Summary
The ventral stream refers to a neural pathway that projects from early visual areas through to anterior temporal cortex, and comprises regions in ventral and lateral occipital-temporal cortex. The ventral stream is critical for recognizing visually presented objects. Functional imaging studies of the human brain have shown that different regions within the ventral stream show differential activation to nonliving (tools, houses) and living stimuli (animals, faces). The causes of these category preferences are widely debated. Using functional magnetic resonance imaging, we find that the same regions of the ventral stream that show category preferences for nonliving stimuli and animals in sighted adults, show the same category preferences in adults who are blind since birth. Both blind and sighted participants had larger blood oxygen-level dependent (BOLD) responses in the medial fusiform gyrus for nonliving stimuli compared to animal stimuli, and differential BOLD responses in lateral occipital cortex for animal stimuli compared to nonliving stimuli. These findings demonstrate that the medial-to-lateral bias by conceptual domain in the ventral stream does not require visual experience in order to develop, and suggest the operation of innately determined domain-specific constraints on the organization of object knowledge.
Introduction
Neuropsychological studies of brain damaged patients (e.g., Capitani, 2003; Warrington and McCarthy, 1987), as well as functional imaging studies of healthy individuals (e.g., Chao et al., 1999), have documented the existence of dissociable neural systems that are specialized for representing knowledge of different conceptual domains. The observation that cognitive and neural systems can dissociate along conceptual domain distinctions has served as an important testing ground for hypotheses about the role of experience in shaping the functional architecture of the brain. It is widely argued that category-specific representations arise due to privileged relationships between specific conceptual domains and specific types of sensory and motor information (e.g., (Haxby et al., 2001; Levy et al., 2001; Op de Beeck et al., 2008; Rogers et al., 2005; Tarr and Gauthier, 2000; Warrington and McCarthy, 1987). An alternative view is that the functional architecture of the brain innately anticipates the different computational requirements for representing and processing items from different conceptual domains, in part, independently of sensory and motor experience (e.g., Caramazza and Shelton, 1998; Carey, 1994; Duchaine, 2006; New et al., 2007).
Recently, interest in these issues has focused on the causes of category-specific neural responses in ventral and lateral occipital-temporal cortex. It is known that ventral and lateral occipital-temporal cortex, or the ‘ventral stream’, subserves high-level visual object processing, and represents the visual form and color of objects. Damage to the ventral stream in sighted individuals can cause difficulties in recognizing visually presented objects (visual agnosia: e.g., Goodale, 1992; Miceli et al., 2001; Ungerleider, 1982). It is also known that occipital-temporal cortex in humans and nonhuman primates contains populations of cells that are specialized for objects from different conceptual domains (e.g., Allison et al., 1994; Martin, 2007; Tsao et al., 2006). In humans, medial regions on the ventral surface of the ventral stream (the medial fusiform gyrus, lingual gyrus, and parahippocampal cortex) show differential blood oxygen-level dependent (BOLD) responses for artifacts, such as tools and nonmanipulable objects, compared to living animate things, such as animals and faces. In contrast, lateral regions on the ventral surface of the ventral stream (the lateral fusiform gyrus, inferior temporal gyrus) show differential neural responses for living things compared to artifacts (e.g., Allison et al., 1994; Chao et al., 1999; Downing et al., 2006; Mahon et al., 2007; Noppeney et al., 2006; for reviews see Martin, 2007; Op de Beeck et al., 2008). There is also articulated structure within lateral occipital cortex, with distinct regions showing functional specialization for body parts, faces, and objects (e.g., Downing et al., 2001; Pitcher et al., 2009).
A widely accepted view is that category preferences in the ventral stream depend only on locally based dimensions of similarity that are defined by visual experience (e.g., Haxby et al., 2001; Levy et al., 2001; Rogers et al., 2005; Tarr and Gauthier, 2000). An alternative view is that category preferences in the ventral stream are determined, in part, by dimensions of similarity that cannot be reduced to the visual experience of individuals (e.g., Caramazza and Mahon, 2003; Mahon et al., 2007). This issue can be resolved using fMRI to study BOLD responses to stimuli from different conceptual domains in congenitally blind adults.
Previous research with blind humans has shown that occipital-temporal cortices are active during tactile exploration of objects (Pietrini et al., 2004), Braille reading (Buchel et al., 1998), as well as during imagery of object shape when participants are presented with the canonical sounds of objects (De Volder, 2001). It is also known, that during tactile exploration of objects, the response properties of the BOLD signal at the voxel-level can be more highly correlated within category than between different categories (Pietrini et al., 2004). However, previous studies with blind participants have not addressed the issue of whether there are differential BOLD responses to items from different conceptual domains in localized regions within the ventral stream, in the absence of visual experience. In particular, it is unknown whether individuals who are blind since birth will show differential BOLD responses in medial regions on the ventral surface of occipital-temporal cortex when thinking about nonliving things. Similarly, it is unknown whether, in the absence of visual experience, stimuli corresponding to living things lead to differential BOLD responses in regions that show the same category preference in sighted participants.
Our goal was not to determine whether there is selectivity in BOLD responses by conceptual domain. We therefore contrasted artifact (hereafter nonliving) with animal stimuli, as this contrast has previously been used to obtain a reliable medial-to-lateral segregation of regions showing category preferences for nonliving and living stimuli (e.g., Chao et al., 1999; Mahon et al., 2007; Noppeney et al., 2006). Our goal was to test whether the medial-to-lateral organization of the ventral stream, reflecting preferences for nonliving and living stimuli, respectively, is present in individuals who have had no visual experience.
Results
In order to test the experimental hypothesis it was necessary to devise a task that could be performed by both sighted and blind individuals. We therefore asked participants to perform size-judgments over stimuli that were presented as auditory words. Stimuli were presented in blocks of six spoken words (spanning 20 seconds), grouped by conceptual category (see Experimental Procedures for details). We chose a size-judgment task because previous functional imaging work has successfully used this task to study the properties of BOLD responses in occipital-temporal cortex (Dobbins et al., 2004). Seven sighted adults and three congenitally blind adults completed the size-judgment task, and twenty sighted participants completed a picture-viewing task involving black and white photographs corresponding to the same stimuli used in the auditory size-judgment task.
We functionally defined the ‘ventral stream’ as all voxels that were significant in the omnibus test for the 20 participants viewing pictures (random effects analysis, threshold: t > |2.87|, p <.05, correcting for false discovery rate, hereafter: FDR corrected). The resulting set of voxels extended from early visual regions to lateral occipital-temporal cortex, as well as ventrally, encompassing the lingual, fusiform, inferior temporal, and parahippocampal gyri (see Figure 1). We refer to this set of voxels as the ‘functionally defined ventral stream.’
Figure 1. Main effects of task in sighted and congenitally blind participants.
The ventral object processing stream was functionally defined as all voxels that were significant in the omnibus test (random effects analysis) in sighted participants viewing pictures, and that were at or below a z coordinate in Talairach space of 6 (resulting mask = 131,602 mm3). The contrast map for the omnibus test for viewing pictures within the functionally defined ventral stream is shown in the right panel. The left and middle panels show the omnibus analysis when sighted (left) and congenitally blind (middle) participants performed the auditory size-judgment task (collapsing across animal and nonliving stimuli). As can be seen, for the auditory size-judgment task there was a pattern of relatively decreased BOLD responses in early visual regions in sighted participants but increased BOLD responses in congenitally blind participants.
In a first analysis, we studied the BOLD response profile throughout the functionally defined ventral stream in the sighted and blind participants who performed the auditory size-judgment task, collapsing across animal and nonliving stimuli. As can be seen in Figure 1, there was a general pattern of relatively decreased BOLD responses in early visual regions in sighted participants, while congenitally blind participants showed increased BOLD responses within the same regions. These data replicate the observation (e.g., Amedi et al., 2004) that congenitally blind adults recruit early visual areas for verbal processing.
In a second analysis, we defined category preferring voxels within the functionally defined ventral stream by contrasting ‘animal’ with ‘nonliving’ stimuli. This contrast was carried out over the group-level dataset for the sighted participants who performed the auditory size-judgment task (threshold: t > |2.77|, p <.05, FDR corrected; see Figure 2 for the contrast maps used to define the regions of interest – ROIs). We then tested the contrast of ‘animal’ vs. ‘nonliving’ stimuli in congenitally blind participants as well as sighted participants viewing pictures (averaging over all voxels within the respective ROIs). This ROI-based approach allows us to define voxels showing differential category effects with a separate dataset (sighted participants performing auditory size-judgments) as that used to test the experimental hypothesis (congenitally blind participants). Furthermore, this approach allows us to confirm the reliability of category preferences, as defined over the auditory size-judgment task in sighted participants, on the dataset from the picture-viewing experiment. Fixed effects analyses were used to analyze the data from the seven sighted participants performing the auditory size-judgment task and the three congenitally blind participants. The critical empirical finding would thus consist in demonstrating that the medial-to-lateral organization of the ventral stream by conceptual domain can be present in individuals who have had no visual experience (see e.g., Dilks et al., 2009 for a study based on similar logic). Random-effects analyses were used to analyze the data from the sighted participants viewing pictures (n = 20), thus allowing confirmation at the population level (of sighted participants) of the category preferences of the ROIs.
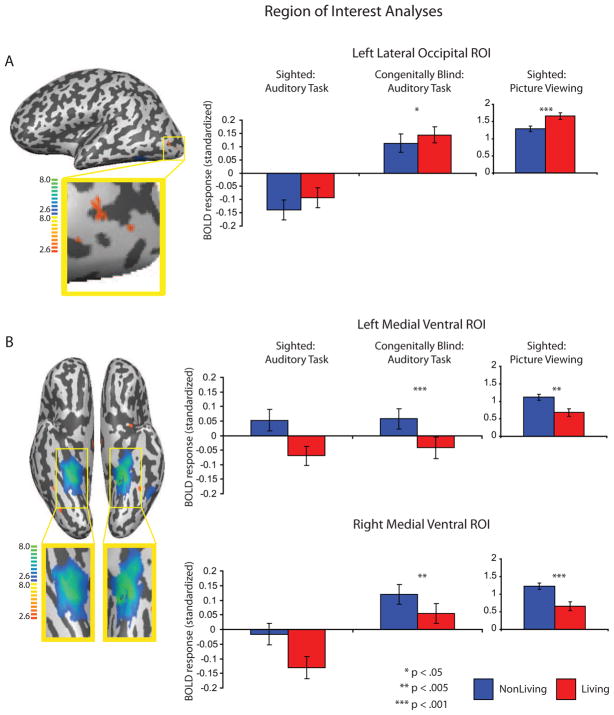
Figure 2. Regions of Interest analyses of category preferences.
Regions of interest (ROIs) were defined contrasting ‘animal’ against ‘nonliving’ stimuli in sighted participants viewing pictures (thresholded at p <.05, FDR corrected). Voxels showing differential BOLD responses for animal stimuli compared to nonliving stimuli are shown on the red-yellow color scale (panel A), while voxels showing differential BOLD responses for nonliving things compared to animals are shown on the blue-green color scale (panel B). The bar graphs depict the estimates for BOLD responses for animal and nonliving stimuli within those ROIs, for all datasets (collapsing across all voxels within the ROI). The left-most graphs (panels a and b) showing BOLD responses for sighted participants performing auditory size-judgments do not have indicators for statistical significance because those data come from voxels that were used to define the ROI. Error bars reflect the standard error of the mean.
The results of the ROI analysis are summarized in Figure 2. An animal-preferring region was identified in left lateral occipital cortex in sighted participants performing auditory size judgments. For that ROI, there were also differential BOLD responses for animal stimuli compared to nonliving stimuli in congenitally blind participants performing the auditory size-judgment task (t = 1.97, p <.05) as well as sighted participants viewing pictures (t = 3.47, p <.0006).
Bilateral medial regions on the ventral surface of occipital-temporal cortex that preferred nonliving stimuli were identified in sighted participants performing auditory size-judgments. Those ROIs encompassed the medial fusiform gyrus, the collateral sulcus, and parahippocampal cortex. The same regions also showed differential BOLD responses for nonliving stimuli compared to animal stimuli in congenitally blind participants (Left: t = −5.99, p <.0001; Right: t = −3.84, p <.0002) as well as in sighted participants viewing pictures (Left: t = −8.99, p <.0001; Right: t = −8.49, p <.0001).
The results of these ROI-based analyses show that regions identified on the basis of sighted participants performing the auditory size-judgment task have the same category preferences in congenitally blind participants as well as in sighted participants viewing pictures. The statistical contrast maps presented in Figure 3 correspond to those obtained when category preferring voxels were identified on the basis of each respective dataset. As can be seen in the enlarged images of Figure 3, there is spatial consistency both in the location and extent of animal and nonliving preferring regions in sighted and congenitally blind participants.
Figure 3. Contrast maps for animal vs. nonliving stimuli for all groups of participants.
Voxels showing differential BOLD responses for animal stimuli compared to nonliving stimuli are shown on the red-yellow color scale (panel A), while voxels showing differential BOLD responses for nonliving things compared to animals are shown on the blue-green color scale (panel B). For visualization purposes, all statistical contrast maps were thresholded at p <.01, uncorrected. Within the left lateral occipital ROI (see Figure 2), the voxels showing the greatest difference between animal and nonliving stimuli were: Sighted participants auditory size-judgments: −43, −76, −5; peak effect: t = 3.61, p <.001; Congenitally Blind: −43, −76, −7; peak effect: t = 2.49, p <.02; Sighted participants viewing pictures: −42, −76, −2; peak effect: t = 5.44, p <.001. Within the bilateral medial ventral stream ROIs (see Figure 2), the voxels showing the greatest difference between animal and nonliving stimuli were: Sighted participants auditory size-judgment: Left: −24, −40, −11; peak effect: t = −9.20, p <.001; Right: 27, −31, −17; t = −7.96, p <.001; Congenitally Blind: Left: −30, −46, −8; peak effect: t = −9.25, p <.001; Right: 30, −37, −11; peak effect: t = −6.21, p <.001; Sighted participants viewing pictures: Left: −24, −40, −11; peak effect: t = −6.88, p <.001; Right: 27, −49, −8; peak effect: t = −13.21, p <.001.
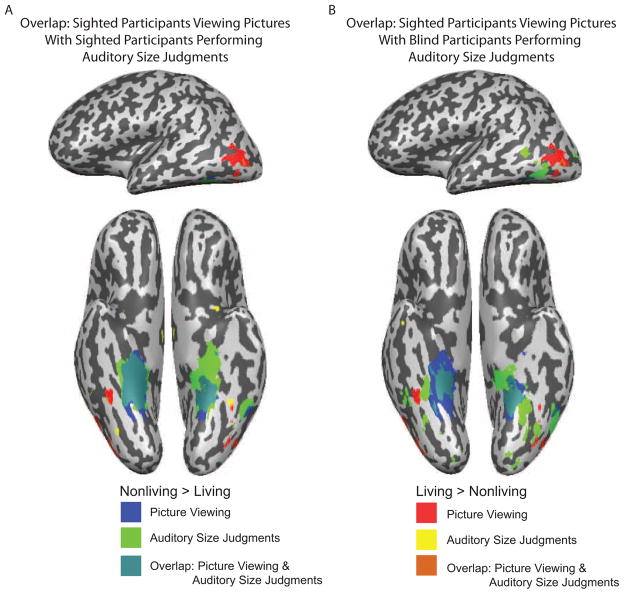
Figure 4 re-plots the data presented in Figure 3 in order to show overlap in category preferences. Separate maps are shown comparing sighted participants viewing pictures with sighted participants performing auditory size-judgments, and sighted participants viewing pictures with the congenitally blind participants performing auditory size-judgments. Those overlap maps summarize the principal finding: the medial-to-lateral bias by conceptual domain on the ventral surface of occipital-temporal cortex does not depend on visual experience. One pattern that emerges is that the differential BOLD responses for nonliving things are both stronger and spatially more extensive than those for living things. This may be due to the fact that congenitally blind participants have disproportionately more sensory experience that is relevant for processing the shapes of nonliving things (e.g., fork, car) than living things (e.g., bird, elephant; see below for further discussion).
Figure 4. Overlay of contrast maps of category preferences in sighted and blind participants.
All statistical contrast maps (nonliving vs. living) were thresholded at p <.01, uncorrected (as in Figure 3). A. Regions showing category preferences are plotted for sighted participants viewing pictures and sighted participants performing the auditory size-judgment task. The greatest overlap is observed for nonliving things in medial regions on the ventral surface of occipital-temporal cortex. Voxels in lateral occipital cortex showing differential BOLD responses for living things in sighted participants performing auditory size-judgments fall within the set of voxels showing the same category preference for the picture viewing experiment. B. Regions showing category preferences are plotted for sighted participants viewing pictures and congenitally blind participants performing the auditory size-judgment task. As was observed for sighted participants performing the auditory size-judgment task (panel A), the regions showing differential BOLD responses for nonliving things were larger than those showing differential BOLD responses for living things. Voxels in blind participants in lateral occipital cortex showing a category preference for living things fall within the region identified in sighted participants viewing pictures.
A further issue that can be addressed is to quantify the relative similarity at the voxel-level, between the blind and the sighted participants, taking as a baseline the similarity among the sighted participants (see Supplemental Discussion). The results of that analysis (see Supplemental Figure S1) showed that the distribution of proportion overlap for the blind participants is within the range established by the sighted participants performing the same task.
As noted above, differential BOLD responses for living stimuli compared to nonliving stimuli, for all participants, were observed in lateral occipital cortex. In other words, the medial-to-lateral bias on the ventral surface of occipital-temporal cortex is principally driven by differential BOLD responses for nonliving things in medial regions. In order to quantify the extent to which differential BOLD responses for nonliving things are biased toward medial regions on the ventral surface of occipital-temporal cortex, we computed a medial-to-lateral index over this region (see Figure 5). The medial-to-lateral index takes the mean contrast-weighted t-value for the comparison of animal stimuli versus nonliving stimuli, averaging along the superior-inferior (z) and anterior-posterior (y) dimensions. The medial-to-lateral index was calculated in sighted participants viewing pictures, sighted participants performing auditory size-judgments, and congenitally blind participants. In all groups of participants, there is a pattern of increasing t-values moving from medial to lateral coordinates within the ventral stream, in both the left and right hemispheres (see Figure 5). Furthermore, there were high levels of similarity in terms of the medial-to-lateral index among all groups of participants (see Figure 5 for details). Thus, across all three data sets differential BOLD responses for nonliving things are biased toward medial regions on the ventral surface of occipital-temporal cortex.
Figure 5. Medial-to-lateral index computed over ventral occipital-temporal cortex.
A. The color overlay shows the extent of the ROI in ventral occipital-temporal cortex (maximum ‘y’ Talairach = −20, minimum ‘y’ Talairach = −70; following Pietrini and colleagues, 2004. Within the ventral occipital-temporal ROI, a medial-to-lateral index was calculated, by averaging the contrast-weighted t-values (animal stimuli – nonliving stimuli) along the anterior-posterior (‘y’) and superior-inferior (‘z’) dimensions, within the range of |25| to |40| on the x axis in Talairach space. The results of this analysis are plotted in panels B for sighted participants viewing pictures, C for sighted participants performing auditory size-judgments, and D for blind participants. Error bars on all graphs represent the standard error of the mean for contrast-weighted t-values, averaged along the z and y axes. Vertical red dotted lines are placed at the mean t-value, in order to indicate the corresponding Talairach coordinate.
Discussion
Using an auditory size-judgment task in sighted and congenitally blind participants, we have shown that the medial-to-lateral bias for nonliving and living stimuli in the ventral stream does not require visual experience. The regions that exhibited category preferences in sighted and congenitally blind participants during the auditory size-judgment task overlapped with regions showing the same preferences when sighted participants viewed pictures corresponding to the auditory stimuli. We further showed that when the analysis is restricted to the ventral surface of occipital-temporal cortex, there is a consistent medial-to-lateral bias in relative category preferences. In particular, differential BOLD responses for nonliving stimuli compared to animals were biased toward medial regions on the ventral surface of occipital-temporal cortex.
Previous research has argued for the role of visually based dimensions in shaping the organization of object representations within the ventral stream (e.g., Haxby et al., 2001; Levy et al., 2001; Op de Beeck et al., 2008; Rogers et al., 2005; Tarr and Gauthier, 2000). The conclusion that visual experience is not necessary for certain aspects of the organization of object knowledge to emerge does not preclude the contribution of such visually based dimensions. Rather, in the context of previous research, our current findings suggest that the organization of the ventral stream reflects a hierarchy of principles working in concert (e.g., Caramazza and Mahon, 2003; Op de Beeck et al., 2008).
One framework that can accommodate our findings views category-specific regions of the ventral stream as parts of broader neural circuits within the brain that are innately disposed to handle information about different domains of objects. We have referred to this view as the distributed domain-specific hypothesis (Mahon and Caramazza, 2009). That hypothesis provides a natural explanation for a range of other findings. For instance, BOLD responses to place and face stimuli in medial and lateral regions of the ventral stream, respectively, were found to be significantly more similar in mono-zygotic than in dizygotic twins (Polk et al., 2007). Other findings that would fit within the distributed domain-specific hypothesis come from neuropsychological studies of brain damaged patients (e.g., Caramazza and Shelton, 1998; Duchaine, 2006; Farah and Rabinowitz, 2003; Miceli et al, 2000), developmental studies of object concepts in infants (e.g., Carey, 1994; Keil, 1981), research with non-human animals (e.g., Gallistel, 1990; Kiani et al., 2007; Kriegeskorte et al., 2008; Tsao et al., 2006), behavioral research with humans (New et al., 2007; Pitcher et al., 2009), and analyses of structural connectivity in congenital prosopagnosia (Thomas et al., 2009).
While an interpretation in terms of innate domain-specific constraints offers an account of both our own and other findings, the adoption of such a strong position is not necessary. The critical open issue that is framed by our results is whether the constraints that determine category preferences for nonliving and living things are expressed over semantically interpreted properties of objects. It may be argued that the basic principle determining the organization of the ventral stream is not the conceptual domain to which an object belongs, but rather a sensory-based dimension of similarity that is highly correlated with the distinction between nonliving and living things. For instance, information about object shape is represented in ventral occipital-temporal cortex, and shape information can be acquired through either the visual or tactile modality. Furthermore, it may be argued that shape information, at least of objects with which we regularly interact, will be similar independently of whether it is acquired through vision or touch. Thus, different regions of the ventral stream may be disposed to represent objects that have different shapes. Such an account cannot at present be excluded on empirical grounds. However, it is important to note that the notion of ‘object shape’ required by such an account would have become so abstract that it would no longer be directly interpretable in terms of specific sensory qualities, either visual or tactile. The account would leave unaddressed the critical issue of what it is about the shapes of nonliving and living things, common to both vision and touch, that determines the observed macro-level organization. In other words, an additional, and pre-existing bias, must also be assumed that would lead to objects with one ‘type’ of shape being represented in one part of the ventral stream, while objects of another ‘type’ would be represented in another part of the ventral stream. Without an account of what the relevant ‘types’ of shapes consist in, it is not obvious that such a proposal is distinguishable from the view that there are innate biases according to distinctions among conceptual domains that determine the organization of the ventral stream.
A related issue that is also framed by our findings concerns the format of information that is represented in occipital-temporal cortex. The fact that congenitally blind participants show category preferences within the ventral stream indicates that the information represented in that region must be accessible through both the visual modality and through other modalities available to blind individuals, such as touch or audition (for discussion see Pietrini et al., 2004). However, this does not sanction the inference that sighted participants do not represent strictly visual, or even visually relevant, information in occipital-temporal cortex. It may be the case that the information that is represented in sighted and congenitally blind individuals in ventral occipital-temporal cortex is radically different. What can be inferred from our findings is that plasticity of function in higher-order visual areas (e.g., Amedi et al., 2004; Kahn and Krubitzer, 2002; Pascual-Leone, 2005) operates within broader constraints that determine category preferences for nonliving and living things.
To date, theoretical accounts of the organization of the ventral object processing stream have generally focused on how the system adapts to constraints that are imposed ‘bottom up.’ A different approach is to view the organization of the ventral stream as satisfying multiple pressures, not all of which are due to the sensory input. Some of the pressures that are satisfied by the organization of the ventral stream may come from other regions of the brain, such as motor or affective systems (Mahon et al., 2007). Ultimately, the utility of sensory information is determined by its role in guiding behavior. The information about a stimulus that is computed in the ventral stream may be channeled to different regions of the brain according to the behavior that is appropriate for that stimulus. Within this framework, the findings reported herein suggest that the organization of the ventral stream innately anticipates the different types of computations that must be carried out over objects from different conceptual domains.
Experimental Procedures
Experimental Stimuli
We selected 24 animals, 24 tools, and 24 nonmanipulable object concepts following the criteria described in a previous study (for details see Mahon et al., 2007). For each concept, a single photograph (black and white grayscale, 400×400 pixels) was selected to be used in the picture-viewing experiment. Each stimulus word was also recorded digitally (22.050 kHz, 16 Bit) by a native Italian speaker (female) to be presented binauraly. We ensured that the three stimulus types were matched on length in Italian (animals mean length = 7.0 letters; tools: 7.6; nonmanipulable: 7.8; one-way Anova: F2,69 < 1). All analyses reported herein collapse tools together with nonmanipulable to form the ‘artifact’ category. Custom software (ASF, Schwarzbach, J., 2008, available from JS) written in Matlab utilizing the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997) was used for stimulus presentation.
Picture-viewing experiment
Stimuli were presented in 20 second blocks, followed by 20 seconds of blank screen (fixation). Each block of stimuli contained 24 pictures, all from the same stimulus type (each stimulus presented for 50 refreshes of the monitor, refresh rate = 60Hz, ISI = 0). All picture stimuli (i.e., blocks of items) were repeated three times throughout the run. The order of items within a block was random, as was the order of blocks. The run lasted approximately 10 minutes. A fourth category of objects (fruit/vegetables) was also included in the picture-viewing experiment (data not shown). Participants viewed the stimuli through a mirror attached to the head coil adjusted to allow foveal viewing of a back-projected monitor.
Size-judgment task with auditorily presented words
Participants (both sighted and blind) were asked to keep their eyes closed throughout the experiment. Stimuli were presented in groups of 6 words, all from the same conceptual category. The duration of the six words spanned 20 seconds. Participants were asked to think about the size of the first item of the block, and then to iteratively compare the size of each subsequent item to the first (i.e., second to the first, third to the first, etc). If all of the objects had, more or less, the same size, participants responded by pushing a button with the index finger of the right hand; if at least one of the last five objects was different in size from the first, participants responded with the index finger of the left hand. Responses were made after the onset of a response cue (auditory tone, duration 200 ms), that was presented a jittered interval (2 to 8 seconds, in steps of .5 seconds, distribution with hyperbolic density) after the offset of the last stimulus from the block. Between the offset of the auditory cue and the onset of the next block of stimuli, there was a 20 second period of silence. The behavioral task (size-judgments) served to ensure that participants were attending to the stimuli in the experiment and was designed so that it could be completed by both sighted and blind participants. Sighted participants judged 25.2%, and blind participants judged 26.5% of the groups of 6 items (i.e., blocks) to be composed of objects that were roughly the same size.
Each of the 72 items was presented once within a run (four blocks of 6 stimuli, for a total of 24 items within each stimulus type). The order of the six items within a block, the assignment of the 6 items (of the 24) to each block, and the order of blocks was random, with the restriction that there were not two blocks in a row from the same stimulus type. The ISI for items within a block consisted of randomly selected intervals in the range of [.5*X], [.75*X], [.9*X], [1.1*X], [1.25*X], and [1.5*X] where ‘X’ corresponds to the duration of the entire block (20 seconds) minus the total duration of all auditory wave files in the block, divided by 6. Each run lasted approximately 10 minutes and constituted a ‘replication’ of the experiment. Sighted participants completed 3 runs (i.e., replications); congenitally blind participant CB1 completed 4 runs; CB2 and CB3 each completed 5 runs.
Participants
Twenty-four participants (21 sighted, 12 female; 3 blind, 2 female) were recruited from the Center for Mind/Brain Sciences volunteer pool and paid for participation in the study. The dataset for one sighted participant for the auditory size-judgment task was excluded because the participant failed to respond properly; the dataset for that participant for the picture-viewing experiment was retained. The datasets for both the auditory size-judgment task as well as the picture-viewing experiment were excluded for another sighted participant due to excessive head motion. All participants who took part in the auditory size-judgment task also participated in the same session in the picture-viewing task – the order (auditory size-judgments, then picture-viewing) was fixed. The remaining participants who participated in the picture-viewing task had participated earlier in the same session in a different auditory task using the same stimuli.
Handedness was assessed with the Edinburgh inventory (Oldfield, 1971). All sighted participants who performed the auditory size-judgment task were right handed; 2 of the 13 (remaining) sighted participants who completed the picture-viewing experiment were left handed (all others right handed). Two of the three congenitally blind participants (CB1 and CB3) were right handed; CB2 was ambidextrous. Sighted participants (mean age: 31.2yrs, standard deviation: 9.5yrs, range: 20yrs to 51yrs) had normal or corrected to normal vision (vision corrected using MR compatible goggles). Participant CB1 (female, age at testing 60yrs) was blind due to Retinitis Pigmentosa, CB2 (male, age at testing 20yrs) due to congenital glaucoma, and CB3 (female, age at testing 31 yrs) due to complete retinal damage at birth.
Informed consent was obtained in writing (sighted participants) and verbally (digitally recorded, blind participants) under approved University of Trento and Harvard University protocols for the use of human participants in research. All participants were examined by a medical doctor (GB) prior to participation in the study.
MR data acquisition and analysis
MR data were collected at the Center for Mind/Brain Sciences, University of Trento, on a Bruker BioSpin MedSpec 4T. Before collecting functional data, a high (1×1×1 mm3) resolution T1-weighted 3D MPRAGE anatomical sequence was performed (sagittal slice orientation, centric Phase Encoding, image matrix = 256×224 (Read × Phase), FoV = 256 mm × 224 mm (Read × Phase), 176 partitions with 1mm thickness, GRAPPA acquisition with acceleration factor = 2, duration = 5.36 minutes, TR = 2700, TE = 4.18, TI = 1020 ms, 7° flip angle). Functional data were collected using an echo planar 2D imaging sequence with phase over-sampling (Image matrix: 70 × 64, TR: 2250ms TE: 33 ms, Flip angle: 76°, Slice thickness = 3 mm, gap =.45mm, with 3×3 in plane resolution). Volumes were acquired in the axial plane in 37 slices. Slice acquisition order was ascending interleaved odd-even.
All MR data were analyzed using Brain Voyager (v. 1.9). The first two volumes of functional data from each run were discarded prior to analysis. Preprocessing of the functional data included, in the following order, slice time correction (sinc interpolation), motion correction with respect to the first (remaining) volume in the run, and linear trend removal in the temporal domain (cutoff: 3 cycles within the run). Functional data were then registered (after contrast inversion of the first remaining volume) to high-resolution de-skulled anatomy on a participant-by-participant basis in native space. For each individual participant, echo-planar and anatomical volumes were transformed into standardized (Talairach and Tournoux, 1988) space. A Gaussian spatial filter with a 4.5 mm full-width at half-maximum was applied to each volume.
All functional data were analyzed using the general linear model in Brain Voyager. Experimental events (duration = 20 seconds) in the picture-viewing experiment were convolved with a standard dual gamma hemodynamic response function. There were 4 regressors or interest (corresponding to the four stimulus types) and 6 regressors of no interest, corresponding to the motion parameters obtained during preprocessing. For the analyses of the auditory size-judgment task, a finite impulse response model (modeling 6 TRs) was used with regressors for all stimulus events, the auditory response cue, and the outputs of motion correction. A random effects analysis was used to analyze the group data in the picture-viewing experiment (n = 20, Degrees of Freedom (DF) = 19) (Figures 1 – 4). Fixed effects analyses with separate study (i.e., run) predictors were used to analyze the data from the sighted participants performing auditory size-judgments (DF = 4907) and the congenitally blind participants (DF = 3261). All functional data were masked with the functionally defined ventral stream (as described in Figure 1) before running the GLM. Beta estimates were standardized (z scores) with respect to the entire time course. The contrast for all analyses, of nonliving stimuli vs. animal stimuli, weighted tools and nonmanipulable objects equally, with respect to animals.
All ROI-based analyses of category contrasts (Figure 2), as well as the ROIs for the functionally defined ventral stream (Figure 1) and ventral occipital-temporal cortex (Figure 5) were thresholded at p < 0.05, FDR corrected. All statistical contrast maps are projected onto the inflated anatomy of a single participant normalized to Talairach space.
Software written in Matlab, using the BVQX Matlab toolbox (by Jochen Weber: http://wiki.brainvoyager.net/BVQX_Matlab_tools) was used for the analyses reported in Figure 5 and Supplemental Figure S1.
Supplementary Material
Acknowledgments
We thank Jorge Almeida, Randy Buckner, Jessica Cantlon, Marc Hauser, Alex Martin, and Ken Nakayama, for their comments, Gianpaolo Basso and Manuela Orsini for technical assistance with the MR, Jessica Cantlon for her insights on analyses, Angelika Lingnau for her help with segmentation, and the Unione Italian Ciechi sezione di Trento for their help in recruiting blind participants. BZM was supported in part by an NSF Graduate Research Grant and an Eliot Dissertation Completion Grant. The research was supported in part by grant DC006842 from the National Institute on Deafness and Other Communication Disorders (AC) and by a grant from the Fondazione Cassa di Risparmio di Trento e Rovereto. BZM is now at the Rochester Center for Brain Imaging and the Department of Brain and Cognitive Sciences, University of Rochester, Rochester, NY, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD. Face recognition in human extrastriate cortex. J Neurophysiol. 1994;71:821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Amedi A, Floel A, Knecht S, Zohary E, Cohen LG. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat Neurosci. 2004;7:1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Buchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998;394:274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Mahon BZ, Caramazza A. What are the facts of category-specific deficits? A critical review of the clinical evidence. Cogn Neuropsychol. 2003;20:213–261. doi: 10.1080/02643290244000266. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Mahon BZ. The organization of conceptual knowledge: the evidence from category-specific semantic deficits. Trends Cogn Sci. 2003;7:354–361. doi: 10.1016/s1364-6613(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain the animate-inanimate distinction. J Cogn Neurosci. 1998;10:1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Carey SSE. Domain specific knowledge and conceptual change. In: Hirschfeld SGL, editor. Mapping the Mind: Domain Specificity in Cognition and Culture. London: Cambridge Univ. Press; 1994. pp. 169–200. [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- De Volder AGT, Kimura H, Kiyosawa Y, Nakano M, Vanlierde H, Wanet-Defalquef A, Mishina M-C, Oda M, Ishiwata K, Senda M. Auditory Triggered Mental Imagery of Shape Involves Visual Association Areas in Early Blind Humans. Neuroimage. 2001;14:129–139. doi: 10.1006/nimg.2001.0782. [DOI] [PubMed] [Google Scholar]
- Dilks DD, Baker CI, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration is not specific to the “preferred retinal locus”. J Neurosci. 2009;29:2768–2773. doi: 10.1523/JNEUROSCI.5258-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Duchaine BC, YG, Butterworth EJ, Nakayama K. Prosopagnosia as an impairment to face specific mechanisms: elimination of the alternative hypotheses in a developmental case. Cogn Neuropsychol. 2006;23:714–747. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Rabinowitz C. Genetic and environmental influences on the organization of semantic memory in the brain: Is “living things” an innate category? Cogn Neuropsychol. 2003;20:401–408. doi: 10.1080/02643290244000293. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge MA: Bradford/MIT Press; 1990. [Google Scholar]
- Goodale MA, Milner D. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proc Natl Acad Sci U S A. 2002;99:11429–11434. doi: 10.1073/pnas.162342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil FC. Constraints on knowledge and cognitive development. Psychological Review. 1981;88:197–227. [Google Scholar]
- Kiani R, Esteky H, Mirpour K, Tanaka K. Object category structure in response patterns of neuronal population in monkey inferior temporal cortex. J Neurophysiol. 2007;97:4296–4309. doi: 10.1152/jn.00024.2007. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Ruff DA, Kiani R, Bodurka J, Esteky H, Tanaka K, Bandettini PA. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron. 2008;60:1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. Concepts and Categories: A Cognitive Neuropsychological Perspective. Ann Rev Psychol. 2009;60:1–15. doi: 10.1146/annurev.psych.60.110707.163532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, Martin A. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Ann Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Miceli G, Capasso R, Daniele A, Esposito T, Magarelli M, Tomaiuolo F. Selective deficit for people’s names following left temporal damage: An impairment of domain-specific conceptual knowledge. Cogn Neuropsychol. 2000;17:489–516. doi: 10.1080/02643290050110629. [DOI] [PubMed] [Google Scholar]
- Miceli G, Fouch E, Capasso R, Shelton JR, Tomaiuolo F, Caramazza A. The dissociation of color from form and function knowledge. Nat Neurosci. 2001;4:662–667. doi: 10.1038/88497. [DOI] [PubMed] [Google Scholar]
- New J, Cosmides L, Tooby J. Category-specific attention for animals reflects ancestral priorities, not expertise. Proc Natl Acad Sci U S A. 2007;104:16598–16603. doi: 10.1073/pnas.0703913104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cereb Cortex. 2006;16:437–445. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Op de Beeck HP, Haushofer J, Kanwisher NG. Interpreting fMRI data: maps, modules and dimensions. Nat Rev Neurosci. 2008;9:123–135. doi: 10.1038/nrn2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet L. The Plastic Human Brain Cortex. Ann Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Pietrini P, Furey ML, Ricciardi E, Gobbini MI, Wu WH, Cohen L, Guazzelli M, Haxby JV. Beyond sensory images: Object-based representation in the human ventral pathway. Proc Natl Acad Sci U S A. 2004;101:5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Charles L, Devlin JT, Walsh V, Duchaine B. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr Biol. 2009;19:319–324. doi: 10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Polk TA, Park J, Smith MR, Park DC. Nature versus nurture in ventral visual cortex: a functional magnetic resonance imaging study of twins. J Neurosci. 2007;27:13921–13925. doi: 10.1523/JNEUROSCI.4001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Mechelli A, Patterson K, Price C. Fusiform activation to animals is driven by the process, not the stimulus. J Cogn Neurosci. 2005;17:434–445. doi: 10.1162/0898929053279531. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung KJ, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle MAGDJ, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: The MIT Press; 1982. pp. 549–586. [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge. Further fractionations and an attempted integration. Brain. 1987;110(Pt 5):1273–1296. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.