Abstract
The psychosis associated with schizophrenia is characterized by alterations in sensory processing and perception1,2. Some antipsychotic drugs were identified by their high affinity for serotonin 5-HT2A receptors (2AR)3,4. Drugs that interact with metabotropic glutamate receptors (mGluR) also show potential for the treatment of schizophrenia5-7. The effects of hallucinogenic drugs, such as psilocybin and lysergic acid diethylamide (LSD), require the 2AR8-10 and resemble some of the core symptoms of schizophrenia10-12. Here we show that the mGluR2 interacts via specific transmembrane helix domains with the 2AR, a member of an unrelated G protein-coupled receptor (GPCR) family, to form functional complexes in brain cortex. The 2AR/mGluR2 complex triggers unique cellular responses when targeted by hallucinogenic drugs, and activation of mGluR2 abolishes hallucinogen specific signalling and behavioural responses. In postmortem human brain from untreated schizophrenic subjects, the 2AR is up-regulated and the mGluR2 is down-regulated, a pattern that could predispose to psychosis. These regulatory changes suggest that the 2AR/mGluR2 complex may be involved in the altered cortical processes of schizophrenia, and represents a promising new target for the treatment of psychosis.
The 2AR and mGluR2/3 show an overlapping distribution in brain cortex in autoradiography studies13. The mGluR2 and mGluR3 are not distinguished by autoradiographic ligands. We used fluorescent in situ hybridization (FISH) to determine whether either of these receptor subtypes are co-expressed by the same neurons. In layer V mouse somatosensory cortex (SCx), 2AR mRNA positive cells were mostly mGluR2 mRNA positive. The level of expression in SCx was much lower for mGluR3 mRNA, which rarely co-localized with 2AR mRNA (Fig. 1a). Control studies validated assay sensitivity and specificity, and similar 2AR/mGluR2 mRNA co-localization was found in cortical primary cultures (Figs. 1a,b,c, and Supplementary Fig. S1). Translation of 2AR protein in cortical pyramidal neurons was found to be necessary for normal mGluR2 expression. Mice with globally disrupted 2AR expression (htr2A−/− mice) showed reduced cortical mGluR2 binding and expression, while mice in which 2AR expression was selectively restored in cortical pyramidal neurons8,14 showed control expression levels (Supplementary Table S1, and Supplementary Fig. S2). The effects of mGluR2/3 activation on 2AR responses have been generally attributed to synaptic mechanisms5,6,13,15. However, the co-localization of 2AR and mGluR2 and the reduction of mGluR2 expression levels in htr2A−/− mice motivated us to examine whether a direct mechanism contributed to cortical crosstalk between these two receptor systems.
Figure 1. 2AR and mGluR2 co-localize and interact.
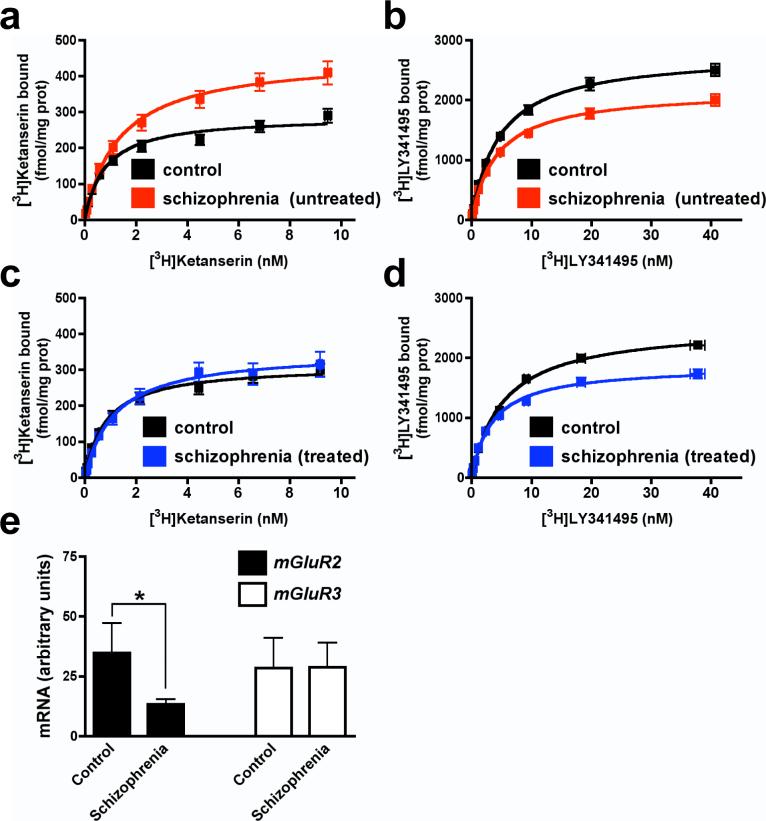
a, 2AR and mGluR2, but not mGluR3, co-express in neurons. Scale, top=50 μm, bottom=10 μm. Nuclei are blue. Inset: co-expressing neuron. b, FISH for mGluR3 in thalamus. Scale, top=25μm, bottom=10μm. c, mRNA levels by real-time PCR (n=6 per group). d, Specific co-immunoprecipitation of 2AR and mGluR2 in duplicate human cortex samples (arrows). e. BRET2 shows specific 2AR and mGluR2 interaction in HEK293 cells. Data are mean±s.e.m. (n=3). The mGluR2/2AR curve is preferably fitted by a saturation curve, F test (p<0.001). The other co-transfection datasets show linear correlations. f, [3H]Ketanserin displacement curves in mouse SCx membranes (top panels). 2AR agonist affinities were higher in the presence of mGluR2/3 agonist 10 μM LY379. [3H]LY341495 displacement curves (bottom panels). mGluR2/3 agonist affinities were lower in the presence of 2AR agonist 10 μM DOI.
Recent studies have demonstrated that some GPCRs belonging to the same sequence classes can form dimers16 or, potentially, higher-order oligomers17. Although the 2AR and mGluR2 belong to different GPCR classes, we established the existence of 2AR/mGluR2 heterocomplexes by several methods: co-immunoprecipitation of human brain cortex samples (Fig. 1d) and of HEK293 cells transfected with epitope-tagged receptors (Fig. 2b), bioluminescence resonance energy transfer (BRET) (Fig. 1e, and Supplementary Fig. S3), and fluorescence resonance energy transfer (FRET) (Fig. 2d) studies in transfected cells.
Figure 2. mGluR2 transmembrane domains 4/5 mediate association with 2AR.
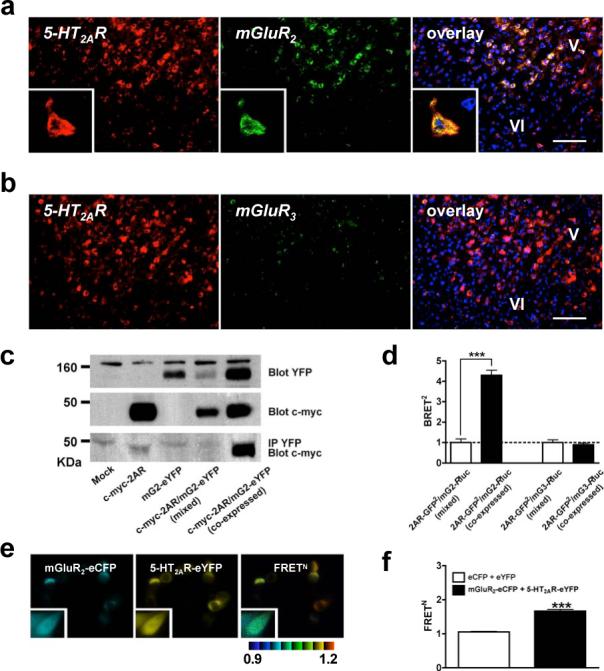
a, mGluR2/mGluR3 chimeras studied. b, c-myc-2AR and HA-mGluR2/mGluR3 chimera co-immunoprecipitations. Cells separately expressing each construct were also mixed. c, 2AR competition binding in cells stably expressing 2AR and transfected with mGluR2/mGluR3 chimeras. d, FRET in cells expressing 2AR-eCFP and either mGluR2, mGluR3 or mGluR3ΔTM4,5 chimera, all tagged with eYFP. Pseudo-colour images represent normalized values (FRETN). eCFP + eYFP (n=19); 2AR-eCFP + mGluR2-eYFP (n=43); 2AR-eCFP + mGluR3-eYFP (n=31); 2AR-eCFP + mGluR3ΔTM4,5-eYFP, (n=27). **p < 0.01; ANOVA with Dunnett's post hoc test. e, DOI-stimulated [35S]GTPγS binding in membranes followed by immunoprecipitation with anti-Gαq/11 (top panels), or anti-Gαi1,2,3 (bottom panels). Cells stably expressing 2AR were transfected with mGluR2, mGluR3 or mGluR3ΔTM4,5. The potency of DOI activating Gαi1,2,3 was significantly increased when the 2AR was co-expressed with either mGluR2 or mGluR3ΔTM4,5, an effect abolished by 10 μM LY379 (p<0.001 by F test). Data are mean±s.e.m. of three experiments performed in triplicate. f, Ribbon backbone representation of the transmembrane helices of the 2AR/mGluR2 heteromer model from the intracellular face.
To determine whether the formation of the 2AR/mGluR2 complex has functional consequences, we first examined the effects in mouse SCx membranes of an mGluR2/3 agonist on the competition binding of several hallucinogenic 2AR agonists (Fig. 1f, top) and of a 2AR agonist on the competition binding of several mGluR2/3 agonists (Fig. 1f, bottom). The agonist affinities for the 2AR and mGluR2/3 were decreased when receptor/G protein complexes were uncoupled by GTPγS (Supplementary Fig. S4, and Supplementary Tables S2 and S3). Notably, the glutamate agonist LY379268 (LY379) increased the affinity of all three hallucinogens studied for the 2AR binding site. Furthermore, the 2AR agonist DOI decreased the affinity of the three mGluR2/3 agonists for the glutamate receptor binding site. The allosteric interactions observed were eliminated by antagonist for each modulator (see Supplementary Tables S2 and S3 and Supplementary Fig. S4 for additional concentrations of DOI and LY379, and elimination of the allosteric effects by antagonists). Although the glutamate agonists studied do not distinguish between the mGluR2 and mGluR3 subtypes18, the rarity of mGluR3 and 2AR mRNA co-expression in cortex, the absence of evidence for 2AR/mGluR3 complex formation by co-immunoprecipitation, BRET and FRET, and the detection of 2AR/mGluR2 complexes by these same assays, suggest that the crosstalk identified results from 2AR/mGluR2 complexes.
The differences in the capacity of the mGluR2 and mGluR3 to interact with the 2AR and their close sequence similarity provided the basis to identify the specific mGluR2 domains responsible for heterocomplex formation. Study of a series of molecular chimeras of the mGluR2 and mGluR3 (see Fig. 2a) demonstrated that the segment containing transmembrane (TM) helices 4 and 5 of the mGluR2 receptor was both necessary and sufficient for complex formation with the 2AR. The mGluR3 receptor chimera containing only this segment from the mGluR2 (mGluR3ΔTM4,5) was capable of co-immunoprecipitating with the 2AR (Fig 2b), mediating allosteric crosstalk (Fig. 2c) and maintaining close proximity with the 2AR as indicated by FRET (Fig. 2d). In contrast, mGluR2ΔTM4,5 did not show evidence of complex formation with the 2AR (Fig. 2, Supplementary Figs. S5, S6, and Supplementary Tables S4 and S5 for complete curves, analysis and evidence of membrane expression of all chimeras). The absolute and relative levels of expression of heterologous constructs were comparable to the physiological levels found in mouse SCx, and in cortical primary cultures (Supplementary Fig. S5 and Supplementary Table S4). Our data do not exclude the possibility that the predicted 2AR/mGluR2 heterodimer, a model of which is shown in Fig. 2f, assembles into tetramers or larger receptor oligomers19,20.
The changes in high affinity binding caused by 2AR/mGluR2 crosstalk suggested that this complex may serve to integrate serotonin and glutamate signalling and modulate G protein coupling21,22. This hypothesis was tested by measuring 2AR regulation of Gαq/11 and Gαi proteins. High-affinity activation of Gαq/11 by the 2AR was reduced by co-expression of mGluR2 (Fig. 2e, and Supplementary Table S6). Interestingly, the activation of Gαi by the 2AR was markedly enhanced by mGluR2 co-expression (Fig. 2e, and Supplementary Table S7). The mGluR2-dependent effects on both Gαq/11 and Gαi regulation by the 2AR were reversed in the presence of mGluR2 agonist (Fig. 2e, and Supplementary Tables S6 and S7). Consonant with the co-immunoprecipitation, allosteric modulation and FRET results, the functional assays of G protein activity also show that the TM4−5 segment of the mGluR2, when substituted into the mGluR3, was sufficient for signalling crosstalk to occur (Fig. 2e). These data support the presence of functional and physiological 2AR/mGluR2 complexes that integrate serotonin and glutamate neurotransmission to specify the pattern of G protein regulation.
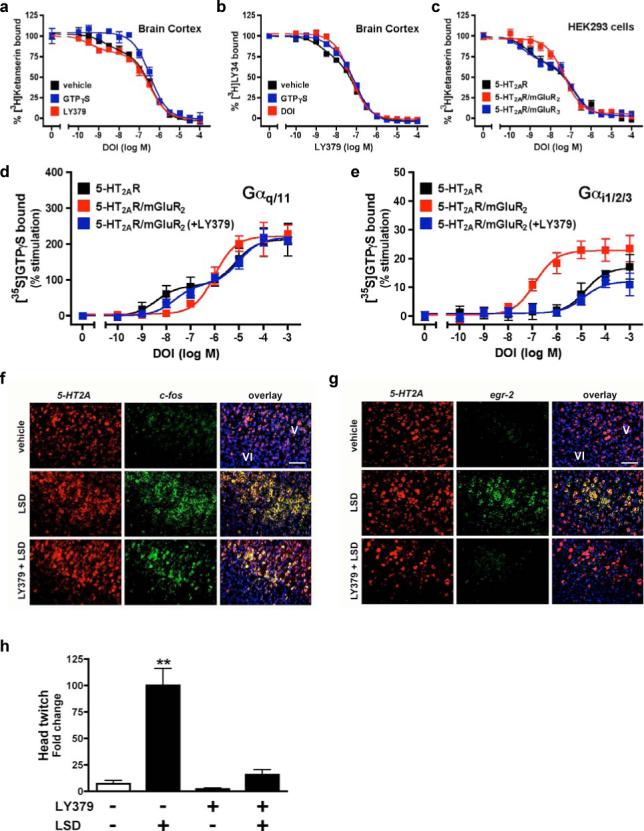
Similar evidence for specification of G protein subtype regulation was also observed by the endogenous brain 2AR/mGluR2 complex with membranes from cortical primary cultures (Fig 3a). The pattern of G protein regulation in cortical pyramidal neurons has been shown to predict specific behavioural responses to 2AR agonists. Hallucinogenic drugs and non-hallucinogenic drugs activate the same population of 2ARs in cortical pyramidal neurons, but differ in the 2AR-dependent pattern of G protein regulation and gene induction they elicit8,9. In brain cortical neurons, the signalling elicited by hallucinogenic and non-hallucinogenic 2AR agonists causes induction of c-fos and requires Gq/11-dependent phospholipase C activation. However, the signalling of hallucinogens such as DOI and LSD acting at the 2AR also induces egr-2, which is Gi/o-dependent. Thus c-fos expression results from any 2AR-signalling, and egr-2 induction is a specific marker for hallucinogen signalling via the 2AR8,9. The finding that mGluR2 modulates the Gi protein coupling of the 2AR (Fig. 3a, and Supplementary Tables S6 and S7) suggested that this complex might be important for hallucinogen signalling. The induction of c-fos by hallucinogenic 2AR agonists or by structurally similar non-hallucinogenic 2AR agonists in vivo in mouse SCx and in cortical primary cultures (Fig. 3b, and Supplementary Figs. S8, S9 and S10) was not affected by the mGluR2/3 agonist LY379. In contrast, the hallucinogen-specific induction of egr-2 was selectively blocked by LY379 in both mouse cortex in vivo and in primary cortical cultures (Fig. 3b, and Supplementary Figs. S8, S9 and S10 for FISH results with LSD treatment, and real-time PCR gene assay results with DOI, DOM, DOB, LSD, lisuride and ergotamine). We also studied the effects of LY379 on the head-twitch response (HTR) behavior, which is hallucinogen-specific8,9. Similar to its effects on G protein activation and gene induction, the glutamate agonist LY379 suppressed the induction of the HTR by either DOI or LSD (Supplementary Fig. S11). These results suggest that LY379 acts at the 2AR/mGluR2 complex to reduce the hallucinogen-specific Gi/o protein signalling and behaviour. To further establish the functional relevance of 2AR/mGluR2 crosstalk, we compared the responses to the mGluR2/3 antagonist LY341494 in htr2A+/+ and htr2A−/− mice. The locomotor and vertical activities elicited by LY341495 were significantly attenuated in the htr2A−/− mice (Fig. 3c), supporting the functional relevance of the 2AR/mGluR2 complex in vivo and suggesting that it also influences the endogenous response to glutamate.
Figure 3. 2AR/mGluR2 complex-dependent modulation of cellular and behavioural responses.
a , DOI-stimulated [35S]GTPγS binding in primary culture membranes followed by immunoprecipitation with anti-Gαq/11 or anti-Gαi1,2,3 antibodies. DOI Gαi1,2,3 activation potency was significantly decreased by 10 μM LY379. Data are mean±s.e.m. of three experiments performed in triplicate. b, FISH in mice injected with vehicle or 2 mg/kg DOI 15 min after being pre-injected with vehicle or 15 mg/kg LY379 (left panels), and in primary cultures treated with 10 μM DOI 15 min after being pre-treated with vehicle or 10 μM LY379 (bottom panels). Nuclei are blue. Scale, left=50 μm, right=10μm. c, Distance and vertical activity induced in htr2A+/+ and htr2A−/− mice by mGluR2/3 antagonist 6 mg/kg LY341495. In htr2A−/− mice, LY341495 effect on distance was reduced (p<0.05, Bonferroni's post hoc test of two-factor ANOVA), and on vertical activity was absent (n=30−32).
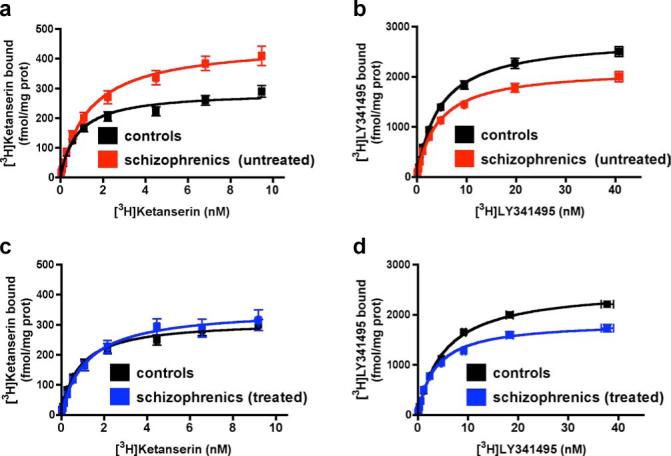
The findings that Gi/o protein regulation, which is necessary for the effects of hallucinogens8, is enhanced by the formation of the 2AR/mGluR2 complex and that activation of the mGluR2 component suppresses hallucinogen-specific signalling implicate this complex in the effects of hallucinogens. The neuropsychological effects of hallucinogenic drugs present commonalities with the psychosis of schizophrenia, and both conditions are accompanied by disruptions of cortical sensory processing10,11,23-27. We investigated whether the components of the 2AR/mGluR2 signalling complex are dysregulated in brain cortex of subjects with schizophrenia. We determined the density of 2AR and mGluR2/3 binding sites in cortex from schizophrenic subjects and controls who were matched by gender, age, and postmortem delay (Supplementary Tables S8 and S9). The receptor densities in cortical membranes from untreated schizophrenic subjects were significantly altered, showing increased 2AR and reduced mGluR2/3 receptor levels (Fig. 4a, b). mRNA assays showed that expression of mGluR2 but not mGluR3 was reduced in schizophrenia cortex (Fig. 4e). The studies in mouse show that activation of the mGluR2 component of the 2AR/mGluR2 complex eliminates the hallucinogen-specific component of the signalling responses to LSD-like drugs. Thus the increased 2AR and decreased mGluR2 found in the brain in schizophrenia may predispose to a hallucinogenic pattern of signalling.
Figure 4. 2AR is increased and mGluR2 is decreased in schizophrenia.
a, b, Frontal cortex membrane receptor binding assays from untreated schizophrenic (n = 13) and matched control subjects (n = 13). In schizophrenia, [3H]ketanserin binding was higher and [3H]LY341495 binding was lower (p< 0.05; Student's t-test). c, d, Receptor binding in antipsychotic-treated schizophrenic (n = 12) and matched control subjects (n = 12). In treated schizophrenia, [3H]ketanserin binding was unaffected and [3H]LY341495 binding was lower (p < 0.05). e, mGluR2 mRNA expression is reduced in untreated schizophrenic subjects (n = 7) compared to matched control subjects (n = 7, p < 0.05, mean±s.e.m).
Many laboratories have attempted to determine the density of 2AR in postmortem brain from subjects with schizophrenia, and some studies have reported decreased or unchanged 2AR densities28. To try to understand the basis for these discrepancies from our results, we first studied the effects of chronic antipsychotic treatment on the 2AR and mGluR2 in mouse. The chronic atypical antipsychotic clozapine specifically down-regulated the level of expression of 2AR and of mGluR2 in mouse SCx (Supplementary Fig. S12). The down-regulation of mGluR2 by clozapine required expression of the 2AR, as it did not occur in htr2A−/− mice (Supplementary Fig. S12), and was not induced by the chronic typical antipsychotic haloperidol (Supplementary Fig. S13). In concordance with the effects of clozapine in murine models, the density of 2AR was reduced to control levels in postmortem human brain cortex of schizophrenics treated with atypical antipsychotic drugs (Fig. 4c), and the mGluR2/3 binding sites were also down-regulated (Fig. 4d). The onset of psychosis in schizophrenia usually occurs in later adolescence or early adulthood1. We studied the relationship of receptor densities with aging and both [3H]ketanserin and [3H]LY341495 binding displayed a highly significant negative correlation with age (Supplementary Fig. S14). Hallucinations and delusions typically attenuate with aging29, which correlates with the lower density of the components of the 2AR/mGluR2 complex that we observed in older subjects. Consequently, the marked dysregulation of both 2AR and mGluR2 expression in schizophrenia would be unlikely to be observed in samples from heterogeneous groups including treated patients28 or in studies including older patients28,30.
These studies identify the 2AR/mGluR2 complex as a possible site of action of hallucinogenic drugs. The glutamate and serotonin systems have both been implicated in psychotic disorders, and the components of this complex are found to be differentially regulated in cortex from individuals with schizophrenia. The results are consistent with the hypothesis that the 2AR/mGluR2 complex integrates serotonin and glutamate signalling to regulate the sensory gating functions of the cortex, a process that is disrupted in psychosis.
METHODS
A detailed Methods section is available in Supplementary Information. Briefly, all reagents were purchased from commercial vendors except for LY379268 (Eli Lilly and Company). Mouse lines, treatment protocols, behavioural studies, dissections, and primary neuronal cultures, approved by Institutional Use and Care Committees, have been previously described8,9. Protocols used for FISH8, binding assays8, real-time PCR8, FRET17 and co-immunoprecipitation17 were performed as previously described or with minor modifications. Epitope tagged, BRET2, FRET and chimera receptor constructs were generated using standard cloning techniques and were confirmed by sequencing. BRET2 using Renilla luciferase and Green Fluorescent Protein (GFP2) was performed in HEK293 cells. Matched schizophrenia and control human brains were obtained from autopsies performed in the Basque Institute of Legal Medicine, Bilbao, Spain in compliance with policies of research and ethical review boards for postmortem brain studies.
Supplementary Material
Acknowledgements
The authors would like to thank Lakshmi Devi and Lidija Ivic for critiquing the manuscript, Susan Morgello and the Manhattan HIV Brain Bank for providing control brain cortex, Itxazne Rodil, Leyre Urigüen and Bernard Lin for assistance with biochemical assays, and the Mount Sinai Microscopy and Microarray, Real-Time PCR and Bioinformatics Shared Research Facilities. We thank the staff members of the Basque Institute of Legal Medicine for their cooperation in the study. We thank James H. Prather of Eli Lilly and Company for a generous gift of LY379268, and Jean-Philippe Pin for graciously providing the signalling peptide sequence of rat mGluR5. This study was supported by the National Institutes of Health, UPV/EHU and Basque Government, Spanish Ministry of Health, REM-TAP Network, the Whitehall Foundation, the Gatsby Foundation, and the American Foundation for Suicide Prevention.
References
- 1.Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–49. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–5. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, et al. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol Psychiatry. 1998;44:1099–117. doi: 10.1016/s0006-3223(98)00187-5. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 5.Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–12. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 6.Marek GJ. Metabotropic glutamate 2/3 receptors as drug targets. Curr Opin Pharmacol. 2004;4:18–22. doi: 10.1016/j.coph.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Patil ST, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Maeso J, et al. Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron. 2007;53:439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Maeso J, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–43. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 11.Gouzoulis-Mayfrank E, et al. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–11. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- 12.Colpaert FC. Discovering risperidone: the LSD model of psychopathology. Nat Rev Drug Discov. 2003;2:315–20. doi: 10.1038/nrd1062. [DOI] [PubMed] [Google Scholar]
- 13.Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- 14.Weisstaub NV, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–40. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 15.Benneyworth MA, et al. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–84. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- 16.Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol. 2002;42:409–35. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Gimenez JF, Canals M, Pediani JD, Milligan G. The alpha1b-adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol Pharmacol. 2007;71:1015–29. doi: 10.1124/mol.106.033035. [DOI] [PubMed] [Google Scholar]
- 18.Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H] LY341495 binding to group II metabotropic glutamate receptors in rat brain. J Pharmacol Exp Ther. 2001;298:453–60. [PubMed] [Google Scholar]
- 19.Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 20.Fotiadis D, et al. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421:127–8. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 21.Kenakin T. Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov. 2002;1:103–10. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ. Quantitative stoichiometry of G-proteins activated by mu-opioid receptors in postmortem human brain. Eur J Pharmacol. 2002;452:21–33. doi: 10.1016/s0014-2999(02)02242-2. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39(Suppl 1):S10–4. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- 24.Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 25.Vollenweider FX, et al. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–72. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 26.Umbricht D, et al. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology. 2003;28:170–81. doi: 10.1038/sj.npp.1300005. [DOI] [PubMed] [Google Scholar]
- 27.Gouzoulis-Mayfrank E, et al. Inhibition of Return in the Human 5HT(2A) Agonist and NMDA Antagonist Model of Psychosis. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300882. [DOI] [PubMed] [Google Scholar]
- 28.Dean B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem. 2003;85:1–13. doi: 10.1046/j.1471-4159.2003.01693.x. [DOI] [PubMed] [Google Scholar]
- 29.Davidson M, et al. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am J Psychiatry. 1995;152:197–207. doi: 10.1176/ajp.152.2.197. [DOI] [PubMed] [Google Scholar]
- 30.Gurevich EV, Joyce JN. Alterations in the cortical serotonergic system in schizophrenia: a postmortem study. Biol Psychiatry. 1997;42:529–45. doi: 10.1016/S0006-3223(97)00321-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.