Abstract
Event-related oscillations (EROs) are rhythmic changes that are evoked by sensory and/or cognitive processes that influence the dynamics of the EEG. EROs are estimated by a decomposition of the EEG signal into phase and magnitude information for a range of frequencies and then changes in those frequencies are characterized over a millisecond time scale with respect to task events. EROs have been demonstrated to be sensitive measures of both normal and abnormal cognitive functioning in humans but have not been fully described in mice. The results of these studies demonstrate that EROs can be generated in cortical sites in mice in the delta, theta, alpha/beta frequency ranges in response to auditory stimuli. Oscillations in the 7.5-40 Hz frequencies were significantly affected in the 0-50 msec time range in response to differences in tone frequency. Whereas, changes in tone loudness produced changes in oscillations in the 7.5-40 Hz frequencies in the 350-800 msec range. No significant changes in EROs were found to differences in tone probability. These studies suggest that EROs are an electrophysiological assay sensitive to tone characteristics and as such may be suitable for the exploration of the effects of genetic or neuropharmacological manipulations on neurosensory processing in mice.
Keywords: Auditory system, EEG, ERO, ERPs, Mice
INTRODUCTION
Event-related potentials (ERPs) are a series of negative and positive voltage deflections that are time locked typically to either sensory or cognitive events. They consist of several components that are averaged from the ongoing EEG that generally occur between 50 and 1000 msec. It has been suggested that certain ERP components, like the P300, are perhaps not the unitary emergent processes suggested by earlier authors; but that they may influence oscillatory changes within the dynamics of ongoing EEG rhythms (see: Basar-Eroglu & Basar, 1991; Schurmann et al., 1995, 2001; Yordanova & Kolev,1996; Karakas et al., 2000a, b; Demiralp et al., 2001). This synchronization or enhancement of ongoing EEG oscillations by a time locked cognitive and/or sensory process is termed an event-related oscillation (see Basar et al., 2000; Begleiter & Porjesz, 2006; Roach & Mathalon, 2008). EROs are thought to arise by a “phase re-ordering” of the background EEG in several frequency bands (Basar, 1980; Makeig et al., 2002). EROs are typically estimated by a decomposition of the EEG signal into phase and magnitude information for a range of frequencies and then changes in those frequencies are characterized over a millisecond time scale with respect to task events.
Event-related oscillations over the spectral range of the EEG (1-50 Hz) have been suggested to underlie a number of different cognitive processes. For instance, event-related alpha oscillations have been attributed to attentional resources, semantic memory, and stimulus processing (Klimesch et al., 1994, 1997a, b; Basar et al., 1997), whereas, beta and gamma oscillations have been associated with sensory integrative processes (Schurmann et al., 1997; Basar et al., 2001a, b). Oscillations in the delta and theta frequency ranges have been associated with signal detection, decision-making, conscious awareness, recognition memory and episodic retrieval (Klimesch et al., 1994, 2001; Doppelmayr et al., 1998; Gevins et al., 1998; Basar et al., 1999, 2001c, d; Schurmann et al., 2001). It has suggested that high frequency oscillations (above 30 Hz) reflect synchronization of neuronal ensembles that are interacting over short distances in response to primarily sensory processes (Bressler & Freeman, 1980; Ohl et al., 2003), whereas, lower frequency oscillations (1-4 Hz) are generated by synchronization of ensembles interacting at longer distances during higher cognitive processing (see Lubar, 1997; Kopell et al., 2000).
Over the last decade animal models of long latency event-related potentials have been developed in order to further explore the behavioral, neuroanatomical and neurochemical basis of the ERP components (see O’Brien, 1982; Harrison & Buchwald, 1985; Paller et al., 1988; Takeuchi et al., 2000). With the rapid advances in molecular genetic techniques many investigators are using mice as subjects. The use of “knock-outs”, transgenics, as well as inbred selected line approaches have established mouse models as very powerful in studies aimed at uncovering the neurobiological basis of behavior and cognitive function (Campbell, 1995; Wehner et al., 1996; Price et al., 1998; Brusa, 1999; Ingram & Jucker, 1999; Sturchler-Pierrrat & Sommer, 1999). While evoked potentials are commonly recorded in mice, ERP paradigms for use in generating late wave responses have been little reported (see Ehlers & Somes, 2002; Siegel et al., 2003; Umbricht et al., 2005). The present study was undertaken to further develop a model of mouse EROs using auditory stimuli. EROs were generated in a paradigm shown to produce P3-like ERP components (Ehlers & Somes, 2002). We previously demonstrated that changes in frequency and loudness of auditory stimuli modified N1 ERP components that occurred in the 50-100 msec range where as P3 components in the 200-400msec range were found to be sensitive to probability but not to tone frequency or loudness (Ehlers & Somes, 2002). The N1 component consists of the N1a component, a large negative component that peaks between 20 and 30 ms, and the N1b component, a broader negative component that peaks between 80 and 90 ms. The P3 component is a broad positive wave that peaks between 200 and 300 ms (Ehlers & Somes, 2002). The specific aim of this study was to evaluate the effects of changes in auditory stimulus probability, loudness, and frequency on mouse EROs.
MATERIALS AND METHODS
The experimental subjects were sixty-five male mice, 37 of the DBA/2 strain and 28 of the C57BL/6 strain obtained from The Scripps Research Institute breeding facility, weighing between 19-30 grams. Mice were housed 3-4 per cage in controlled temperature and lighting conditions, with food and water ad libitum, for the duration of the study. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1985) and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
The purpose of the study was to test the sensitivity of mouse EROs to changes in stimulus probability, frequency and loudness. To accomplish this aim, mice were implanted with stainless steel screws (Small Parts, Miami Lakes, FL; size: 1/16″ in length) in three sites in the calvarium under Halothane anesthesia (1.5-2 liters/min). One screw was placed over more frontal areas (1.5 mm lateral of midline, 1.7 mm anterior to bregma, area M1, primary motor), one over the parietal cortex (2mm lateral to midline,2 mm posterior to bregma, area PPtA, posterior parietal association area). A ground screw was also placed in the thick bony area of the calvarium 5 mm posterior to bregma which obliquely overlies the cerebellum. Each screw was secured to the skull using dental acrylic (Dual Cure Geristore by Den-Mat, Santa Maria, CA). Soldered to each of the three screws was a silver 34 gauge insulated wire (Cooner wire, Chatsworth, CA), to which a pin contact (amphenol) was crimped. The three pins were adhered together with dental cement at an angle perpendicular to the saggital suture and shrink wrap was applied around each pin. Additional dental cement was applied outside the shrink wrap to secure it to the skull. Mice were then returned to their home cage which was housed within a recording chamber and allowed 3-9 days to recover before recording procedures.
Recording Procedures
For ERO recordings, all animals were in electrically shielded light, sound and temperature controlled recording chambers where the animals are unrestrained but are spatially restricted. EEG signals were recorded on a polygraph. The bandpass for recordings was set at 0.3 - 35 Hz with a 60 Hz notch filter in. The signals were amplified (75 microvolts/mm) and the EEG, as well as calibration signals, were transferred from the Nihon-Kohden polygraph on-line to a Macintosh computer which also controlled the presentation of the auditory stimuli. Free field auditory stimuli were presented through a small speaker centered approximately 18 cm above the mouse’s head. Five minutes of resting, awake EEG was additionally collected from each mouse.
Event-Related Oscillations
EROs were elicited with acoustic oddball paradigms, these paradigms are similar to those demonstrated to successfully generate ERPs in human, monkey, rat, and mouse studies (Ehlers, 1988; Kaneko et al., 1996; Ehlers et al., 1999; Ehlers & Somes, 2002). The tones were generated by a programmable multiple-tone generator, the characteristics of which have been described previously (Polich et al., 1983). Three different stimulus change paradigms were used. In these paradigms the loudness of the tones, the frequency of the tones, and the probability of their occurrence, were varied. This allowed for the assessment of how changes in characteristics of the auditory stimulus would modify EROs at the two brain sites. The stimulus characteristics for the three paradigms were: paradigm (1) standard tone: 80% probability, 1kHz frequency, 70 dBSPL loudness, rare tone: 20% probability, 2kHz frequency 70 dBSPL loudness; paradigm (2) standard tone: 50% probability, 1kHz frequency, 70 dBSPL loudness, rare tone: 50% probability, 2kHz frequency 70 dBSPL loudness; paradigm (3) standard tone: 80% probability, 1kHz frequency, 70 dBSPL loudness, rare tone: 20% probability, 2kHz frequency 85 dBSPL loudness.
In all paradigms, rare tones were interspersed with standard tones such that no two rare tones occurred successively. The digitizing epoch for each trial was one second and a .5 - 1 second intertrial interval was used (to reduce habituation). In the 3 paradigms, 2 tones (standard, rare) were presented and the total number of trials in a recording session was 150. Animals were only tested on a single paradigm per test day and at least 24 hours lapsed between testing sessions. The order of presentation of each paradigm over time as well as which paradigm each mouse participated in was randomized. There were equivalent numbers of DBA’s and C57’s in all paradigms. Each mouse participated over a 4-week period. Mice were between 10 and 14 weeks of age at the time of the electrophysiological recordings.
Event-Related Oscillation Analysis
The ERO trials were digitized at a rate of 256 Hz. Trials containing excessive artifact were eliminated prior to averaging (<5% of the trials). An artifact rejection program was utilized to eliminate individual trials in which the EEG exceeded ± 400 μV. ERO analyses were accomplished from the same datasets that were used to generate the ERP data reported in previous publications (Ehlers & Somes, 2002). Since previously published ERP data suggested limited differences in morphology between frontal and parietal sites, only frontal sites were used to analyze ERO data. Data from single trials generated by the stimuli were entered into the time frequency analyses algorithm. The S-transform (ST), a generalization of the Gabor transform (Gabor, 1946), was used (Stockwell et al., 1996).
The S transform mathematically resembles the continuous wavelet transform but it uses Gaussian windows which do not meet a requirement of wavelet analysis, and it includes a “phase correction” that is not part of wavelet analysis. The actual use of the S transform was simplified by performing first a forward Fourier transform of the time series. Then, for each frequency of the Fourier transform, summing the results of multiplication by a set of Fourier transforms of Gaussian windows of varying width. Finally, for each of these sums, taking the inverse Fourier transform. The equation for calculation of the S transform of discrete time series h(kT) at time jT and frequency n/NT is where T is the sample period of the discrete time series, j is the sample index, N is the number of samples in the time series, n is the frequency index, and H[ ] is the Fourier spectrum of the discrete time series. The S-transform results in a time-frequency representation of the data. The exact code we used is a C language, S transform subroutine available from the NIMH MEG Core Facility web site (http://kurage.nimh.nih.gov/meglab/). This code is specifically for use with real time series, so it sets the input imaginary values, required by the S transform, to zero, and it always uses the Hilbert transform so that each of the complex output time series is an analytic signal.
To reduce anomalies in the S transform output at the beginning and the end of the output time series, we used a Hanning window over the initial and final 100 msec of the input time series. The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time-frequency energy distributions. To quantify S transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI. Total volume was determined as the measure of the energy values in the ROI. These analyses are similar to what has been previously described (Jones et al., 2004). Total volume was defined as the volume of the ROI in energy summed over time and frequency. The ROI frequencies were: delta (1-4 Hz), theta (4-8 Hz), and alpha/beta (8-35 Hz). The ROI time intervals correspond to the P1/N1 ERP component (0 - 50 ms), N2/P3a components (50 - 350 ms) and the P3b component (350-800 ms). ROI frequencies and time intervals are consistent with our previous ERP studies (Ehlers & Somes, 2002; Slawecki et al., 2003).
Statistical Analysis
Analysis of variance (ANOVA) was utilized to statistically evaluate the data. In order to compare differences between the total volume in the 3 regions of interest for the three frequency ranges following the presentation of the three different stimulus paradigms, a within subjects design was utilized. To account for multiple comparisons, p-value was set at p< 0.01 to determine the levels of statistical significance.
RESULTS
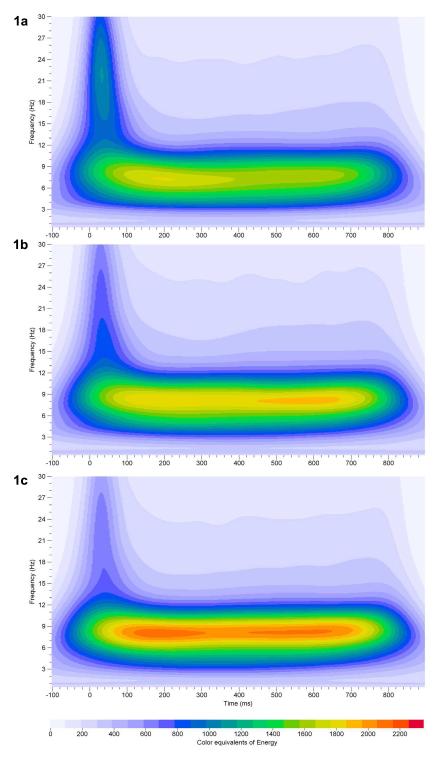
Changes in total volume in the frontal cortex were estimated for regions of interest in the three frequencies bands in three stimulus paradigms where the frequency of the tone, the loudness of the tone and the probability of the tone occurring were varied. Results of these analyses are presented in figure 1. In the first stimulus paradigm, the rare tone occurred 20% of the time and the standard (Freq=1 kHz) and rare (Freq=2 kHz) tones were of equivalent loudness In this paradigm there was a significant volume difference found between the standard and rare tones within a specific frequency range and region of interest. Following the rare tone a reduction in total volume of the 50-350 ms region of interest in the 0.75-3.5 Hz frequencies was found (rare tone= 320 ± 130, standard tone= 338 ± 125; F=7.2, df=1,64, p<0.009). This reduction in total volume may be related to the N1a component as previously published data has demonstrated that the N1a component of the ERP recorded at this site in this latency range is significantly larger following the standard tone when compared to the rare, whereas the N1b and the P3 components are larger following the rare tone (Ehlers & Somes, 2002).
Figure 1.
Grand averages of event—related oscillations (EROs) across 64 mice recorded from frontal cortex. Time-frequency representation of evoked delta, theta and alpha/beta bands energy distribution of rare tone in three different stimulus paradigm conditions. In Fig. 1a the loudness of the rare tone was 85 dBSPL occurring 20% of the time with a frequency of 2 kHz (paradigm 3). In Fig. 1b the loudness of the rare tone was 70 dBSPL occurring 20% of the time with a frequency of 2 kHz (paradigm 1). In Fig. 1c the loudness of the rare tone was 70 dBSPL occurring 50% of the time with a frequency of 2 kHz (paradigm 2). A significant increase in the total volume in the 0-50 msec region of interest and a significant decrease in the 350-800 msec region of interest in the 7-34 Hz frequency band was found in Fig. 1a (paradigm 3), compared to Fig. 1b (paradigm 1). Comparison of the total volume of ERO regions of interest between Fig. 1b (paradigm 1) and Fig. 1c (paradigm 2) did not show statistically significant results. In each graph ERO frequency (Hz) is presented on the Y-axis, time regions of interest on the X-axis (msec) and energy is presented as color equivalents as indicated on the bar at the bottom of the graph.
The second ERP stimulus paradigm differed from the first in that the probability of the presentation of the rare and standard tone was equivalent; this meant that the only difference in stimuli characteristics between the standard and rare tones was the frequency of the tone. Never-the-less a significant reduction was found in the total volume of the 0-50 ms region of interest in the 0.75-3.5 Hz frequencies in response to the rare tone as compared to the standard (rare tone=1004 ± 528, standard tone 1107 ± 526; F=28.8, df=1,53, p<0.0001). A comparison of the total volume of ERO regions of interest between the rare tone presented in paradigm 1 (20% probability) and that presented in paradigm 2 (50% probability), both of which had the same frequency and loudness, did not produce any statistically significant results.
In the third paradigm, the loudness of the rare tone was increased from 70 dBSPL to 85 dBSPL while all other parameters were kept equal to paradigm 1. This change in stimulus loudness was found to cause significant increases in the total volume of the rare tone in the 0-50 msec region of interest in the 7-34 Hz frequencies as compared to the standard (rare tone=1280 ± 575, standard tone 1117 ± 547; F=23.3, df=1,64, p<0.0001). A comparison of the total volume of ERO regions of interest between the rare tone presented in paradigm 1 (70 dBSPL, loudness) and that presented in paradigm 3 (85 dBSPL loudness), both of which had the same frequency and probability, revealed that a significant increases in the total volume of the rare tone in paradigm 3 in the 0-50 msec region of interest in the 7-34 Hz frequencies as compared to paradigm 1(rare tone paradigm 3=1280 ± 575, rare tone paradigm 1 1087 ± 626; F=9.8, df=1,64, p<0.003) and a decrease in the total volume of the 350-800 msec region of interest in the 7-34 Hz frequencies (rare tone paradigm 3=5007 ± 1962, rare tone paradigm 1=5785 ± 2554; F=9.9, df=1,64, p<0.002). Again, the reduction in total volume may be related to the N1a component as previously published data has demonstrated that the N1a component of the ERP recorded at this site in this latency range is significantly larger following the rare tone when compared to the standard for this paradigm; whereas, no changes were observed in the late positive components in this paradigm as a function of difference in stimulus loudness (Ehlers & Somes, 2002).
DISCUSSION
A number of investigators have used mice for subjects to explore sensory evoked potentials recorded from mouse cortex. These studies typically use auditory, visual or somatosensory stimuli and have primarily focused on early component responses (e.g. < 100msec) (see Mazzucchelli et al., 1995; Porciatti et al., 1999; Tebano et al., 1999; Frenkel et al., 2000; Troncoso et al., 2000; Metzger et al., 2007). More recent investigations extend that literature by providing evidence to suggest that several late ERP components, including a late positivity, can be obtained from mice that differ in their latencies, amplitudes, polarities and morphology depending on the recording site, and stimulus characteristics (see Ehlers & Somes 2002; Siegel et al., 2003; Umbricht et al., 2005). Like most ERP studies using rodents as subjects a “passive” oddball ERP paradigm has been used to generate ERPs in mice. The advantages of a passive paradigm are that it can be administered to human and other animal subjects without extensive prior training, and it does not require the subject to respond to the stimuli. Such paradigms have been particularly important in clinical studies of young children, or subjects with limited attentional capacity (see Jodo et al., 1995). Passive paradigms are also efficiently used in mouse studies where large numbers of subjects are necessary for instance, in the screening of drugs or testing line/sex differences (Slawecki et al., 2003; Connolly et al., 2004; Maxwell et al., 2004; Amann et al., 2008; Ehrlichman et al., 2008; Gandal et al., 2008; Rudnick et al., 2008).
While ERPs have been successfully recorded in a number of animal species the use of ERO technology to study brain function in animal models has been less applied. Schurmann et al.(2000) reported that event-related alpha oscillations could be observed in cats in response to visual stimuli in specific sensory pathways and also in the hippocampus. Evoked gamma oscillations have been reported in mice that were enhanced by nicotine and blocked by pretreatment with mecamylamine (Phillips et al., 2007). In the present report the effects of stimulus characteristics on the generation of EROs in the .5-35 Hz frequencies was evaluated. The results of these studies demonstrate that EROs can be generated in cortical sites in mice in the delta, theta, alpha/beta frequency ranges in response to auditory stimuli. Oscillations in the 7.5-40 Hz frequencies were significantly affected in the 0-50 msec time range in response to differences in tone frequency. Whereas, changes in tone loudness produced changes in oscillations in the 7.5-40 Hz frequencies in the 350-800 msec range. No significant changes in EROs were found to differences in tone probability. In previous investigations changes in frequency and loudness of auditory stimuli were found to modify N1 ERP components that occurred in the 10-50 msec (N1a) and 50-150 msec (N1b) ranges, whereas P3 components in the 200-400 msec range were found to be sensitive to probability, but not to tone frequency or loudness (Ehlers & Somes, 2002). For instance, while shifts upward in tone frequency produced decreases in N1a amplitude, it had no effect on the amplitude of the N1b component. Moreover, increasing the loudness of the tone produced an increase in the amplitudes of both N1a and N1b components. In contrast, mouse N1 components were not sensitive to changes in stimulus probability (Ehlers & Somes, 2002). Previous studies also found that P3 amplitudes were larger following the less probable or infrequently occurring tone, compared to the frequent tone, but were not affected by changes in tone frequency and loudness (Ehlers & Somes, 2002). These findings suggest that mouse EROs may be indexing different aspects of neurocognitive functioning than ERP components.
Molecular genetics techniques have provided the means for targeted germ line mutations and as a result a proliferation of mice with null mutations of specific proteins exists. Such animals provide the opportunity to further study the mechanisms underlying ERP/ERO components. In human studies EROs have been demonstrated to be important endophenotypes that have been linked to select genes as well as neuropsychiatric conditions such as disinhibition and alcoholism (see Jones et al.,2004; 2006; Kamarajan et al., 2004, 2006; Porjesz et al., 2005; Begleiter & Porjesz, 2006; Rangaswamy et al., 2007; Chen et al., 2008). While rodents with their limited cortical development do not simulate the human condition, nevertheless, the mouse ERO models can eventually provide endophenotypes that may be useful as animal models of a number of neuropsychiatric disorders.
ACKNOWLEDGMENTS
This work was supported by NIAAA grants RO1 AA006059 to Cindy L. Ehlers. The authors would like to thank Ms. Chris Somes, Mr. Philip Lau and Ms. Shirley Sanchez for help in data collection, data analyses and manuscript preparation. Software for ERO analyses was developed by Dr. James Havstad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amann LC, Phillips JM, Halene TB, Siegel SJ. Male and female mice differ for baseline and nicotine-induced event related potentials. Behav. Neurosci. 2008;122:982–90. doi: 10.1037/a0012995. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E. A compound P300-40 Hz response of the cat hippocampus. Int. J. Neurosci. 1991;60:227–37. doi: 10.3109/00207459109167035. [DOI] [PubMed] [Google Scholar]
- Basar E. EEG-brain dynamics- Relation between EEG and Brain evoked potentials. Elsevier/North-Holland Biomedical Press; Amsterdam: 1980. [Google Scholar]
- Basar E, Yordanova J, Kolev V, Basar-Eroglu C. Is the alpha rhythm a control parameter for brain responses? Biol. Cybern. 1997;76:471–80. doi: 10.1007/s004220050360. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–8. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int. J. Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. 2001b;39:241–8. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Demiralp T, Basar-Eroglu C, Ademoglu A. Event-related oscillations are ‘real brain responses’--wavelet analysis and new strategies. Int. J. Psychophysiol. 2001a;39:91–127. doi: 10.1016/s0167-8760(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Sakowitz O. The selectively distributed theta system: functions. Int. J. Psychophysiol. 2001c;39:197–212. doi: 10.1016/s0167-8760(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Demiralp T. Selectively distributed gamma band system of the brain. Int. J. Psychophysiol. 2001d;39:129–35. doi: 10.1016/s0167-8760(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int. J. Psychophysiol. 2006;60:162–71. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr. Clin. Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Brusa R. Genetically modified mice in neuropharmacology. Pharmacol. Res. 1999;39:405–19. doi: 10.1006/phrs.1998.0457. [DOI] [PubMed] [Google Scholar]
- Campbell IL. Neuropathogenic actions of cytokines assessed in transgenic mice. Int. J Dev. Neurosci. 1995;13:275–84. doi: 10.1016/0736-5748(94)00073-c. [DOI] [PubMed] [Google Scholar]
- Chen AC, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, Edenberg HJ, Hesselbrock V, Nurnberger J, Jr., Kuperman S, O’Connor SJ, Schuckit MA, Bauer LO, Tischfield J, Rice JP, Bierut L, Goate A, Porjesz B. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. Am J Med. Genet. B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30818. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem. Res. 2004;29:1179–88. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topogr. 2001;13:251–67. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neurosci. Lett. 1998;257:41–4. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL. ERP responses to ethanol and diazepam administration in squirrel monkeys. Alcohol. 1988;5:315–20. doi: 10.1016/0741-8329(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurontensin studies in alcohol naive, preferring and non- preferring rats. Neuroscience. 1999;93:227–36. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C. Long latency event-related potentials in mice: effects of stimulus characteristics and strain. Brain Res. 2002;957:117–28. doi: 10.1016/s0006-8993(02)03612-0. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J Cogn Neurosci. 2008;20:1403–14. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- Frenkel M, Sherman GF, Bashan KA, Galaburda AM, LoTurco JJ. Neocortical ectopias are associated with attenuated neurophysiological responses to rapidly changing auditory stimuli. Neuroreport. 2000;11:575–9. doi: 10.1097/00001756-200002280-00029. [DOI] [PubMed] [Google Scholar]
- Gabor D. Theory of Communication. J. Inst. Elec. Eng. 1946;93:429–57. [Google Scholar]
- Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157:95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Hum. Factors. 1998;40:79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Harrison J, Buchwald J. Aging changes in the cat P300 mimic the human. Electroencephalogr. Clin. Neurophysiol. 1985;62:227–34. doi: 10.1016/0168-5597(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Jucker M. Developing mouse models of aging: a consideration of strain differences in age-related behavioral and neural parameters. Neurobiol. Aging. 1999;20:137–45. doi: 10.1016/s0197-4580(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Jodo E, Takeuchi S, Kayama Y. P3b-like potential of rats recorded in an active discrimination task. Electroencephalogr. Clin. Neurophysiol. 1995;96:555–60. doi: 10.1016/0013-4694(95)00067-9. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr., O’Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int. J. Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr., O’Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav. Genet. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int. J. Psychophysiol. 2004;51:155–80. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol. Psychiatry. 2006;59:625–34. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko WM, Ehlers CL, Phillips EL, Riley EP. Auditory event-related potentials in fetal alcohol syndrome and Down’s syndrome children. Alcohol Clin. Exp. Res. 1996;20:35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci. Lett. 2000a;285:45–8. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin. Neuropathol. 2000b;111:1719–32. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr. Clin. Neurophysiol. 1994;91:428–41. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997b;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997a;34:169–76. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Stadler W, Pollhuber D, Sauseng P, Rohm D. Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neurosci. Lett. 2001;302:49–52. doi: 10.1016/s0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U. S. A. 2000;97:1867–72. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubar JF. Neocortical dynamics: implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Appl. Psychophysiol. Biofeedback. 1997;22:111–26. doi: 10.1023/a:1026276228832. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–4. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, Turetsky BI, Bilker WB, Lenox RH, Siegel SJ. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004;29:739–46. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli A, Conte S, D’Olimpio F, Ferlazzo F, Loizzo A, Palazzesi S, Renzi P. Ultradian rhythms in the N1-P2 amplitude of the visual evoked potential in two inbred strains of mice: DBA/2J and C57BL/6. Behav. Brain Res. 1995;67:81–4. doi: 10.1016/0166-4328(94)00107-q. [DOI] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, Liang Y, Siegel SJ. Effects of nicotine vary across two auditory evoked potentials in the mouse. Biol. Psychiatry. 2007;61:23–30. doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- O’Brien JH. P300 wave elicited by a stimulus-change paradigm in acutely prepared rats. Physiol Behav. 1982;28:711–3. doi: 10.1016/0031-9384(82)90056-7. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Deliano M, Scheich H, Freeman WJ. Analysis of evoked and emergent patterns of stimulus-related auditory cortical activity. Rev. Neurosci. 2003;14:35–42. doi: 10.1515/revneuro.2003.14.1-2.35. [DOI] [PubMed] [Google Scholar]
- Paller KA, Zola-Morgan S, Squire LR, Hillyard SA. P3-like brain waves in normal monkeys and in monkeys with medial temporal lesions. Behav. Neurosci. 1988;102:714–25. doi: 10.1037//0735-7044.102.5.714. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Ehrlichman RS, Siegel SJ. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event-related potential and evoked gamma. Neuroscience. 2007;144:1314–23. doi: 10.1016/j.neuroscience.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich JM, Fischer A, Starr A. A programmable multiple-tone generator. Behav. Res. Methods. 1983;15:39–41. [Google Scholar]
- Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Res. 1999;39:3071–81. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin. Neuropathol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Alzheimer’s disease: genetic studies and transgenic models. Annu. Rev. Genet. 1998;32:461–93. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int. J Psychophysiol. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bull. 2008;34:907–26. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick ND, Koehler C, Picciotto MR, Siegel SJ. Role of beta2-containing nicotinic acetylcholine receptors in auditory event-related potentials. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1358-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. A new metric for analyzing single-trial event-related potentials (ERPs): application to human visual P300 delta response. Neurosci Lett. 1995;197:167–70. doi: 10.1016/0304-3940(95)11912-g. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Basar E. Gamma responses in the EEG: elementary signals with multiple functional correlates. Neuroreport. 1997;8:531–4. doi: 10.1097/00001756-199701200-00030. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Demiralp T, Basar E, Basar EC. Electroencephalogram alpha (8-15 Hz) responses to visual stimuli in cat cortex, thalamus, and hippocampus: a distributed alpha network? Neurosci. Lett. 2000;292:175–8. doi: 10.1016/s0304-3940(00)01456-7. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int. J. Psychophysiol. 2001;39:229–39. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–82. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Grahame NJ, Roth J, Katner SN, Ehlers CL. EEG and ERP profiles in the high alcohol preferring (HAP) and low alcohol preferring (LAP) mice: relationship to ethanol preference. Brain Res. 2003;961:243–54. doi: 10.1016/s0006-8993(02)03959-8. [DOI] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S Transform. IEEE Trans on Signal Processing. 1996;44:998–1001. [Google Scholar]
- Sturchler-Pierrat C, Sommer B. Transgenic animals in Alzheimer’s disease research. Rev. Neurosci. 1999;10:15–24. doi: 10.1515/revneuro.1999.10.1.15. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Jodo E, Suzuki Y, Matsuki T, Hoshino KY, Niwa SI, Kayama Y. ERP development in the rat in the course of learning two-tone discrimination task. Neuroreport. 2000;11:333–6. doi: 10.1097/00001756-200002070-00022. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Luzi M, Palazzesi S, Pomponi M, Loizzo A. Effects of cholinergic drugs on neocortical EEG and flash-visual evoked potentials in the mouse. Neuropsychobiology. 1999;40:47–56. doi: 10.1159/000026596. [DOI] [PubMed] [Google Scholar]
- Troncoso E, Muller D, Czellar S, Zoltan KJ. Epicranial sensory evoked potential recordings for repeated assessment of cortical functions in mice. J Neurosci. Methods. 2000;97:51–8. doi: 10.1016/s0165-0270(00)00164-3. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotki D, Latanov A, Nitsch R, Lipp HP. Deviance-related electrophysiological activity in mice: is there mismatch negativity in mice? Clin. Neurophysiol. 2005;116:353–63. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Bowers BJ, Paylor R. The use of null mutant mice to study complex learning and memory processes. Behav. Genet. 1996;26:301–12. doi: 10.1007/BF02359386. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Brain theta response predicts P300 latency in children. Neuroreport. 1996;8:277–80. doi: 10.1097/00001756-199612200-00055. [DOI] [PubMed] [Google Scholar]