Abstract
Unraveling the energy metabolism and the hemodynamic outcomes of excitatory and inhibitory neuronal activity is critical not only for our basic understanding of overall brain function, but also for the understanding of many brain disorders. Methodologies of magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI) are powerful tools for the non-invasive investigation of brain metabolism and physiology. However, the temporal and spatial resolution of in vivo MRS and MRI is not suitable to provide direct evidence for hypotheses that involve metabolic compartmentalization between different cell types, or to untangle the complex neuronal micro-circuitry which results in changes of electrical activity. This review aims at describing how the current models of brain metabolism, mainly built on the basis of in vitro evidence, relate to experimental findings recently obtained in vivo by 1H MRS, 13C MRS and MRI. The hypotheses related to the role of different metabolic substrates, the metabolic neuron-glia interactions, along with the available theoretical predictions of the energy budget of neurotransmission, will be discussed. In addition, the cellular and network mechanisms that characterize different types of increased and suppressed neuronal activity will be considered within the sensitivity-constraints of MRS and MRI.
Keywords: brain, energy metabolism, neuronal activation, neuron-glia coupling, BOLD, lactate, malate aspartate shuttle, NMR, inhibition
This review focuses on some open debates which concern the investigation of brain hemodynamics and energy metabolism during increased and decreased neuronal activity. Emphasis is given to controversies pertaining to the assessment of the main metabolic fuel of neurons, the metabolic neuron-glia interactions, the neurovascular coupling, the energetic outcomes of inhibition, and the sensitivity of magnetic resonance spectroscopy (MRS) and imaging (MRI) to investigate changes in neuronal activity. With this goal in mind, we describe the available hypotheses related to the utilization of metabolic substrates and to the compartmentalization of metabolism (section A); we then discuss results recently obtained in vivo with 1H MRS (section B) and 13C MRS (section C), giving particular emphasis to how these findings relate to the formulated metabolic hypotheses. Following this is a section dedicated to the sensitivity of functional MRI (fMRI) techniques (section D). While references are made to literature on other imaging methodologies where relevant, such as positron emission tomography (PET), this article is not intended to be a review of the large body of work that exist with these methodologies. With the intent of establishing a connection with the MRI and MRS data, insights are then provided into the available theoretical estimates of the energy budgets of neurotransmission (section E). Finally, we focus on the complex mechanisms, which can lead to suppression of neuronal activity, describing how this can be revealed by functional investigations of the brain (section F). Concluding remarks will be summarized in section G.
A. Energy metabolism of neuronal activation: the available hypotheses
The human brain has a high energy requirement. Even though it constitutes only 2% of the body’s weight, the brain consumes almost 20% of the global resting metabolism (Sokoloff, 1960). On the other hand, the glucose consumption rate increases not more than ∼50% when the brain responds to external stimuli, and the increases in oxygen consumption are even lower (for review, see Shulman et al, 2001a; Giove et al, 2003). Such increases are remarkably small, especially if compared, for example, to muscle, in which oxygen consumption during intense physical activity may increase as much as 70 fold (Gibala et al, 1998) largely due to the fact that energy requirements of resting muscle is minimal.
These stimulation or task-evoked energy requirements and accompanying hemodynamic responses form the basis of most current neuroimaging approaches (e.g. fMRI and PET) employed for “functional” mapping in the brain. Yet, direct measurement of metabolic changes during neuronal activation in the working human brain remains a great challenge and in vitro evidence still plays a dominant role in defining our understanding of specific metabolic events related to neurotransmission.
A.1 Metabolic substrates for neurons at work
Glucose has been traditionally considered as the primary energy source for neurons (Sokoloff, 1992), but several lines of in vitro and in vivo evidence have demonstrated that a number of compounds, such as lactate, pyruvate, acetate, glutamate and glutamine, can also be oxidized by neurons (Zielke et al, 2007 and reference therein). Among the substrates for energy generation that can serve as an alternative to glucose, lactate is the one that has received the greatest attention from the scientific community. Lactate can be efficiently oxidized by neurons, as extensively reviewed by Pellerin (2003). In certain cell cultures, lactate was reported to be equivalent to glucose with regard to its access to the neuronal tricarboxylic acid (TCA) cycle (Waagepetersen et al, 1998). Furthermore, lactate has been proposed to have some advantages compared to glucose during neuronal activation, since it can be converted to pyruvate in the absence of ATP (Bliss and Sapolsky, 2001). This hypothesis is consistent with the mandatory role of lactate for the recovery of synaptic activity after acute episodes that lead to abnormal levels (up to 10 mM) of tissue lactate concentration [Lac], as hypoxia (Schurr et al, 1997a; 1997b) or traumatic brain injury (Chen et al, 2000).
A.2 The astrocyte-neuron lactate shuttle hypothesis (ANLSH)
Currently, one of the most debated hypotheses to describe metabolism of neuronal activation is the so-called astrocyte-neuron lactate shuttle hypothesis (ANLSH), which was introduced by Pellerin and Magistretti (1994) on the basis of experimental findings obtained in their cell cultures. Briefly, the ANLSH relies on a functional metabolic coupling between glutamatergic neurons and astrocytes, and postulates a major role for lactate as metabolic substrate for the activated neurons (Figure 1). The ANLSH suggests that glutamate, released by neurons, stimulates astrocytic glycolysis, thus producing lactate which is then exported to neurons to be converted back to pyruvate and enter the neuronal TCA cycle. Partly supporting this hypothesis, Itoh et al. (2003) found that the activation of pyruvate dehydrogenase (PDH) reduced glial lactate release in vitro, and increased brain glucose consumption in vivo. The authors interpreted these results as suggestive of an increase in neuronal glucose consumption in the presence of reduced lactate released by glia; however, their results also indicated that the compartmentalization between glia and neurons with respect to glucose and lactate metabolism might be neither complete nor entirely obligatory. Results obtained from the retrotrapezois nucleus of intact rats (Elrichman et al, 2008) indicated that neurons increased glucose uptake when neuronal monocarboxylate transporters (MCT2s) were inhibited by treatment with α-cyano-4-hydroxycinnamate (4-CIN). This finding was considered to support the ANLSH as neurons in vivo would metabolize a combination of glucose and lactate. However, in the past other authors have criticized the use of 4-CIN to inhibit the plasmalemmal lactate transport (Chih et al, 2001), since this compound is known to affect glucose metabolism by blocking pyruvate entry into mitochondria (Cox et al, 1985; Halestrap et al, 1974). The idea that glial glutamate transport represents a critical step for triggering enhanced glucose utilization was supported by Voutsinos-Porche et al, (2003), who found a reduced metabolic response to whisker stimulation in knockout mice for glial glutamate transporters. Similar findings were reported by Cholet et al (2001) in rats with down-regulated expression of one of the astrocytic glutamate transporters.
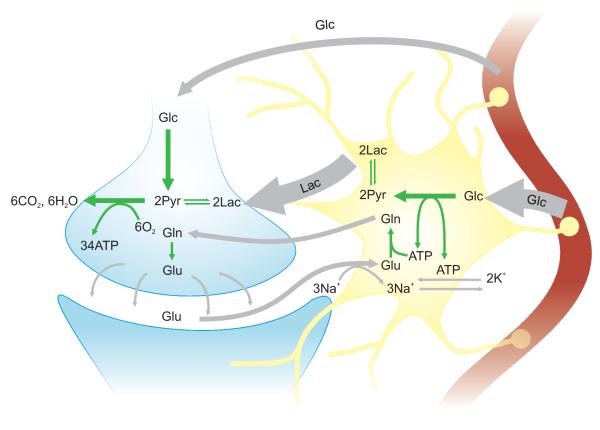
Figure 1.
Schematics of the metabolic neuron-glia coupling hypothesis as suggested by Pellerin and Magistretti (1994). During depolarization, glutamate (Glu) is released in the intersynaptic space, and subsequently recycled by the Glu-Gln cycle. The transport of glutamate into astrocytes occurs with a concomitant inflow of Na+, leading to activation of Na+/K+-ATPase. The pump activates astrocytic glycolysis, which results in lactate (Lac) production. Sibson et al (1998) further emphasized that astrocytic glycolysis is needed to quickly satisfy the energy demands of the neurotransmitter-recycling. Lactate, once released, can be taken up by neurons and then converted to pyruvate (Pyr) before entering into the TCA cycle. Even if direct neuronal uptake of glucose is not excluded by the ANLSH, lactate is considered to be the major fuel of the neuronal oxidative metabolism linked to neurotransmission.
The metabolic coupling between neurons and astrocytes is thought not to occur in the case of inhibitory neuronal activity, given that GABA uptake into astrocytes does not induce a metabolic response in terms of increased glucose utilization or decreased ATP levels (Chatton et al, 2003). Notably, given that glutamate is a precursor of GABA, the metabolic response to GABA uptake, if any, may not be direct.
Since its introduction, many experimental and theoretical efforts have been devoted to examine the ANLSH, providing evidence both in favor (reviewed in Pellerin et al, 2007; Pellerin and Magistretti, 2003) and against the ANLSH (Chih et al, 2001; Chih and Roberts, 2003; Dienel and Cruz, 2003; 2004).
A.3 Findings from in vitro studies relevant for addressing the utilization of glucose and lactate
Consistent with the ANLSH, results obtained with microautoradiographic imaging have shown that [14C]2-deoxyyglucose is taken up almost evenly between astrocytes and neurons in cell preparations obtained from moving rats (Nehlig et al, 2004). Similar conclusions applied for the rat vagus nerve, where ∼80% of glucose appeared to be taken up by the Schwann (glia) cells (Vega et al, 2003). Additionally, studies performed with real-time confocal microscopy indicated that glutamate stimulates glucose transporters in cultured hippocampal astrocytes (Loaiza et al, 2003), and inhibits glucose transporters in hippocampal neurons (Porras et al, 2004). Finally, the selective distribution of specific subtypes of the lactate dehydrogenase (LDH) within neurons and glia has been interpreted as an evidence for a kinetic preference of neurons to oxidize lactate and of astrocytes to produce lactate (Lovatt et al, 2007). In addition, after acute isolation, astrocytes were found to produce large quantities of lactate (Lovatt et al, 2007), thus supporting the concept that lactate metabolism might be compartmentalized.
Regarding the amount of lactate used by cultured neurons, current results are inconsistent. The relative contributions of lactate and glucose to neuronal oxidative metabolism during non-depolarizing conditions were estimated as ∼70% and ∼30%, respectively, when the concentration of substrates simulated the physiological in-situ environment (Bouzier-Sore et al, 2006). Lactate metabolism was confirmed to be higher than glucose metabolism in cultures of cerebellar neurons from neonatal mice under non-depolarizing conditions, but when neurons were exposed to pulses of N-methyl-D-aspartate, known to induce depolarization, only glucose and not lactate metabolism was up-regulated (Bak et al, 2006). Finally, findings from Ramirez et al (2007) did not confirm a preferential usage of lactate by neurons. When glucose and lactate were present in physiological concentrations in the extracellular fluid, neurons consumed 81% glucose and 19% lactate. Neurons preferentially consumed lactate (60%) only when lactate was several-fold more concentrated than glucose, in agreement with previous findings by Bliss and Sapolsky (2001).
Different experimental procedures in preparing the cell cultures might explain the different outcomes of the afore mentioned in vitro studies (Bak et al, 2006; Bouzier-Sore et al, 2006; Ramirez et al, 2007). From a more general perspective, a key component about cellular function that should be kept in mind is that electrical excitability is compromised under in vitro conditions because cell membranes are in an unusual environment. Additionally, using cultured cells to infer the metabolic fates of glucose and lactate in vivo has been questioned by some authors (Dienel and Cruz, 2004). Cultured cells are indeed prepared from the brain of neonatal animals, and their metabolism does not necessarily reflect the in vivo metabolism of neurons of adult brains. The ratio between glycolysis and aerobic oxidization changes with development (Bilger and Nehlig, 1991; Lust et al, 2003). Although oxidative metabolism is relatively small in the early developmental stages, the oxidative pathway is mainly fueled by lactate during the early neonatal period (Medina, 1985). Both in the cortex and in the striatum of rat, the levels of lactate are about 10-fold higher in the fetus than in the neonate, and decrease by a further 50% from neonate to adult, while the glucose concentrations in the same regions remain comparable, and the ATP levels decrease only slightly (Lust et al, 2003). An analogous change in energy metabolism is also suggested by the increase from prenatal to adult rat brain of the activity of the mitochondrial enzyme cytochrome oxidase (5-19 fold) which reflects oxidative capacity; in contrast, the activity increase of cytosolic LDH is only 2-3 fold for the same period (Bilger and Nehlig, 1991).
A.4 The redox-switch/redox-coupling hypothesis
Further elements to the neuron-glia metabolic coupling have been proposed by Cerdan et al (2006), who put the emphasis on the fact that both lactate and pyruvate can return back to the extra-cellular space from both neurons and astrocytes (Cerdan et al, 2006 and references therein). Thus, extracellular lactate and pyruvate is in exchange with both neuronal and astrocytic pyruvate and lactate (Figure 2). By considering that the monocarboxylate recycling depends on the cytosolic NAD+/NADH of both cell types, the authors suggested that neurons and glia choose their metabolic substrates (glucose and/or lactate) by “sensing” the cytosolic redox state of each other. Monocarboxylates released in the extracellular space would act as trans-cellular couplers of the cellular cytosolic redox states. Furthermore, it is suggested that, following glutamatergic neurotransmission, the increased metabolic activity leads to a reduced glial cytosolic NAD+/NADH. Reducing equivalents are then transferred to the extracellular medium and to neurons, leading to a reduced redox state in neurons as well. This would inhibit neuronal glycolysis, favoring the neuronal oxidation of extracellular lactate. Part of the pyruvate derived from neuronal lactate oxidation, instead of entering into the TCA cycle, would finally return to the extracellular space and back to glia.
Figure 2.
Schematics of the redox-switch/redox-coupling hypothesis as suggested by Cerdan et al (2006). The model emphasizes the bi-directional shuttling of lactate and pyruvate between neurons and astrocytes. Glucose enters in both neurons and astrocytes. Monocarboxylate transporters regulate the flux of lactate and pyruvate from neurons and astrocytes into the extra-cellular space, and vice-versa from the extra-cellular space into neurons and astrocytes. Monocarboxylate concentrations are related to the cytosolic cellular redox state (NAD+/NADH ratio), and their release into the extracellular space act as trans-cellular couplers of the cellular cytosolic redox states of neurons and astrocytes. Whenever the cellular redox state is reduced in one cell type, glycolysis of that cell type is inhibited favoring the oxidation of extracellular lactate. The cellular cytosolic redox state is also regulated by the malate-aspartate shuttle. A description of the malate-aspartate shuttle is provided in Figure 6.
Whereas a recycling of lactate has been convincingly demonstrated in cell cultures in the time-scale of several hours (Rodrigues et al, 2005), the contribution of monocarboxylate recycling seems to be less significant in the in vivo activated cortex (section B.5).
A.5 Alternative hypothesis about the role of lactate in energy metabolism
Another idea emphasizing the role of lactate in metabolism suggests that lactate instead of pyruvate is the end product of glycolysis potentially in all kind of tissues and in any condition, even in the presence of ample oxygen supplies (Schurr, 2006; Schurr and Payne, 2007). In this picture, cytosolic glycolysis always leads to lactate formation, mainly in order to regenerate NAD+, which is consumed during the conversion of glucose to pyruvate. NAD+ needs to be replenished by reoxidation of NADH, because depletion of NAD+ reservoir would ultimately inhibit glycolysis. If lactate production occurs under aerobic conditions, lactate is subsequently shuttled into mitochondria and finally converted to pyruvate to enter the TCA cycle (Figure 3). The shuttling of lactate into mitochondria (Hashimoto et al, 2008) and the presence of mitochondrial LDH have been recently supported by experimental findings obtained in mitochondria extracted from cerebellar granule cells (Atlante et al, 2007).
Figure 3.
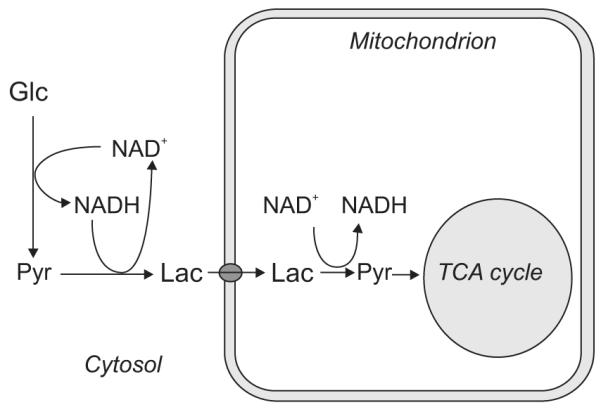
Schematics of the hypothesis about lactate as suggested by Schurr (2006). Cytosolic glycolysis leads to lactate formation, which regenerates NAD+. Lactate is then transported into mitochondria where it is converted to pyruvate to enter the TCA cycle.
B. Interpretation of experimental findings obtained with in vivo 1H MRS
1H MRS allows the non-invasive measurement of metabolite concentrations in the human brain, along with their changes induced by variations in neurotransmission activity (for review, see Giove et al, 2003; Mangia et al, 2006). This capability provides unique and valuable information to understand the metabolism of the brain at work. In fact, changes of metabolite concentrations during activation imply changes in metabolic patterns, such as preferential use of different pathways or alteration of fluxes, even though changes in metabolic fluxes can be accomplished without changes in metabolite concentrations.
B.1 The technical challenges for a reliable quantification of metabolites
The afore-discussed metabolic events associated with brain activity can in principle be probed by 1H MRS since some of the metabolites in question are detectable by this approach. However, the reliable quantification of changes in metabolite concentrations during studies of functional MRS (fMRS) is a demanding task, and indeed studies reported in literature have often produced inconsistent results (reviewed in Mangia et al, 2006). Due to the limited chemical shift range of the proton spectra (∼ 5 ppm), resonances of different brain metabolites are largely overlapped, thus challenging quantification especially for those metabolites present in low concentrations, such as lactate, glucose, or GABA. This difficulty likely contributed to the inconsistency of results reported in the literature about [Lac] changes during activation. The feasibility of detecting [Lac] changes under stimulation has been challenged by some authors: a high variability of the basal [Lac] was underlined by Merboldt et al (1992), who did not detect any reproducible time-course during several kinds of visual stimulation. In addition, unwanted contributions from lipids outside the selected area of interest can contaminate spectra obscuring lactate resonances. Boucard et al (2005) did not observe any significant alteration of the spectra during prolonged stimulation; in their setup, the region of the spectrum around 1.33 ppm was indeed affected by unstable signals presumably coming from lipids of the scalp. In other works, significant increases in [Lac] have been reported in healthy subjects during prolonged visual stimulations (Frahm et al, 1996; Mangia et al, 2007a; Prichard et al, 1991; Sappey-Marinier et al, 1992), but not during 32-sec visual stimulations (Katz-Brull et al, 2006).
Optimizing the sensitivity and accuracy of the methodology for fMRS is especially critical not only because metabolites are present in low concentration, but also because concentration changes can be relatively small (Riera et al, 2008). One of the findings provided by MRS of intact organs in or ex vivo has been the demonstration that metabolite concentrations are likely to be homeostatically controlled under physiological conditions (e.g. Balaban et al, 1986; From et al, 1986; Hochachka 2003; Robitaille et al, 1990). In this context, ultra-high magnetic field (B0) systems can help in obtaining robust and accurate time-courses of metabolites. Similar to MRI, 1H MRS benefits from the gain in signal-to-noise ratio (SNR) at higher B0 (Gruetter et al, 1998b; Vaughan et al, 2001). In addition, the chemical shift dispersion measured in Hz increases with B0, thus emphasizing the characteristic spectral patterns of metabolites and decreasing spectral overlaps. These gains are realized despite increases in linewidths with higher B0 (Tkac et al, 2001). These features allow more accurate assignments and reliable quantification of an increased number of metabolites at ultra-high B0. The detection of metabolites by 1H MRS, nonetheless, remains restricted to those compounds with concentrations on the order of μmol/g.
Whereas high resolution NMR spectrometers operating up to 900 MHz (21 T) are routinely used for in vitro biochemistry studies, the development of in vivo 1H MRS is challenged by the construction of large bore-size magnets operating at ultra-high B0. In vivo 1H MRS at ultra-high B0 has specific requirements on adjustment of B0 homogeneities (shimming) and on pulse sequence design (reviewed in Tkac and Gruetter, 2005). Efficient shimming methods (Gruetter and Tkac, 2000; Hetherington et al, 2006) and powerful second-order shimming system are essential to take advantage of the increased chemical shift dispersion at ultra-high B0 and thus to acquire highly resolved spectra. Additionally, in order to guarantee reliable quantification of an extended set of brain metabolites, pulse sequences must minimize chemical shift displacement errors, must reduce the contamination by unwanted signals from outside the voxel (such as subcutaneous lipids), and must efficiently suppress the water signal.
Magnetic fields up to 7 T have now been utilized for human spectroscopy applications (Michaeli et al, 2002; Terpstra et al, 2002; Tkac et al, 2001), leading to the reliable quantification of a high number of brain metabolites. The concentrations of 17 brain metabolites were measured in 12 subjects in 36 separate studies with unprecedented sensitivity and temporal resolution at 7 T in the human primary visual cortex during three different paradigms of visual stimulation (Mangia et al, 2007a; 2007b). Among all measured brain metabolites, minute (∼ 0.2 μmol/g) but significant changes were observed for [Lac], glutamate concentration [Glu] and aspartate concentration [Asp]; in addition, glucose concentration [Glc] manifested a tendency to decrease during stimulation periods (Mangia et al, 2007a). The temporal changes found for these four metabolites were validated by the analysis of the difference spectrum (Figure 4). The peaks in this difference spectrum (Figure 4C) were attributed to concentration changes of metabolites as well as to linewidth changes ascribed to the blood-oxygenation level dependent (BOLD) effect (Ogawa et al, 1990), which is known to alter the T2* of both water and metabolite signals. This phenomenon results in a small narrowing of all signals in the spectrum during brain activation (Mangia et al, 2006; Zhu and Chen, 2001), and is discernible mainly on the strong singlets of the spectrum. The linewidth changes introduced by the BOLD effect can be minimized or even eliminated by applying appropriate line-broadening to the stimulation spectrum prior to subtraction (Figure 4D). The residuals in this “BOLD-free” difference spectrum correspond to concentration changes.
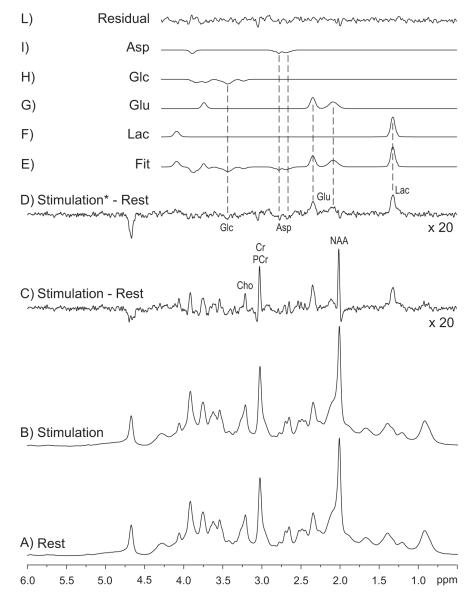
Figure 4.
Changes of metabolite concentrations during sustained visual stimulation, as revealed by the analysis of the difference (C and D) between spectra acquired during rest (A) and stimulation (B). D same as C, but the spectrum acquired during stimulation was line-broadened by 0.4 Hz in order to match the linewidth of the spectrum acquired at rest (elimination of the BOLD effect on metabolites). (E-L): LCModel fit (Provencher, 1993) of the difference spectrum D. Spectra were summed from different subjects (N = 12). Minute but significant changes in metabolite concentrations (∼0.2 μmol/g) were observed for lactate, aspartate and glutamate. In particular, [Lac] increased by 23% ± 5% (p < 0.0005), [Glu] increased by 3% ± 1% (p < 0.01), whereas [Asp] decreased by 15% ± 6% (p < 0.05). Finally, [Glc] showed a tendency to decrease during activation periods. From Mangia et al (2007a).
B.2 Decreases of glucose concentration during sensory stimulation: a link to increased glucose consumption rates
Among the metabolites quantifiable with 1H MRS, glucose is undoubtedly one of the most problematic, since its low concentration and the spread of its resonances in several multiplets can impair detection. Therefore, particular caution needs to be applied when assessing changes in [Glc] by 1H MRS. Significant [Glc] decreases (Chen et al, 1993; Frahm et al, 1996; Merboldt et al, 1992), or indications of gradual reductions in [Glc] (Mangia et al, 2007a), have been reported during sensory stimulations. Reduction of [Glc] can be interpreted in terms of increased rates of glucose consumption (CMRglc), as suggested for example by Chen et al (1993). The maximum rate at which [Glc] decrease upon onset of stimulation is given by the difference between CMRglc under stimulation and rest, ΔCMRglc. Considering CMRglc = 0.4 μmol/g/min in basal condition (Gruetter et al, 2001 and references therein) and assuming an increase by ∼30% during visual stimulation (Marrett and Gjedde, 1997; Newberg et al, 2005), brain [Glc] cannot decrease faster than ∼0.1 μmol/g/min, which is consistent with published data (Chen et al, 1993; Frahm et al, 1996; Merboldt et al, 1992; Mangia et al, 2007a). During stimulation, a new constant steady-state [Glc] should occur when the net influx of glucose trough the blood brain barrier (BBB) is equal to CMRglc. Reversibility of glucose transport through the BBB (Gruetter et al, 1998a) suggests that the rate at which the new steady-state is approached depends on the reverse transport of glucose from the brain to plasma, which must decrease when brain [Glc] is reduced. However, detailed and quantitative information about glucose transport parameters during activation cannot be obtained from the available 1H MRS data. After 25 min of visual stimulation, Chen et al (1993) observed an indication of recovery towards the initial baseline of [Glc], but the temporal resolution of their study was not sufficient to assess if a steady-state [Glc] was approached during stimulation. A time independent steady-state [Glc] during stimulation was not observed either in the studies by Frahm et al (1996), Merboldt et al (1992) and Mangia et al (2007a), likely due to the insufficient duration of the stimulation epochs or to insufficient sensitivity of the measurement to determine the [Glc] trajectory more precisely.
B.3 Increases of lactate concentration during sensory stimulation: a link to increased oxidative metabolism
Findings obtained at 7 T showed that [Lac] increased in the first minute of visual activation by ∼0.2 μmol/g (corresponding to almost 20% [Lac] increase), reached a new steady-state during the on-going stimulation period, and came back to baseline after the end of the stimulus (Mangia et al, 2007a). These observations were in qualitative agreement with earlier studies (Frahm et al, 1996; Prichard et al, 1991) which also reported [Lac] increases during similar paradigms. However, previous findings indicated transient and larger increases of [Lac], which might be ascribed to different experimental conditions adopted, as discussed by Mangia et al (2007a).
The small increase in [Lac] observed by Mangia et al (2007a) could not be due to insufficient oxygen supply, since blood is known to be hyperoxygenated during activation, a phenomenon which is at the basis of the BOLD contrast (Ogawa et al, 1990). The observed increase in [Lac] was interpreted to be consistent with increased oxidative metabolism (Figure 5). Indeed, lactate being in dynamic equilibrium with pyruvate, changes in the steady-state [Lac] imply an increase in pyruvate levels for a given cytosolic redox potential and pH. On the other hand, pyruvate levels have been suggested to rise during increased oxidative metabolism (Dienel and Cruz, 2003; Gjedde and Marrett, 2001); therefore, the small changes of [Lac] observed during physiological stimulation (Mangia et al, 2007a) can reflect increased oxidative metabolism. Due to the permeability of the BBB to lactate, an increased brain [Lac] during stimulation may result in a net lactate efflux, considering that the plasma [Lac] is unlikely to change. This lactate efflux was estimated to account for a drop of the oxygen to glucose index from 5.5 to 5.1, and at least 97% of the increased ATP synthesis during activation was calculated to be powered by oxidative processes (Mangia et al, 2007a). This is in disagreement with the concept that anaerobic processes are largely responsible for sustaining increased neuronal activation, a hypothesis that was suggested after initial studies conducted with PET (e.g., Blomqvist et al, 1994; Fox et al, 1988). However, it is consistent with other data obtained with MRS, MRI and PET that significant increases in oxygen consumption occur during stimulation (e.g., Chen et al, 2001; Hoge et al, 1999; Marrett and Gjedde, 1997), albeit proportionately less than the increases in glucose consumption (for review, see Giove et al, 2003; Shulman et al, 2001a). In any case, it is important to emphasize that in order to evaluate how much energy is accounted by oxidative or non-oxidative processes, the energy budget must be discussed in terms of ATP produced and consumed, and not in terms of glucose consumption alone. Aerobic glucose consumption yields ∼34 ATP molecules per glucose consumed versus only 2 when glucose is converted to pyruvate and lactate without requiring oxygen. Thus, even if stimulus-induced increases of oxygen consumption rate are very small (∼ 5%) while CMRglc increases by 50%, as suggested by the original PET data (Fox et al, 1988), non-oxidative glucose consumption would account for only 40% of the increase in ATP requirements induced by alterations in neuronal activity. This leads to the conclusion that even a relatively small increase in oxygen consumption rate translates into most of the stimulation or task induced ATP being produced oxidatively. In this conclusion, we refer to metabolism by all cell types (astrocytes and neurons) without making a distinction between them.
Figure 5.
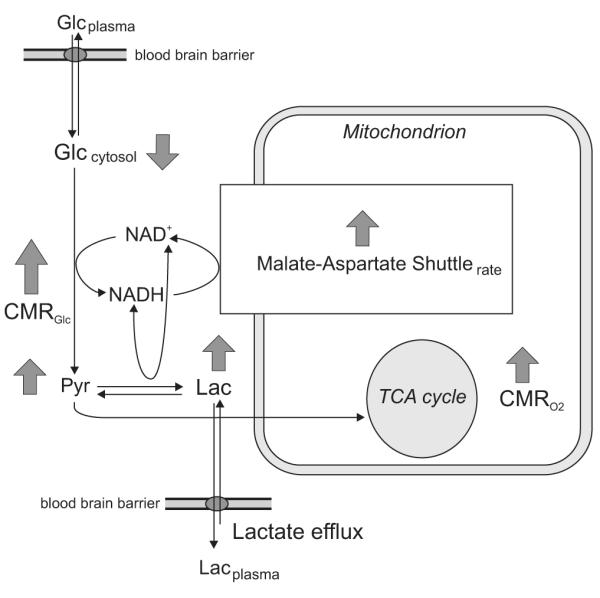
Schematics of metabolic events following increased neuronal activity as suggested by Mangia et al (2007a). Increased CMRglc during activation leads to slight decreased [Glc]. The small augment of the steady-state level of [Lac] results from an increased rate of glycolysis and TCA cycle that is accompanied by an increased steady-state [Pyr]. Lactate efflux from the brain to the plasma occurs since plasma and brain [Lac] are supposed to be different. An increased rate of the malate-aspartate shuttle is expected for increase oxidative metabolism (details about the malate-aspartate shuttle are provided in Figure 6).
B.4 Changes of glutamate and aspartate concentrations during sensory stimulation: a possible link to the malate-aspartate shuttle
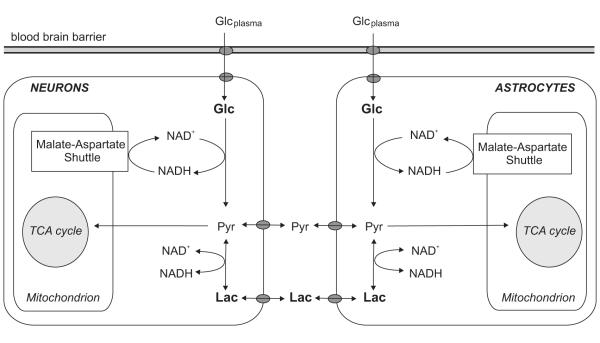
The small [Lac] increases (Mangia et al, 2007a) described in the previous section can potentially be linked with the rise of oxidative metabolism also within the context of the hypothesis formulated by Schurr (2006). However, this hypothesis does not specify whether and how lactate formation in the cytosol competes with the malate-aspartate shuttle (MAS), which is the commonly accepted mechanism that neurons have to guarantee the continuation of aerobic glycolysis (for review, see McKenna et al, 2006; for a brief description, refer to Figure 6). An increase of the MAS rate during stimulation, expected with increased oxygen consumption, has been suggested based on the observations that [Glu] slightly increased during prolonged visual stimulation, while [Asp] decreased by a comparable amount (Mangia et al, 2007a). Indeed, since most of the tissue glutamate is in the cytosol, the increased [Glu] along with a similar decrease in [Asp] were consistent with an increased flux through the MAS and the fact that the Glu-Asp antiporter at the inner mitochondrial membrane is rate limiting (LaNoue and Tischler, 1974).
Figure 6.
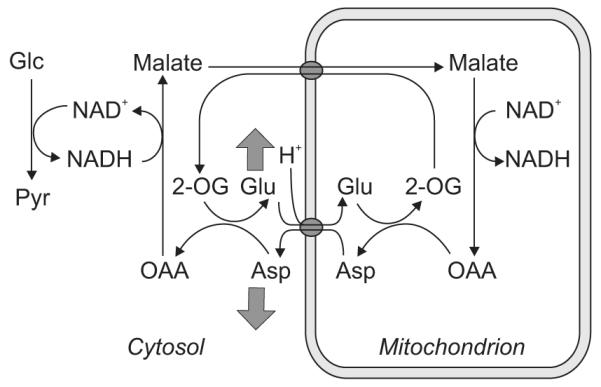
Schematics of the malate-aspartate shuttle (MAS). The MAS transfers reducing equivalents from NADH in the cytosol to the mitochondria by relying on the following steps: electron transfer from NADH to oxaloacetate in the cytosol forming malate (replenishing NAD+); transport of malate into mitochondria in exchange for α-ketoglutarate; conversion of malate to oxaloacetate, with formation of NADH; transamination of oxaloacetate to aspartate in conjunction with the conversion of glutamate to α-ketoglutarate; transport of aspartate from the mitochondria to the cytosol in cotransport of cytosolic glutamate to the mitochondria. Grey arrows indicate the changes in [Glu] and [Asp] observed during stimulation (Mangia et al, 2007a); such changes were interpreted in agreement with an increased flux through the MAS expected for increased oxidative metabolism.
B.5 Changes of lactate concentration in vivo: an evidence for inhibition of neuronal glycolysis?
To date there are no direct in vivo measurements of the amounts of lactate and glucose that are specifically used by neurons and astrocytes during activation, and the interpretation of experimental data regarding [Lac] changes in vivo is anything but straightforward (Dienel and Cruz, 2003; Korf, 2006).
A question one can address is whether or not the [Lac] increases observed in vivo during stimulation (Frahm et al, 1996; Mangia et al, 2007a; Prichard et al, 1991; Sappey-Marinier et al, 1992) are high enough to inhibit neuronal glucose consumption, leading to a preferential usage of lactate as in pathological conditions (Chen et al, 2000; Schurr et al, 1997a; 1997b). Results by Ramirez et al (2007) suggested that ∼3.6 mM extra-cellular [Lac] is needed to inhibit neuronal glucose consumption by 50%. However, tissue [Lac] has been measured to increase no more than 1 μmol/g during stimulation (Dienel et al, 2002; Frahm et al, 1996; Madsen et al, 1999; Mangia et al, 2007a; 2007b; Prichard et al, 1991); even the increases in [Lac] measured in the extra-cellular medium did not overcome this limit (Hu and Wilson, 1997). Ramirez et al (2007) suggested that [Lac] could still be significantly higher in the constrained space of the synaptic cleft. However, there are no available measurements of [Lac] in the synaptic cleft. A much higher density of monocarboxilate transporters (MCTs) in the synaptic zone areas would be supportive of the assumption that [Lac] levels are high in the synaptic cleft. Unfortunately, available evidence about MCT distributions (Bergersen et al, 2005; Debernardi et al, 2003; Hertz and Dienel, 2005; Pellerin et al, 2005; Pierre et al, 2000; 2002) are not conclusive either, because localization of MCTs in presynaptic or postsynaptic elements is specific to certain (but not all) synapses, and species differences may exist (Debernardi et al, 2003; Pierre et al, 2002).
B.6 Changes of lactate concentration in vivo: an evidence for a preferential usage of lactate glycolitically produced by astrocytes?
Another question of interest is whether or not the functional [Lac] changes measured in vivo are consistent with a preferential usage by neurons of lactate produced glycolitically by astrocytes. The interpretation of tissue [Lac] changes in the context of compartmentalized metabolism requires the formulation of mathematical models, a goal that has been tackled by Aubert and Costalat (Aubert and Costalat, 2005; Aubert et al, 2005; 2007). The main common conclusion drawn by these simulation studies was that astrocytic release and neuronal uptake of lactate occur in the initial 30 sec of increased neurotransmission (Aubert and Costalat, 2005; Aubert et al, 2005; 2007). However, when considering the evolution of [Lac] during the rest of the prolonged stimulation (up to 6-minutes), the transient increases of tissue [Lac] observed experimentally by Frahm et al (1996) were consistent with a neuronal, rather than astrocytic, release of lactate in the extracellular space, as shown by the Fig. 4 of the paper by Aubert and Costalat (2005). Overall, these results indicate that the ANSL would be active only for the early response to stimulation, possibly for reasons hypothesized previously (Shulman et al, 2001b), but not during the rest of on-going increased neuronal activity.
Recently, other investigators (Simpson et al, 2007) have suggested that neurons would be responsible for the generation of the transients changes in [Lac] observed during stimulation. Such a conclusion was based on the results of a mathematical model which included the concentration and kinetic properties of glucose and monocarboxylate transporters. Simulation based on this model predicted that neurons, and not astrocytes, would generate the transient increase of extracellular [Lac] as measured in the dentate gyrus of the hippocampus of the rat brain after electrical stimulations of the perforant pathway (Hu and Wilson, 1997). Neuronal rather than astrocytic glycolysis (e.g., the conversion of glucose to pyruvate) was previously suggested by Gjedde and Marrett (2001). The authors predicted two time courses of oxygen consumption based on the kinetic properties of the lactate dehydrogenase subtypes LD1 and LD5, which are preferentially distributed in neurons and astrocytes respectively (Bittar et al, 1996). By comparing the time-course of oxygen consumption changes measured by PET with the predicted ones, Gjedde and Marrett (2001) concluded that the conversion of glucose to pyruvate occurs mainly in neurons, both at rest and during stimulation.
These modeling considerations, in conjunction with the reported time-courses of [Lac] in vivo suggest that neurons may not necessarily rely on the lactate glycolytically produced by astrocytes as their main metabolic fuel although such lactate produced by astrocytes can indeed be a carbon substrate for neurons.
C. Interpretation of experimental findings obtained with in vivo 13C MRS
13C MRS is less affected by resonance overlaps compared to 1H MRS, due to much wider chemical shift range, but carbon isotope 13C has low natural abundance (1%) and, therefore, the intrinsic sensitivity of in vivo 13C MRS to detect brain metabolites is very low. However, when a highly enriched 13C-labeled substrate is administrated (for instance [1-13C]glucose), 13C MRS can detect the incorporation of 13C label into different carbon positions of various brain metabolites, such as glutamate, glutamine, lactate or aspartate. From these time-courses, metabolite fluxes (e.g. neurotransmitter recycling and TCA cycle rates) can be extracted using one- or two-compartments metabolic models (for review, see de Graaf et al, 2003; Henry et al, 2006). The increased sensitivity offered by ultra-high B0 results in more reliable estimates of metabolic fluxes.
At the moment, 13C MRS has been used for investigating the energy cost of neuronal activity mostly in animal models; only a couple of pilot functional human studies have been published so far (Chen et al, 2001; Chhina et al, 2001). The conclusions of both studies were limited to the definition of upper limits for functional changes of the TCA cycle rate due to modeling constraints; these upper limits suggested significantly more fractional increase in oxidative glucose consumption (∼30% in Chen et al, 2001 and ∼60%-in Chhina et al, 2001) compared with the early PET data but more consistent with those reported using BOLD modeling. In the following paragraphs, we will discuss the main results and the interpretation of the findings obtained by 13C MRS studies aimed at investigating the relationship between metabolism and neurotransmission in animal models.
C1. The relationship between glucose consumption and neurotransmitter recycling rates
Experiments conducted with 13C MRS in animals under different level of anesthesia lead to the conclusion that the majority of brain energy consumption is associated with neuronal activity as measured by neurotransmitter recycling (Choi et al, 2002; Sibson et al, 1998). The energy consumption measured in rats under pentobarbital anesthesia, where electroencephalographic (EEG) measurements showed an iso-electricity state of the cortex, was found to be 20-50% of total energy consumption (Choi et al, 2002; Oz et al, 2004; Shulman et al, 2004). In addition, a 1:1 slope was reported for the relationship between aerobic glucose consumption rates and neurotransmitter cycling rates (Sibson et al, 1998). Other authors have reported this relationship to be different from this ratio (Choi et al, 2002; Gruetter et al, 2001). However, Hyder et al (2006) have suggested that differences between these reports are negligible.
Sibson et al (1998) proposed a mechanistic explanation for a 1:1 stoichiometry, involving basic aspects of the metabolic neuron-glia coupling model. Specifically they suggested that glycolysis is used by astrocytes because it provides the two “fast” ATPs for the increased activity of Glu-Gln cycle after the neuronal release of glutamate in the intersynaptic cleft. This process links the increased metabolic needs of glucose by astrocytes in a 1:1 way with the energy demands of the Glu-Gln cycle. If one further assumes that neurons use the lactate which is exported in the extra-cellular space by astrocytes, without increasing their own glycolysis, then a 1:1 relationship should be expected between overall aerobic glucose consumption and Gln-Gln cycle rates. Hyder et al (2006) subsequently included the GABA cycling (Patel et al, 2005) and the glial oxidative fluxes (Cruz et al, 2005; Oz et al, 2004), but they retained the basic idea of a lactate flow from glia to neurons, and maintained the basic hypothesis that anaerobic glycolysis is needed in glia. It is noteworthy that including the GABA-glutamine cycling in the neuron-glia coupling implies that GABA uptake by astrocytes should increase glycolysis, in contrast with experimental findings from Chatton et al (2003). Hyder et al (2006) concluded that a relationship still close enough (even if not exactly equal) to 1:1 could be predicted on the basis of this up-dated version of the model, estimating that neurons use 70% of glucose equivalents from lactate produced by astrocytic glycolysis, with the remaining 30% coming from exogenous glucose.
C.2 The Glu-Gln cycle as a measure of neuronal electrical activity
Along with estimates of metabolic fluxes obtained with 13C MRS, Sibson et al (1998) reported electrical measurements of the EEG activity of the rat brain under the different anesthesia used in their study. However, a quantitative correlation between the EEG activity and the neurotransmitter cycling rate was not established.
EEG is mainly sensitive to synaptic (rather than spiking) activity. The synaptic activity is linked to both glutamatergic (excitatory) and GABAergic (inhibitory) neurotransmitter recycling activity. On the other hand, the spiking output critically depends not only on the global neurotransmitter recycling, but also on the dynamic balance between excitatory and inhibitory activity. Therefore, the relationship between spiking and synaptic activity is not always obvious or predictable. Given this consideration, it would be critical to establish the energy consumptions also based on the firing rates. Some experiments conducted with [14C]deoxyglucose method (reviewed in Sokoloff, 1999) have demonstrated a proportionality between the rates of glucose utilization and spike frequency in the superior cervical ganglion or dorsal horn of the spinal cord during electrical stimulation. Proportionality of energy consumption with spiking rates have been suggested also in the cortex (Smith et al, 2002), but this issue remain an open debate. We will discuss the matter related to the contribution of synaptic versus spiking activity in greater details in sections E and F.
C.3 The challenges of the anesthetized and awake states for metabolic studies
Anesthetics or drugs such as bicuculline are widely used to modulate neurotransmission. Correlating the energy demands under different anesthetics with neuronal activity levels is nonetheless challenged by the complexity of the anesthetics action (Hemmings et al, 2005), even the majority of anesthetics likely affect similar pathways to induce loss of consciousness (Franks et al, 2008). In principle, observed changes in metabolism could be due to secondary effects induced by anesthetics rather than merely to a decrease in neurotransmission activity. Awake alert brain studies also face numerous challenges; they can be confounded by genetic, hormonal, or stress factors. In addition, in the awake state, one is confronted with the problem of the high rate of basal neuronal activity and energy consumption and, likely, relatively small perturbations imposed on this ongoing activity. The alterations induced by physiological interventions, such as external stimuli, on metabolic turnover rates used for modeling may be too small to be detected by the current methods. This is indicated in the raw data for glutamate C4 carbon labeling kinetics in the human brain (Chen et al, 2001); in this study the two labeling curves were obtained simultaneously in the two different hemispheres, one maximally stimulated and the other not stimulated at all; they display a very small difference requiring highly accurate measurements to quantitate this small difference.
C.4 Reliability of the estimates of metabolic fluxes
Metabolic fluxes are not directly measured by 13C MRS, but are obtained on the basis of metabolic modeling. Therefore their estimates critically depend on the adopted models and experimental conditions. Unfortunately, all of the currently published results have been analyzed by a variety of different models that make comparisons difficult or, given the complexity of the modeling involved, provide grounds for questioning the validity of the comparisons. Normally, new approaches can be validated by performing a set of measurements under conditions where the answer is a priori known, and/or by comparison with results obtained using other, preferably well-established, techniques. In case of 13C modeling, this is possible for the measurement of the TCA cycle rate which must be stochiometrically related to CMRO2. For example, Zhu et al (2002) found that, when matched for cerebral blood flow, CMRO2 measured by 17O MR in the anesthetized rat brain agreed well with TCA cycle rate determined from 13C metabolic modeling (Hyder et al, 2000) as well as with CMRO2 calculated from measurements of glucose consumption using invasive but robust autoradiographic technique (Nakao et al, 2001). However, such an evaluation is not feasible with Glu-Gln cycling rate. No other approach for the measurement of this process exists. Several observations demonstrate that the rates for Glu-Gln cycling derived from 13C MRS data behave as expected; e.g. they decrease with deeper anesthesia (Choi et al, 2002; Sibson et al, 1998), and are significantly lower in the anesthetized state relative to the awake state (Oz et al, 2004), as previously discussed. However, a direct validation of the absolute rates remains elusive. Recently, Shestov et al (2007) have utilized Monte Carlo simulations to investigate the performance of a two-compartment model (Gruetter et al, 2001) to fit positional 13C enrichment curves obtained during infusion of 13C-labeled glucose. Monte Carlo simulations are widely used to predict the variance of the estimates obtained by mathematical models; this approach estimates the probability of certain outcomes by running multiple trials with random variables. The authors explored the effects of different simulated experimental conditions, varying parameters such as the number of fitted turnover curves (from 3 to 7), the number of experimental data points per turnover curve (from 20 to 100), or the noise level as identified by the standard deviation of a Gaussian white noise (from 0 to 0.25 μmol/g). In addition, the authors investigated how changes in metabolic fluxes affect the turnover curves. They concluded that the determination of Glu-Gln cycling rate, using the specific model they were testing (Gruetter et al, 2001), is relatively unreliable under the experimental conditions typical for in vivo 13C MRS studies (generally 2-3 turnover curves, number of points < 30, noise level: 0.1-0.3 μmol/g). On the contrary, the neuronal TCA cycle rate is determined with a much higher precision. The applicability of these results to other formulation of the metabolic modeling employed for the analysis of the 13C MRS data remains unknown. However, these results have already stimulated the inclusion of metabolic processes some of which were previously observed but not consistently utilized in modeling, such as glutamine dilution (e.g. Shen et al 1999) and the additional use of [2-13C]acetate substrate which is known to be specific to glia (e.g. Lebon et al, 2002), for achieving significant improvements in the accuracy of the 13C based Glu-Gln cycling rates. Thus, metabolic modeling of 13C MRS data is still evolving. It is anticipated that further developments will lead to better models and improved accuracy of the determination of neurotransmitter cycling.
C.5 The (∼) 1:1 relationship and the ANLSH
Since the main function of neurons is neurotransmission, it is not surprising that glucose energy consumption and neurotransmitter recycling rates have been observed to be correlated (Choi et al, 2002; Sibson et al, 1998). In principle, this proportionality could be 1:1 as well as any other ratio. A 1:1 relationship (Sibson et al. 1998) or close to 1:1 relationship (Hyder et al. 2006) are consistent with the ANLSH only on the basis of additional assumptions, namely that 1) astrocytes strictly need glycolysis for the Glu-Gln cycle and 2) neurons consume the lactate produced by astrocytes and utilize ∼17 aerobically produced ATP per molecule of lactate transported to neurons in order to fulfill the energy demands of neurotransmission-related events. Notably, linking glucose consumptions in a ∼ 1:1 way with the Glu-Gln cycle in the context of the ANLSH implicitly neglects the intervention of neuronal glutamate uptake, which occurs during neurotransmission (Bak et al, 2006; Danbolt, 2001; Drejer et al, 1982; Waagepetersen et al, 2005). Finally, a ∼ 1:1 relationship could be explained by other more conventional hypothesis of brain metabolism. As emphasized by Chih and Roberts (2003), a model where astrocytic glucose consumption is just 20% aerobic predicts that neurons would account for 75% of the total glucose consumption in the study by Sibson et al (1998).
D. Interpretation of experimental findings obtained with fMRI
fMRI techniques have been developed on the basic notion that brain activity is strongly and constantly coupled with hemodynamic and metabolic phenomena. fMRI reveals neuronal activation in terms of an increase in measured signal during the execution of a task or an input of a signal, when compared to a control state with lower or different functional involvement. This response reflects the task- or stimulus-induced switch between two different levels of neuronal activity. fMRI techniques generally acquire a series of consecutive brain images using fast acquisition schemes. The effective resolution of activation maps is mainly determined by the characteristics of the functional signals and not by the intrinsic resolution of the acquired images (Ugurbil et al 2003 and references therein). The hemodynamic/metabolic alterations detected by fMRI during neuronal activity indeed spread on a wider area than the actual neuronal site of activation. The choice itself of the acquisition sequence and the magnetic field can be of outstanding importance in modulating signal dynamics. For instance, spin-echo and gradient-echo approaches exploit very different sensitivity to micro- and macro-vasculature. These sensitivities are field-dependent, and the signal behavior can only be explained on the basis of an accurate physical modeling of the static and dynamic contributions to the NMR signal originating from blood vessels (Ogawa et al, 1993; Ugurbil et al, 2006 and references therein).
Ultra-high B0 are beneficial for fMRI, because they enhance SNR, contrast-to-noise and specificity (Ugurbil et al, 2003; 2006, and references therein). Spatial resolutions in the range of 0.15 × 0.15 × 2 mm3 (Fukuda et al, 2006; Harel et al, 2006; Logothetis et al, 2002, and references therein), 0.2 × 0.2 × 2 mm3 (Silva and Koretsky 2002) or 0.2 × 0.2 × 0.2 mm3 (Kida et al, 2002) can be reached in high field animal studies, and up to 0.5 × 0.5 × 2 mm3 in high field human studies (Yacoub et al, 2007, 2008 and references therein). At these resolutions and with appropriate imaging strategies, detection of neuronal activity at a columnar or laminar level can be attained. However, the differentiation of the local cellular microcircuitry of single columns or the differentiation of different cell types (neurons/astrocytes, excitatory/inhibitory neurons) remain intrinsically not accessible by fMRI.
Finally, the specificity and sensitivity of hemodynamic signals to inhibitory neurotransmission is nowadays an active area of debate, and it will be discussed in section D.3 and later in section F. Since fMRI techniques rely on the so-called neurovascular coupling, for the sake of completeness some relevant aspects of the latter are briefly discussed in section D.1; the current knowledge of the link between measured fMRI signals and neuronal activity is summarized in section D.2.
D.1 The mechanism of vasodilatation
Both neurons and glia play a critical role to the regulation of cerebral blood flow (CBF); this regulation relies on the orchestrated work of endothelial cells, pericytes, and smooth muscle cells (for review, see Girouard and Iadecola, 2006 and reference therein). Numerous factors, such as extracellular ions (e.g. Ca2+ and K+), metabolic factors (e.g. lactate or CO2) and neurotransmitters (e.g. glutamate and GABA), have been shown to be either directly or indirectly implicated in the neurovascular coupling. Even if CBF is correlated to metabolic activity in the activated cortex (Hoge et al, 1999), absolute level of energy usage does not directly regulate CBF, as hypoglycemia (Powers et al, 1996) and hypoxia (Mintun et al, 2001) have been shown to have no effects on the local CBF responses to physiological brain stimulation. This implies that it is not the lack of nutrients that drives directly the increase of CBF with stimulation.
D.2 The neuronal origin of the BOLD contrast
The most commonly used fMRI strategy relies on the BOLD contrast (Ogawa et al, 1990), in which signal changes in T2*—weighted or T2—weighted images result from the local variations in the deoxyhemoglobin (dHb) content of the blood. As such, the BOLD signal is not directly related to electrical neuronal activity, but rather depends on the interplay of metabolic—vascular parameters which affect the dHb content in response to neurotransmission events. Measurements of intracortical electrical activity and BOLD-fMRI in monkeys (Logothetis et al, 2001, Rauch et al, 2008) have convincingly demonstrated that the BOLD contrast reflects the input and intracortical processing identified by local field potentials (LFPs), rather than the cortical spiking output identified by multi unit activity (MUA). A similar conclusion applies also for CBF in the cerebellum (Lauritzen, 2001; Mathiesen et al, 1998). Earlier studies with simultaneous EEG recordings, which are expected to reflect synaptic activity hence LFPs, and BOLD based fMRI studies had also shown proportionality during suppression of neuronal activity during inhibitory interactions (Ogawa et al, 2000). These evidences are consistent (even if not conclusive) with the release of neurotransmitters and extracellular ions playing a critical role in the mediation of the neurovascular coupling. Notably, recent findings obtained with two-photon imaging of calcium signals in the ferret visual cortex showed that inhibiting glutamate transporters of astrocytes inhibits the hemodynamic response (Schummers et al, 2008). Some authors argue that BOLD actually reflects spiking activity as well (on this topic, see the review by Raichle and Mintun, 2006). However, it is worth noting that if the firing rate is increased (not always the case) during certain behavioral or stimulation conditions, these increases are always correlated with LFPs and of course with BOLD. This is why BOLD signals show significant temporal correlations with both LFP and MUA during increased neuronal activity (Logothetis et al, 2001). Of additional interest are the cases of dissociation in cortex, where converging inputs in an area result in cross-inhibition and abolishment of spiking (no output). As later emphasized by Logothetis and Wandell (2004), the conclusion drawn about the origin of the BOLD signal relied on the observation that recording sites characterized by strong adaptation showed that, in the absence of any change in the MUA and single spikes, the LFPs were the only regressor that could be used to estimate BOLD (Logothetis et al, 2001). Supporting this line of thought, Viswanathan and Fremman (2007) have found that changes in tissue oxygen concentration were well correlated with LFP even in absence of spikes.
D.3 The interpretation of task induced decreases of the fMRI signal
Areas showing a significant decrease of the fMRI signal correlated with a task are usually observed during functional experiments. These signal decreases are often interpreted as task-induced “deactivation” or negative deviations from a basal state (“default mode”) of brain function under conditions of minimal sensory information processing (Binder et al, 1999; Lawrence et al, 2003; McKiernan et al, 2006; Pochon et al, 2002).
However, the notion of reduction of neuronal activity in some regions while other regions may be involved in sensory or motor processing does not exclude the possibility of hemodyanmic signal-reductions due to vascular steal effects (see discussion in Shmuel et al, 2002). In the rat primary somatosensory cortex, it has been shown that suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative BOLD signal (Devor et al, 2007). Therefore caution has to be used when predicting the outcome of suppressed neuronal activity on the fMRI signal, and, vice-versa, when deducing a suppressed neuronal activity based on signal decreases. For instance, no measurable changes in BOLD were detected when the motor cortex was inhibited (as confirmed by transcranial magnetic stimulation experiments) during the no-go condition of a two-choice reaction time task, where go and no-go visual stimuli were presented randomly (Waldvogel et al, 2000). Robust negative BOLD signals were instead observed in the inhibited ipsilateral motor cortex during unimanual motor tasks (Hamzei et al, 2002; Stefanovic et al, 2004). On the other hand, Harel et al (2002) observed a reduction of both the BOLD signal and cerebral blood volume in higher-order visual areas of the anaesthetized cat in response to full-field drifting gratings, whereas such regions are known to increase their neural activity during that task. Although vascular effects cannot be excluded as contributors to signal reduction, evidence suggest that they are unlikely to be the predominant cause in humans. For one, vascular shunts, which by themselves would generate unpredictable vascular-contrasts, do not exist in the primate cortex (Duvernoy et al, 1981). Second, hemodynamic artifacts have been shown to be inconsequential in the human early visual cortex. The evoked hemodynamic response induced by a fixed visual stimulus was indeed observed to have different amplitudes depending on the baseline conditions, likely reflecting different neuronal responses required to reach the same peak amplitude (Pasley et al, 2007). Most importantly, fMRI and CBF measurements during retinotopic stimulations in the primary visual cortex of humans (Shmuel et al, 2002) as well as combined electrophysiology-fMRI investigations in non-human primates (Shmuel et al, 2006) have demonstrated the existence of clear decreases in all measured parameters. Such signal reductions were directly related to decreased energy consumption, decreased local synaptic inputs and intracortical processing as measured by LFP, and decreased spiking output as measured by MUA. Nevertheless, the mechanisms leading to neuronal suppression are not fully understood yet, and several explanations are possible (Shmuel et al, 2002). The BOLD response of the human primary visual cortex has been also shown to be modulated by attention (Smith et al, 2006a) and by auditory information (Laurienti et al, 2002).
E. Energy usage during neuronal activity: theoretical predictions
The modest increase of the brain energy consumption observed during functional studies is not surprising when considering that the alert brain is never really resting (Shulman and Rothman, 1998), being involved in an intense organized baseline processing which has been referred to as “default mode” of brain function (Gusnard et al, 2001; Raichle et al, 2001; Raichle, 2006).
Cortex is thought to be a link in the chain of afferent-efferent signal processing, and can be understood as a cooperative network that acts as a nonlinear, adaptive (e.g. plasticity, memory) filter of modality-dependent properties, continuously transforming the afferent signal flow (Rockel et al, 1980; Szentagothai, 1978). Studies combining intracellular recording and labeling, iontophoresis, and circuit modeling have long suggested that the elementary operational units of this network, the so-called canonical microcircuits, are universal for all neocortex (for review, see Douglas and Martin, 2004). The central doctrine of the canonical microcircuit of the neo-cortex is weak excitatory input (Garey and Powell, 1971; Winfield and Powell, 1983), and strong local excitatory and inhibitory recurrence (Douglas et al, 1995; Douglas and Martin, 1991; 2004). The proper function of the canonical microcircuits relies on the tight control of excitation-inhibition balance, which provides a controlled amplification of the incoming signals and it fine-tunes the physiological properties of the cortical input. Transient deviations from this balance may occur as a result of neuromodulatory or driver input, leading to feed-forward signaling which usually represents the output of an area (Haider et al, 2006).
Cortical microcircuits operate in a “high-input” regime since a cortical neuron in the primate can integrate ca. 10,000 synapses and receives a continuous bombardment of synaptic input. This feature implies intense neurotransmission and perturbation of ionic gradients in the pre and postsynaptic membrane, both processes being of high energy demand. Experimental findings reviewed by Sokoloff (1999) demonstrate that energy requirements of increased neuronal activity are mostly localized to the neuropil (regions of cortex with high synaptic density) rather that to the perikarya (cell bodies), consistent with the fact that the soma membrane of neurons are known to be not very excitable because of a relative paucity of voltage-gated Na+ channels.
E.1 Available estimations on the energy budget of neurotransmission
The resting membrane potential of neurons is generated by strong ion concentration gradients that are actively kept far from equilibrium through the operation of ionic pumps. This situation allows a very efficient and energetically inexpensive propagation of synaptic potentials (namely excitatory and inhibitory postsynaptic potentials, EPSPs and IPSPs) and action potentials. In fact, when voltage gated ionic channels open in response to neurotransmitter binding, ions passively move following the concentration gradients without consuming ATP. However, an increased work of the active pumps is required to prevent the concentration gradients from collapsing due to the leaking of ions, and for the re-establishment of ion gradients after action potential.
While at present there is general consensus that ion trafficking is the most energetically demanding process in the central nervous system, the exact contributions of this and the other cellular processes to the global cerebral energy consumption are still unclear. Part of the uncertainty is related to the difficulty of integrating results obtained with different approaches and different models. Estimating the contribution of those processes that show activity-related variations of the energy consumption is controversial, because the neural processing involves the modulation of processes active also in resting conditions, and because of the difficulty of defining a “resting” state at cellular level.
The energy cost of the so-called housekeeping activities, i.e. not directly related to neurotransmission (anabolic processes, intracellular signaling and transport, resting potentials, other processes), has been roughly calculated to be over 50% of the total brain energy consumption (for review, see Ames, 2000). On the other hand, the energy consumption at the iso-electricity state of the cortex has been estimated to be either ∼50% (Choi et al, 2002; Du et al, 2006; Du et al, 2008) or as low as ∼20% (Shulman et al, 2004) of total energy consumption, as mentioned in section C.1. Part of the discrepancy can probably be attributed to the reference state taken before inhibition of activity associated with neurotransmission and achieving a state where the energy requirements can be characterized as being dominated by housekeeping functions. This reference state is often a lightly anesthetized state where the energy requirements may be diminished relative to the awake state, depending on the anesthesia type and/or dosage. However, even when comparing pentobarbital anesthetized state (Choi et al, 2002) to awake animals (Oz et al, 2004), as much as ∼40% of the total energy consumption appears to be devoted to functions not directly involved in neurotransmission.
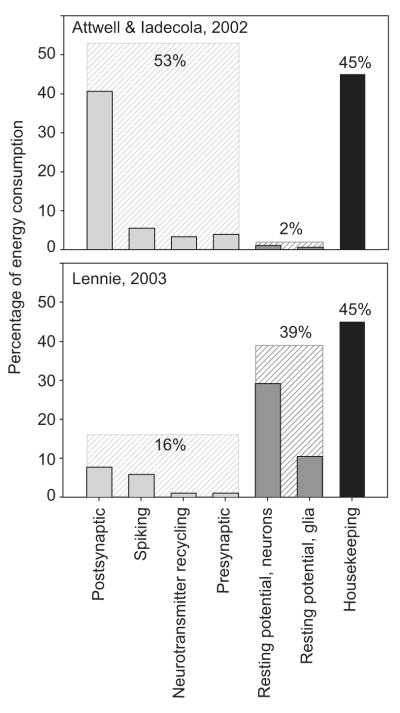
In particular, the energy expended on resting potentials (unrelated to neurotransmission) is markedly unclear. Assuming that housekeeping activities (excluding resting potentials) use 45% of total energy consumption, calculations based on the electric properties of rodent brain cells suggest that the energy used to maintain the resting potential is about 2% for the human brain (Attwell and Iadecola, 2002; Attwell and Laughlin, 2001). However, a correction based on the calculated difference of the membrane conductivity between rodents and humans and on the total number of glia and neurons in the human cortex, suggests that this value can be larger, up to 38% (Lennie, 2003).
Lennie (2003) calculated that the energy required for Na+ pumping related to neurotransmission is only 12% of the global value. The total cost of neurotransmission including presynaptic Ca2+ fluxes and neurotransmitter recycling was estimated by Lennie to amount to 16%. This value is in stark contrast with the value suggested for the human brain by Attwell and Iadecola, ∼50%. From this calculation, Lennie estimated that the sustainable average spike rate is only 0.16 spikes/neuron/s for the whole human cortex, based on axon length and synaptic density measurements in non-human primates (Amit and Brunel, 1997; Davies et al, 2006).
In the calculation of Attwell and Iadecola (2002), the larger contribution to the energy cost of neurotransmission is, by far, the neuronal postsynaptic response to glutamate (41%), followed by ion fluxes related to spiking (5%). This is a marked difference with the estimations on rats (Attwell and Laughlin, 2001), where the relative contributions of these factors are inverted (33% and 47%, respectively, without including the housekeeping component). This is mainly due to the larger number of synapses per neuron in the human brain. In comparison, Lennie (003) estimated that contributions of neuronal postsynaptic and spiking activity are comparable (8% vs 6%). For a global outline of the estimations here discussed, see Figure 7.
Figure 7.
Two estimations of energy consumption of the human brain cortex. For uniformity, the housekeeping component (intracellular transport and signaling, vegetative metabolism) has been set to 45% (in black). Postsynaptic: postsynaptic ion fluxes induced by glutamate. Spiking: pumping out of the Na+ influx during action potentials. Neurotransmitters recycling: actions (mainly astrocytic) related to glutamate recycling. Presynaptic: extrusion of presynaptic Ca2+ influx related to Glu extrusion, exocytosis of neurotransmitter vescicles. The total values of the components related to neurotransmission (light gray) and to resting potential (dark gray) are shown. Values are rounded. Above: adapted from the estimation by Attwell and Iadecola (2002). Below: adapted from the estimation by Lennie (2003).
E.2 The role of astrocytes
Both previous calculations (Attwell and Laughlin, 2001; Lennie, 2003) estimated that the removal of neurotransmitter by astrocytes accounts for no more than 5% of the total energy needs of neurotransmission. However, the energetics of astrocytes during neurotransmission might be underestimated if based only on the glutamate cycling (Hertz et al, 2007). Astrocytes indeed play an important role for the clearance of extracellular K+, in the generation of Ca2+ wave and in signaling processes. The energy demands of these functions are nonetheless quantitatively unknown. As reviewed by Hyder et al (2006) and by Hertz et al (2007), in the awake human brain, the relative contribution of astrocytes to the total oxidative metabolism has been estimated to be ∼25%, similar to their volume densities in the cortex. This estimate suggests comparable oxidative capacities of astrocytes and neurons.
E.3 The energy cost of changes in neurotransmission
The previous estimates (Attwell and Laughlin, 2001; Lennie, 2003) were based on the energy cost of the most important cellular events occurring during depolarization of one neuron firing at an average firing rate. The results were then extended to an ensemble of neurons by considering an average neuronal density, and by assuming the entire population of neurons being glutamatergic. In other words the system was modeled as a linear combination of isolated neurons. However, in order to predict the energy cost of increased/decreased neurotransmission, one should consider that the neural network connecting inhibitory and excitatory neurons is far from being linear. In principle, without modeling the network of input/output signals, a proper quantitative estimate of the energy demands following changes in neuronal activity cannot be provided.
F. The challenging prediction of energy demands during decreased neuronal activity
In spite of the quantitative disagreements between the energy calculations described above (Attwell and Laughlin, 2001; Lennie, 2003), both models qualitatively predict that an increase of spiking rates and consequent postsynaptic actions drives increased energy consumption. Similarly, decreased spiking rates due to decreased feedforward inputs and consequent decreased postsynaptic actions are expected to lower the energy demands of the area investigated. In general, however, the prediction of the energetic outcome of suppressed neuronal activity depends on how firing suppression is accomplished. The estimates of the energy budget of neuronal firing suppression are challenged by the fact that inhibition can occur via different cellular and/or network mechanisms that are not only activity dependent, but also region specific. The organization and segregation of synaptic inputs are indeed highly heterogeneous in the brain. This complexity is presumably at the origin of the controversial results that have been obtained with neuroimaging methods during deactivation tasks (discussed in section D.3).
F.2 The activity of inhibitory interneurons
Inhibitory GABAergic interneurons are fast spiking (McCormick et al, 1985) and are metabolically more active than excitatory neurons (McCasland and Hibbard, 1997). Inhibitory interneurons respond more strongly than excitatory neurons to thalamic inputs (Cruikshank et al, 2007), thus generating very powerful feedforward inhibition which sculpts the response of excitatory neurons to have a tighter tuning than the thalamic inputs. The relative contribution of GABAergic neurons to neuronal oxidative metabolism has been estimated to be ∼20%, similar to their relative synaptic densities (Patel et al, 2005). When firing suppression is accomplished by an increased spiking of inhibitory neurons, brain regions with a high density of afferent nerve terminals of GABAergic neurons were found to increase energy consumption (Ackermann et al, 1984). This finding was further confirmed by investigating the energy changes in the lateral superior olive (LSO) during activation (Nudo and Masterton, 1986). This is a tonotopic structure in the auditory system of the cat that receives excitatory afferents from the ipsilateral ear and inhibitory afferents from the contralateral ear. When stimulated with pure tones, both the excited ipsilateral and the inhibited contralateral LSO increased metabolism due to the increase activity of the afferent inhibitory terminals.
F.3 The inhibitory microcircuitry
Inhibitory interneurons selectively target distinct subcellular domains of neurons (axon, perisomatic region, and distal dendrites), thus establishing intricate microcircuits which counteract different sets of excitatory synapses. This complex microcircuitry is becoming experimentally accessible thanks to recent developments of wire and micro-machined silicon electrode arrays, which allow the massive parallel recording of the electrical activity of large neuronal ensembles (Buzsaki, 2004).
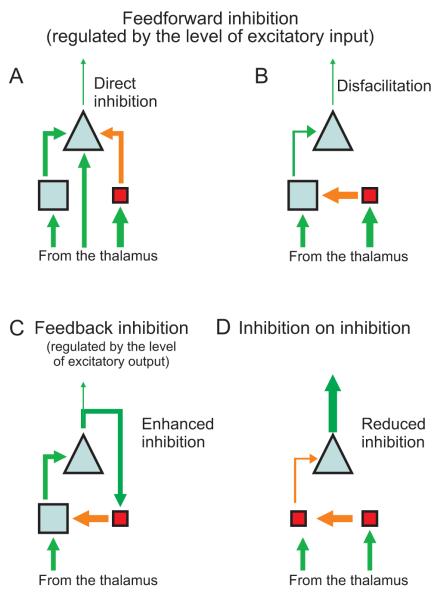
Inhibition can be generally classified in terms of 1) feedforward inhibition, triggered by excitatory inputs on inhibitory interneurons, thus regulated by the level of the input; 2) feedback inhibition, triggered by the collaterals of pyramidal cells, thus regulated by the level of the output (Tepper et al, 2004). Feedforward inhibition can occur via “active inhibition” by local interneurons on pyramidal cells, or via “disfacilitation”, where inhibitory interneurons indirectly suppress the firing of pyramidal cells by suppressing their afferent excitatory synaptic activity (Timofeev et al, 2001). In addition, feedback modulatory connections are established between different regions of the cortex, and between the cortex and the thalamus (Callaway, 2004). Finally, collaterals of excited inhibitory neurons themselves can inhibit other inhibitory interneurons, thus producing inhibition on inhibition (Tsodyks et al, 1997).
In the neocortex, the vast majority of inhibitory and excitatory synapses come from neurons in the immediate vicinity of the synapse (Binzegger et al, 2004; Sillito and Jones, 2002; Stettler et al, 2002); notably, the thalamic inputs to the primary visual cortex involve only 5% of synapses (Peters and Payne, 1993; Peters et al, 1994). Therefore, suppression presumably occurs mainly through recurrent local inhibition (i.e., disfacilitation or direct active inhibition on pyramidal cells). However, it is often unclear how these microcircuits are involved in different patterns of activity in the brain, even for the primary structures of the neo-cortex. For instance, several sources of suppression (intracortical inhibitory circuits, depression of thalamocortical synapses, or nonlinear responses from the lateral geniculate nuclei) have been suggested as putative mechanisms when cross-orientation or surround suppression induce a reduction of the optimal response of neurons in the primary visual cortex to their classical receptive field (Smith et al, 2006b). For a global schematic of the different pathways which generate neuronal firing suppression, see Figure 8.
Figure 8.
Schematics representing different pathways which result in neuronal firing suppression (Tepper et al, 2004; Timofeev et al, 2001). Triangles represent pyramidal cells, blue squares represent glutamatergic interneurons, pink squares represent GABAergic interneurons; size corresponds to proportion of the cortical population. Green connecting lines represent excitatory connections, red lines indicate inhibitory inputs; line thickness indicates either firing rates (connecting lines) or strength of excitatory postsynaptic potentials (EPSPs) in cells. A) Direct inhibition: IPSPs from inhibitory interneurons compete on pyramidal cells with the EPSPs coming from excitatory interneurons and from the thalamus. B) Disfacilitation: inhibitory interneurons primarily act on the excitatory interneurons, which reduce their firing on pyramidal cells. C) Feedback inhibition: collaterals of pyramidal cells excite inhibitory interneurons, which conversely inhibit excitatory interneurons. D) Inhibition on inhibition: inhibitory neurons inhibit other inhibitory neurons, overall reducing inhibition on pyramidal cells (Tsodyks et al, 1997).
F.4 A working hypothesis about the energetic outcomes of neuronal firing suppression
Summarizing what we have discussed in section F.3, suppression of neuronal activity in the neocortex can be achieved through 1) decreased afferent inputs, which, for the neocortex, are always relayed by excitatory neurons (not the case, for example, for the LSO described in the previous section) 2) decreased intracortical processing due to disfacilitation; 3) increased active inhibition on pyramidal cells. The last two cases involve increased local inhibitory activity. In the first case, a reduction of metabolism can be predicted on the basis of energy budget considerations (Attwell and Laughlin, 2001; Lennie, 2003; Sibson et al, 1998); this prediction agrees with several experimental findings (Pasley et al, 2007; Shmuel et al, 2002; 2006). Here we suggest that the global energy needs in the neocortex decrease also when firing is suppressed by increased inhibitory activity (second and third cases) rather than decreased afferent inputs. This working hypothesis is based on two main considerations. Firstly, inhibitory synapses constitute a minor percentage of the synapses population, no more than 20% in average (Fairen et al, 1984). The findings of increased metabolism in the stratum pyramidal of the hippocampus during long-duration recurrent inhibition (Ackermann et al, 1984) likely do not apply to neo-cortical areas, because not even the input layer of the neocortex presents dense plexus of inhibitory synapses like the stratum pyramidal of the hippocampus does. Secondly, IPSPs should be markedly less expensive than EPSPs. Inhibitory and excitatory neuronal activity indeed share several electrical phenomena, but, postsynaptically, the flux of chloride involved in hyperpolarizing the membrane is determined by smaller electrochemical gradients compared to the EPSPs. In addition, the ‘Cl pump’, responsible for reestablishing the concentration gradient of Cl-, is a secondary active transporter which utilizes the energy stored in a pre-existing concentration gradient for a different ion, without directly requiring hydrolysis of ATP (Payne et al, 1996). Overall, these two considerations imply that increased inhibition should drop energy request compared to a non-inhibited state: the energy savings due to decreased spiking rate of the majority of excitatory cells overcomes the negligible energy increases of IPSPs and the energy increases of the minority of GABAergic neurons. The neo-cortical energy demands would then ultimately reflect the spiking activity of the area.
The above mentioned working hypothesis can be utilized for guiding and interpreting combined fMRI/fMRS studies which aim at investigating neuronal firing suppression in vivo. In a preliminary study of this kind, the time-courses of the BOLD effect and of [Lac] have been measured upon repeated identical 2-min long visual stimuli (Mangia et al, 2007b). The average amplitude of these increases of [Lac] was found to be reduced over time, whereas the amplitude of the BOLD effect was persistent during the whole observation period. This finding can be interpreted to suggest a differential adaptation of the cortical output that is not reflected at the level of the global excitation-inhibition activity of the cortical canonical circuits.
G. Concluding remarks
So far, many theoretical and experimental efforts have been devoted to investigate the energy need of the functioning brain. Some conclusions can be safely drawn, but other important issues remain open. In summary:
Astrocytes play many functions during neurotransmission, but the hypothesis about their major “feeding role” as providers of lactate to neurons remains controversial. It is still under debate whether or not lactate is the preferential substrate of neurons for the neurotransmission-related energy needs in vivo.
Quantitative measurements of changes of local metabolism in vivo are problematic. 13C MRS can potentially estimate changes in fluxes, but technical limitations do not yet allow extensive applications in functional human studies. On the other hand, 1H MRS is feasible for functional investigations of the human brain, but the technique is not sensitive to changes in metabolic fluxes that are not reflected in changes in metabolite concentrations.
The interpretation of in vivo MRS results in the context of compartmentalized metabolic models is challenged by the lack of the suitable spatial resolution of the available in vivo experimental methodologies.
Energy needs are correlated with neurotransmission. However, predicting the energy cost of the specific aspects of neurotransmission remains a challenging task, which has been approached only partly so far.
Signals which rely on hemodynamic phenomena have been demonstrated to be associated with the input and intracortical processing of the system: whenever the global synaptic activity increases (no matter if excitatory or inhibitory), CBF is expected to increase.
Methodologies of MRS and MRI are sensitive to different aspects of neuronal activation, thus providing valuable complementary tools for investigating the human brain at work.
Acknowledgments
Supporting grants and Institutes: NIH P41RR08079; P30 NS057091; R01NS38672; the W. M. Keck Foundation and the MIND Institute.
References
- Ackermann RF, Finch DM, Babb TL, Engel J., Jr. Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. J Neurosci. 1984;4:251–64. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Amit DJ, Brunel N. Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb Cortex. 1997;7:237–52. doi: 10.1093/cercor/7.3.237. [DOI] [PubMed] [Google Scholar]
- Atlante A, de Bari L, Bobba A, Marra E, Passarella S. Transport and metabolism of L-lactate occur in mitochondria from cerebellar granule cells and are modified in cells undergoing low potassium dependent apoptosis. Biochim Biophys Acta. 2007;1767:1285–99. doi: 10.1016/j.bbabio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–5. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R. Interaction between astrocytes and neurons studied using a mathematical model of compartmentalized energy metabolism. J Cereb Blood Flow Metab. 2005;25:1476–90. doi: 10.1038/sj.jcbfm.9600144. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci U S A. 2005;102:16448–53. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Pellerin L, Magistretti PJ, Costalat R. A coherent neurobiological framework for functional neuroimaging provided by a model integrating compartmentalized energy metabolism. Proc Natl Acad Sci U S A. 2007;104:4188–93. doi: 10.1073/pnas.0605864104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab. 2006;26:1285–97. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986;232:1121–3. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Magistretti PJ, Pellerin L. Selective postsynaptic co-localization of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cereb Cortex. 2005;15:361–70. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- Bilger A, Nehlig A. Quantitative histochemical changes in enzymes involved in energy metabolism in the rat brain during postnatal development. I. Cytochrome oxidase and lactate dehydrogenase. Int J Dev Neurosci. 1991;9:545–53. doi: 10.1016/0736-5748(91)90015-e. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–53. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab. 1996;16:1079–89. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Bliss TM, Sapolsky RM. Interactions among glucose, lactate and adenosine regulate energy substrate utilization in hippocampal cultures. Brain Res. 2001;899:134–41. doi: 10.1016/s0006-8993(01)02218-1. [DOI] [PubMed] [Google Scholar]
- Blomqvist G, Seitz RJ, Sjogren I, Halldin C, Stone-Elander S, Widen L, Solin O, Haaparanta M. Regional cerebral oxidative and total glucose consumption during rest and activation studied with positron emission tomography. Acta Physiol Scand. 1994;151:29–43. doi: 10.1111/j.1748-1716.1994.tb09718.x. [DOI] [PubMed] [Google Scholar]
- Boucard CC, Mostert JP, Cornelissen FW, De Keyser J, Oudkerk M, Sijens PE. Visual stimulation, 1H MR spectroscopy and fMRI of the human visual pathways. Eur Radiol. 2005;15:47–52. doi: 10.1007/s00330-004-2494-y. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24:1687–94. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–51. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Cakir T, Alsan S, Saybaşili H, Akin A, Ulgen KO. Reconstruction and flux analysis of coupling between metabolic pathways of astrocytes and neurons: application to cerebral hypoxia. Theor Biol Med Model. 2007;4:48. doi: 10.1186/1742-4682-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. Feedforward, feedback and inhibitory connections in primate visual cortex. Neural Netw. 2004;17:625–32. doi: 10.1016/j.neunet.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Cerdan S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, Garcia-Martin ML. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–30. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci U S A. 2003;100:12456–61. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Qian YZ, Rice A, Zhu JP, Di X, Bullock R. Brain lactate uptake increases at the site of impact after traumatic brain injury. Brain Res. 2000;861:281–7. doi: 10.1016/s0006-8993(00)01992-2. [DOI] [PubMed] [Google Scholar]
- Chen W, Novotny EJ, Zhu XH, Rothman DL, Shulman RG. Localized 1H NMR measurement of glucose consumption in the human brain during visual stimulation. Proc Natl Acad Sci U S A. 1993;90:9896–900. doi: 10.1073/pnas.90.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu XH, Gruetter R, Seaquist ER, Adriany G, Ugurbil K. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using (1)H-[(13)C] MRS and fMRI. Magn Reson Med. 2001;45:349–55. doi: 10.1002/1522-2594(200103)45:3<349::aid-mrm1045>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Chhina N, Kuestermann E, Halliday J, Simpson LJ, Macdonald IA, Bachelard HS, Morris PG. Measurement of human tricarboxylic acid cycle rates during visual activation by (13)C magnetic resonance spectroscopy. J Neurosci Res. 2001;66:737–46. doi: 10.1002/jnr.10053. [DOI] [PubMed] [Google Scholar]
- Chih CP, Lipton P, Roberts EL., Jr. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24:573–8. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23:1263–81. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–51. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Welker E, Lacombe P, Seylaz J, Magistretti P, Bonvento G. Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. J Cereb Blood Flow Metab. 2001;21:404–12. doi: 10.1097/00004647-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Cox DW, Drower J, Bachelard HS. Effects of metabolic inhibitors on evoked activity and the energy state of hippocampal slices superfused in vitro. Exp Brain Res. 1985;57:464–70. doi: 10.1007/BF00237833. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–8. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem. 2005;92:934–47. doi: 10.1111/j.1471-4159.2004.02935.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davies RM, Gerstein GL, Baker SN. Measurement of time-dependent changes in the irregularity of neural spiking. J Neurophysiol. 2006;96:906–18. doi: 10.1152/jn.01030.2005. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–55. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–57. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–9. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Neighborly interactions of metabolically-activated astrocytes in vivo. Neurochem Int. 2003;43:339–54. doi: 10.1016/s0197-0186(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int. 2004;45:321–51. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. J Cereb Blood Flow Metab. 2002;22:1490–502. doi: 10.1097/01.WCB.0000034363.37277.89. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–5. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–69. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–51. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Drejer J, Larsson OM, Schousboe A. Characterization of L-glutamate uptake into and release from astrocytes and neurons cultured from different brain regions. Exp Brain Res. 1982;47:259–69. doi: 10.1007/BF00239385. [DOI] [PubMed] [Google Scholar]
- Du F, Zhang Y, Friedman M, Zhu XH, Ugurbil K, Chen W. Study of correlation between brain activity and ATP metabolic rates by means of 31P magnetization transfer and electroencephalograph measurement. Proceedings Meeting International Society Magnetic Resonance in Medicine; Seattle. 2006. p. 2125. [Google Scholar]
- Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105:6409–14. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519–79. doi: 10.1016/0361-9230(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Hewitt A, Damon TL, Hart M, Kurascz J, Li A, Leiter JC. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte-neuron lactate-shuttle hypothesis. J Neurosci. 2008;28:4888–96. doi: 10.1523/JNEUROSCI.5430-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairen A, DeFelipe J, Regidor J. Nonpyramidal neurons. In: Peters A, Jones E, editors. Cerebral cortex. Plenum; New York: 1984. pp. 201–53. [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–4. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frahm J, Kruger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med. 1996;35:143–8. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- From AH, Petein MA, Michurski SP, Zimmer SD, Ugurbil K. 31P-NMR studies of respiratory regulation in the intact myocardium. FEBS Lett. 1986;206:257–61. doi: 10.1016/0014-5793(86)80992-9. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Moon CH, Wang P, Kim SG. Mapping iso-orientation columns by contrast agent-enhanced functional magnetic resonance imaging: reproducibility, specificity, and evaluation by optical imaging of intrinsic signal. J Neurosci. 2006;26:11821–32. doi: 10.1523/JNEUROSCI.3098-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Powell TP. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971;179:41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Gibala M, MacLean D, Graham T, Saltin B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Physiol. 1998;275:E235–E42. doi: 10.1152/ajpendo.1998.275.2.E235. [DOI] [PubMed] [Google Scholar]
- Giove F, Mangia S, Bianciardi M, Garreffa G, Di Salle F, Morrone R, Maraviglia B. The physiology and metabolism of neuronal activation: in vivo studies by NMR and other methods. Magn Reson Imaging. 2003;21:1283–93. doi: 10.1016/j.mri.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–35. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Marrett S. Glycolysis in neurons, not astrocytes, delays oxidative metabolism of human visual cortex during sustained checkerboard stimulation in vivo. J Cereb Blood Flow Metab. 2001;21:1384–92. doi: 10.1097/00004647-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281:E100–12. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echoplanar techniques. Magn Reson Med. 2000;43:319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Ugurbil K, Seaquist E. Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem. 1998a;70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Weisdorf SA, Rajanayagan V, Terpstra M, Merkle H, Truwit CL, Garwood M, Nyberg SL, Ugurbil K. Resolution improvements in in vivo 1H NMR spectra with increased magnetic field strength. J Magn Reson. 1998b;135:260–4. doi: 10.1006/jmre.1998.1542. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–45. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Denton RM. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974;138:313–6. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Dettmers C, Rzanny R, Liepert J, Buchel C, Weiller C. Reduction of excitability (“inhibition”) in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage. 2002;17:490–6. doi: 10.1006/nimg.2002.1077. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–17. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Harel N, Lin J, Moeller S, Ugurbil K, Yacoub E. Combined imaging-histological study of cortical laminar specificity of fMRI signals. Neuroimage. 2006;29:879–87. doi: 10.1016/j.neuroimage.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE. 2008;3:e2915. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Henry PG, Adriany G, Deelchand D, Gruetter R, Marjanska M, Oz G, Seaquist ER, Shestov A, Ugurbil K. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn Reson Imaging. 2006;24:527–39. doi: 10.1016/j.mri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res. 2005;79:11–8. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–49. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Chu WJ, Gonen O, Pan JW. Robust fully automated shimming of the human brain for high-field 1H spectroscopic imaging. Magn Reson Med. 2006;56:26–33. doi: 10.1002/mrm.20941. [DOI] [PubMed] [Google Scholar]
- Hochachka PW. Intracellular convection, homeostasis and metabolic regulation. J Exp Biol. 2003;206:2001–9. doi: 10.1242/jeb.00402. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96:9403–8. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–90. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–98. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–77. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A. 2003;100:4879–84. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Brull R, Alsop DC, Marquis RP, Lenkinski RE. Limits on activation-induced temperature and metabolic changes in the human primary visual cortex. Magn Reson Med. 2006;56:348–55. doi: 10.1002/mrm.20972. [DOI] [PubMed] [Google Scholar]
- Kida I, Xu F, Shulman RG, Hyder F. Mapping at glomerular resolution: fMRI of rat olfactory bulb. Magn Reson Med. 2002;48:570–6. doi: 10.1002/mrm.10248. [DOI] [PubMed] [Google Scholar]
- Korf J. Is brain lactate metabolized immediately after neuronal activity through the oxidative pathway? J Cereb Blood Flow Metab. 2006;26:1584–6. doi: 10.1038/sj.jcbfm.9600321. [DOI] [PubMed] [Google Scholar]
- LaNoue KF, Tischler ME. Electrogenic characteristics of the mitochondrial glutamate-aspartate antiporter. J Biol Chem. 1974;249:7522–8. [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Wallace MT, Yen YF, Field AS, Stein BE. Deactivation of sensory-specific cortex by cross-modal stimuli. J Cogn Neurosci. 2002;14:420–9. doi: 10.1162/089892902317361930. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J Cereb Blood Flow Metab. 2001;21:1367–83. doi: 10.1097/00004647-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–38. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–31. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–7. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–42. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N, Merkle H, Augath M, Trinath T, Ugurbil K. Ultra high-resolution fMRI in monkeys with implanted RF coils. Neuron. 2002;35:227–42. doi: 10.1016/s0896-6273(02)00775-4. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–69. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–66. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust WD, Pundik S, Zechel J, Zhou Y, Buczek M, Selman WR. Changing metabolic and energy profiles in fetal, neonatal, and adult rat brain. Metab Brain Dis. 2003;18:195–206. doi: 10.1023/a:1025503115837. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van De Moortele PF, Giove F, Maraviglia B, Ugurbil K. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn Reson Imaging. 2006;24:343–8. doi: 10.1016/j.mri.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007a;27:1055–63. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Logothetis NK, Gruetter R, Van de Moortele PF, Ugurbil K. Dynamics of lactate concentration and blood oxygen level-dependent effect in the human visual cortex during repeated identical stimuli. J Neurosci Res. 2007b;85:3340–6. doi: 10.1002/jnr.21371. [DOI] [PubMed] [Google Scholar]
- Marrett S, Gjedde A. Changes of blood flow and oxygen consumption in visual cortex of living humans. Adv Exp Med Biol. 1997;413:205–8. doi: 10.1007/978-1-4899-0056-2_22. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgoren N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512(Pt 2):555–66. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Hibbard LS. GABAergic neurons in barrel cortex show strong, whisker-dependent metabolic activation during normal behavior. J Neurosci. 1997;17:5509–27. doi: 10.1523/JNEUROSCI.17-14-05509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71:399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–91. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JM. The role of lactate as an energy substrate for the brain during the early neonatal period. Biol Neonate. 1985;48:237–44. doi: 10.1159/000242176. [DOI] [PubMed] [Google Scholar]
- Merboldt KD, Bruhn H, Hanicke W, Michaelis T, Frahm J. Decrease of glucose in the human visual cortex during photic stimulation. Magn Reson Med. 1992;25:187–94. doi: 10.1002/mrm.1910250119. [DOI] [PubMed] [Google Scholar]
- Michaeli S, Garwood M, Zhu XH, DelaBarre L, Andersen P, Adriany G, Merkle H, Ugurbil K, Chen W. Proton T2 relaxation study of water, N-acetylaspartate, and creatine in human brain using Hahn and Carr-Purcell spin echoes at 4T and 7T. Magn Reson Med. 2002;47:629–33. doi: 10.1002/mrm.10135. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci U S A. 2001;98:6859–64. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci U S A. 2001;98:7593–8. doi: 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: high-resolution microautoradiographic imaging by cellular 14C-trajectography combined with immunohistochemistry. J Cereb Blood Flow Metab. 2004;24:1004–14. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wang J, Rao H, Swanson RL, Wintering N, Karp JS, Alavi A, Greenberg JH, Detre JA. Concurrent CBF and CMRGlc changes during human brain activation by combined fMRI-PET scanning. Neuroimage. 2005;28:500–6. doi: 10.1016/j.neuroimage.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol. 1986;245:553–65. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Stepnoski R, Chen W, Zhu XH, Ugurbil K. An approach to probe some neural systems interaction by functional MRI at neural time scale down to milliseconds. Proc Natl Acad Sci U S A. 2000;97:11026–31. doi: 10.1073/pnas.97.20.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–12. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–9. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–76. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, de Graaf R, Mason G, Rothman D, Shulman R, Behar K. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–93. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative KCl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem. 1996;271:16245–52. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43:331–8. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005;79:55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–62. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23:1282–6. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- Peters A, Payne BR. Numerical relationships between geniculocortical afferents and pyramidal cell modules in cat primary visual cortex. Cereb Cortex. 1993;3:69–78. doi: 10.1093/cercor/3.1.69. [DOI] [PubMed] [Google Scholar]
- Peters A, Payne BR, Budd J. A numerical analysis of the geniculocortical input to striate cortex in the monkey. Cereb Cortex. 1994;4:215–29. doi: 10.1093/cercor/4.3.215. [DOI] [PubMed] [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:586–95. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience. 2000;100:617–27. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci U S A. 2002;99:5669–74. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci. 2004;24:9669–73. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Hirsch IB, Cryer PE. Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation. Am J Physiol. 1996;270:H554–9. doi: 10.1152/ajpheart.1996.270.2.H554. [DOI] [PubMed] [Google Scholar]
- Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991;88:5829–31. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Neuroscience. The brain’s dark energy. Science. 2006;314:1249–50. [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Ramirez BG, Rodrigues TB, Violante IR, Cruz F, Fonseca LL, Ballesteros P, Castro MM, Garcia-Martin ML, Cerdan S. Kinetic properties of the redox switch/redox coupling mechanism as determined in primary cultures of cortical neurons and astrocytes from rat brain. J Neurosci Res. 2007;85:3244–53. doi: 10.1002/jnr.21386. [DOI] [PubMed] [Google Scholar]
- Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc Natl Acad Sci U S A. 2008;105:6759–64. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera JJ, Schousboe A, Waagepetersen HS, Howarth C, Hyder F. The micro-architecture of the cerebral cortex: functional neuroimaging models and metabolism. Neuroimage. 2008;40:1436–59. doi: 10.1016/j.neuroimage.2007.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille PM, Merkle H, Lew B, Path G, Hendrich K, Lindstrom P, From AH, Garwood M, Bache RJ, Ugurbil K. Transmural high energy phosphate distribution and response to alterations in workload in the normal canine myocardium as studied with spatially localized 31P NMR spectroscopy. Magn Reson Med. 1990;16:91–116. doi: 10.1002/mrm.1910160110. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TP. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–44. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Rodrigues TB, Gray HL, Benito M, Garrido S, Sierra A, Geraldes CF, Ballesteros P, Cerdan S. Futile cycling of lactate through the plasma membrane of C6 glioma cells as detected by (13C, 2H) NMR. J Neurosci Res. 2005;79:119–27. doi: 10.1002/jnr.20308. [DOI] [PubMed] [Google Scholar]
- Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992;12:584–92. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–43. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Schurr A. Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab. 2006;26:142–52. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res. 1997a;744:105–11. doi: 10.1016/s0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS. Lactate, not pyruvate, is neuronal aerobic glycolysis end product: an in vitro electrophysiological study. Neuroscience. 2007;147:613–9. doi: 10.1016/j.neuroscience.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia. Brain Res. 1997b;774:221–4. doi: 10.1016/s0006-8993(97)81708-8. [DOI] [PubMed] [Google Scholar]
- Shestov AA, Valette J, Ugurbil K, Henry PG. On the reliability of (13)C metabolic modeling with two-compartment neuronal-glial models. J Neurosci Res. 2007;85:3294–303. doi: 10.1002/jnr.21269. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci U S A. 1998;95:11993–8. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R, Hyder F, Rothman D. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001a;14:389–96. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci U S A. 2001b;98:6417–22. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–95. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–21. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B Biol Sci. 2002;357:1739–52. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci U S A. 2002;99:15182–7. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–91. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A. 2002;99:10765–70. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AT, Cotillon-Williams NM, Williams AL. Attentional modulation in the human visual cortex: the time-course of the BOLD response and its implications. NeuroImage. 2006a;29:328–34. doi: 10.1016/j.neuroimage.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Smith MA, Bair W, Movshon JA. Dynamics of suppression in macaque primary visual cortex. J Neurosci. 2006b;26:4826–34. doi: 10.1523/JNEUROSCI.5542-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. The metabolism of the central nervous system in vivo. In: Field J, Magoun H, Hall V, editors. Handbook of Physiology. Section I, Neruophysiology. American Physiological Society; Washington D.C.: 1960. pp. 1843–64. [Google Scholar]
- Sokoloff L. The brain as a chemical machine. Prog Brain Res. 1992;94:19–33. doi: 10.1016/s0079-6123(08)61736-7. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–9. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22:771–8. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Das A, Bennett J, Gilbert CD. Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron. 2002;36:739–50. doi: 10.1016/s0896-6273(02)01029-2. [DOI] [PubMed] [Google Scholar]
- Szentagothai J. The Ferrier Lecture, 1977. The neuron network of the cerebral cortex: a functional interpretation. Proc R Soc Lond B Biol Sci. 1978;201:219–48. doi: 10.1098/rspb.1978.0043. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–9. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med. 2002;47:1009–12. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–9. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–6. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- Tkac I, Gruetter R. Methodology of H-1 NMR spectroscopy of the human brain at very high magnetic fields. Appl Magn Reson. 2005;29:139–57. doi: 10.1007/BF03166960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL. Paradoxical effects of external modulation of inhibitory interneurons. J Neurosci. 1997;17:4382–8. doi: 10.1523/JNEUROSCI.17-11-04382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Adriany G, Andersen P, Chen W, Garwood M, Gruetter R, Henry PG, Kim SG, Lieu H, Tkac I, Vaughan T, Van De Moortele PF, Yacoub E, Zhu XH. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn Reson Imaging. 2003;21:1263–81. doi: 10.1016/j.mri.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Chen W, Harel N, Van De Moortele PF, Yacoub E, Zhu XH, Uludag K. Brain function, magnetic resonance imaging of. In: Akay M, editor. WIley Encyclopedia of Biomedical Engineering. John Wiley & Sons, Inc; Hoboken, NJ: 2006. pp. 647–88. [Google Scholar]
- Ugurbil K, Toth L, Kim DS. How accurate is magnetic resonance imaging of brain function? Trends Neurosci. 2001;26:108–14. doi: 10.1016/S0166-2236(02)00039-5. [DOI] [PubMed] [Google Scholar]
- Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46:24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- Vega C, Martiel JL, Drouhault D, Burckhart MF, Coles JA. Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve. J Physiol. 2003;546:551–64. doi: 10.1113/jphysiol.2002.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007;10:1308–12. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–86. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A. Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci. 1998;20:310–20. doi: 10.1159/000017326. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Qu H, Sonnewald U, Shimamoto K, Schousboe A. Role of glutamine and neuronal glutamate uptake in glutamate homeostasis and synthesis during vesicular release in cultured glutamatergic neurons. Neurochem Int. 2005;47:92–102. doi: 10.1016/j.neuint.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Waldvogel D, van Gelderen P, Muellbacher W, Ziemann U, Immisch I, Hallett M. The relative metabolic demand of inhibition and excitation. Nature. 2000;406:995–8. doi: 10.1038/35023171. [DOI] [PubMed] [Google Scholar]
- Winfield DA, Powell TP. Laminar cell counts and geniculo-cortical boutons in area 17 of cat and monkey. Brain Res. 1983;277:223–9. doi: 10.1016/0006-8993(83)90929-0. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A. 2008;105:10607–12. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage. 2007;37:1161–77. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Chen W. Observed BOLD effects on cerebral metabolite resonances in human visual cortex during visual stimulation: a functional (1)H MRS study at 4 T. Magn Reson Med. 2001;46:841–7. doi: 10.1002/mrm.1267. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of (17)O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A. 2002;99:13194–9. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Oxidation of (14)C-labeled compounds perfused by microdialysis in the brains of free-moving rats. J Neurosci Res. 2007;85:3145–9. doi: 10.1002/jnr.21424. [DOI] [PubMed] [Google Scholar]