicated an increase in the NMDA receptor expression or recombination of new spinal-NMDA receptor expression (such as NR1111 and NR1 011) in the T10-L1 and L4-S1 of hypersensitive rats. Another unique finding of our study was the presence of NR1-splice variants (NR1-N1, NR1-C1, NR1-C2 plus) positive cell expression predominantly located in lamina I & II and mild expression in lamina V in the T10-L1 and L4-S1 spinal cord of hypersensitive rats, but not present in the saline control or recovered rats. These findings are similar to our findings in the enteric nervous system of the colon where TNBS inflammation induced the expression of NR1111 and NR1011 (Zhou et al 2006).
The NMDA NR1 subunit forms eight functional splice variants based on the presence or absence of three alternatively spliced exons, Exon 5 (N1), Exon 21 (C1) and Exon 22 (C2) (Durand et al 1992; Hollmann et al 1993; Nakanishi et al 1992; Sugihara et al 1992; Zhou et al 2006; Zhou & Verne 2008). Splicing out the exon segment that encodes the C2 cassette removes the first stop codon, resulting in a new open reading frame that encodes an unrelated sequence of 22 amino acids (C2 minus) before a second stop codon is reached (Price et al 2006; Petrenko et al 2003; Qin et al 2002; Ren & Dubner 1999; Rumbaugh et al 2000). The presence of N1 enhances the current flow through the NMDA receptors and prevents glycine independent stimulation of the receptors by spermine (Kashiwagi et al 2001; Zheng et al 1994). The C1 cassette contains four serines that are known phosphorylation sites and an ER retention signal. Phosphorylation of the serines blocks the ER retention signal and allows transport of the receptors to the plasma membrane (Carroll & Zukin 2002; Scott et al 2001; Xia et al 2001). The presence of the C2 cassette alters the C-terminus of the protein and changes the targeting of the protein for different cell structures (Durand et al 1993; Zukin & Bennett 1995).
The NR1001 has N1 (N terminal) and C1 (C terminal) spliced out, while the C2-plus (C terminal) is spliced in; NR1011 has the N1 spliced out, while the C1 and C2-plus are spliced in; NR1100 has C1 and C2-plus spliced out, while the N1 is spliced in; NR1000 has N1, C1 and C2-plus spliced out, while C2-minus spliced in; NR1 111 has all three exons present (Durand et al 1993; Zukin & Bennett 1995).
The functional properties of NMDA receptors depend on the NR1 splice variant combinations. NR1 receptors, lacking the N-terminal exon, exhibit a high affinity for NMDA and marked potentiation by spermine (Durand et al 1993). Presence of the N1 insert reduces the apparent affinity of homomeric NR1 receptors for NMDA and almost abolishes potentiation by spermine at saturating glycine (Durand et al 1993). The N1 insert also increases current amplitude (Hollmann et al 1993; Zheng et al 1994). In the current study, we found NR1001 expression in spinal cord T10-L1 and L4-S1 of saline control and recovered rats. Durand et al. has shown that NR1001 does not generate significant current (Durand et al 1993), which suggests that the spinal NR1001 may not be involved in nociceptive processing or be part of a functional receptor. An early study demonstrated that the presence of the N1 cassette caused a decrease in the open time of the NMDA receptor channel (Rumbaugh et al 2000) and decreased the ability of spermine to potentiate the NMDA-mediated current (Mott et al 1998). Without the N1 cassette, there was an increased pH, Zn++, and spermine (Traynelis et al 1995). Our current findings suggest that the NMDA receptors expressed in the spinal cord of hypersensitive rats may be more responsive to glutamate released from primary afferent neurons resulting in enhanced synaptic connections. The enhanced synapses may be one mechanism leading to central sensitization as we found that NR1111 has the N1 insert present in the spinal cord of the hypersensitive group of rats. Splicing in the N1 insert increases current amplitude (Hollmann et al 1993; Zhou & Verne 2008). Therefore, NR1111 may be a key protein to increase the NMDA receptor activity leading to hypersensitivity in these rats. In our immunohistochemistry study of the spinal cord, the NR1-N1 was present in lamina I, II and lamina V in hypersensitive rats, but was not detected in recovered and normal control rats.
Phosphorylation of NMDA receptors is thought to be an important factor for cell modulation, regulation, and neuronal plasticity in response to a variety of stimuli. It may also play a critical role in long term potentiation (LTP) underlying memory formation. A number of residues that undergo phosphorylation are contained within a single alternatively spliced exon in the C-terminal domain, the C1 cassette (Tingley et al 1993; Zukin & Bennett 1995). Our immunohistochemistry results revealed that the NR1-C1 protein was also expressed in lamina I, II and mildly expressed in lamina V of the spinal cord from T10-L1 and L4-S1 in hypersensitive rats. NR1-C1 contains an endoplasmic reticulum (ER) retention signal suggesting that the presence of C1 may alter translocation of NMDA receptors. The ER works as a control center in coordinating the sequential assembly of multi-subunit protein complexes within the ER and in defining the number of receptors expressed at the plasma membrane (Bichet et al 2000; Blount et al 1990; Brodsky & McCracken 1999). Scott et al (2001) found that the ER regulates plasma membrane delivery of NMDA receptors. Therefore, the presence of the C1 cassette in the NR1 splice variants found in hypersensitive rats following TNBS inflammation may indicate that the NMDA receptors are targeted for dispersal to synapses that are responsible for processing nociceptive information.
NR1-C2-plus expression was located in lamina III, IV of the spinal cord in recovered rats, and normal control rats. This result suggested that the NR1-C2-plus labeling was primarily a response to afferent input carried by low-threshold large fibers. The NR1-C2-plus expression was also found in the ventral horn areas suggesting that the NR1-C2-plus may be involved in some motor neuron activity and /or possibly involved in the viscera-sympathetic reflex.
Enhanced expression of the NR1 subunits enhances current flow through NMDA receptors and therefore is likely to be an integral mechanism of peripheral and central sensitization (Kashiwagi et al 1997; Zheng et al 1994; Zhou et al 2008). These molecular changes may occur on presynaptic terminals of primary visceral afferents that terminate in the dorsal horn and/or on post-synaptic neurons. Reasons for enduring changes in NR1 subunits in some rats and not others are topics for future investigation.
Regardless of the exact mechanisms involving NR1 NMDA receptor subunits, increased impulse activity in afferents innervating the colon and rectum is likely to increase the excitability of dorsal horn neurons. The chronicity of the tonic afferent input from the viscera to the spinal cord may then lead to sensitization of dorsal horn nociceptive neurons and would be associated with visceral hypersensitivity. Somatic hypersensitivity could also develop as a consequence of long term tonic impulse activity and convergence of visceral and somatic primary afferent impulse inputs onto the same dorsal horn nociceptive neurons. In other words, somatic hypersensitivity would develop over time as a result of increased sensitization of visceral/somatic convergent neurons.
NMDA receptors are important in the induction and maintenance of central sensitization. Even when the peripheral inflammatory injury to the colonic myenteric plexus is healed, changes in central NMDA receptor subunits in response to transient colonic inflammation may have profound implications and could be involved in the pathophysiology of chronic visceral hypersensitivity seen in some patients with post-infectious IBS and other chronic visceral pain disorders (Chaudhary & Truelove 1962; Spiller 2003; McRoberts et al 2001; Traub et al 2002; Willert et al 2004; Zhou & Verne 2008).
In our current study, increased NMDA receptor expression or recombined new NMDA receptor expression (such as NR1011, NR1111) in hypersensitive rats is considered to enhance, prolong and alter activity in nociceptive circuitry in the spinal cord. This enhanced activity may play a critical role in more prolonged and maintained pain states, such as visceral and somatic hypersensitivity.
Conclusions and Summary
Persistent alterations in the spinal-NMDA receptor as a result of transient colonic inflammation may be an underlying mechanism of visceral and somatic hypersensitivity in a subset of rats that recovered histologically from colitis. These chronic changes in the NMDA receptors in the central nervous system may be ideal targets for the development of new pharmacologic agents to treat chronic hypersensitivity. This may lead to new therapeutic approaches with which to treat difficult functional bowel disorders such as IBS.
4. Experimental Procedures
Animals and Experimental Design
Male Sprague-Dawley rats (200g-250g) were treated with either 20 mg/rat trinitrobenzene sulfonic acid (TNBS, Sigma Chemical Co.) in 50% ethanol (n=27); or an equivalent volume of saline (n=10). The rats were housed in pairs under constant temperature and humidity with 12-hour light-dark cycles, and were given free access to food and water. Prior to administration of TNBS in the colon, the animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). TNBS or saline was delivered with a 24 gauge catheter inserted into the lumen of the colon 3-4cm proximal to the anus. The rats were kept in a vertical position for several minutes to avoid leakage of the instilled intracolonic solutions. Rats were monitored daily for changes in body weight, body condition, physical appearance, and behavior during the 16 weeks following treatment. No adverse events were observed in any of the rats. All procedures were approved by the University of Florida, Ohio State University, and North Florida/South Georgia Veterans Health System Institutional Animal Care & Use Committees. Somatic and visceral pain testing were performed 16 weeks following administration of TNBS or saline under blinded conditions and the order of testing was counterbalanced across groups. Behavioral testing was done following a 12 hour fast. The rats were euthanized after all behavior tests were completed and the spinal cord (T10-L1; L4-S1) was removed for 2-D western blotting and immunohistochemistry studies. The colon was removed from each rat for histopathological study.
Visceral/Somatic Pain Testing
Mechanical and thermal behavioral tests were performed using an automated Von-Frey and Hargreaves device to evaluate somatic hyperalgesia. The colonic distension test was performed using an automated distension device to evaluate visceral pain thresholds.
Visceral Pain Testing
Colonic Distension
A balloon (3 cm-long, 1.5 cm max diameter) made of polyethylene was secured to tubing and attached to an automated distension device (G&J Electronic Inc.,Toronto, Canada) to perform colonic distension. The balloon was lubricated and placed into the rat's distal colon so that the tip of the balloon was 1 cm from the anus. The rats were allowed 5-10 minutes to acclimatize before behavior testing began. The rats were restrained in a plastic containment device and received phasic distension (0-80 mmHg in 5 mmHg ascending increments) of the colon until the first contraction of the testicles, tail, or abdominal musculature occurred. This threshold response was considered to reflect a behavioral index of visceral sensitivity in response to a nociceptive stimulus as previously described (Ness & Gebhart 1998; Wesselmann et al 1998; Zhou et al 2008a). The colonic distensions were repeated 4 times with 10 minute inter-stimulus intervals and the mean pressures at response threshold were recorded for each rat.
Somatic Pain Testing
Mechanical Stimulation
Mechanical hypersensitivity was measured using an electronic Von Frey device (Dynamic Plantar Aesthesiometer; Electronic Unit/Filaments and Calibration Weights, from Ugo Basile S.R.L. Biological Research Apparatus, Italy). Rats were placed on a wire mesh floor in a plastic enclosure. A computer driven filament was then extended up through the mesh floor and exerted an increasing amount of pressure (maximum 50g) onto the rat's hindpaw. The force in grams required for the rat to withdraw its hind-paw was defined as the mechanical pain threshold. Both hindpaws were tested in each rat. The stimulus was repeated 4 times following a 5 minute interstimulus interval and the mean was calculated for each rat's hindpaw.
Thermal Stimulation
A thermal stimulus was delivered using the Hargreave's technique (7371 Plantar Test, Ugo Basile S.R.L. Biological Research Apparatus, Italy) (Hargreaves et al 1998). Rats were placed in a plastic enclosure on a clear plastic glass surface and the heat stimulus was applied underneath the chamber. The time in seconds (latency) until the rat withdrew its hindpaw was recorded for each rat. Both hindpaws were tested in each rat. The stimulus was repeated 4 times following a 5 minute interstimulus interval and the mean was calculated for each rat's hindpaw.
Spinal-NMDA NR1 Splice Variant Expression
Tissues preparation for Two-dimensional polyacrylamide gel electrophoresis
Following the visceral/somatic pain testing, rats were euthanized using sodium pentobarbital (120mg/kg, IP) and perfused transcardially with 150-200 ml cold saline. Following perfusion, the dorsal spinal cord (T10-L1 and L4-S1) was retrieved from control rats, recovered rats, and hypersensitive rats respectively, to detect the expression / distribution of NMDA NR1 receptor splice variants.
Two-dimensional polyacrylamide gel electrophoresis
Sample preparation of Two-dimensional polyacrylamide gel electrophoresis
Samples were homogenized in 1.3 ml of osmotic lysis buffer containing protease inhibitor stock and nuclease stock. A portion of each sample was removed and protein concentration determined using the BCA Assay (Pierce Chemical Co., Rockford, IL). Samples were then lyophilized, redissolved to 4 mg/ml in SDS Boiling Buffer and heated in boiling water bath for 5 minutes before performing two-dimensional (2D) electrophoresis.
Two-dimensional polyacrylamide gel electrophoresis
2D electrophoresis was performed according to the carrier ampholine method of isoelectric focusing (Kendrick Labs, Inc., Madison, WI) as follows: Isoelectric focusing was carried out in a glass tube of inner diameter 2.0 mm using 2% pH 3.5-10 ampholines (GE Healtcare, Piscataway, NJ) for 9600 volt-hrs. One mg of an IEF internal standard, tropomyosin, was added to the sample. This protein migrates as a doublet with lower polypeptide spot of MW 33,000 and pI 5.2. Following equilibration for 10 minutes in ‘O’ buffer (10% glycerol, 50mM dithiothreitol, 2.3% SDS and 0.0625 M tris, pH 6.8), the tube gel was sealed to the top of a stacking gel that overlaid a 10% acrylamide slab gel (0.75mm thick). SDS slab gel electrophoresis was carried out for about 4 hrs at 12.5 mA/gel. After slab gel electrophoresis the gel was placed in transfer buffer (12.5mM Tris, pH 8.8, 8.6 mM Glycine, 10% MeOH) and transblotted onto a PVDF membrane overnight at 200mA and approximately 100 volts/two gels. The following proteins (Sigma Chemical Co.) were added as molecular weight standards to a well in the agarose that sealed the tube gel to the slab gel: myosin (220,00), phosphorylase A (94,000), catalase (60,000), actin (43,000) carbonic anhydrase (29,000) and lysozyme (14,000).
Western blot procedure
Individual membranes from each group (saline control rats, recovered rats and hypersensitive rats) were used throughout the entire 2-D experiment. Primary antibodies (Courtesy of Dr. Caudle's lab) of NMDA NR1-C1 (1:500), NR1-N1 (1:500), NR1-C2-plus (1:1000) and NR1-C2-minus (1: 400) were used and incubated overnight at 4°C. Each antibody was repeated 3 times by using 3 individual rats with 3 individual 2-D Western blot membranes. The western blots were developed using enhanced Chemiluminescent detection and radiographic film. Primary antibodies were removed with western blot stripping buffer (Pierce Co.) for multiple antibody probings. The efficiency of the stripping procedure was verified by using the secondary antibody and re-exposing the membrane to film.
Immunohistochemistry
Sample preparation of immunohistochemistry
Following the visceral/somatic pain testing, the rats were euthanized using sodium pentobarbital (120mg/kg, IP) and perfused transcardially with 150-200ml cold saline. The T10-L1 and L4-S1 spinal cord were retrieved from control rats, recovered rats and hypersensitive rats, to detect the distribution / location of NMDA NR1 receptor splice variants in spinal T10 – L1 and L4-S1. Following cryoprotection, six-micron sections were cut with a cryostat at −20°C. Spinal cord tissue from T10-L1 and L4-S1 were cut and 4-5 slices were collected at every 70th-100th section serially and mounted on bond-rite slides.
Staining procedure for immunohistochemistry study
All staining procedures were performed according to the Histology Tech Service (Gainesville, FL) protocol as follows: The slices were removed from freezer and placed in acetone for 10 minutes at 4°C. Slices were rinsed in Tris buffer 3 times for 5 minutes each. Then, 3% H2O2 was applied and then the slides were rinsed in Tris buffer again. Protein block (10% normal goat serum in Tris buffer) was applied for 10 minutes, then the slides were rinsed again in Tris buffer. Biotin solution (Avidin Biotin Blocking Kit-Vector Laboratories) was applied for 10 minutes and then the slides were rinsed in Tris buffer again. Primary antibody NR1-N1, dilution 1:600; NR1-C1, dilution 1:1000; NR1-C2-plus, dilution 1:1500; NR1-C2-minus, dilution 1:400) was applied overnight at 4°C. For negative controls, 10% normal serum was applied in place of primary antibody. Following 3 rinses, secondary antibody was applied for 45 minutes to 1 hour, and then ABC reagent (Vector Laboratories) was applied for 60 minutes, and rinsed with Tris buffer. DAB was applied under the microscope and moved to water once staining was sufficient. The counterstaining was performed as follows: slices were submerged in hematoxylin 7211 (RAS Inc) for 30 seconds. Slides were then rinsed in tap water for 1 minute. Then, slides were dipped 5 times in 95% alcohol, followed by 3 changes in 100% alcohol for 3 second each, and then cleared in 3 changes of Xylene (RAS Inc) for 30 second each. Coverslips were used to Cytoseal (RAS Inc).
Quantitative Immunohistochemistry- Image Capture
All photomicrogaphs were obtained using a SPOT RT digital Scanning Camera from Diagnostic Instruments (Sterling Heights, MI) attached to a Nikon E600 microscope system at 1000X magnification, and then were saved in TIFF format for further evaluation (Matkowskyi et al 2000, 2003a, 2003b). The NR1 subunits abundance was quantified by determining the cumulative signal strength of the digital image file of a histologically relevant region of interest. The image of the spinal cord dorsal horn neurons were measured by groups, which they were divided from lamina I&II, lamina III & IV and lamina V from T10 to L1 and from L4 to S1. A total of 20-30 spinal sections from each group (group of T10-L1; group from L4-S1) were selected from each rat for each NR1 subunit antibody (NR1-N1; NR1-C1; NR1-C2 plus; NR1-C2 minus) and positive cell expression of quantification (Image Capture) was obtained.
Histopatholgical Evaluation of Colonic Tissues
Following euthanasia, the colon was also removed from all rats and processed for histopathology. Cross sections of the colons were fixed in formalin, dehydrated in xylene and alcohol, and embedded in paraffin. All of the colonic sections were cut into 50μm sections and evaluated using standard techniques for H & E staining. Microscopic evaluation of tissue was done in a blinded fashion by a single pathologist.
Statistical Analysis
All image data of immunohistochemistry reported here are expressed as energy units per pixel (eu/pix); the two ways AVOVA follow by Bonferroni Post-tests. The behavioral studies data classified the groups of rats by using Frequency distribution and one way AVOVA follow by Tukey post test. We performed a correlation between the splice variant expression and the behavioral tests. Pearson correlations between NMDA NR1-C1, NR1-N1, and NR1-C2 plus receptor expression as measured by energy units/pixel (eu/pix) and colonic distension, mechanical stimuli (Von Frey) and thermal stimuli (Hargreaves test) were analyzed. All statistics were run using Prism version 6 and SAS system. In all instances, data are expressed as the mean ± SD.
Acknowledgements
Dr. Verne is supported by a NIH RO1-NS053090 award (PI: GN Verne) and a VA Merit Review Award (PI: GN Verne) from the Medical Research Service at the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog Neurobiol. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. The III loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- Blount P, Smith MM, Merlie JP. Assembly intermediates of the mouse muscle nicotinic acetylcholine receptor in stably transfected fibroblasts. J Cell Biol. 1990;111:2601–2611. doi: 10.1083/jcb.111.6.2601. 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. 2002. [DOI] [PubMed] [Google Scholar]
- Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Semin Cell Dev Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Castroman PJ, Ness TJ. Ketamine, an N-methyl-D-aspartate receptor antagonist, inhibits the spinal neuronal responses to distension of the rat urinary bladder. Anesthesiology. 2002;96(6):1410–9. doi: 10.1097/00000542-200206000-00021. [DOI] [PubMed] [Google Scholar]
- Cervero F. Visceral nociception: peripheral and central aspects of visceral nociceptive systems. Philos Trans R Soc Lond B Biol Sci. 1985;308:325–337. doi: 10.1098/rstb.1985.0033. [DOI] [PubMed] [Google Scholar]
- Cervero F, JM Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol. 2004;61:45–54. doi: 10.1002/neu.20084. [DOI] [PubMed] [Google Scholar]
- Chaudhary NA, Truelove SC. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med. 1962;31:307–322. [PubMed] [Google Scholar]
- Cross SA. Pathophysiology of pain. Mayo Clin Proc. 1994;69:375–383. doi: 10.1016/s0025-6196(12)62225-3. 1994. [DOI] [PubMed] [Google Scholar]
- Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51(Suppl 1):i67–i71. doi: 10.1136/gut.51.suppl_1.i67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Dunphy RC, Bridgewater L, Price DD, Robinson ME, Zeilman CJ, 3nd, Verne GN. Visceral and cutaneous hypersensitivity in Persian Gulf War veterans with chronic gastrointestinal symptoms. Pain. 2003;102:79–85. doi: 10.1016/s0304-3959(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Durand GM, Gregor P, Zheng X, Bennett MV, Uhl GR, Zukin RS. Cloning of an apparent splice variant of the rat N-methyl-D-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc Natl Acad Sci U S A. 1992;89:9359–9363. doi: 10.1073/pnas.89.19.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci U S A. 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1998;32:77–88. doi: 10.1016/0304-3959(88)90026-7. 1998. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Holzer P, Michl T, Danzer M, Jocic M, Schicho R, Lippe IT. Surveillance of the gastrointestinal mucosa by sensory neurons. J Physiol Pharmacol. 2001;52:505–521. [PubMed] [Google Scholar]
- Kashiwagi K, Pahk AJ, Masuko T, Igarashi K, Williams K. Block and modulation of Nmethyl-D-aspartate receptors by polyamines and protons: role of amino acid residues in the transmembrane and pore-forming regions of NR1 and NR2 subunits. Mol Pharmacol. 1997;52:701–713. doi: 10.1124/mol.52.4.701. [DOI] [PubMed] [Google Scholar]
- Klatt S, Böck W, Rentschler J, Beckh K, Adler G. Effects of tropisetron, a 5-HT3 receptor antagonist, on proximal gastric motor and sensory function in nonulcer dyspepsia. Digestion. 1999;60(2):147–52. doi: 10.1159/000007640. [DOI] [PubMed] [Google Scholar]
- Lu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: implications for the treatment of mechanical allodynia. Pain. 1995;61:383–390. doi: 10.1016/0304-3959(94)00195-K. [DOI] [PubMed] [Google Scholar]
- T Mach T. The brain-gut axis in irritable bowel syndrome--clinical aspects. Med Sci Monit. 2004;10:RA125–RA131. [PubMed] [Google Scholar]
- Matkowskyi KA, Schonfeld D, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software Photoshop and Matlab. J Histochemistry Cytochem. 2000;48:303–311. doi: 10.1177/002215540004800216. [DOI] [PubMed] [Google Scholar]
- Matkowskyi KA, Cox R, Jensen RT, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochemistry Cytochem. 2003a;51(2):205–214. doi: 10.1177/002215540305100209. [DOI] [PubMed] [Google Scholar]
- Matkowskyi KA, Keller K, Glover S, Lori Kornberg, Tran-Son-Tay R. Expression of GRP and its receptor in well-differentiated colon cancer cells correlates with the presence of Focal adhesion Kinase phosphorylated at Tyrosines 397-407. J Histochemistry Cytochem. 2003b;51(8):1041–1048. doi: 10.1177/002215540305100807. [DOI] [PubMed] [Google Scholar]
- Mayer EA, GF Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Mayer EA, T Lembo T, L Chang L. Approaches to the modulation of abdominal pain. Can J Gastroenterology. 1999;13(Suppl A):65A–70A. [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizón JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Gepetti P, Bunnett NW, Mayer EA. Role of peripheral n-methyl-d-aspartate (nmda) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Mertz H. Review article: visceral hypersensitivity. Aliment Pharmacol Ther. 2003;17:623–633. doi: 10.1046/j.1365-2036.2003.01447.x. [DOI] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci. 1998;1(8):659–67. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Axel R, Shneider NA. Alternative splicing generates functionally distinct N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1992;89:8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41(4):505–12. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distention as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudoaffective reflexes in the rat. Brain Res. 1998;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7(8):529–35. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- Qin C, Chandler MJ, Foreman RD, Farber JP. Upper thoracic respiratory interneurons integrate noxious somatic and visceral information in rats. J Neurophysiol. 2002;88:2215–2223. doi: 10.1152/jn.00120.2002. [DOI] [PubMed] [Google Scholar]
- Ren K, R Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain. 1999;13:155–163. [PubMed] [Google Scholar]
- Rumbaugh G, Prybylowski K, Wang JK, Vicini S. Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol. 2000;83(3):1300–6. doi: 10.1152/jn.2000.83.3.1300. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992;185:826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- Sun L, FL Margolis FL, MT Shipley MT, MS Lidow MS. Identification of a long variant of mRNA encoding the NR3 subunit of the NMDA receptor: its regional distribution and developmental expression in the rat brain. FEBS Lett. 1998;441(3):392–6. doi: 10.1016/s0014-5793(98)01590-7. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Roche KW, Thompson AK, Huganir LR. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993;364:70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Zhai Q, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression in the spinal cord and the visceromotor reflex. Neuroscience. 2002;113(1):205–11. doi: 10.1016/s0306-4522(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268(5212):873–6. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Central mechanisms in pain. Med Clin North Am. 1999a;83:585–596. doi: 10.1016/s0025-7125(05)70125-5. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999b;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain3. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Wesselmann U, Czakanski PP, Affaitat G, Giamberardino MA. Uterine inflammation as a noxious visceral stimulus: behavioral characterization in the rat. Neurosci Lett. 1998;246:73–76. doi: 10.1016/s0304-3940(98)00234-1. [DOI] [PubMed] [Google Scholar]
- Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126:683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology. 2001;41:714–723. doi: 10.1016/s0028-3908(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Durand GM, Bennett MV, Zukin RS. Mutagenesis rescues spermine and Zn2+ potentiation of recombinant NMDA receptors. Neuron. 1994;12:811–818. doi: 10.1016/0896-6273(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats of rats following TNBS-Induced colitis. Pain. 2008a;134(12):9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Caudle RM, Price DD, Del Valle-Pinero AY, Verne GN. Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain. 2006;2:3. doi: 10.1186/1744-8069-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain. 2008b;139(1):218–224. doi: 10.1016/j.pain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Verne GN. NMDA receptor and colitis: basic science and clinical implications. Reviews in Analgesia. 2008;10(1):33–43. doi: 10.3727/154296108783994013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]