Abstract
Objectives:
We previously reported that saturated fat (SAT) enriched diets increase arterial cholesteryl ester (CE) deposition, especially from LDL-selective uptake (SU), and this was associated with increased arterial lipoprotein lipase (LpL). We now questioned how n-3 fatty acid rich diets influence arterial cholesterol delivery and arterial LpL levels.
Methods and Results:
C57BL/6 mice were fed chow or eucaloric high fat diets enriched in SAT or fish oil (n-3) for 12 weeks, and then injected with double radiolabeled or fluorescent-labeled human LDL to separately trace LDL-CE and LDL-apoB uptake. SAT and n-3 diets increased plasma cholesterol levels similarly; n-3 diets lowered plasma triglyceride concentrations. SAT increased arterial LDL-SU with significantly higher CE infiltration into aortic media. In contrast, n-3 markedly reduced total LDL uptake and CE deposition and abolished SU with LDL localized only in aortic intima. Disparate patterns of CE deposition between diets were consistent with distribution of arterial LpL - SAT diets induced higher LpL levels throughout the aorta; n-3 diets decreased LpL levels and limited LpL expression to the aortic intima.
Conclusions:
n-3 rich diets decrease arterial total LDL delivery and abrogate LDL-SU in parallel with changing arterial wall LpL expression and distribution.
Keywords: fatty acids, fish oil, lipoprotein lipase, LDL, atherosclerosis
Arterial cholesterol uptake and deposition are major contributors to atherogenesis. LDL retention in atherosclerosis-susceptible sites results from increased flux into the arterial wall, as well as from reduced lipoprotein efflux. While LDL uptake via LDL receptors (LDLR) is a major pathway and is critical for cholesterol delivery to many cell types 1, significant amounts of LDL are delivered to certain tissues, such as the arterial wall, independent of LDLR. These “non-classical” pathways include scavenger receptors which may play important roles in pathological arterial lipid accumulation 2. We and others have previously demonstrated that LDL-core lipids, particularly cholesteryl esters (CE), can be delivered to cells without concomitant cellular uptake and internalization of whole LDL particles by a process known as selective uptake (SU) 3-5. SU from HDL via scavenger receptor type B I (SR-BI) has been well characterized and is involved in steroidogenesis and reverse cholesterol transport. LDL SU was observed in mice, and among the sites of selective uptake of LDL lipid were tissues that express abundant LpL such as muscle, heart and adipose 6. We previously demonstrated in cultured cells that LDL-SU was mediated via non-SR-BI-mediated pathways and that SU was markedly increased by LpL 7. Consistent with these findings, muscle LDL SU was significantly increased in mice over-expressing human LpL in muscle 5. Moreover, feeding high saturated fat (SAT) diets markedly increased the contribution of SU to total arterial LDL-CE deposition 4- in apolipoproteinE (apoE) knockout mice, as much as 50% or more of total LDL-CE uptake was accounted for by SU. Thus, we suggest that this mechanism contributes an additional pathway for CE delivery in LpL-expressing cells, e.g., macrophages, and may contribute to pathological accumulation of CE.
It is of interest that Corey and Zilversmit and others reported a direct correlation between arterial LpL and extent of aortic cholesterol deposition 8, 9. In humans, atherosclerotic lesions are rich in LpL 10. In contrast to the well-documented adverse effects of saturated fats on cardiovascular disease (CVD) and atherosclerosis, consumption of unsaturated fats, particularly those enriched in very long chain omega-3 (n-3) fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can reduce the risk of CVD 11. Although a number of mechanisms have been postulated for anti-atherogenic roles of n-3 fatty acids, little is known about how n-3 fatty acids might affect pathways in the early development and progression of atherosclerosis, i.e., cholesterol delivery at the level of the arterial wall.
In the current studies, we show that the n-3 fatty acid rich diet markedly reduces arterial LDL whole particle uptake and LDL-CE SU relative to a SAT diet and that these effects are independent of plasma cholesterol levels. These changes in arterial LDL delivery and deposition were related to changes in LpL expression and distribution in the arterial wall. Our studies suggest that n-3 fats decrease arterial CE deposition by reducing whole LDL particle uptake and abolishing arterial SU from LDL.
METHODS
For expanded Materials and Methods please see http://atvb.ahajournals.org.
Feeding Protocols
Four-week-old male C57BL/6 mice weighing 15-18 g were placed on a normal chow (4.5% fat, w/w), or a high fat diet (21% fat, w/w) enriched in either n-3 or saturated fats (SAT) for 12 weeks (n > 10). n-3 and SAT diets were eucaloric with 42% of total calories from fat (see supplemental Table 1 for fatty acid composition of each diet). The fat content of the SAT diet consisted of 71% saturated fat from coconut oil, 19% monounsaturated fat from olive oil, and 9% polyunsaturated fats from safflower and corn oil. In the n-3 diet, half of the total fat was from menhaden oil enriched in EPA and DHA with saturated, mono- and poly-unsaturated fat contributing 8%, 8%, and 6%, respectively, w/w. During the feeding period, blood was collected weekly and measured for free fatty acids (FFA), triglycerides (TG) and total cholesterol (Chol) using enzymatic assays following the manufacturer's procedures as previously detailed 4.
LDL preparation and labeling
LDL (d = 1.025-1.055 g/ml) was isolated from normolipidemic humans by sequential ultracentrifugation. Isolated LDL was labeled with two nonhydrolyzable markers, [3H] cholesteryl oleoyl ether (CEt) and [125I] tyramine cellobiose (TC), to trace LDL-CE core and whole LDL particle uptake, respectively 5. The calculated cholesterol/protein weight ratios of LDL ranged from 1.6-1.8 (n=20). Typical specific activities were 10-15 and 52-230 dpm/ng LDL protein for [3H] CEt and [125I] TC, respectively. For fluorescent microscopy studies, isolated LDL was labeled with BODIPY-C12 and Alexa to independently trace LDL-CE and whole LDL particle uptake. Preparation and characterization of fluorescent LDL were previously described 4. Notably, our labeling procedures did not modify LDL physical properties or induce LDL oxidation.
LDL uptake in aorta
At the end of each feeding period, mice were injected with double radiolabeled human LDL. Twenty-four hours after injection, animals were sacrificed and radioactivity derived from accumulated 3H and 125I was determined in whole aorta. SU was determined from the differences in actual LDL-CE delivery based on [3H] CEt (actual CE uptake) and CE uptake from LDL whole particle uptake estimated from 125I-TC, and LDL composition 4.
Alternatively, mice were injected with 200 μg double BODIPY-C12- and Alexa-labeled LDL after feeding. BODIPY-C12 and Alexa fluorescence were recorded with a laser scanning confocal microscope (Zeiss LSM-510) as previous described. To determine BODIPY-C12/Alexa ratios, we quantitated fluorescence intensity in the intima-medial layers of the aortic arch using ImageJ version 1.41. (We previously reported that changes in LDL and CE uptake as a result of diets were much more evident in proximal as compared to distal aorta and these results reported herein are from the proximal aorta 4.) The patterns of Alexa/BODIPY dual channel colocalization were confirmed by a threshold-based overlap analysis-a binary test of whether the 2 signals occur in the same or in different regions, by using ImageJ 1.41 with the Colocalisation plug-ins 12, 13.
LpL localization and quantification in the arterial wall
Arterial LpL content was analyzed using immunohistochemical and immunofluorescent staining of mouse aorta. For both procedures, aortal sections were fixed, permeabilized and blocked from non-specific background staining before antibody application, as described in expanded Methods. Aorta sections from mice that were injected with double fluorescent-labeled LDL were analyzed by immunohistochemistry for arterial LpL levels. This was performed by using rabbit polyclonal anti-human LpL antibodies and ExtrAvidin Alkaline Phosphatase staining kit (Sigma-Aldrich) as described previously 4. The microscope settings and intensity analyses for arterial LpL are described in detail in expanded Methods.
In separate analyses, arterial LpL content was assayed by immunofluorescence. Pre-stained aorta sections, processed as described in expanded Methods, were incubated with a primary anti-LpL antibody (Santa Cruz) and a secondary antibody conjugated with Alexa Fluor 647 (Molecular Probes), followed by counterstaining with a cell nucleus marker-SYTOX Green (Molecular Probes). Labeled nuclei and LpL were visualized using a laser scanning confocal microscope, LSM-510 META, as described in expanded Methods.
To quantitate arterial immunofluorescent stained LpL content on each optical section, regions of interests (ROI), either aortic intima or the aortic media, were defined based primarily on the location of cell nucleus staining (SYTOX Green), which helps to define arterial morphological structure. All LpL markers within each ROI were semi-automated counted utilizing the ImageJ built-in function Analyze Particle 14. Detection, counting and analysis of LpL are described in detail in expanded Methods.
Statistical analyses
Student t-tests of group means were used for comparing endpoints and analysis of variance (ANOVA) was used to evaluate potential interactions between the diets. Statistical significance was determined at the level of p<0.05.
RESULTS
Effects of diet on body weight and plasma lipid profiles
Mice fed SAT diets showed higher and more rapid weight gain compared to mice fed a chow or n-3 diet; this effect was noted in the first 2-3 weeks of the feeding (p<0.05). After 12 weeks of feeding, SAT fed mice weighed 27.7 ± 1.3 g, while chow and n-3 fed mice weighed less, 23.0 ± 0.9 g and 21.6 ± 0.6 g, respectively (p<0.001 for each vs. SAT, supplemental Figure 1).
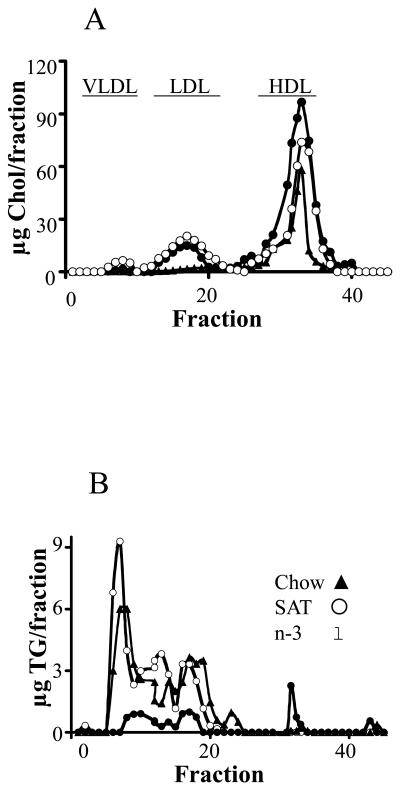
Each diet achieved the expected differences in fatty acyl composition in plasma (see supplementary Results, and supplemental Table 2). Plasma lipid profiles were determined at the end of the feeding periods (Table 1). The SAT diets led to 2-fold greater plasma FFA and TG levels (p<0.01) in mice compared to chow diets, a finding consistent with our previous report 4. In contrast, mice fed the n-3 diet had ∼ 40 % lower plasma fatty acid and ∼70% lower TG levels than chow fed mice. Both SAT and n-3 diets led to similar increases in plasma total cholesterol levels compared to mice fed chow (p<0.01), presumably due to higher cholesterol content in these two diets (0.2% w/w) compared to the chow diet (0.02% w/w). Changes in TG levels were entirely related to increase or to loss of VLDL pools as determined by fast performance liquid chromatography (FPLC) in SAT vs. n-3 fed mice (Figure 1). These results are in agreement with other studies demonstrating that n-3 diets inhibit TG synthesis and lower plasma TG levels 15. We previously reported that plasma clearance of injected radiolabeled LDL was not different in SAT or chow fed mice 4 – using similar methodology in out current studies, LDL fractional catabolic rates (FCR) were not significantly changed in n-3 feeding as compared to chow or SAT diets (0.12 ± 0.04, 0.12 ± 0.04 and 0.11 ± 0.03 pools/ h for chow, SAT and n-3, respectively).
Table 1.
Plasma lipid profiles of mice fed a chow, SAT or n-3 diet. Four-week-old mice were fed chow, SAT and n-3 diets for 12 weeks (n>8). Blood samples were collected and determined for total cholesterol (Chol), triglyceride (TG) and free fatty acids (FFA) levels as described in Methods. The results are expressed as the mean ± SD
| TG (mg/dl) |
Chol (mg/dl) |
FFA (mM) |
|
|---|---|---|---|
| Chow (n=12) |
56.65±5.84 | 67.52±3.06 | 0.42±0.26 |
| SAT (n=12) |
130.96±6.97 * | 102.24±11.10 * | 0.82±0.2 * |
| n-3 (n=8) |
21.39±4.68 †,‡ | 111.78±11.24 † | 0.25±0.10 ‡ |
Significant differences between chow- and SAT- fed mice (p<0.01)
significant difference between n-3- and chow-fed mice (p<0.001)
significant difference between SAT-and n-3-fed mice (p<0.01).
Figure 1.
Plasma lipoprotein profiles in mice fed chow, SAT and n-3 diets. Total cholesterol (Chol) (A), and triglycerides (TG) (B) were determined for each fraction after separation by FPLC. Elution fractions 2-10, 12-22, and 25-35 represent elution zones for VLDL, LDL, and HDL, respectively.
Dietary effects on arterial LDL uptake
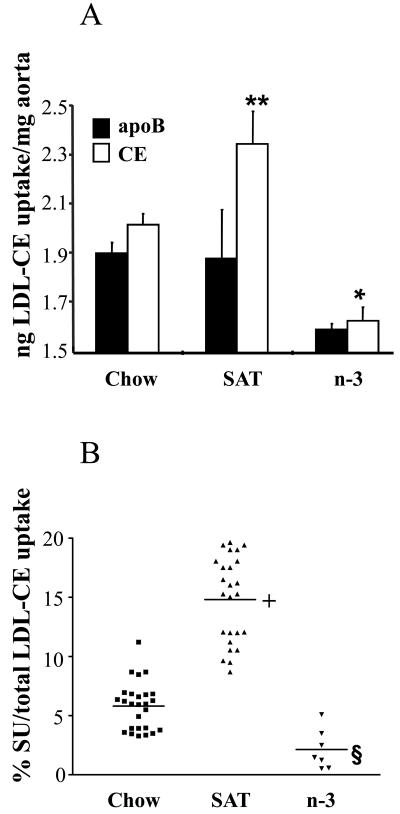
Since in contrast to SAT diets, n-3 fatty acid rich diets can diminish CVD risk 11, we compared, in the mouse model, differences in arterial LDL uptake and SU on each diet (Figure 2). The SAT diet markedly increased arterial LDL-CE uptake traced by [3H] CEt compared to the chow diet (Figure 2A), where there was no apparent significant increase in uptake of 125I-apoB (or whole LDL particles; this can be accounted for by dilution of the injected labeled LDL with endogenous lipoproteins (Figure 1, supplemental Table 2)). Thus, as judged by differences in whole LDL particle CE estimated uptake as actual measured CE uptake, the markedly increased LDL-CE uptake relative to LDL-apoB was associated with a 4- to 5-fold increase in SU. In contrast, mice fed a n-3 diet had a marked reduction of whole particle LDL uptake and LDL-CE uptake in the arterial wall. There was no obvious SU from LDL in mice fed the n-3 diet.
Figure 2.
Arterial LDL-CE calculated from apoB uptake and CE uptake measured directly (A). *, p<0.05, chow vs. n-3; **. p<0.05, chow vs. SAT. (B) Percent contribution of SU to total CE delivery in individual mice. Horizontal bars show means. +, p<0.001, SAT vs. chow; §, p<0.001, n-3 vs. SAT and chow.
When changes in SU induced by the different diets were plotted as the percent of total arterial LDL-CE delivery that is accounted for by SU in individual animals (Figure 2B), SU contributed ∼ 5-6% of total arterial LDL-CE delivery in mice fed a chow diet. Mice fed a SAT diet on average had a 4-fold increase of CE-uptake attributed to SU accounting for up to ∼20% of total LDL-CE delivery. However, in mice that were fed n-3 diets, contribution of SU to arterial CE mass was absent or almost absent; arterial LDL-CE delivery by SU only contributed a non-significant 2.3% of total LDL-CE uptake.
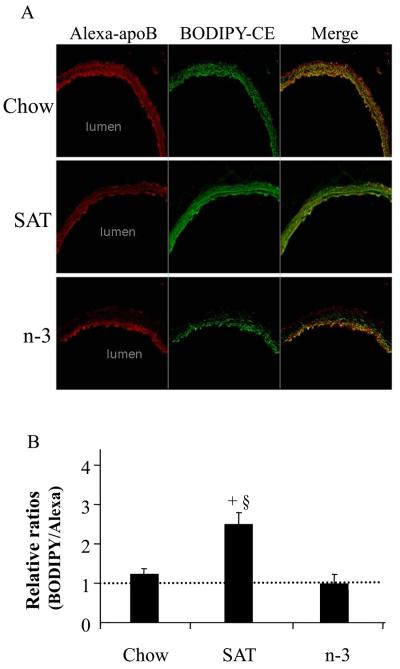
In parallel studies, mice were injected with fluorescent BODIPY/Alexa labeled LDL to trace LDL-CE and -apoB, respectively (Figure 3). We previously reported that use of fluorescent labeled LDL produced very similar data compared to measuring SU by isotopic studies 4. Fluorescent labeling also allowed localization of both whole LDL particle uptake and LDL-CE SU in specific layers of the arterial wall. Of note, there was no evidence of atherosclerotic lesions or fatty streaks in any of the arteries when examined by dissecting microscopy.
Figure 3.
Effects of diet on fluorescent LDL-apoB (Alexa) and LDL-CE (BODIPY) uptakes in aorta (A). (B) Ratios of arterial uptake of total LDL-CE to that of CE measured from whole LDL particle uptake (mean ± SE). A ratio >1 indicates SU. +, p<0.001, SAT vs. chow; §, p<0.001, SAT vs. n-3.
LDL-Alexa apoB uptake appeared slightly reduced in mice fed SAT diets compared to chow fed mice (likely due to dilution from non-labeled endogenous apoB lipoproteins) (Figure 3A). Still, the SAT diet strongly increased labeled LDL-CE uptake, indicating LDL-CE delivery via SU. SAT caused a higher arterial LDL infiltration compared to the chow diet, and this was also associated with much higher quantitative levels of LDL-CE deposition via SU (Figure 3B). In contrast, uptake of LDL-CE traced by BODIPY, and of LDL-apoB traced by red Alexa was markedly reduced in mice fed a n-3 diet (Figure 3A), in keeping with the observation that net LDL influx was diminished by n-3 feeding as observed with the isotopic studies. Again, there was no arterial SU in mice fed the n-3 diet (Figure 3B). Of interest, as shown in Figure 3A, whole LDL particle (Alexa-apoB) and LDL-CE (BODIPY) in arteries of chow and SAT fed groups were mostly in the intima-media regions of the aorta. However, in mice fed a n-3 diet, overall arterial LDL infiltration was much less; furthermore, nearly all LDL-CE and apoB co-localized strictly within in the intimal and/or sub-endothelial area with much less deposition in the media.
Patterns of fluorescence colocalization were confirmed by Colocalisation Plus-ins of ImageJ version 1.41 13. Fluorophore patterns did not change after extensive washing with heparin containing perfusate indicating that the fluorophores were located inside the arterial wall and associated with cells present in the arterial wall. Similar to our previous report 4, we found almost no SU, and little or no effects of diet on SU in distal aorta (data not shown). Thus, changes in SU are prominent in regions of aortic atherosclerosis susceptibility.
Dietary fat changes arterial LpL levels and distribution
Our previous studies showed that increased LpL in the arterial wall was linked to increased arterial LDL uptake and SU 4. We and others have demonstrated that LpL associates with lipoproteins and binds to cell surface proteoglycans, especially heparan sulfate proteoglycans (HSPG), to increase cellular uptake of LDL and other lipoproteins 5, 16. In our current studies, using densitometry of Western analyses, we found that the different diets resulted in absolute differences in arterial LpL protein expression. The SAT diet increased arterial LpL levels 2.6 fold compared to chow as previously reported 4. In contrast, the n-3 diet reduced arterial LpL compared to chow fed mice by 33% as averaged in two sets of experiments.
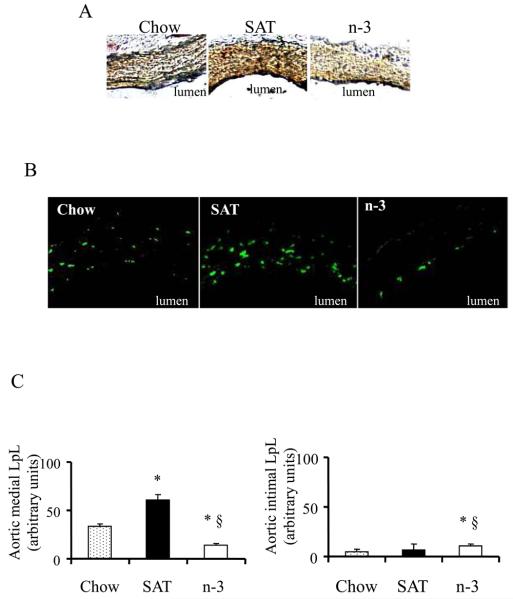
Because our data (Figure 3) indicate that SAT and n-3 diets strongly affect distribution of LDL and CE deposition within the arterial wall, we examined whether these differences correlated with changes in arterial LpL distribution (Figure 4). Arterial LpL content and distribution were analyzed both by immunohistochemistry and immunofluorescence. Consistent with Western blot analyses, densitometric analyses of both assays showed that SAT significantly increased while n-3 fed diets decreased intensity of arterial LpL staining when compared to chow (Figure 4A). While LpL was located consistently throughout the whole arterial section in chow and even more intensely in SAT fed mice. The n-3 diet led to a marked decrease in total LpL levels and markedly decreased aortic media LpL levels with remaining LpL located in the intimal/subendothelial layer (Figure 4A and 4B).
Figure 4.
Effects of diet on arterial LpL content and distribution. (A) Reddish-brown immumohistochemical staining shows LpL. (B) Confocal fluorescence of LpL. LpL is seen as the green dots. (C) Quantification of LpL in aortic media and intima (mean ± SE). *, p<0.05, chow vs. SAT or n-3; §, p<0.001, n-3 vs. SAT.
To further elucidate the effects of dietary fatty acids on arterial LpL distribution, arterial LpL was detected by rabbit polyclonal antibody with a secondary antibody conjugated with Alexa Fluor 647 (Figure 4B). Quantitation of fluorophore-tagged LpL showed that, in contrast to a more than 2-fold increase of aortic medial LpL in SAT (p<0.05), the n-3 diet markedly decreased aortic medial LpL levels by 80% as compared to SAT (p<0.01) (Figure 4C). Although aorta sections from n-3 fed mice had less LpL content on each optical section when compared to chow, there was a slight increase of intimal LpL in n-3 fed mice (p<0.05, Figure 4C), indicating arterial LpL can be disproportionally changed within different aortic layers (intima vs. media) after SAT vs. n-3 feedings. Thus, distribution patterns of LpL in the arterial wall of mice fed different diets were very similar to those of LDL-CE uptake traced by BODIPY, suggesting a role for LpL and its distribution in the quantity and the location of LDL deposition and LDL-SU in the arterial wall.
DISCUSSION
Atherosclerosis correlates with increased lipid deposition and lipoprotein retention in the arterial wall 17. In humans, excess cholesterol delivery occurs primarily through LDL, the predominant cholesterol carrying lipoprotein. Higher LDL blood concentrations with concomitant increased arterial wall cholesterol deposition are associated with increased risk of CVD. Moreover, extent and severity of atherosclerosis, a major contributor to CVD, is modulated by type of dietary fats. Diets enriched in saturated fat promote CVD; diets enriched in n-3 fats lower CVD morbidity and mortality 18, 19. Thus, to study an early event in atherosclerosis, we examined how different dietary fats directly influence delivery of LDL-CE to the arterial wall, differentiating between whole particle LDL and selective LDL-CE uptake before development of atherosclerotic lesions. We demonstrate that a SAT rich diet increases arterial LDL-CE uptake and markedly increase SU compared to mice fed a chow diet, and that a n-3 rich diet significantly reduces both arterial apoB and LDL-CE uptake, with relatively little or no SU. Our studies show that mice fed the n-3 diet resulted in a substantial reduction of plasma FFA and TG levels that were associated with a decrease in plasma triglyceride-rich particle lipoprotein pools. Plasma cholesterol levels, however, were increased by both SAT and n-3 diets to a similar degree compared to a chow diet, suggesting that the observed changes in LDL-SU were not secondary to differences in plasma cholesterol levels.
We previously demonstrated that plasma cholesterol levels and advancing atherosclerosis could explain, in part, changes in LDL uptake and SU in mice fed a SAT diet. In fact, using apoE null mice as a model, we found that with developing arterial atherosclerosis SU could contribute to almost 60% of total LDL cholesterol delivery to the arterial wall at high plasma cholesterol levels. These observations are consistent with other studies of atherosclerosis in humans and in animal models 4, 20. Interestingly, our current studies evaluating the effects of n-3 fatty acid diets did not follow this paradigm – even with increased levels of plasma cholesterol, the n-3 diet decreased LDL uptakes. Our results are consistent with studies by Weiner et al., who demonstrated that n-3 fatty acids reduced development of coronary atherosclerosis in pigs after a catheter injury, regardless of plasma lipid profiles 21. Since in the current study the effects of decreased total LDL uptake and SU in mice on n-3 diets compared to SAT cannot be attributed to differences in plasma cholesterol levels, other factors must underlie the effects of n-3 fatty acids. Our studies suggest that atherogenic effects of LDL could relate to the level and localization of arterial LpL. In our studies, we utilized both radioisotopes and fluorescent tags to trace LDL-CE and whole particle uptake in mouse artery and documented similar results obtained by both methods. Our novel approach of using double fluorescent-labeled LDL now enabled us to localize LDL-CE and LDL whole particle distribution in the arterial wall before development of atherosclerosis.
Arterial LpL content has been recognized to correlate with accumulation of arterial wall cholesteryl ester in rabbit models for atherosclerosis 8. Increases in arterial LpL link to the presence of macrophages and smooth muscle cells in atherosclerotic lesions. LpL enhances proliferation of vascular smooth muscle cells in vitro 22. Babaev et al. 23 demonstrated in mouse bone marrow transplant models, that focal expression of macrophage LpL stimulates atherosclerosis in mice. In vitro studies have demonstrated that LpL associates with lipoproteins and binds to cell surface proteoglycans, especially heparan sulfate proteoglycans (HSPG) 24, to promote the cellular uptake of lipoproteins by acting as a “bridging” molecule 25. Likely LpL-LDL complexes bind to HSPG to increase the internalization of LDL both by receptor-mediated and receptor-independent pathways 26. Our own in vitro and in vivo studies further showed that elevation of LpL levels increased accumulation of LDL-CE via non-SR-B1- or LDLR-mediated pathways in different cells 4, 5. This is in agreement with other studies, which have shown that LDL-CE SU occurs in LpL-rich tissues such as muscle, heart, and adipose tissues 6.
What are possible mechanisms for the differential effects of dietary fatty acids on cholesterol deposition in the arterial wall? A high fat or western diet has been shown to increase LpL levels in rabbit adipocytes and muscle 27. LpL itself enhances cellular proteoglycan production 22, 28. Camejo et al. 29 reported that higher concentrations of saturated fatty acid induced smooth muscle cell extracellular matrix alterations with more trapping of LDL with longer retention time; a condition which would favor SU 5. Thus increasing arterial proteoglycans together with increasing LpL levels will lead, we propose, to synergistic effects in increasing early aortic cholesterol deposition via anchoring and retention of LDL; similar to interactions recently reported in atherosclerotic lesions in apoB transgenic mice 30. High levels or intake of saturated fatty acids, respectively, in vitro or in vivo, can thus lead to a “vicious cycle” of increased LpL levels, increased numbers of arterial wall macrophages and smooth muscle cells, and this combined with increased proteoglycans, will result in increased cholesterol deposition in the artery.
In contrast to saturated fatty acids, n-3 fatty acids have effects quite different on arterial LpL and on cholesterol deposition. n-3 fatty acids, in vitro, can decrease LpL secretion in human macrophages, and induce macrophage and smooth muscle cell apoptosis, thus inhibiting atherogenesis 31. n-3 fatty acids, such as EPA, can inhibit LpL activity by 50-60%. Adding EPA to media of cultured J774 macrophages markedly decreased LpL transcription and translation while opposite effects were observed after addition of saturated fatty acids 32. Likely an n-3 rich diet also inhibits structural changes in vascular smooth muscle cells and inhibits macrophage accumulation to decrease SU.
In the current studies, similar to others, we suggest that LDL-cholesterol accumulation is one key pathogenic event and requirement for lesion development in early atherosclerosis progression. However, there are many other factors that can affect binding uptake and the flux of the lipoproteins to the artery. One such factor is shear stress. Although, we have not accounted for changes in shear stress in terms of LDL uptake, the hemodynamic stress is a prerequisite, as atherosclerosis does not develop within the venous system due to a low pressure-lower shear stress environment. Also pulmonary arteries do not develop atherosclerosis unless pulmonary hypertension is present. Another possibility that might influence LDL accumulation in the arterial wall is the changes in vascular permeability. There is a significant body of evidence that supports the involvement of LpL in lipoprotein interactions within the arterial wall. It has been demonstrated that LpL associated lipolysis increased arterial permeability to lipoproteins 33, although lipolysis was not associated in their studies herein.
It is of interest that the distribution patterns of LpL and LDL-CE from immunohistology and immunofluorostaining are highly comparable; there is deeper infiltration of LDL with increased medial LpL in SAT fed mice. In contrast, LpL and LDL remain in the intimal layer with less lipid penetration in mice fed with the n-3 diet. At this point, mechanisms behind redistribution of arterial LpL almost entirely to the intima seen in animals fed the n-3 rich diet are not known; however, the n-3 diet may be beneficial in preventing deeper LDL infiltration into the arterial wall. We reported that addition of LpL to perfusates of triglyceride-rich particles markedly diminished their penetration into arterial wall media 34. These conditions were different from our current studies, where LpL intimal concentration would result from redistribution of endogenous arterial LpL or derived from exogenous LpL from other tissues. LpL as suggested above, at the intimal level might indeed prevent deeper LDL infiltration into the arterial wall. Taken together, LpL facilitates the binding of lipoproteins to extracellular proteoglycans by acting as a bridging molecule, and modulates arterial LDL-cholesterol accumulation. n-3 fatty acids can reduce total arterial LpL levels and, we hypothesize, at the intima decrease vascular permeability to LDL and its penetration into arterial wall.
It is possible that increased LDL in arteries from mice fed SAT diets might be derived from the vasa vasorum. In our current studies, we have not observed any vascular remodeling and neovascularization of the intima or media. Although it is unlikely that increased cholesterol delivery in SAT fed mice can be directly linked to vasa vasorum, we cannot exclude this possibility.
Our current studies were carried out in mice with no identifiable atherosclerotic lesions (e.g., fatty streaks). In our previous reports, we showed that events associated with atherosclerosis progression such as increasing plasma cholesterol levels and lesions themselves modulate SU 4, 35. Now we demonstrate that SU can be an important contributor to accelerated arterial CE uptake even prior to the appearance of atherosclerotic lesions. Moreover, cholesterol delivery to the arterial wall is modified by diet, in part, through regulating arterial LpL levels even before atherosclerosis develops. While a SAT diet increases total LDL-CE uptake and SU, a n-3 diet inhibits these pathways of early arterial cholesterol accumulation. We hypothesize that the effect of n-3 fatty acids in decreasing arterial wall cholesterol delivery is one important mechanism by which these bioactive n-3 fatty acids may decrease the risk of cardiovascular disease.
Acknowledgments
We thank Inge H. Hansen for assisting in the GLC analyses to determine plasma fatty acyl compositions.
Sources and Funding
This work was supported by NIH grants HL40404 (R. J. Deckelbaum) and DK60497 (T. Seo) and American Heart Association Grants-in-Aid 0255656N (T. S. Worgall). C. L. Chang is supported by NIH grant T32-DK007647.
Footnotes
Disclosures
None.
REFERENCES
- 1.Brown MS, Goldstein JL. A receptor mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinninger F, Brundert M, Jäckle S, Kaiser T, Greten H. Selective uptake of low-density lipoprotein-associated cholesteryl esters by human fibroblasts, human HepG2 hepatoma cells and J774 macrophages in culture. Biochim Biophys Acta. 1995;1255:141–153. doi: 10.1016/0005-2760(94)00228-q. [DOI] [PubMed] [Google Scholar]
- 4.Seo T, Qi K, Chang C, Liu Y, Worgall TS, Ramakrishnan R, Deckelbaum RJ. Saturated fat-rich diet enhances selective uptake of LDL cholesteryl esters in the arterial wall. J Clin Invest. 2005;115:2214–2222. doi: 10.1172/JCI24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo T, Al-Haideri M, Treskova E, Worgall TS, Kako Y, Goldberg IJ, Deckelbaum RJ. Lipoprotein lipase-mediated selective uptake from low density lipoprotein requires cell surface proteoglycans and is independent of scavenger receptor class B type 1. J Biol Chem. 2000;275:30355–30362. doi: 10.1074/jbc.M910327199. [DOI] [PubMed] [Google Scholar]
- 6.Green SR, Pittman RC. Selective uptake of cholesteryl esters from low density lipoproteins in vitro and in vivo. J Lipid Res. 1991;32:667–678. [PubMed] [Google Scholar]
- 7.Seo T, Velez-Carrasco W, Qi K, Hall M, Worgall TS, Johnson RA, Deckelbaum RJ. Selective uptake from LDL is stimulated by unsaturated fatty acids and modulated by cholesterol content in the plasma membrane: role of plasma membrane composition in regulating non-SR-BI-mediated selective lipid transfer. Biochemistry. 2002;41:7885–7894. doi: 10.1021/bi011949g. [DOI] [PubMed] [Google Scholar]
- 8.Corey JE, Zilversmit DB. Effect of cholesterol feeding on arterial lipolytic activity in the rabbit. Atherosclerosis. 1977;27:201–212. doi: 10.1016/0021-9150(77)90057-0. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien KD, Gordon D, Deeb S, Ferguson M, Chait A. Lipoprotein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest. 1992;89:1544–1550. doi: 10.1172/JCI115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Goldberg IJ, Steinberg D, Witztum JL. Macrophages and smooth muscle cells express lipoprotein lipase in human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:10143–10147. doi: 10.1073/pnas.88.22.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Fay FS, Taneja KL, Shenoy S, Lifshitz L, Singer RH. Quantitative digital analysis of diffuse and concentrated nuclear distributions of nascent transcripts, SC35 and poly(A) Exp Cell Res. 1997;231:27–37. doi: 10.1006/excr.1996.3460. [DOI] [PubMed] [Google Scholar]
- 13.Lachmanovich E, Shvartsman DE, Malka Y, Botvin C, Henis YI, Weiss AM. Co-localization analysis of complex formation among membrane proteins by computerized fluorescence microscopy: application to immunofluorescence co-patching studies. J Microsc. 2003;212:122–131. doi: 10.1046/j.1365-2818.2003.01239.x. [DOI] [PubMed] [Google Scholar]
- 14.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 15.Harris WS. n-3 fatty acids and serum lipoproteins: animal studies. Am J Clin Nutr. 1997;65:1611S–1616S. doi: 10.1093/ajcn/65.5.1611S. [DOI] [PubMed] [Google Scholar]
- 16.Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J Clin Invest. 1992;90:1504–1512. doi: 10.1172/JCI116018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deckelbaum RJ, Akabas SR. n-3 Fatty acids and cardiovascular disease: navigating toward recommendations. Am J Clin Nutr. 2006;84:1–2. doi: 10.1093/ajcn/84.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 20.Rinninger F, Kaiser T, Mann WA, Meyer N, Greten H, Beisiegel U. Lipoprotein lipase mediates an increase in the selective uptake of high density lipoprotein-associated cholesteryl esters by hepatic cells in culture. J Lipid Res. 1998;39:1335–1348. [PubMed] [Google Scholar]
- 21.Weiner BH, Ockene IS, Levine PH, Cuenoud HF, Fisher M, Johnson BF, Daoud AS, Jarmolych J, Hosmer D, Johnson MH. Inhibition of atherosclerosis by cod-liver oil in a hyperlipidemic swine model. N Engl J Med. 1986;315:841–846. doi: 10.1056/NEJM198610023151401. [DOI] [PubMed] [Google Scholar]
- 22.Mamputu JC, Levesque L, Renier G. Proliferative effect of lipoprotein lipase on human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:2212–2219. doi: 10.1161/01.atv.20.10.2212. [DOI] [PubMed] [Google Scholar]
- 23.Babaev VR, Patel MB, Semenkovich CF, Fazio S, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J Biol Chem. 2000;275:26293–26299. doi: 10.1074/jbc.M002423200. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg S, Sehayek E, Olivecrona T, Vlodavsky I. Lipoprotein lipase enhances binding of lipoproteins to heparan sulfate on cell surfaces and extracellular matrix. J Clin Invest. 1992;90:2013–2021. doi: 10.1172/JCI116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams KJ, Fless GM, Petrie KA, Snyder ML, Brocia RW, Swenson TL. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267:13284–13292. [PubMed] [Google Scholar]
- 26.Obunike JC, Sivaram P, Paka L, Low MG, Goldberg IJ. Lipoprotein lipase degradation by adipocytes: receptor-associated protein (RAP)-sensitive and proteoglycan-mediated pathways. J Lipid Res. 1996;37:2439–2449. [PubMed] [Google Scholar]
- 27.Sartippour MR, Renier G. Upregulation of macrophage lipoprotein lipase in patients with type 2 diabetes: role of peripheral factors. Diabetes. 2000;49:597–602. doi: 10.2337/diabetes.49.4.597. [DOI] [PubMed] [Google Scholar]
- 28.Obunike JC, Pillarisetti S, Paka L, Kako Y, Butteri MJ, Ho YY, Wagner WD, Yamada N, Mazzone T, Deckelbaum RJ, Goldberg IJ. The heparin-binding proteins apolipoprotein E and lipoprotein lipase enhance cellular proteoglycan production. Arterioscler Thromb Vasc Biol. 2000;20:111–118. doi: 10.1161/01.atv.20.1.111. [DOI] [PubMed] [Google Scholar]
- 29.Camejo G, Olsson U, Hurt-Camejo E, Baharamian N, Bondjers G. The extracellular matrix on atherogenesis and diabetes-associated vascular disease. Atheroscler Suppl. 2002;3:3–9. doi: 10.1016/s1567-5688(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 30.Gustafsson M, Levin M, Skalen K, Perman J, Friden V, Jirholt P, Olofsson SO, Fazio S, Linton MF, Semenkovich CF, Olivecrona G, Boren J. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ Res. 2007;101:777–783. doi: 10.1161/CIRCRESAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 31.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 32.Michaud SE, Renier G. Direct regulatory effect of fatty acids on macrophage lipoprotein lipase: potential role of PPARs. Diabetes. 2001;50:660–666. doi: 10.2337/diabetes.50.3.660. [DOI] [PubMed] [Google Scholar]
- 33.Rutledge JC, Woo MM, Rezai AA, Curtiss LK, Goldberg IJ. Lipoprotein lipase increases lipoprotein binding to the artery wall and increases endothelial layer permeability by formation of lipolysis products. Circ Res. 1997;80:819–828. doi: 10.1161/01.res.80.6.819. [DOI] [PubMed] [Google Scholar]
- 34.Mullick AE, Deckelbaum RJ, Goldberg IJ, Al-Haideri M, Rutledge JC. Apolipoprotein E and lipoprotein lipase increase triglyceride-rich particle binding but decrease particle penetration in arterial wall. Arterioscler Thromb Vasc Biol. 2002;22:2080–2085. doi: 10.1161/01.atv.0000040221.70377.19. [DOI] [PubMed] [Google Scholar]
- 35.Arai T, Rinninger F, Varban L, Fairchild-Huntress V, Liang CP, Chen W, Seo T, Deckelbaum RJ, Huszar D, Tall AR. Decreased selective uptake of high density lipoprotein cholesteryl esters in apolipoprotein E knock-out mice. Proc Natl Acad Sci U S A. 1999;96:12050–12055. doi: 10.1073/pnas.96.21.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]