Abstract
Objective
To assess associations between baseline values of four different circulating markers of inflammation and future risk of coronary heart disease, potential triggers of systemic inflammation (such as persistent infection), and other markers of inflammation.
Design
Nested case-control comparisons in a prospective, population based cohort.
Setting
General practices in 18 towns in Britain.
Participants
506 men who died from coronary heart disease or had a non-fatal myocardial infarction and 1025 men who remained free of such disease until 1996 selected from 5661 men aged 40-59 years who provided blood samples in 1978-1980.
Main outcome measures
Plasma concentrations of C reactive protein, serum amyloid A protein, and serum albumin and leucocyte count. Information on fatal and non-fatal coronary heart disease was obtained from medical records and death certificates.
Results
Compared with men in the bottom third of baseline measurements of C reactive protein, men in the top third had an odds ratio for coronary heart disease of 2.13 (95% confidence interval 1.38 to 3.28) after age, town, smoking, vascular risk factors, and indicators of socioeconomic status were adjusted for. Similar adjusted odds ratios were 1.65 (1.07 to 2.55) for serum amyloid A protein; 1.12 (0.71 to 1.77) for leucocyte count; and 0.67 (0.43 to 1.04) for albumin. No strong associations were observed of these factors with Helicobacter pylori seropositivity, Chlamydia pneumoniae IgG titres, or plasma total homocysteine concentrations. Baseline values of the acute phase reactants were significantly associated with one another (P<0.0001), although the association between low serum albumin concentration and leucocyte count was weaker (P=0.08).
Conclusion
In the context of results from other relevant studies these findings suggest that some inflammatory processes, unrelated to the chronic infections studied here, are likely to be involved in coronary heart disease.
Introduction
Circulating concentrations of C reactive protein, serum amyloid A protein, and serum albumin and the leucocyte count can fluctuate widely during acute responses to tissue damage or infection. Plasma concentrations of C reactive protein and serum amyloid A protein can each rise 10 000-fold; the leucocyte count can increase about threefold; and the concentration of serum albumin can fall by about 20%.1,2 In recent years these “acute phase reactants” have been studied as potential markers of more subtle and persistent systemic alterations that may be loosely called low grade inflammation. If the sharp short term fluctuations are ignored, then long term circulating concentrations of these factors show a similar year-to-year consistency within individuals to levels of some more extensively studied risk factors such as blood cholesterol concentration and blood pressure.2,3 Moreover, highly sensitive assays for C reactive protein and serum amyloid A protein are now available that can detect low grade inflammation that would previously have been unnoticed.4,5
Several reports have suggested that plasma C reactive protein and other possible markers of low grade inflammation can predict increased risks of coronary heart disease, but it is not known whether the associations are causal.2,6 A variety of mechanisms by which C reactive protein might directly promote vascular disease have been proposed,7 but none is proved. These markers of inflammation might, however, be indicators of chronic infective processes possibly correlated with risk of coronary heart disease, such as infection by Chlamydia pneumoniae or chronic gastric infection with Helicobacter pylori.8 Alternatively, the markers might be mainly indicators of classic vascular risk factors (such as smoking and obesity) or of the extent of pre-existing disease (since atherosclerosis may be partly an inflammatory lesion6). If so, the markers themselves would not be of causal relevance to disease. Long term prospective studies with data collected on many possible confounders or mediators can help to distinguish among these different possibilities since this approach is generally less influenced than retrospective studies by any effect of pre-existing disease itself on the factors being investigated.9
We report one of the largest and most prolonged prospective studies of “inflammatory” factors and coronary heart disease so far, with updated meta-analyses of previous relevant studies to put our findings in context. We measured four circulating markers of inflammation (C reactive protein, serum amyloid A protein, leucocyte count, and albumin10), which should help to determine whether there is some underlying process related to inflammation (rather than any one marker by itself) that might be relevant to disease. Most previous studies could not make such assessments because they typically reported on only one marker of inflammation.
Methods
Cases and controls
During 1978-80, 7735 men aged 40-59 (response rate 78%) were randomly selected from general practice registers in each of 24 British towns. Nurses administered questionnaires, made physical measurements, recorded an electrocardiogram, and, for 5661 men in 18 of the towns, collected non-fasting venous blood samples, which were stored at −20°C for subsequent analysis.11 Additional questionnaires on car ownership and childhood social circumstances (father's social class and childhood household amenities) were posted to participants five years (98% response among survivors) and 12 years (90% response among survivors) after entry.
All men have been monitored subsequently for all cause mortality and for cardiovascular morbidity, with a loss to follow up of less than 1% to date. A total of 507 men with available samples had major coronary heart disease events between the beginning of follow up and December 1995 (223 deaths and 284 non-fatal myocardial infarctions). Deaths from coronary heart disease were ascertained through NHS central registers on the basis of a death certificate with ICD-9 codes 410-414, and the diagnosis of non-fatal myocardial infarction was based on reports from general practitioners, supplemented by regular reviews of general practice records, and diagnosed in accordance with World Health Organization criteria. We selected 1026 controls who were “frequency” matched to cases on town of residence and age in five year bands from among men who had survived to the end of the study period without a myocardial infarction. One potential case and one potential control were found not to have a serum sample available. In previous interim analyses data were reported on albumin concentration and coronary heart disease in only 97 of the 507 cases included here12 and on leucocyte count and coronary heart disease in only 235 cases.13
Laboratory methods
Laboratory workers blind to the case-control status of participants measured concentrations of C reactive protein and serum amyloid A protein using sensitive enzyme immunoassays.4,5 The C reactive protein assay was standardised on the WHO international reference standard, and the results of the serum amyloid A protein assay were used to establish the WHO international reference standard for this protein.14 Albumin was measured with bromocresol green, and leucocyte counts were done with Coulter counters at the time of blood collection.11 The coefficients of variation within each of these assays was 2-4%; the variation between the assays was about 6-8%. Measurements were also made of concentrations of serum IgG antibodies to Helicobacter pylori (by enzyme linked immunoassay, Meridian Diagnostics, Cincinatti, Ohio), IgG antibodies to Chlamydia pneumoniae (whole organism antigen by time resolved fluorimetry15) and serum lipids, plasma homocysteine, glucose, insulin, and markers of renal function (by standard assays11).
Statistical methods
Case-control comparisons were made by unmatched stratified logistic regression fitted by unconditional maximum likelihood (Stata Corporation, Texas, USA). Analysis of concentrations of C reactive protein, serum amyloid A, and albumin and of leucocyte count was prespecified to be by thirds of values in the controls. Adjustment was made for age; cigarette smoking (never, former, current); daily cigarette consumption; non-fasting blood concentrations of total cholesterol, high density lipoprotein cholesterol, and triglycerides; current social class (registrar general's 1980 classification with separate category for armed forces11); housing tenure (owner, private rent, council rent); marital status; current car ownership; and father's occupation (manual, non-manual), family car ownership, bathroom in house, hot water tap in house, and bedroom sharing. H pylori seropositivity, C pneumoniae IgG titres, and concentrations of non-fasting blood lipids, plasma homocysteine, and other blood components were investigated as possible correlates of levels of each of the acute phase reactants.
We have previously described the methods for meta-analyses of prospective studies of non-fatal myocardial infarction or death from coronary heart disease with over a year's follow up.2 The meta-analysis was updated to include all studies identified with data available in March 2000. Most studies related risk of coronary heart disease to baseline measurement of risk factors, even though the levels of each factor can fluctuate within individuals over time. Our comparisons of those in the top third of baseline values with those in the bottom third have therefore been corrected for such regression dilution16 by use of self-correlation coefficients derived from other studies.2 Results of studies were combined by using inverse variance weighted averages of log odds ratios. Heterogeneity was assessed by standard χ2 tests. Odds ratios are given with 95% confidence limits, and two sided probability values are used.
Results
The mean age at coronary heart disease event among cases was 62 years (mean duration of follow up 9.5 years). There were highly significant differences between cases and controls with respect to known vascular risk factors such as smoking, obesity, blood pressure, and blood lipids concentrations (table 1).
Table 1.
Baseline characteristics of men with coronary heart disease and of controls matched for age and town. Values are mean (SD) unless stated otherwise
| Characteristic | Cases (n=507) | Controls (n=1026) | P value |
|---|---|---|---|
| Questionnaire | |||
| Age (years) | 52.2 (5.3) | 52.2 (5.3) | Matched |
| No (%) current smokers | 268 (53) | 436 (42) | <0.0001 |
| No (%) with evidence of coronary disease* | 177 (35) | 204 (20) | <0.0001 |
| No (%) with treated diabetes | 12 (2) | 15 (1) | NS |
| No (%) >2 drinks alcohol/day | 110 (22) | 232 (23) | NS |
| No (%) in non-manual occupation | 112 (22) | 280 (27) | 0.03 |
| No (%) of home owners† | 275 (64) | 667 (69) | 0.03 |
| Physical measurements | |||
| Body mass index (kg/m2) | 25.8 (3.4) | 25.3 (3.3) | 0.008 |
| Height (cm) | 171 (6.4) | 172 (6.5) | 0.002 |
| Weight (kg) | 76.3 (11.4) | 75.8 (11.2) | NS |
| Systolic blood pressure (mm Hg) | 151 (21) | 147 (21) | <0.0001 |
| Diastolic blood pressure (mm Hg) | 86 (14) | 83 (13) | <0.0001 |
| Forced expiratory volume (l) | 308 (68) | 326 (77) | <0.0001 |
| Blood sample | |||
| Total cholesterol (mmol/l) | 6.63 (1.10) | 6.20 (0.99) | <0.0001 |
| HDL cholesterol (mmol/l) | 1.10 (0.28) | 1.15 (0.29) | 0.0003 |
| Triglyceride (mmol/l) | 2.26 (1.33) | 1.93 (1.22) | <0.0001 |
Evidence of ischaemia on baseline electrocardiogram or reported history of angina or myocardial infarction.
Information on home ownership was available for only 431 cases and 964 controls.
Associations among different acute phase reactants
In controls without evidence of coronary heart disease at baseline, the correlation coefficients of the associations between plasma C reactive protein and serum amyloid A protein, leucocyte count, and albumin were 0.58, 0.33, and −0.19 respectively (P<0.0001 for each), and the correlation coefficients between serum amyloid A protein and leucocyte count and albumin were 0.19 and 0.14 respectively (P<0.0001 for each). These associations among the four acute phase reactants were not adjusted for values of one another to avoid overadjustment since they may reflect a common underlying process (see tables on BMJ 's website). Leucocyte count and serum albumin concentration were not strongly related (R=−0.06, P=0.08).
Acute phase reactants, classic vascular risk factors, and other characteristics
C reactive protein concentration was strongly associated with cigarette smoking, body mass index, and low forced expiratory volume in one second (P<0.0001 for each), and the associations were little changed by adjustments for age, town, smoking, and indicators of socioeconomic status. Associations of C reactive protein with high density lipoprotein cholesterol, triglyceride, urate, and insulin, however, weakened substantially after such adjustments (table A on BMJ 's website). There were strong and highly significant associations of serum amyloid A protein with body mass index and total cholesterol (P<0.0001) and with low forced expiratory volume (P<0.001) which remained significant after adjustment for possible confounders, but such adjustment substantially weakened the association with high density lipoprotein cholesterol (table B on website). Strong adjusted associations were observed between leucocyte count and cigarette smoking and packed cell volume (P<0.0001 for each, table C on website) and between low albumin and age and cigarette smoking (P<0.0001 for each, table D on website) and between albumin and blood pressure, total cholesterol, and packed cell volume (P<0.0001 for each). Possible associations existed between albumin and high density lipoprotein cholesterol, triglycerides, and serum markers of renal function. No consistent associations were observed of these acute phase proteins with H pylori seropositivity, C pneumoniae IgG titres, plasma total homocysteine concentrations, or a range of childhood or adult indicators of socioeconomic status.
Acute phase reactants and incident coronary heart disease
The odds ratio for coronary heart disease was 3.46 (95% confidence interval 2.59 to 4.62; χ2 =71, df=1) in men in the top third compared with those in the bottom third of baseline C reactive protein concentration (tertile cut offs, >2.4 v <0.9 mg/l). The odds ratio fell to 2.13 (1.38 to 3.28;χ2 =11.8, df=1) after smoking, vascular risk factors, and indicators of socioeconomic status were adjusted for (table 2). Comparisons between the top and bottom thirds of the other factors gave the following adjusted odds ratios for coronary heart disease: 1.65 (1.07 to 2.55) for serum amyloid A protein (>9 v <6 mg/l); 1.12 (0.71 to 1.77) for leucocyte count (>7.8 v 6.4×109/l); and 0.67 (0.43 to 1.04) for albumin (>46 v <44 g/l). These results were not materially changed in analyses restricted to the 329 cases and 820 controls with no evidence of coronary heart disease at baseline (table 2) or in analyses restricted to the 227 cases and 779 controls who had complete information on childhood socioeconomic status. The findings were also unaffected by varying the prespecified cut-off levels for analysis of each factor.
Table 2.
Odds of coronary heart disease in men who had values of inflammatory factors in top third of distribution of controls relative to those who had values in bottom third of this distribution
| Factor (tertile cut offs) | No of cases
|
No of controls
|
Odds ratio (95% CI) adjusted for:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Top | Middle | Bottom | Top | Middle | Bottom | Age and town | Age, town, and smoking | Age, town, smoking, and risk factors* | Age, town, smoking, risk factors, socioeconomic status† | |||
| All 506 cases and 1025 controls | ||||||||||||
| C reactive protein (2.4 and 0.9 mg/l) | 268 | 150 | 88 | 347 | 298 | 380 | 3.46 (2.59 to 4.62) | 3.32 (2.47 to 4.47) | 2.92 (2.13 to 4.01) | 2.13 (1.38 to 3.28) | ||
| Serum amyloid A protein (9 and 6 mg/l) | 255 | 144 | 107 | 362 | 351 | 312 | 2.12 (1.60 to 2.80) | 2.13 (1.61 to 2.82) | 1.66 (1.22 to 2.26) | 1.65 (1.07 to 2.55) | ||
| Leucocyte count (7.8 and 6.4×109/l) | 212 | 168 | 110 | 332 | 329 | 330 | 1.89 (1.43 to 2.51) | 1.69 (1.25 to 2.28) | 1.46 (1.06 to 2.00) | 1.12 (0.71 to 1.77) | ||
| Albumin (46 and 44 g/l) | 174 | 141 | 191 | 348 | 347 | 327 | 0.83 (0.63 to 1.10) | 0.90 (0.68 to 1.20) | 0.61 (0.44 to 0.83) | 0.67 (0.43 to 1.04) | ||
| 329 cases and 820 controls without evidence of coronary heart disease at entry | ||||||||||||
| C reactive protein (2.4 and 0.9 mg/l) | 164 | 95 | 70 | 265 | 234 | 321 | 2.93 (2.10 to 4.09) | 2.74 (1.95 to 3.85) | 2.61 (1.81 to 3.77) | 2.31 (1.42 to 3.76) | ||
| Serum amyloid A protein (9 and 6 mg/l) | 163 | 87 | 79 | 284 | 288 | 248 | 1.90 (1.37 to 2.64) | 1.93 (1.38 to 2.68) | 1.50 (1.05 to 2.16) | 1.67 (1.02 to 2.74) | ||
| Leucocyte count (7.8 and 6.4×109/l) | 142 | 104 | 71 | 253 | 261 | 277 | 2.19 (1.56 to 3.07) | 1.87 (1.30 to 2.68) | 1.57 (1.07 to 2.30) | 1.34 (0.80 to 2.27) | ||
| Albumin (46 and 44 g/l) | 115 | 95 | 118 | 282 | 266 | 269 | 0.90 (0.64 to 1.26) | 1.0 (0.71 to 1.41) | 0.65 (0.45 to 0.95) | 0.75 (0.45 to 1.25) | ||
Blood pressure, total cholesterol, high density lipoprotein cholesterol, triglycerides, body mass index
Occupation, housing tenure, marital status, car ownership, and childhood socioeconomic factors (father's occupation, family car ownership, bathroom in house, hot water tap in house, bedroom sharing, height).
Analyses of acute phase reactants and coronary heart disease were not adjusted for values of other inflammatory markers to avoid possible overadjustment (see results).
Discussion
Our prospective study in the general British male population shows that baseline values of four acute phase reactants are associated with one another as well as with future risk of coronary heart disease. These data support the idea that there are some underlying processes related to inflammation that are relevant to coronary heart disease. To determine what factor(s) might be triggering such low grade inflammation years in advance of disease, we studied classic risk factors, plasma homocysteine concentration, serological evidence of chronic infection with C pneumoniae or H pylori, and a range of possible other triggers. None, however, was strongly related to inflammatory markers, suggesting that these factors cannot fully account for the associations observed with coronary heart disease. Even though the strength of the association of coronary heart disease with baseline concentrations of C reactive protein and serum amyloid A protein seems comparable with that for some more extensively studied risk factors (such as blood fibrinogen concentration2), several uncertainties remain. We discuss below the strengths and limitations of the available evidence on coronary heart disease and each of these factors in the context of updated meta-analyses of other relevant studies.
C reactive protein
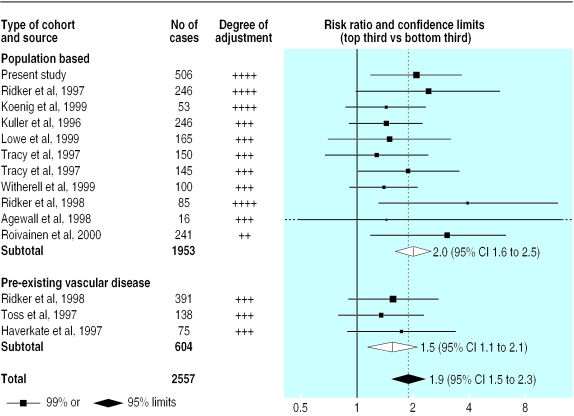
A previous meta-analysis of seven prospective studies of C reactive protein, with a total of 1053 cases, indicated a risk ratio for coronary heart disease of 1.7 (95% confidence interval 1.4 to 2.1) in individuals in the top third compared with those in the bottom third of baseline measurements.2 Since the publication of that review, six additional prospective studies of C reactive protein have been identified, including an additional 994 cases of non-fatal myocardial infarction or death from coronary heart disease.17–22 All used sensitive assays and adjusted for smoking and some standard vascular risk factors. In aggregate, the 14 available prospective reports (including the present study of 506 cases) include 2557 cases with a weighted mean age at entry of 58 and weighted mean follow up of eight years.17–28 There was no significant heterogeneity among them (χ2=6.9, df=13; P>0.1), and a comparison of individuals with C reactive protein concentrations in the top third with those in the bottom third at baseline gave a combined risk ratio of 1.9 for coronary heart disease (1.5 to 2.3; fig 1) with a similar risk ratio of 2.0 (1.6 to 2.5) in the 11 studies of the general population (test for heterogeneity, χ2=5.8, df=10; P>0.1). The estimated mean usual log C reactive protein values in the top third and bottom thirds were 0.38 and 0.02 mg/l in the general population, which correspond to mean estimated usual values of 2.4 and 1.0 mg/l. Hence, this updated meta-analysis indicates a similar risk ratio to the previous review, but the scope for the play of chance is now substantially reduced because there are more than twice as many cases reported.
Figure 1.
Prospective studies of C reactive protein and coronary heart disease. Risk ratios compare top and bottom thirds of baseline measurements. Black squares indicate the risk ratio in each study, with the size of square proportional to number of cases. The combined risk ratio and its 95% confidence interval are indicated by unshaded diamonds for subtotals and shaded diamond for total. All studies adjusted for age, sex, smoking, and some other standard vascular risk factors; the first three studies also adjusted for markers of socioeconomic status
For two main reasons, however, it remains uncertain whether C reactive protein is an independent risk factor for coronary heart disease. Firstly, the odds ratio in our study was reduced from 3.46 to 2.13 after baseline confounding factors were adjusted for. This substantial reduction suggests that more exact adjustment for the usual long term concentrations of these and other factors might produce a greater reduction—for example, we did not measure blood fibrinogen concentration. Secondly, although experimental studies suggest that C reactive protein might directly contribute to vascular damage (such as its frequent detection in atherosclerotic plaques,29 its ability to stimulate tissue factor production by macrophages,30 and its enhancement of complement activation after binding partly degraded, non-oxidised low density lipoprotein cholesterol31,32), no direct evidence exists for such involvement.7 Additional studies are also needed to determine the relevance of apparently sustained falls in C reactive protein concentrations after the use of lipid lowering statin drugs3 as well as apparently sustained increases after postmenopausal hormone replacement therapy.33
Serum amyloid A protein
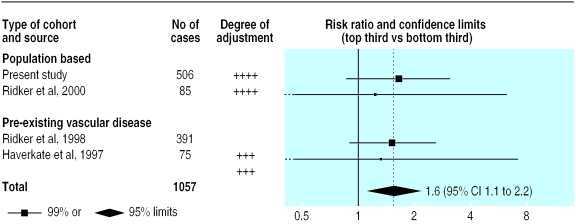
Only three previous studies have reported on serum amyloid A protein and incident coronary heart disease,22,28,34 including two cohorts defined on the basis of pre-existing coronary heart disease. They comprised 551 cases in total (fig 2). Our 506 cases in the general population almost double the available evidence. In aggregate, these four studies include 1057 cases, with a weighted mean age at entry of 56 and weighted mean follow up of 10 years. There was no significant heterogeneity among them (χ2=0.3, df=3; P>0.1), and a comparison of individuals with values in the top third with those in the bottom third at baseline gave a combined risk ratio of 1.6 for coronary heart disease (1.1 to 2.2; fig 2). The estimated mean usual log values in these groups were 1.00 and 0.68 mg/l, which correspond to mean values of 10.0 and 4.7 mg/l. Further studies are needed to determine whether this association is independent of possible confounders.
Figure 2.
Prospective studies of serum amyloid A protein concentration and coronary heart disease. Risk ratios compare top and bottom thirds of baseline measurements. Black squares indicate the risk ratio in each study, with the size of square proportional to number of cases. All studies adjusted for age, sex, smoking, and some other standard vascular risk factors; our study also adjusted for markers of socioeconomic status
Leucocyte count and serum albumin
The adjusted odds ratio for coronary heart disease in our study was 1.12 (0.71 to 1.77) in men with leucocyte counts in the top third compared with those in the bottom third of baseline measurements. Although this odds ratio is not significant, it is consistent with a combined risk ratio for coronary heart disease of 1.4 (1.3 to 1.5) reported in a previous meta-analysis of 19 prospective studies of leucocyte count including a total of 7229 cases, with top and bottom thirds corresponding to mean usual leucocyte counts of 8.4 and 5.6×109/l, respectively.2 We also observed an adjusted odds ratio for coronary heart disease of 0.67 (0.43 to 1.04) in men in the top third compared with those in the bottom third of baseline albumin measurements. Again, despite a lack of conventional significance, this odds ratio is compatible with a combined risk ratio of 0.7 (0.6 to 0.8) reported in a meta-analysis of eight prospective studies including a total of 3770 cases, with top and bottom thirds corresponding to mean usual albumin concentrations of 42 and 38 g/l, respectively.2 The strongly positive associations of serum albumin values with blood pressure and serum lipid concentrations indicate that these variables cannot account for the association between low serum albumin and coronary heart disease—rather the reverse in fact.
What is already known on this topic
Plasma concentrations of C reactive protein and other sensitive markers of systemic inflammation may be correlated with future risk of coronary heart disease in the general population
It is not known whether these associations are causal or merely due to confounding by classic risk factors, chronic infective processes, or early disease
What this study adds
Baseline values of C reactive protein, serum amyloid A protein, leucocyte count, and serum albumin were associated with one another as well as with future risk of coronary heart disease
Values of these factors were not associated with markers of chronic infective processes
These findings suggest that low grade inflammatory processes may be relevant to coronary heart disease
Conclusions
We found that baseline levels of four circulating markers of low grade inflammation were associated with one another and with future risk of coronary heart disease. The markers, however, were not associated with some chronic infective processes possibly related to coronary heart disease. These findings—in the context of results from other relevant studies—suggest that low grade inflammatory processes may be relevant to coronary heart disease.
Supplementary Material
Acknowledgments
We thank H Refsum and P Ueland for the homocysteine assays; M Thomas, Yuk-ki Wong and M Ward for C pneumoniae serology; J Atherton and C Hawkey for H pylori serology; and J John for valuable help. Professor AG Shaper established the British Regional Heart Study
Editorial by Koenig
Footnotes
Funding: The British Regional Heart Study is a British Heart Foundation research group and also receives support from the Department of Health. JD is supported by Merton College and the Frohlich Trust. MBP is supported by the UK Medical Research Council.
Competing interests: None declared.
Tables showing distribution of risk factors in the control population are available on the BMJ's website
References
- 1.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive and related proteins (pentaxins) and serum amyloid A protein. Adv Immun. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 2.Danesh J, Collins R, Appleby P, Peto R. Fibrinogen, C-reactive protein, albumin or white cell count: meta-analyses of prospective studies of coronary heart disease. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins J, Gallimore JR, Moore EG, Pepys MB. Rapid automated high sensitivity enzyme immunoassay of C-reactive protein. Clin Chem. 1998;44:1358–1361. [PubMed] [Google Scholar]
- 5.Wilkins J, Gallimore JR, Tennent GA, Hawkins PN, Limburg PC, van Rijswijk MH, et al. Rapid automated enzyme immunoassay of serum amyloid A. Clin Chem. 1994;40:1284–1290. [PubMed] [Google Scholar]
- 6.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–121. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Lagrand WK, Visser CA, Hermens W, Niessen WH, Verheugt FW, Wolbink GJ, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- 8.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J. Smouldering arteries? Low-grade inflammation and coronary heart disease. JAMA. 1999;282:2169–2171. doi: 10.1001/jama.282.22.2169. [DOI] [PubMed] [Google Scholar]
- 10.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–455. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 11.Shaper AG, Pocock SJ, Walker M, Cohen NM, Wale CJ, Thomson AG. British regional heart study: cardiovascular risk factors in middle-aged men in 24 towns. BMJ. 1981;283:179–186. doi: 10.1136/bmj.283.6285.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;ii:1434–1436. doi: 10.1016/s0140-6736(89)92042-4. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AN, Neaton JD, Cook DG, Grimm RH, Shaper AG. Leukocyte count and risk of coronary heart disease. Am J Epidemiol. 1992;136:59–70. doi: 10.1093/oxfordjournals.aje.a116421. [DOI] [PubMed] [Google Scholar]
- 14.Poole S, Walker D, Gaines Das RE, Gallimore JR, Pepys MB. The first international standard for serum amyloid A protein (SAA). Evaluation in an international collaborative study. J Immunol Methods. 1998;214:1–10. doi: 10.1016/s0022-1759(98)00057-x. [DOI] [PubMed] [Google Scholar]
- 15.Wong Y-K, Sueur JM, Fall CHD, Orfila J, Ward ME. The species specificity of the microimmunofluorescence antibody test and comparisons with a time resolved fluoroscopic immunoassay for measuring antibodies against Chlamydia pneumoniae. J Clin Pathol. 1999;2:99–103. doi: 10.1136/jcp.52.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 17.Lowe GDO, Rumely A, Sweetnam PM, Yarnell JWG. Fibrin D-dimer, C-reactive protein and risk of ischaemic heart disease: the Speedwell study. Blood Coagul Fibrin. 1999;10 (suppl 1):S92–S93. [Google Scholar]
- 18.Agewall S, Wikstrand J, Fagerberg B. Prothrombin fragment 1+2 is a risk factor for myocardial infarction in treated hypertensive men. J Hypertension. 1998;16:537–541. doi: 10.1097/00004872-199816040-00016. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 20.Witherell HL, Smith KL, Ley C, Freidman GD, Orentreich N, Vogelman JH. Helicobacter pylori infection, C-reactive protein, and risk for myocardial infarction: a prospective study. Gastroenterology. 1999;116:A355. [Google Scholar]
- 21.Roivainen M, Viik-Kajarnder M, Palosuo T, Sacks F, Braunwald E. Infections, inflammation, and the risk of coronary heart disease. Circulation. 2000;101:257. doi: 10.1161/01.cir.101.3.252. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Rifai N, Pfeffer MA, Toivanen P, Leinonen M, Saikku P, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. New Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 24.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA-Augsburg cohort study, 1984-1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Am J Epidemiol. 1996;144:537–547. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 26.Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly: results from the cardiovascular health study and the rural health promotion project. Arterioscler Thromb Vasc Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 27.Toss H, Lindahl B, Siegbahn A, Wallentin L. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. Circulation. 1997;96:4204–4210. doi: 10.1161/01.cir.96.12.4204. [DOI] [PubMed] [Google Scholar]
- 28.Haverkate F, Thompson SG, Pyke SDM, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YX, Cliff WJ, Schoefl GI, Higgins G. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375–379. doi: 10.1016/s0021-9150(99)00105-7. [DOI] [PubMed] [Google Scholar]
- 30.Cermak JC, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- 31.de Beer FC, Soutar AK, Baltz ML, Trayner I, Feinstein A, Pepys MB. Low density and very low density lipoproteins are selectively bound by aggregated C-reactive protein. J Exp Med. 1982;156:230–242. doi: 10.1084/jem.156.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhakdi S, Torzewski M, Klouche M, Hemmes M. Complement activation and atherogenesis. Binding of CRP to degraded, nonoxidised LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999;19:2348–2354. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- 33.Cushman M, Legault C, Barrett-Connor EL, Stefanick ML, Kessler C, Judd HL, et al. Effect of postmenopausal hormones on inflammation-sensitive proteins: the postmenopausal estrogen/ progestin interventions (PEPI) study. Circulation. 1999;100:717–722. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Hennekens CH, Buring JE, Rifai N. C reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.