Abstract
Synaptic ribbons are organelles that tether vesicles at the presynaptic active zones of sensory neurons in the visual, auditory and vestibular systems. These neurons generate sustained, graded electrical signals in response to sensory stimuli, and fidelity of transmission therefore requires their synapses to release neurotransmitter continuously at high rates. It has long been thought that the ribbons at the active zones of sensory synapses accomplish this task by enhancing the size and accessibility of the readily releasable pool of synaptic vesicles, which may represent the vesicles attached to the ribbon. Recent evidence suggests that synaptic ribbons immobilize vesicles in the resting cell and coordinate the transient, synchronous release of vesicles in response to stimulation, but it is not yet clear how the ribbon can efficiently mobilize and coordinate multiple vesicles for release. However, detailed anatomical, electrophysiological and optical studies have begun to reveal the mechanics of release at ribbon synapses, and this multidisciplinary approach promises to reconcile structure, function, and mechanism at these important sensory synapses.
Keywords: Retina, Inner Ear, Synaptic Transmission, Photoreceptors, Hair Cells
The primary receptor cells of the visual, auditory, and vestibular systems generate graded changes in membrane potential in response to sensory stimuli, and to faithfully transmit these signals, their synapses must be capable of releasing quanta of neurotransmitter at a high rate for sustained periods. The active zone of these specialized synapses bears an enigmatic structure called the synaptic ribbon, which positions an abundance of synaptic vesicles close to release sites, within nanometers of calcium channels and directly across from postsynaptic glutamate receptors (Matsubara and others, 1996; Morigiwa and Vardi, 1999; Khimich and others, 2005). The ribbon is thought to enhance the size and accessibility of the readily releasable pool of vesicles in order to achieve the sustained neurotransmitter release required for signal transmission, but the precise way it does so is unknown. Based on the anatomical characteristics and vesicle release properties of ribbon-type synapses, the ribbon has been variously described as a priming device, a calcium diffusion barrier, an endocytic apparatus, a safety belt for synaptic vesicles, a conveyor belt for vesicles, a device for compound fusion of vesicles with other vesicles, and a mechanism to coordinate multivesicular fusion. Presently, none of these hypotheses can be dismissed, though evidence indicates that some are more feasible than others. In this article, we assess these varied perspectives on how synaptic ribbons might contribute to the rapid and continuous transmitter release characteristic of ribbon-containing neurons.
Morphology of synaptic ribbons
Synaptic ribbons are proteinaceous organelles that are anchored to the plasma membrane at the active zone and extend into the cytoplasm, perpendicular to the membrane (Fig. 1A). In addition to the large cytoplasmic population of synaptic vesicles in the terminal, a sizeable pool of vesicles is tethered to the surface of the ribbon via fine filaments of unknown composition. Across cell types and species, ribbons are anatomically diverse, varying in size, shape, and capacity to tether vesicles (see Fig. 1). Photoreceptor ribbons (Fig. 1A,B) are typically planar, ~30 nm thick, and extend vertically >0.5 μm into the cytoplasm. They can be up to several μm in length, parallel to the membrane. In a typical cone terminal, multiple ribbons tether ~3000 vesicles and dock ~600 at the plasma membrane (Sterling and Matthews, 2005). Mammalian rod photoreceptor ribbons are crescent-shaped sheets that dock ~130 vesicles (green subset of vesicles in Fig. 1) and tether ~640 vesicles. Like photoreceptors, the second-order bipolar cells of the retina produce graded changes in membrane potential and have ribbon synapses. Generally, photoreceptor ribbons tend to be larger and less numerous than bipolar cell synaptic ribbons, which appear as spheres or flattened ellipsoids and can tether ~110 vesicles. Hair-cell synaptic ribbons also called dense bodies are among the most morphologically diverse. Depending on the cell type, they range from ~0.1 to ~0.4 μm in length and can be ellipsoidal, plate-like, barrel-shaped, or spherical (e.g., Fig. 1C,D). Accordingly, their vesicle capacities are quite varied, with the smallest ribbon tethering 20 vesicles and the largest tethering up to 400 vesicles (Moser and others, 2006). In the chick cochlea, hair cell ribbons vary in size along the tonotopic axis, with cells in high-frequency regions having larger ribbons that tether more vesicles (Martinez-Dunst and others, 1997). Interestingly, many synapses in arthropod nervous systems have a T-shaped structure at the active zone (Prokop and Meinertzhagen, 2006; Fig. 1E,F), which may be similar in function to ribbons of vertebrate sensory synapses.
Fig. 1.
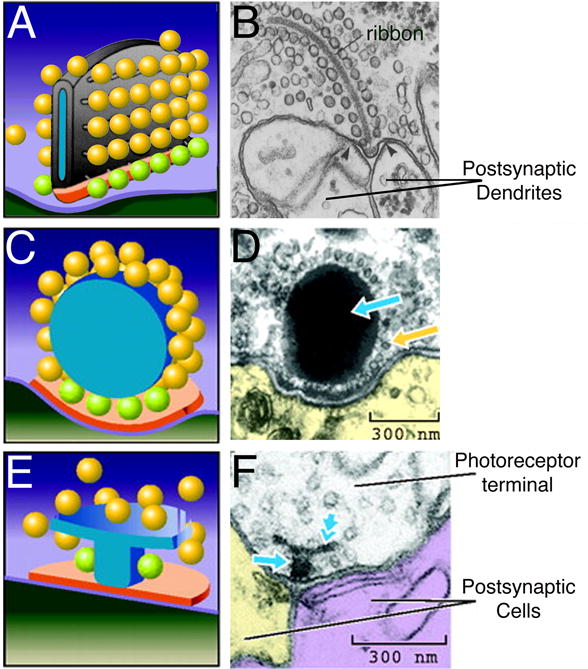
Synaptic ribbons are morphologically diverse organelles. A, C, E, Schematic representations of the electron micrographs shown to their right. Green vesicles represent the docked, ribbon-associated pool. Modified from Zhai and Bellen (2004) with permission of the American Physiological Society. B, Electron micrograph showing a ribbon-like projection surrounded by synaptic vesicles in a salamander rod photoreceptor terminal. Modified from Townes-Anderson and others (1985) with permission of the Rockefeller University Press. D, Electron micrograph showing a spherical synaptic body in a frog saccular hair cell. Yellow arrow points to vesicles tethered to the synaptic body (blue arrow). Modified from Lenzi and von Gersdorff (2001) with permission from John Wiley & Sons, Inc. F, Electron micrograph showing a T-bar shaped “ribbon” in a drosophila photoreceptor terminal. The T-bar is made up of a platform (double arrow) and a pedestal (single arrow). Modified from Meinertzhagen (1996) with permission of Elsevier Inc.
Molecular composition of the synaptic ribbon complex
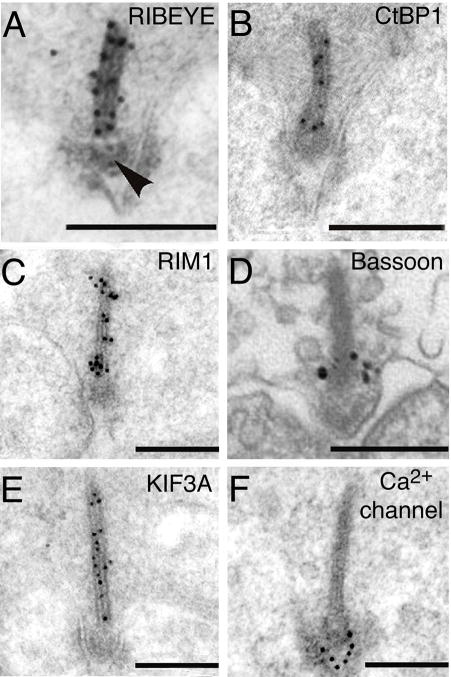
The only known component exclusive to synaptic ribbons is RIBEYE, a protein composed of a novel N-terminal A-domain that is unique to ribbons and a C-terminal B-domain that is identical to all but the N-terminal 20 amino acids of the transcriptional co-repressor CtBP2 (Schmitz and others, 2000). RIBEYE self-assembles into complexes and accounts for most of the mass of the ribbon (Zenisek and others, 2004). Therefore, it is thought to form the structural backbone of the ribbon (Fig. 2A). Both A- and B-domains contain sites for RIBEYE-RIBEYE interactions that potentially construct and stabilize synaptic ribbons (Magupalli and others, 2008). In addition to RIBEYE, several proteins first identified as part of the cytomatrix at the active zone (CAZ) of conventional synapses are also present at ribbon synapses, including CtBP1 (Fig. 2B), RIM1 (Fig. 2C), RIM2, Bassoon (Fig. 2D), and Piccolo (tom Dieck and others, 2005, 2006). Bassoon is located at the boundary between the ribbon and the plasma membrane (Fig. 2D; Brandstatter and others, 1999; tom Dieck and others, 2005; Deguchi-Tawarada and others, 2006), and a direct interaction between Bassoon and RIBEYE is necessary for anchoring of the ribbon at the active zone (tom Dieck and others, 2005).
Fig. 2.
Electron micrographs showing the ultrastructural localization of photoreceptor synaptic ribbon-complex proteins. A–C, E, F, Postembedding immunogold labeling. A, RIBEYE labeling decorates the ribbon but is absent from the arciform density (arrow). B, CtBP1 is found along the length of the ribbon. C, RIM1 is found along the length of the ribbon. D, Immunolabeling reveals that Bassoon is located at the base of the ribbon but not along its length. Modified from Deguchi-Tawarada and others (2006) with permission of John Wiley & Sons, Inc. E, KIF3A is found along the length of the ribbon. F, Ca2+ channel α1 subunits are located beneath the ribbon at the active zone. Scale bars in A–F represent 200 nm. A–C, E, F, modified from tom Dieck and others (2005), with permission of the Rockefeller University Press.
By binding to the vesicle-associated GTPase Rab3A, RIM recruits synaptic vesicles to the priming protein Munc13-1, which is crucial for preparing vesicles for rapid exocytosis at conventional synapses (Betz and others, 2001). At ribbon synapses, RIM1 is associated with the ribbon itself, while RIM2 is thought to be a component of the CAZ, located between the ribbon and the plasma membrane (tom Dieck and others, 2005). It has been suggested that all of the vesicles tethered to the ribbon, not just those docked at the plasma membrane, have undergone ATP-dependent priming (Heidelberger and others, 2002). If this is correct, then an attractive hypothesis—as yet untested is—that RIM1 may mediate priming of ribbon-tethered vesicles, whereas RIM2 primes vesicles docked at the plasma membrane.
Another protein localized to ribbons is KIF3A (Fig. 2E; Muresan and others, 1999), which is a component of the ATP-dependent kinesin II motor that moves cargo along microtubules. This led to the idea that vesicles might actively move down the ribbon, using a kinesin-like motor, to fuse at the plasma membrane. However, other kinesin II components, KIF3B and KAP3, and components of microtubules have not been detected at ribbons, and turnover of the releasable pool has been shown not to require ATP hydrolysis, which would be necessary for the function of a kinesin motor complex. Therefore, the possible function of KIF3A at ribbons remains uncertain.
The monoclonal antibody B16 was the first to specifically label ribbons (Balkema, 1991), and its antigen has been shown to exhibit homology to the μ chain of an adaptor protein that facilitates the binding of clathrin to vesicles during endocytosis (Balkema and Nguyen 1999). This suggests that adaptor proteins may be distributed along the ribbon—perhaps to mediate vesicle—binding or that the ribbon may be involved in the endocytic limb of the vesicle cycle. When ribbons are absent from inner hair cells of Bassoon knockout mice, large tubular structures accumulate at the active zones, similar to the large endosomes observed at ribbon synapses of goldfish bipolar cells and frog saccular hair cells (Lenzi and others, 2002; Paillart and others, 2003; Holt and others, 2003). This might be another indication that the ribbon facilitates membrane turnover at the active zone by clearing endocytosed material.
The voltage-gated calcium channels that drive neurotransmitter release are located in the plasma membrane beneath the ribbon (Fig. 2F). Unlike conventional synapses, ribbon-containing synapses express the L-type CaV1.3 (Kollmar and others, 1997; Sidi and others, 2004; Brandt and others, 2005; LoGiudice and others, 2006) or CaV1.4 isoforms (Bech-Hansen and others, 1998; Strom and others, 1998). These channels activate at relatively negative voltages and inactivate slowly, thereby permitting sustained calcium entry during tonic depolarization. Synaptic ribbons and calcium channels are tightly correlated in space, with the channels situated along docking sites beneath the ribbon (Fig. 2F) (Roberts and others, 1990; Issa and Hudspeth, 1994; Llobet and others, 2003; Zenisek and others, 2003, 2004; Midorikawa and others, 2007). Consequently, the vesicles docked at the base of the ribbon are poised for rapid release when calcium channels open. The functional significance of the ribbon is further indicated by its direct apposition to dense clusters of postsynaptic glutamate receptors (Matsubara and others, 1996; Morigiwa and Vardi 1999; Khimich and others, 2005).
Disruption of ribbons
Knockout of the CAZ protein Bassoon in mice causes dislocation of synaptic ribbons from their normal position at the active zone in photoreceptors (Dick and others, 2003) and cochlear inner hair cells (Khimich and others, 2005). Ribbons are still present, but they float free in the cytoplasm of the terminal. The amplitude of the b-wave of the electroretinogram—an index of synaptic transmission from photoreceptors to bipolar cells—is reduced in these mice, indicating impaired efficiency of photoreceptor synaptic transmission. However, dendrites of postsynaptic bipolar cells branch abnormally and extend past the terminal to the photoreceptor cell body, where they form ectopic synapses, complete with anchored ribbons on the presynaptic side and postsynaptic glutamate receptors (Dick and others, 2003). Also, ribbons in bipolar cell terminals remain anchored at active zones in Bassoon knockout retina. Since anchored ribbons still occur at ectopic photoreceptor synapses and bipolar cell synapses, alternative mechanisms for ribbon attachment must exist that do not involve Bassoon. It is not clear why this alternative mechanism is able to anchor ribbons at the plasma membrane in the photoreceptor soma, but not at their proper location in the synaptic terminal.
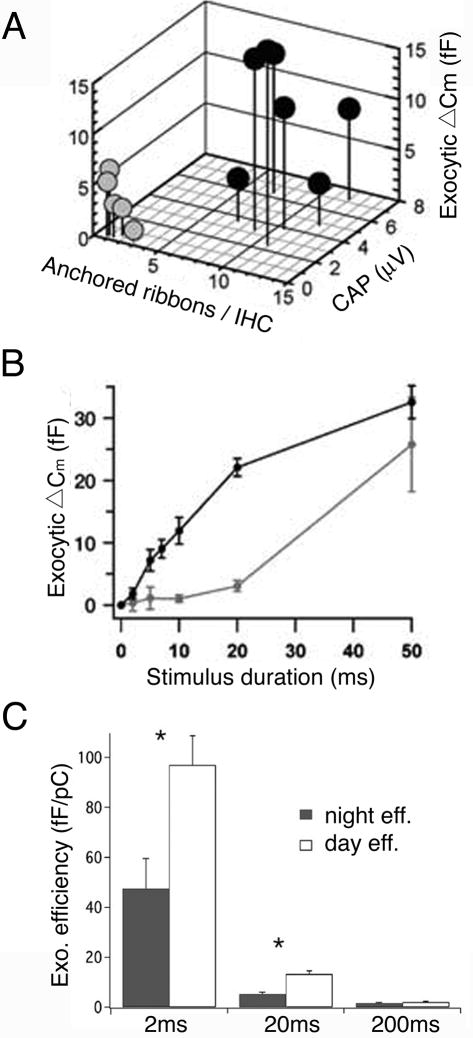
In inner hair cells lacking Bassoon, few anchored ribbons are observed, although active zones still formed, and synchronous afferent transmission to second-order neurons is impaired (Khimich and others, 2005; Fig. 3A). Interestingly, the fast component of exocytosis triggered by brief depolarization is strongly reduced without ribbons, but the slower, sustained response to longer depolarizations is largely unaffected (Fig. 3B). This result is surprising, because the canonical view is that ribbons support sustained release, whereas the mutant synaptic phenotype indicates that hair cell ribbons are essential for fast vesicle fusion but are less critical for sustained release. Perhaps this indicates that the primary purpose of ribbons in inner hair cells is to coordinate the release of multiple vesicles during the initial response to sound onset, and that other mechanisms can apparently compensate for the absence of ribbons and support sustained release. However, this does not necessarily mean that ribbons play no role in sustained neurotransmitter release when they are present.
Fig. 3.
Synaptic ribbons are essential for fast, synchronous release. A, Bassoon mutant mice (grey) have impaired fast exocytosis and synchronous release compared to wild type mice (black). The diagram correlates the mean number of anchored synaptic ribbons per inner hair cell (IHC), fast exocytosis in response to 10-ms depolarizations measured by a change in IHC membrane capacitance (ΔCm), and spiral ganglion neuron compound action potential amplitude. B, Graph showing the kinetics of exocytosis assembled from ΔCm of the same inner hair cells in response to depolarization of varying lengths. In mutant mice, ΔCm was significantly reduced for 5-, 10-, and 20-ms depolarizations compared to wild-type mice. A, B: Modified from Khimich and others (2005) with permission of the Nature Publishing Group. C, Bar graph illustrating the efficiency of exocytosis per pC of calcium entry. Exocytosis is significantly more efficient during the daytime for short depolarizations. The efficiency of exocytosis in response to longer depolarizations was not significantly different from day to night. Modified from Hull and others (2006) with permission of the American Physiological Society.
Synaptic ribbons are dynamic organelles whose size, shape, and number change on a circadian cycle. These cyclical changes in ribbon number and morphology provide an alternative way to examine ribbon function without altering the cell’s native protein components. For instance, in goldfish bipolar cells, the number of ribbons is reduced by ~65% at night, yet the number of active zones remains constant, and many active zones therefore lack ribbons at night (Hull and others, 2006). Recordings of membrane capacitance revealed a correlation between ribbon number and the fast component of exocytosis (Hull and others, 2006). At night, exocytosis in response to a 20-ms depolarization is reduced compared to evoked release during the day, when ribbons occupy the active zones. However, the exocytic response to a 200-ms depolarization was the same in day or night (Fig. 3C). As with the results from inner hair cells lacking Bassoon discussed above (Khimich and others, 2005), this surprising result again suggests that ribbons are more important for fast, synchronous release than for sustained release.
The synaptic ribbon as a scaffold for releasable vesicles
The mobility of reserve vesicles at conventional synapses is highly constrained (Henkel and others, 1996; Kraszewski and others, 1996), most likely because vesicles bind to proteins such as synapsin (Hilfiker and others, 1999), which is not expressed at ribbon-type synapses (Mandell and others, 1990). Perhaps because of the absence of synapsin, cytoplasmic vesicles in ribbon-type terminals are more mobile than in conventional boutons. In cone photoreceptor terminals, for example, ~85% of vesicles are mobile, which may contribute to the fast replenishment of vesicles at tonically active release sites (Rea and others, 2004). Vesicles in goldfish bipolar cell terminals diffuse freely in regions both proximal and distal to the plasma membrane, and random collision of cytoplasmic vesicles is considered sufficient to refill the synaptic ribbon after depletion (Holt and others, 2004). In terminals of mouse bipolar cells, approximately half of the cytoplasmic vesicles are mobile in the resting state (LoGiudice and others, 2008). Therefore, a large pool of cytoplasmic vesicles is freely mobile within ribbon-containing terminals. How, then, are these mobile vesicles stabilized near the active zone? A variety of lines of evidence indicate that the ribbon carries out this job, acting as a trap or “safety belt” for releasable vesicles (Parsons and Sterling, 2003).
In the static snapshot provided by EM, a halo of vesicles is attached to the surface of the ribbon, and it is natural to suppose that these ribbon-tethered vesicles are immobile. Indeed, measurements in living neurons provide solid evidence that this pool is in fact stationary in the resting cell. Total internal reflection fluorescence microscopy (TIRFM) shows that vesicles labeled with fluorescent FM dye in bipolar cell terminals preferentially reside at “hot spots” near the plasma membrane, where they appear to be spatially fixed (Zenisek and others, 2000). Resident vesicles at these sites rarely disappeared without undergoing exocytosis and releasing their dye, unlike vesicles at other locations, which often left the membrane without fusing. The presumed substrate for vesicle immobilization and preferential fusion at these hot spots is the synaptic ribbon. To determine directly the mobility of vesicles associated with ribbons in mouse bipolar cell terminals, LoGiudice and others (2008) used a fluorescent peptide that labels ribbons (Zenisek and others, 2004), in conjunction with FM dye to label vesicles. Fluorescence recovery after photobleaching revealed that only ~20% of vesicles at ribbons are mobile, compared with ~50% of cytoplasmic vesicles. Therefore, ribbon-tethered vesicles are not in dynamic equilibrium with cytoplasmic vesicles and are tightly bound to the ribbon in the resting neuron. TIRFM in goldfish bipolar neurons corroborate this conclusion. Single synaptic vesicles were preferentially captured and locked into position at the base of fluorescently labeled ribbons, whereas vesicles at non-ribbon locations were less stable (Zenisek, 2008). These studies provide compelling evidence that ribbons execute at least one key task at the synapse: to immobilize a pool of vesicles at the active zone.
Ribbon-associated vesicles contribute to the releasable pool
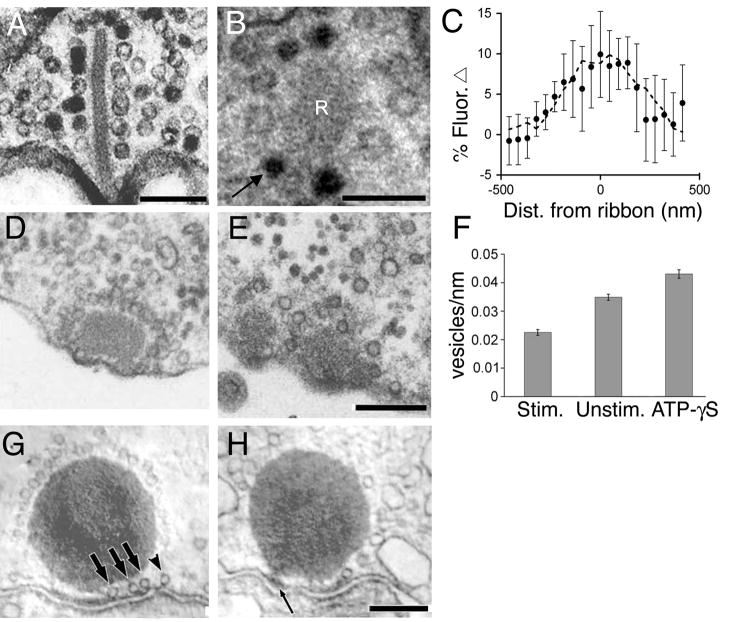
Several lines of evidence indicate that the vesicles immobilized on synaptic ribbons participate in neurotransmitter release. First, EM images of photoreceptors, bipolar cells, and hair cells reveal that ribbon-bound vesicles can contain extracellular markers such as horseradish peroxidase (Fig. 4A) or photoconverted FM dye (Fig. 4B), clearly demonstrating turnover of at least some of the ribbon-associated pool (Schaeffer and Raviola, 1978; Siegel and Brownell, 1986; LoGiudice and others, 2008). Second, EM reveals that in stimulated goldfish bipolar cells, ribbons are significantly denuded of their vesicles compared to ribbons in inhibited neurons (Figure 4D-F; Holt and others, 2004; Matthews and Sterling, 2008). Third, in frog saccular hair cells, vesicles associated with synaptic ribbons are depleted in response to strong stimulation, with depletion being strongest on the part of the ribbon nearest the plasma membrane (Figure 4G, H; Lenzi and others, 2002). Fourth, turnover of ribbon-associated vesicles has also been observed directly in response to depolarization in living mouse bipolar neurons (LoGiudice and others, 2008). Photobleached vesicles immobilized at ribbons were released upon stimulation and replaced by unbleached labeled vesicles, resulting in an increase in FM dye fluorescence specifically at the ribbon location (Fig. 4C; LoGiudice and others, 2008). Thus, it seems clear that vesicles on ribbons contribute to the pool of vesicles that are released upon depolarization.
Fig. 4.
Synaptic ribbons release and recapture vesicles in response to stimulation. A, Electron micrograph of the turtle cone synapse reveals that vesicles labeled with horseradish peroxidase (HRP) appear on the ribbon after a 1-hr HRP incubation in the dark. Scale bar represents ~125 nm. Modified from Schaeffer and Raviola (1978) with permission of the Rockefeller University Press. B, Electron micrograph reveals that vesicles labeled with photoconverted FM1-43 (arrow) appear on the mouse bipolar cell synaptic ribbon after 5 min of repetitive high K+ stimulation (3 sec puff/15 sec). Scale bar represents 125 nm. R denotes the ribbon. C, Fluorescence profile of the change in previously bleached FM4-64 fluorescence (filled circles) with respect to ribbon position (dashed line) in response to a 250ms pulse from 60mV to 0mV (mouse bipolar cells). Modified from LoGiudice and others (2008) with permission of the Society for Neuroscience. D, E, Electron micrographs of goldfish bipolar cells reveal that calcium spiking reduces the number of vesicles attached to synaptic ribbons (E) compared to cells inhibited by muscimol (D). Scale bar in E represents 200 nm and is applicable to D as well. F, Bar graph showing the density of vesicles attached to a ribbon in a single EM slice. ATP-γS further inhibits cells from releasing vesicles. D, F: Modified from Matthews and Sterling (2008) with permission of the Society for Neuroscience. G. Transmission electron micrographs of frog saccular hair cells exposed to 0 Ca2+ saline. Long arrows indicate vesicles that are docked beneath the synaptic body and arrowhead marks a vesicle that is docked nearby. H, Transmission electron micrographs of frog saccular hair cells exposed to high K+. Thin arrow points to a region of presynaptic density. Scale bar in H represents 200 nm and is applicable to G as well. Modified from Lenzi and others (2002) with permission of Elsevier, Inc.
When synaptic vesicles fuse with the plasma membrane, the resulting increase in surface area can be monitored using membrane capacitance, allowing the kinetics of fusion and retrieval to be observed with fine temporal resolution at living synapses. Capacitance measurements in several sensory neurons, including retinal bipolar cells, rod and cone photoreceptors, cochlear hair cells, and frog saccular hair cells, indicate the presence of at least two kinetically distinct components of release (Parsons and others, 1994; Spassova and others, 2004; Mennerick and Matthews 1996; von Gersdorff and others, 1998; Moser and Beutner, 2000; Thoreson and others, 2004; Khimich and others, 2005; Innocenti and Heidelberger, 2008). The first component reflects a rapidly depleted vesicle pool that is released within milliseconds, while the second component is delayed and can last for several hundred milliseconds or more. What are the morphological correlates of the fast and slow components and to what extent do ribbon-associated vesicles contribute to each? In a variety of cell types, there is good agreement between the size of the fast component and the number of synaptic vesicles at the base of the ribbon, in close proximity to the plasma membrane (the docked pool; green vesicles in Fig. 1). In frog saccular hair cells, rapid release of this group of docked, tethered vesicles is also predicted by a model of release that incorporates calcium channel properties, calcium diffusion, and anatomical relationships of ribbons and vesicles (Wittig and Parsons, 2008). Also, without this pool, the modeled response of the afferent fiber was delayed and more variable, in keeping with the effect of loss of ribbons on transmission in the cochlea (Khimich and others, 2005). Therefore, the conclusion seems reasonable that these docked vesicles at the ribbon likely account for the very rapid, synchronous burst of exocytosis when calcium channels open.
But what about the correspondence between the slower component of release and the non-docked vesicles in higher rows on the ribbon? Here, things get murkier. On the one hand, in rod photoreceptors (Thoreson and others, 2004), goldfish bipolar cells (von Gersdorff and others, 1996), and chick cochlear hair cells (Spassova and others, 2004), the total population of ribbon-attached vesicles corresponds well with the size of the entire releasable pool. This would be consistent with a “ribbon hypothesis” that attributes the majority of functional vesicle release to the ribbon. On the other hand, in other instances the anatomically defined, ribbon-tethered pool is either smaller than the physiologically defined releasable pool (saccular hair cells; Lenzi and others, 1999) or larger (mouse cochlear hair cells; Khimich and others, 2005). Also, as discussed earlier, disruption of ribbons suggests a more important role for the ribbon in fast, synchronous release than in sustained, slower release.
As an alternative, the “docked outlier hypothesis” offers a less ribbon-centric point of view with regard to sustained vesicle release. This idea similarly attributes the fast component to the fusion of vesicles docked near synaptic ribbons, but credits the delayed component to the fusion of outlier vesicles positioned at a distance from synaptic ribbons (e.g., Midorikawa and others, 2007). In goldfish bipolar cells, TIRF imaging reveals that stimulation evokes the fast, reliable fusion of vesicles docked beneath synaptic ribbons, as well as delayed vesicle release at outlying sites, perhaps uncovering the physiological correlates of the fast and slow components of exocytosis in the bipolar cell (Zenisek and others, 2000; Midorikawa and others, 2007; Zenisek 2008). While fast transient events predominantly occurred in ribbon-containing regions, both ribbon-associated and ribbon-free zones maintained a low rate of vesicle fusion during prolonged depolarization (Midorikawa and others, 2007). Furthermore, activation of protein kinase C, which is known to selectively increase the size of the slowly released component (Minami and others, 1998; Berglund and others, 2002), significantly enhanced the number of fusion events outside of the ribbon region (Midorikawa and others, 2007), which is again consistent with the delayed component being partially due to the fusion of outliers. Are some of these outlier sites authentic ribbon-free active zones? In support of this possibility, Midorikawa and others (2007) showed EM images documenting the presence of two different types of active zone in goldfish bipolar neurons: ribbon-associated synapses with dyad processes, and ribbon-free synapses contacted by single postsynaptic processes. However, given the plasticity of ribbons described above, it is unclear whether ribbons once occupied these active zones, or if these non-ribbon vesicles actually contribute to functional neurotransmission.
The physiological relevance of glutamate release from sites distal to postsynaptic receptors is a potential issue for the docked outlier hypothesis. AMPA receptors are selectively apposed to synaptic ribbons (Matsubara and others, 1996; Morigiwa and Vardi, 1999; Qin and Pourcho 1999; Khimich and others, 2005), but extrasynaptic receptors may participate in sensory transmission as well. For instance, immunogold labeling of retinal ganglion cells confirms that NMDA receptors are located perisynaptically, ~100–300nm from the postsynaptic density (Chen and Diamond, 2002; Zhang and Diamond, 2006), and NMDA receptors may also be located extrasynaptically on AII amacrine cell dendrites (Matsui and others, 2001; Zhou and Dacheux, 2004). Therefore, release events hundreds of nanometers away from synaptic ribbons may have postsynaptic targets. In bipolar cells and frog saccular hair cells, synaptic vesicles dock at the plasma membrane peripheral to the central ribbon region and so may sense incoming calcium, albeit less rapidly than those docked beneath the ribbon (Roberts and others, 1990; Burrone and others, 2002). In addition to these docked vesicles, cytoplasmic vesicles appear to cluster near synaptic ribbons in mouse and goldfish bipolar cells and in frog saccular hair cells (Lenzi and others, 1999; LoGiudice and others, 2008; Matthews and Sterling, 2008). These non-docked vesicle clusters may provide a mechanism to quickly refill the ribbon (Spassova and others, 2004) or perhaps they are directly released at the active zone and contribute to the sustained component without ever contacting the ribbon itself.
Mechanisms of release from the ribbon: The synaptic ribbon as a conveyor belt
The ribbon is often likened to a conveyor belt, dynamically moving vesicles toward the active zone in response to a depolarizing stimulus (Parsons and Sterling, 2003). In this context, the presence on ribbons of the kinesin motor protein KIF3A is of interest. Additionally, the actin-based motor molecule Myosin Va (Mehta and others, 1999) is found in photoreceptors, and mutation of this protein leads to abnormal visual function, malformed ribbons, and ectopic vesicle clustering (Libby and others, 2004). Myosin VIIa is also found in photoreceptors (Liu and others, 1997) and has been implicated in Usher Syndrome type 1, a heritable condition that causes deafness, balance problems, and visual defects (Weil and others, 1995). However, while the presence of both microtubule- and actin-based motor proteins is suggestive, actin and components of microtubules, though present within the terminal, are believed to be absent from synaptic ribbons. Furthermore, using TIRFM, Zenisek and others (2000) showed that vesicles at active zones and at outlying positions approach the membrane at the same speed (~0.8 μm s−1). This suggests that a ribbon-based motor, if it exists, does not deliver vesicles any faster than the means of delivery (most likely diffusion) used by ribbon-free vesicles. By tethering vesicles on its surface, the synaptic ribbon effectively constrains vesicle movement to two dimensions, thus increasing their probability of docking at the active zone. Therefore, diffusion may be a viable mechanism for delivering vesicles to the active zone. It is unclear how vesicles would diffuse on the ribbon, however. Do tethered vesicles diffuse freely on the ribbon or are they fixed in place until a release-triggering event? Precise optical measurements of fluorescent vesicles on ribbons could potentially resolve this question.
Mechanisms of release from the ribbon: Coordinated release
Several lines of evidence indicate that ribbon synapses are able to coordinate the simultaneous fusion of multiple synaptic vesicles. Recordings from afferent boutons postsynaptic to cochlear hair cells show that multiquantal release occurs from single presynaptic active zones (Glowatzki and Fuchs, 2002). Also, analysis of fluctuations in capacitance responses of cochlear inner hair cells suggests that elementary fusion events are often larger than predicted for single synaptic vesicles (Neef and others, 2007), which is also consistent with synchronized fusion of multiple vesicles. In the auditory system, this synchronized release by cochlear hair cells requires ribbons and is the basis for precisely timed activation of the postsynaptic neuron (Khimich and others, 2005). Such precision is in turn necessary for brainstem auditory neurons to detect slight differences in interaural arrival time of acoustic stimuli. Large postsynaptic events consistent with synchronized release of multiple quanta have also been reported at the ribbon synapse between bipolar neurons and AII amacrine cells in the retina (Singer and others, 2004), but the functional requirement for such synchrony is not as readily apparent in this system.
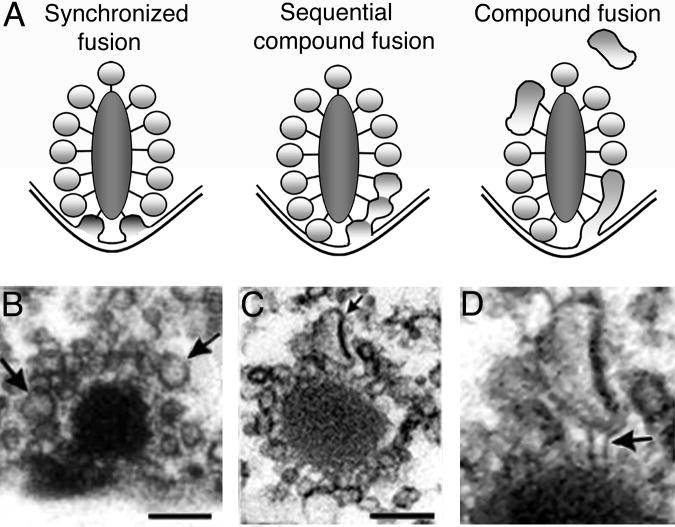
What mechanisms might account for synchronized fusion of multiple vesicles? If multivesicular events reflect the coordinated release of multiple docked vesicles (Glowatzki and Fuchs, 2002), then vesicles may be coupled in some manner, enabling them to fuse in unison, as suggested by Singer and others (2004). (Figure 5A, synchronized fusion). One could imagine this to be the case if the filamentous tethers of distinct vesicles were physically coupled, or if the synaptic ribbon somehow coordinated the fusion machinery of separate vesicles. Alternatively, multivesicular release may occur via the homotypic “compound” fusion of several vesicles associated with the same release site (Heidelberger and others, 1994; Parsons and Sterling, 2003; Edmonds and others, 2004; Matthews and Sterling, 2008). Compound fusion could be a cumulative event triggered by depolarization, in which a docked ribbon-associated vesicle fuses with the membrane and other vesicles sequentially fuse with the one below (Figure 5A, sequential compound fusion). This type of mechanism was proposed by Edmonds and others (2004) to explain their observation that a brief depolarization of frog saccular hair cells releases ~10 times more vesicles than are docked at the active zone. Alternatively, vesicles could fuse with each other either in the cytoplasm or on the ribbon prior to their contact with the plasma membrane (Figure 5A, Compound fusion). Such homotypic fusion could then result in larger quantal events like those reported by Glowatzki and Fuchs (2002) and Singer and others (2004).
Fig. 5.
Multivesicular release at a ribbon-type synapse. A, Schematic representation of three different scenarios where the contents of multiple vesicles are released in a highly synchronized manner. B–D, Electron micrographs of goldfish bipolar cell synaptic ribbons. Large vesicles are often seen attached to synaptic ribbons (arrows, B). C, Large pleomorphic structures appear to be attached by tether-like filaments to the base of the ribbon in repetitively stimulated neurons. Scale bars represent 100 nm. D, Magnified view of cistern-like structure in C. Arrow points at the electron dense filaments that contact the ribbon and the structure. B–D: Modified from Matthews and Sterling (2008) with permission of the Society for Neuroscience.
Recently, Matthews and Sterling (2008) reported two types of ultrastructural evidence favoring compound fusion at ribbons of goldfish bipolar cells. First, depolarization increased the percentage of unusually large vesicles attached to ribbons (Fig. 5B), consistent with possible calcium-dependent fusion among non-docked vesicles tethered to the face of the ribbon. Second, in cells fixed during repetitive stimulation, large, cistern-like membrane structures were observed at the base of the ribbon, attached by tethers like those that bind synaptic vesicles to the ribbon (Fig. 5C,D). Moreover, the attached cisternal structures were continuous with the plasma membrane, as if in the process of fusing with the membrane. A simple interpretation is that the cisterns arise from exocytosis rather than endocytosis, because 1) they occupy the base of the ribbon, where synaptic vesicles are found in unstimulated terminals, 2) they are continuous with the plasma membrane at a location where exocytosis is thought to occur, and 3) they are attached to the ribbon by filaments like those that tether normal synaptic vesicles. Therefore, these structures may represent compound fusion of synaptic vesicles during neurotransmitter release. Although the evidence for vesicle-vesicle fusion at synaptic ribbons is by no means conclusive, compound fusion would neatly account for the functional characteristics of transmitter release at ribbons, including the rapid, synchronous release of multiple vesicles and, to the degree it occurs at ribbon vs. non-ribbon sites, the slower sustained release during prolonged depolarization.
Concluding Remarks
Synaptic ribbons are found in cells that must release neurotransmitter tonically, and it has long been assumed that the ribbon therefore provides a steady supply of synaptic vesicles to the active zone to allow for continuous release of transmitter quanta. Consistent with this view, the size of ribbons and the number of ribbon-associated vesicles correlate across cell types with the physiological needs of the synapse. For example, in the retina, rod and cone photoreceptors have the largest ribbons, in keeping with their high need for ongoing exocytosis at a rapid rate. Also, there is a tonotopic map of ribbon surface area in the cochlea, with hair cells in high-frequency regions having larger ribbons that tether more vesicles. It is surprising, then, that recent work points to greater importance of ribbons for transient, synchronous release than for the sustained release that is, after all, the hallmark of ribbon-containing synapses. There is at present no satisfactory resolution to this contradiction, but to us, it seems unlikely that the large pools of ribbon-tethered vesicles are just bystanders to synaptic transmission, with the real work of continuous release being carried out elsewhere. This issue aside, one thing that does seem clear is that the ribbon facilitates synchronous fusion of multiple synaptic vesicles, and it will also be interesting to see if this high throughput is achieved via vesicle-vesicle fusion or by coordinated exocytosis of two or more separate vesicles. Fortunately, optical tools are at hand that may soon allow ribbon researchers to settle these questions regarding the important role of the ribbon and its tethered vesicles at sensory synapses.
Acknowledgments
Supported by NIH grant EY003821.
Contributor Information
Lisamarie LoGiudice, Program in Neuroscience, State University of New York, Stony Brook, NY 11794-5230.
Gary Matthews, Department of Neurobiology and Behavior, State University of New York, Stony Brook, NY 11794-5230.
References
- Balkema GW. A synaptic antigen (B16) is localized in retinal synaptic ribbons. J Comp Neurolc. 1991;312:573–83. doi: 10.1002/cne.903120408. [DOI] [PubMed] [Google Scholar]
- Balkema GW, Nguyen TH. Co-localization of clathrin adapter proteins with photoreceptor synaptic ribbons in the OPL of the mouse. Soc Neurosci Abstr. 1999;25:135. [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, et al. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–7. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- Berglund K, Midorikawa M, Tachibana M. Increase in the pool size of releasable synaptic vesicles by the activation of protein kinase C in goldfish retinal bipolar cells. J Neurosci. 2002;22:4776–85. doi: 10.1523/JNEUROSCI.22-12-04776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, et al. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–96. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wassle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–93. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005;25:11577–85. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron. 2002;33:101–12. doi: 10.1016/s0896-6273(01)00565-7. [DOI] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–73. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi-Tawarada M, Inoue E, Takao-Rikitsu E, Inoue M, Kitajima I, Ohtsuka T, Takai Y. Active zone protein CAST is a component of conventional and ribbon synapses in mouse retina. J Comp Neurol. 2006;495:480–96. doi: 10.1002/cne.20893. [DOI] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–86. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Edmonds BW, Gregory FD, Schweizer FE. Evidence that fast exocytosis can be predominantly mediated by vesicles not docked at active zones in frog saccular hair cells. J Physiol. 2004;560:439–50. doi: 10.1113/jphysiol.2004.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–54. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–5. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Sterling P, Matthews G. Roles of ATP in depletion and replenishment of the releasable pool of synaptic vesicles. J Neurophysiol. 2002;88:98–106. doi: 10.1152/jn.2002.88.1.98. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Simpson LL, Ridge RM, Betz WJ. Synaptic vesicle movements monitored by fluorescence recovery after photobleaching in nerve terminals stained with FM1-43. J Neurosci. 1996;16:3960–7. doi: 10.1523/JNEUROSCI.16-12-03960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–79. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Cooke A, Neef A, Lagnado L. High mobility of vesicles supports continuous exocytosis at a ribbon synapse. Curr Biol. 2004;14:173–83. doi: 10.1016/j.cub.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23:1329–39. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol. 2006;96:2025–33. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti B, Heidelberger R. Mechanisms contributing to tonic release at the cone photoreceptor ribbon synapse. J Neurophysiol. 2008;99:25–36. doi: 10.1152/jn.00737.2007. [DOI] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca(2+)-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci U S A. 1994;91:7578–82. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, et al. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–94. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ. Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken’s cochlea. Proc Natl Acad Sci U S A. 1997;94:14883–8. doi: 10.1073/pnas.94.26.14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K, Daniell L, Mundigl O, De Camilli P. Mobility of synaptic vesicles in nerve endings monitored by recovery from photobleaching of synaptic vesicle-associated fluorescence. J Neurosci. 1996;16:5905–13. doi: 10.1523/JNEUROSCI.16-19-05905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Crum J, Ellisman MH, Roberts WM. Depolarization redistributes synaptic membrane and creates a gradient of vesicles on the synaptic body at a ribbon synapse. Neuron. 2002;36:649–59. doi: 10.1016/s0896-6273(02)01025-5. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci. 1999;19:119–32. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, von Gersdorff H. Structure suggests function: the case for synaptic ribbons as exocytotic nanomachines. Bioessays. 2001;23:831–40. doi: 10.1002/bies.1118. [DOI] [PubMed] [Google Scholar]
- Libby RT, Lillo C, Kitamoto J, Williams DS, Steel KP. Myosin Va is required for normal photoreceptor synaptic activity. J Cell Sci. 2004;117:4509–15. doi: 10.1242/jcs.01316. [DOI] [PubMed] [Google Scholar]
- Liu X, Vansant G, Udovichenko IP, Wolfrum U, Williams DS. Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil Cytoskeleton. 1997;37:240–52. doi: 10.1002/(SICI)1097-0169(1997)37:3<240::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Llobet A, Cooke A, Lagnado L. Exocytosis at the ribbon synapse of retinal bipolar cells studied in patches of presynaptic membrane. J Neurosci. 2003;23:2706–14. doi: 10.1523/JNEUROSCI.23-07-02706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice L, Henry D, Matthews G. Identification of calcium channel alpha1 subunit mRNA expressed in retinal bipolar neurons. Mol Vis. 2006;12:184–9. [PMC free article] [PubMed] [Google Scholar]
- LoGiudice L, Sterling P, Matthews G. Mobility and turnover of vesicles at the synaptic ribbon. J Neurosci. 2008;28:3150–8. doi: 10.1523/JNEUROSCI.5753-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J Neurosci. 2008;28:7954–67. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990;5:19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Martinez-Dunst C, Michaels RL, Fuchs PA. Release sites and calcium channels in hair cells of the chick’s cochlea. J Neurosci. 1997;17:9133–44. doi: 10.1523/JNEUROSCI.17-23-09133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–67. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Hasegawa J, Tachibana M. Modulation of excitatory synaptic transmission by GABA(C) receptor-mediated feedback in the mouse inner retina. J Neurophysiol. 2001;86:2285–98. doi: 10.1152/jn.2001.86.5.2285. [DOI] [PubMed] [Google Scholar]
- Matthews G, Sterling P. Evidence that vesicles undergo compound fusion on the synaptic ribbon. J Neurosci. 2008;28:5403–11. doi: 10.1523/JNEUROSCI.0935-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE. Myosin-V is a processive actin-based motor. Nature. 1999;400:590–3. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA. Ultrastructure and quantification of synapses in the insect nervous system. J Neurosci Methods. 1996;69:59–73. doi: 10.1016/S0165-0270(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–9. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Midorikawa M, Tsukamoto Y, Berglund K, Ishii M, Tachibana M. Different roles of ribbon-associated and ribbon-free active zones in retinal bipolar cells. Nat Neurosci. 2007;10:1268–76. doi: 10.1038/nn1963. [DOI] [PubMed] [Google Scholar]
- Minami N, Berglund K, Sakaba T, Kohmoto H, Tachibana M. Potentiation of transmitter release by protein kinase C in goldfish retinal bipolar cells. J Physiol. 1998;512 (Pt 1):219–25. doi: 10.1111/j.1469-7793.1998.219bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigiwa K, Vardi N. Differential expression of ionotropic glutamate receptor subunits in the outer retina. J Comp Neurol. 1999;405:173–84. doi: 10.1002/(sici)1096-9861(19990308)405:2<173::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–8. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Brandt A, Lysakowski A. Hair cell ribbon synapses. Cell Tissue Res. 2006;326:347–59. doi: 10.1007/s00441-006-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J Neurosci. 1999;19:1027–37. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef A, Khimich D, Pirih P, Riedel D, Wolf F, Moser T. Probing the mechanism of exocytosis at the hair cell ribbon synapse. J Neurosci. 2007;27:12933–44. doi: 10.1523/JNEUROSCI.1996-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23:4092–9. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–83. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Sterling P. Synaptic ribbon. Conveyor belt or safety belt? Neuron. 2003;37:379–82. doi: 10.1016/s0896-6273(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Prokop A, Meinertzhagen IA. Development and structure of synaptic contacts in Drosophila. Semin Cell Dev Biol. 2006;17:20–30. doi: 10.1016/j.semcdb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Localization of AMPA-selective glutamate receptor subunits in the cat retina: a light- and electron-microscopic study. Vis Neurosci. 1999;16:169–77. doi: 10.1017/s0952523899161121. [DOI] [PubMed] [Google Scholar]
- Rea R, Li J, Dharia A, Levitan ES, Sterling P, Kramer RH. Streamlined synaptic vesicle cycle in cone photoreceptor terminals. Neuron. 2004;41:755–66. doi: 10.1016/s0896-6273(04)00088-1. [DOI] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–84. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer SF, Raviola E. Membrane recycling in the cone cell endings of the turtle retina. J Cell Biol. 1978;79:802–25. doi: 10.1083/jcb.79.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–72. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci. 2004;24:4213–23. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JH, Brownell WE. Synaptic and Golgi membrane recycling in cochlear hair cells. J Neurocytol. 1986;15:311–28. doi: 10.1007/BF01611434. [DOI] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–33. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Avissar M, Furman AC, Crumling MA, Saunders JC, Parsons TD. Evidence that rapid vesicle replenishment of the synaptic ribbon mediates recovery from short-term adaptation at the hair cell afferent synapse. J Assoc Res Otolaryngol. 2004;5:376–90. doi: 10.1007/s10162-004-5003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–9. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–3. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Rabl K, Townes-Anderson E, Heidelberger R. A highly Ca2+-sensitive pool of vesicles contributes to linearity at the rod photoreceptor ribbon synapse. Neuron. 2004;42:595–605. doi: 10.1016/s0896-6273(04)00254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, et al. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–36. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Brandstatter JH. Ribbon synapses of the retina. Cell Tissue Res. 2006;326:339–46. doi: 10.1007/s00441-006-0234-0. [DOI] [PubMed] [Google Scholar]
- Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina: ultrastructure and uptake of horseradish peroxidase. J Cell Biol. 1985;100:175–88. doi: 10.1083/jcb.100.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–88. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–7. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–1. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Wittig JH, Jr, Parsons TD. Synaptic ribbon enables temporal precision of hair cell afferent synapse by increasing the number of readily releasable vesicles: a modeling study. J Neurophysiol. 2008;100:1724–39. doi: 10.1152/jn.90322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci U S A. 2008;105:4922–7. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–48. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. J Neurosci. 2004;24:9752–9. doi: 10.1523/JNEUROSCI.2886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–37. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–70. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006;498:810–20. doi: 10.1002/cne.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Dacheux RF. All amacrine cells in the rabbit retina possess AMPA-, NMDA-, GABA-, and glycine-activated currents. Vis Neurosci. 2004;21:181–8. doi: 10.1017/s0952523804042099. [DOI] [PubMed] [Google Scholar]