Abstract
Menopausal hot flushes compromise the quality of life for the majority of women. The physiological mechanisms underlying hot flushes remain poorly understood and the absence of an animal model to investigate hot flushes hinders investigations in this field. We have developed the sheep as a model to study peripheral skin temperature changes. Subjecting sheep to fever-inducing treatments with lipopolysaccharide, a significant (P<0.01) change in ear skin temperature was observed. As a strong correlation between luteinizing hormone pulses and hot flushes has previously been reported, we then determined whether intravenous gonadotropin-releasing hormone (GnRH), at doses sufficient to elevate CSF GnRH concentrations, could modulate ear skin temperature. No effect was observed, suggesting that GnRH per se dose not play a role in the etiology of hot flashes.
Keywords: hot flush, GnRH, thermoregulation, menopause
Introduction
Menopause occurs when the ovaries cease causing menstrual cyclicity due to the loss of follicular function. This cessation occurs around the age of fifty for most women (1). The relatively young age of onset can make symptoms associated with menopause especially problematic as they may significantly interfere with the lifestyles of those afflicted. Perhaps the most problematic of these symptoms is the hot flash. Approximately 75% of women going through menopause experience hot flashes (or hot flushes). Hot flashes usually occur multiple times per day and can persist for several years following the onset of menopause. Flashes are characterized by rises in skin temperature caused by abnormal vasodilation (2). It is also noteworthy that most men who undergo either chemical or anatomical orchidectomy experience hot flashes (3).
The dearth of information on the mechanisms driving hot flashes (4) necessitates further research. As stated at a 2004 NIH workshop on hot flashes (5), few animal models have been developed to study hot flashes. Furthermore, it has recently been argued that serious risks, such as a greater incidence of cardiac pathologies and ischemia (6, 7), are associated with steroid replacement therapy. These potential risks serve to amplify the need for research into the pathology of hot flashes with the intent of finding non-steroidal treatments. The need for extensive study, paired with the practical and ethical restraints of human research, clearly define the need for an effective, easy-to-use animal model to study hot flashes. Specifically, an animal model in which peripheral skin temperature measurements can be taken without stress to the animal during various experimental manipulations is vital. Ovariectomized ewes also show cyclical changes in subcutaneous skin temperature (8). These rises in skin temperature may be considered hot flashes and thus the ewe may be an appropriate model for research on hot flashes. Accordingly, our first objective was to develop a model system in sheep that could reliably detect peripheral temperature changes using a known febrile response.
It is clear that hot flashes are related to the loss of the gonadal steroids (9), which accompanies either menopause or ovariectomy, and steroid replacement therapy is the most effective treatment for hot flashes (10). However, declining levels of steroids do not appear to be directly causative of the vasomotor symptoms associated with hot flashes (11). Indeed, while several hypotheses exist, the causative mechanism(s) behind hot flashes remains unknown. Most hot flashes are accompanied by an abnormal rise in core body temperature. This core temperature change is hypothesized to initiate vasodilation, leading to the sensation of heat that is associated with flashes (12, 13). The cause of the initial rise in core temperature remains unidentified. Abnormalities with several hormonal and neurotransmitter systems, known to be regulated by gonadal steroids, have been hypothesized to play a role. These systems include the gonadotropins (14), endogenous opioids (15), and catecholamines (2).
Gonadotropin releasing hormone (GnRH) may also be involved. GnRH has an established role in stimulating the pituitary gland to cause the biosynthesis and release of the gonadotropins. There is also a striking correlation between luteinizing hormone pulses and hot flashes (16–18), which raises the possibility that hot flashes may be causally related to the pulsatile secretion of GnRH. It is noteworthy that luteinizing hormone per se is not involved in hot flashes: hypophysectomy (19) and GnRH agonist therapy (20, 21) both eliminate endogenous LH, but hot flashes persist or are induced. Few studies have investigated the precise role of GnRH. There is increasing evidence that GnRH has a variety of extra-pituitary roles, especially within the brain. Support for this role in mammals includes the detection of GnRH binding sites (22), GnRH receptor mRNA (23) and GnRH receptor protein (24, 25), the ability of GnRH to elicit behavioral changes (26) and evidence that GnRH can affect the electrical properties of neurons (27). Few studies have investigated the human brain. However, GnRH receptors are clearly evident in the human brain (28, 29) and in neuronal cell lines of human origin (30). Initial studies have shown GnRH receptor expression in hippocampal and neocortical neurons; specifically in the entorhinal cortex and occipitotemporal gyrus (31) but, apart from these data, the precise distribution of GnRH receptors in the human brain remains undetermined. The high prevalence of GnRH receptors within the mammalian medial septum and pre-optic region (24), both temperature regulating areas of the brain, adds further credence to the hypothesis that GnRH may play a role in hot flashes (32, 33). Furthermore, GnRH secreting neurons known to be regulated by estrogen are highly present in both of these regions (34); and gonadotropin secretion is known to be altered in women following menopause suggesting an alteration in GnRH secretion (35). GnRH secreted into the hypophyseal portal system has also been directly shown to differ between older and younger primates (36). It is therefore possible that deregulation of local GnRH secretion into the preoptic region and medial septum following loss of estrogen feedback after menopause or gonadectomy may be responsible for deviant actions of these neural regions. We hypothesized that deviant actions of GnRH may cause the core temperature changes associated with hot flashes. Thus, the second objective of the study was to determine the effect of GnRH on peripheral temperature changes.
Materials and Methods
Experiment 1: Development of a model to study peripheral temperature changes
Adult Rambouillet X Columbia ewes (n=6) that were in the anestrous season (April–July) were housed in outdoor pens under natural photoperiod and fed a mixture of hay and concentrate. Animals were fed daily with hay and had free access to water. During temperature measurements, animals were moved in pairs to indoor pens under a 12L/12D photoperiod (lights off: 18:00) and the indoor temperature was maintained at 18°C. Procedures were approved by the University of Wyoming Animal Care Committee (IACUC #A-3126-01).
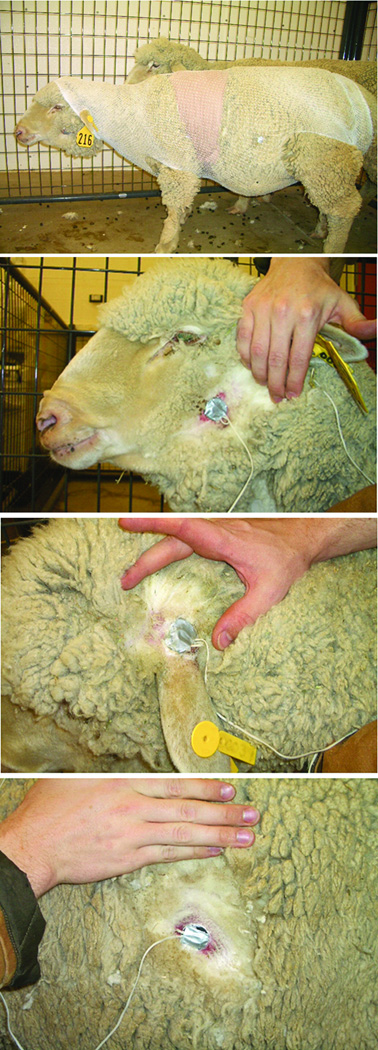
Portable data loggers (Mini-Mitter Inc, Bend, OR) were wired to three small temperature sensitive probes. These data loggers have been previously used to record bovine tympanic temperatures (37). Loggers were programmed to record temperatures every 30 seconds. A 4×4cm section of wool was shaved off each ewe at the lower half of the back the ear, the upper section of the cheek, and the middle of the abdomen. Temperature probes were super-glued to these sections of bare skin (Fig. 1). The wires and data loggers were secured by wrapping the sheep in lightweight netting and athletic wrap. An additional probe recorded heart rate.
Figure 1.
The sheep as a model for studying peripheral temperature changes. The locations of the thermocouples on the ear, cheek and abdomen are shown.
After mounting of loggers and temperature leads, sheep were left alone for at least 4 hours prior to any recording. A cross-over design was used so that each ewe received each treatment: either intravenous saline (control to observe temperature changes in response to handling) or lipopolysaccharide (LPS; 200µg/kg). LPS is a well known pyrogen (38, 39), and was used to determine if the sensors were capable of detecting skin temperature changes. Preliminary observations recorded a slight decrease in skin temperature each evening as the animals began to rest, indicative of a circadian rhythm. Thus, all injections were performed at approximately 9pm. Baseline temperature was recorded every 30 seconds for 5h pre-injection and then measurements were recorded for a further 10h post-injection.
Experiment 2: Effect of GnRH on peripheral temperature
Adult Rambouillet X Columbia ewes in the anestrous season (April–July) were ovariectomized (n=6) or ovariectomized and simultaneously administered a subcutaneous 1cm Silastic estradiol implant (n=6), which produces a basal circulating concentration of 2–4 pg/ml of estradiol (40). Temperature probes were prepared as for Experiment 1. Baseline temperature was recorded every 30 seconds for 1h and then ewes were injected with either GnRH (1mg bolus) or saline. A cross-over design was used so that each ewe received each treatment. Temperature measurements were recorded for 5h following the injection. As before, injections were performed at approximately 9pm. We have recently shown that a 1mg bolus injection of GnRH is required to elevate CSF-GnRH concentrations to physiological concentrations (41). Specifically, peak physiological concentrations of GnRH range from 5pg/ml during pulses to over 100pg/ml during the LH surge (41–43) and 1mg intravenous GnRH elevates CSF-GnRH concentrations to (38.5 ± 10.6 pg/ml) (41).
Data Analysis
Skin temperatures varied according to animal and location (Fig. 2). Thus for data analysis, all temperature measurements for each ewe were standardized relative to the mean temperature in the 1h preceding the injection. Data were pooled in 1h periods and statistically analyzed by two-way repeated measures ANOVA.
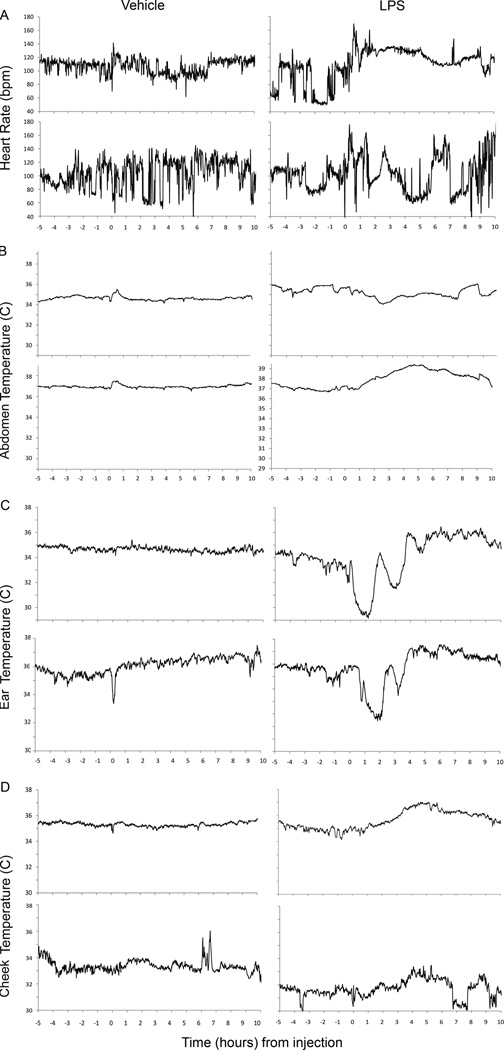
Figure 2.
Changes in (A) heart rate, (B) abdomen temperature, (C) ear temperature and (D) cheek temperature in two representative ewes after an intravenous injection of 0.9% saline (vehicle; n=6; left) or LPS (n=6; right).
Results
Experiment 1: Development of a model to study peripheral temperature changes
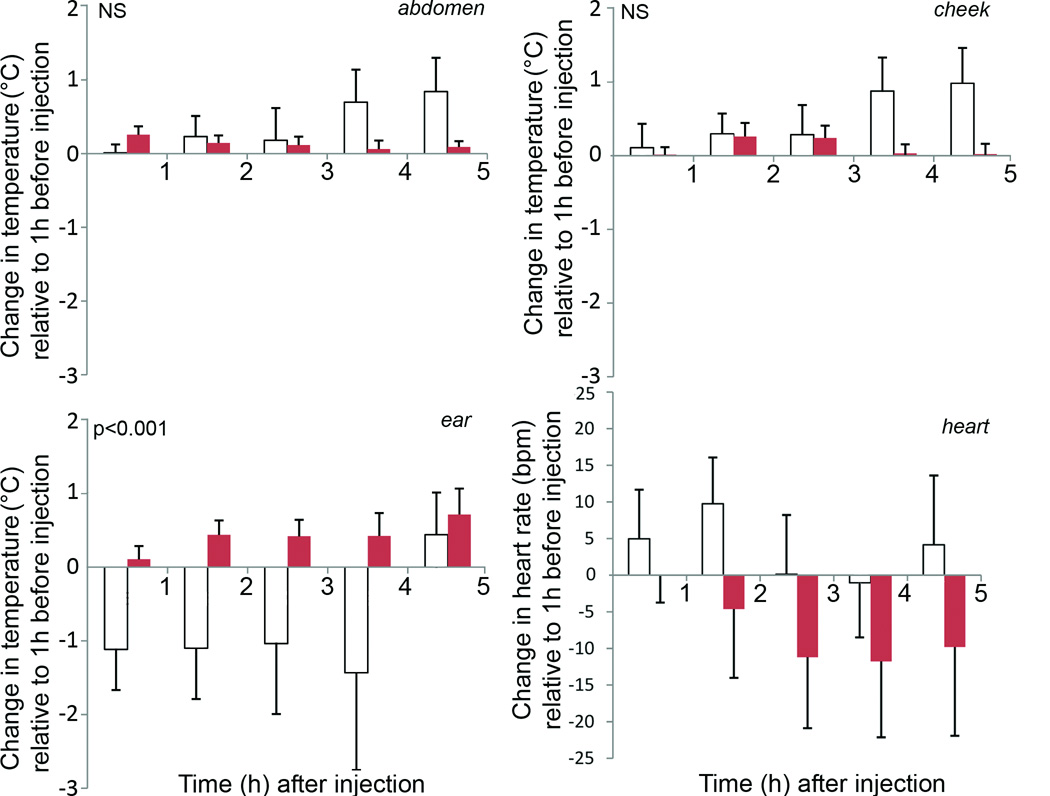
Although occasional responses to LPS were noted in heart rate (Fig. 1A, upper left panel) and abdominal temperatures (Fig. 1B, lower right panel), for the group, there was no significant effect on heart rate (Fig. 3: bottom right) or abdominal skin temperature (Fig. 3: top left). There was also no significant effect of LPS on cheek temperature (Fig. 2D; Fig. 3: top right). In contrast, LPS injection caused a significant (p<0.001) and consistent change in skin temperature at the ear (Fig. 2C; Fig. 3: bottom left). The LPS-induced temperature changes followed a consistent pattern involving a series of transient falls and rises, which were initiated almost immediately following the LPS injection.
Figure 3.
Mean (+SEM) changes in abdomen, cheek and ear temperature and heart rate in 6 ewes after an intravenous injection of 0.9% saline (solid columns) or LPS (open columns). A significant effect (p<0.001) of LPS was evident on ear temperature.
Experiment 2: Effect of GnRH on peripheral temperature
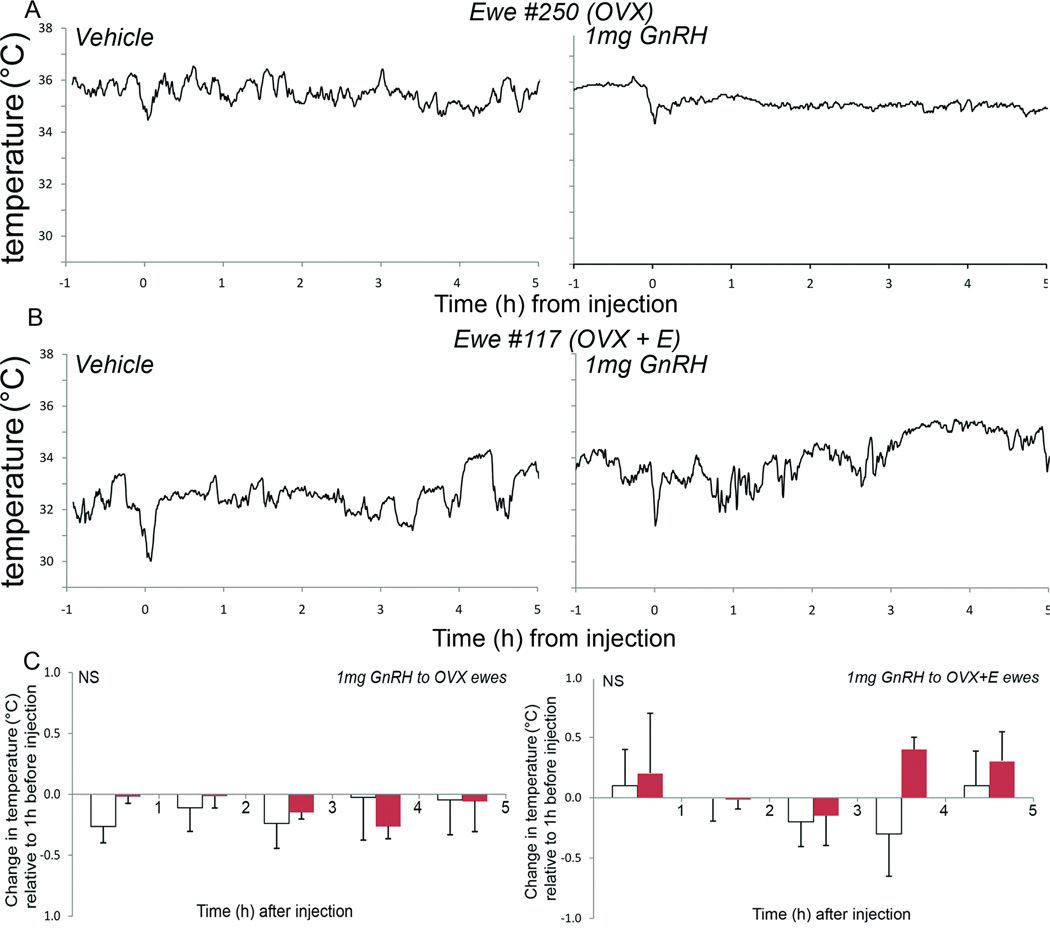
The ear temperatures showed no significant changes in response to the injection of GnRH relative to control injections in both the no estrogen and low estrogen groups of animals (Fig. 4). Cheek and abdomen skin temperatures also remained unchanged (data not shown).
Figure 4.
Representative ewes showing no change in ear temperature following vehicle (0.9% saline; left panels A and B; n=6) and either a 1mg GnRH injection to ovariectomized ewes (A right panel; n=6) or a 1mg GnRH injection to ovariectomized ewes bearing subcutaneous estradiol implants (B right panel; n=6). This was confirmed in the group analysis for both the ovariectomized (C left panel) and the ovariectomized+estradiol (C right panel) groups following 0.9% saline (solid columns) or 1mg GnRH (open columns).
Discussion
LPS has been well established as a reliable fever inducer acting through the actions of interleukin-1 to activate the arachidonic acid/prostaglandin pathway (38, 39). The mechanism employed by LPS to induce skin temperature changes is most likely different than the mechanisms which cause hot flashes. However, the ability of the portable data loggers to detect LPS induced changes in skin temperature demonstrates their potential ability to detect experimentally induced vasodilatory events. Thus, the model system that we have developed may be useful to study the etiology of skin temperature changes.
As recently noted (5), there are few available animal models to study hot flashes. Ovariectomized, morphine-dependent rats exhibit rises in tail temperature during antagonist induced opiate withdrawal (44). Additionally, ovariectomized mice forced to exercise exhibit similar changes (45). Temperature effects in both models are dependent on ovariectomy and are tempered by exogenous estrogen. Non-human primates have also been used to study menopause and hot flashes. They hold the advantage of possessing reproductive cycles and basic physiology that is similar to humans. Indeed, several species appear to undergo a natural menopause (46). In a study on 2 ovariectomized female monkeys, cyclical rises in forehead skin temperature resembling hot flashes were observed. This effect was reduced in response to exogenous estrogen (47). While monkeys hold great potential as model systems to study hot flashes, few facilities are available for this research. It is noteworthy that ovariectomized ewes have already been used as a model of post-menopausal bone loss, an effect partially corrected through estrogen therapy (48). Our system could improve on detection of skin temperature changes in ewes in several ways. First, our system measures external temperature, and is therefore more relevant to human hot flashes. Second, in the previous study examining potential hot flashes in ewes (8), loggers were placed on the inside of the axilla and thigh. Our system allows placement of temperature sensors in more appropriate heat-loss areas such as the cheek and ear. Finally, the loggers in the previous study could only take measurements once every 150 seconds, whereas the system used in the current study can acquire data every 30 seconds allowing for more potential data points within the time course of hot flashes.
LH pulses, which are induced by GnRH, are precisely correlated with the occurrence of hot flashes in humans (16–18). Lomax et al (49) detected a significant effect of high dose GnRH (1µg) injections into the preoptic area on skin tail temperature in the rat (49). Similarly, Hosono et al (50) reported that GnRH administered into the hypothalamic septal area affects rat tail and paw vasodilation in response to warming of the preoptic area. We tested the hypothesis that GnRH may play a critical role in thermoregulatory events in sheep by measuring skin temperature changes in ewes with low levels of serum estrogen following large bolus injections of GnRH. In contrast to the significant changes we observed in response to LPS, GnRH was without effect.
Some caveats between the present experiments and the actual occurrence of menopause must be considered before GnRH, as a potential candidate, is excluded in the sheep. First, the number of ewes used in the current study is small (n=6 per condition) and it is possible that only substantial changes, such as those induced by LPS, may be statistically detectable. However, given the complete absence of any perturbation in temperature following the 1mg GnRH injection, we consider this unlikely. Second, it is arguable that hormonal changes occur at vastly different rates during menopause in comparison to the rate at which those changes would have occurred during our experiments. The perimenopausal period appears to be associated with gradual changes in the levels of several hormones including a gradual rise before a decline in the level estrogen (51). Estrogen acts on a wide variety of neural systems, many of which may interact with GnRH neurons (52). Thus, during gradual estrogen loss, cellular changes within many different neuronal systems would take place slowly. Some of these changes may cause gradual downstream effects on the secretion of GnRH onto temperature regulating neurons. Within the ewes examined estrogen was suddenly lost; therefore it did not properly imitate the estrogen profile of peri- and post-menopausal women. However, individuals who undergo gonadectomy often still experience hot flashes (3). The experiments performed in the absence of supplemental estrogen may have more closely resembled hot flashes occurring from this kind of steroid loss. Third, there may be a difference between our experiments and what may occur in vivo in the exposure of the medial septum and pre-optic regions to GnRH. While we have shown recently that iv injected GnRH crosses the blood-brain barrier (41), we do not know how much of this GnRH reaches specific neural tissues. In this context, it was shown in the rat that high doses (2µg) of GnRH administered into the septal area lowered the threshold hypothalamic temperature for skin vasodilation, whereas icv GnRH had no effect (50). Furthermore, it is unknown whether the level or rate at which GnRH accesses these regions resembles that of menopausal women or gonadectomized individuals.
In summary, we have developed a model system in the ewe that can accurately detect small changes in peripheral skin temperature. This system has the potential to be extremely useful in future studies investigating the pathology of hot flashes and holds several advantages over previous models systems used for this work. Our study does not support the hypothesis that GnRH per se is involved in thermoregulatory events.
Acknowledgements
This publication was made possible by Grant Number RR15640 from the National Center for research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. AJA was funded, in part, by a grant from NSF #EPS-9983278.
Financial support: Grant Number NIH NCRR RR15640 and NSF #EPS-9983278
References
- 1.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet. 1999;353:571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- 2.Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:117–125. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- 3.Spetz AC, Hammar ML. Hot flushes in men: prevalence and possible mechanisms. J Brit Menopause Soc. 2002:57–62. [Google Scholar]
- 4.Sturdee DW. The menopausal hot flush--anything new? Maturitas. 2008;60:42–49. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Bethesda, Maryland: National Institute of Health Workshop; Assessing and Improving Measures of Hot Flashes. 2004:1–54.
- 6.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed]
- 8.MacLeay JM, Lehmer E, Enns RM, Mallinckrodt C, Bryant HU, Turner AS. Central and peripheral temperature changes in sheep following ovariectomy. Maturitas. 2003;46:231–238. doi: 10.1016/s0378-5122(03)00196-8. [DOI] [PubMed] [Google Scholar]
- 9.Ratka A, Miller V, Brown K, Jenschke M, Simpkins JW. Association of various dimensions of hot flashes with systemic levels of gonadal steroids. Exp Biol Med (Maywood) 2009 doi: 10.3181/0811-RM-334. [DOI] [PubMed] [Google Scholar]
- 10.Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610–1620. doi: 10.1001/jama.291.13.1610. [DOI] [PubMed] [Google Scholar]
- 11.Aksel S, Schomberg DW, Tyrey L, Hammond CB. Vasomotor symptoms, serum estrogens, and gonadotropin levels in surgical menopause. Am J Obstet Gynecol. 1976;126:165–169. doi: 10.1016/0002-9378(76)90270-2. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RR, Woodward S. Core body temperature during menopausal hot flushes. Fertil Steril. 1996;65:1141–1144. [PubMed] [Google Scholar]
- 13.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 14.Tataryn IV, Meldrum DR, Lu KH, Frumar AM, Judd HL. LH, FSH and skin temperaure during the menopausal hot flash. J Clin Endocrinol Metab. 1979;49:152–154. doi: 10.1210/jcem-49-1-152. [DOI] [PubMed] [Google Scholar]
- 15.Lightman SL, Jacobs HS, Maguire AK, McGarrick G, Jeffcoate SL. Climacteric flushing: clinical and endocrine response to infusion of naloxone. Br J Obstet Gynaecol. 1981;88:919–924. doi: 10.1111/j.1471-0528.1981.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 16.Casper RF, Yen SSC. Neuroendocrine changes during menopausal flushes. In: Norman RL, editor. Neuroendocrine Aspects of Reproduction. New York: Academic Press; 1983. pp. 359–378. [Google Scholar]
- 17.Casper RF, Yen SS. Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin Endocrinol (Oxf) 1985;22:293–312. doi: 10.1111/j.1365-2265.1985.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 18.Casper RF, Yen SS, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteinizing hormone secretion. Science. 1979;205:823–825. doi: 10.1126/science.462193. [DOI] [PubMed] [Google Scholar]
- 19.Mulley G, Mitchell JR, Tattersall RB. Hot flushes after hypophysectomy. Br Med J. 1977;2:1062. doi: 10.1136/bmj.2.6094.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J Obstet Gynaecol Res. 2000;26:325–331. doi: 10.1111/j.1447-0756.2000.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodgers AK, Falcone T. Treatment strategies for endometriosis. Expert Opin Pharmacother. 2008;9:243–255. doi: 10.1517/14656566.9.2.243. [DOI] [PubMed] [Google Scholar]
- 22.Jennes L, Dalati B, Conn PM. Distribution of gonadotropin releasing hormone agonist binding sites in the rat central nervous system. Brain Res. 1988;452:156–164. doi: 10.1016/0006-8993(88)90020-0. [DOI] [PubMed] [Google Scholar]
- 23.Jennes L, Eyigor O, Janovick JA, Conn PM. Brain gonadotropin releasing hormone receptors: localization and regulation. Rec Progr Horm Res. 1997;52:475–491. [PubMed] [Google Scholar]
- 24.Albertson AJ, Navratil A, Mignot M, Dufourny L, Cherrington B, Skinner DC. Immunoreactive GnRH type I receptors in the mouse and sheep brain. J Chem Neuroanat. 2008;35:326–333. doi: 10.1016/j.jchemneu.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albertson AJ, Talbott H, Wang Q, Jensen D, Skinner DC. The gonadotropin releasing hormone type I receptor is expressed in the mouse cerebellum. The Cerebellum. 2008;7:379–384. doi: 10.1007/s12311-008-0038-8. [DOI] [PubMed] [Google Scholar]
- 26.Riskind P, Moss RL. Midbrain central gray: LHRH infusion enhances lordotic behavior in estrogen-primed ovariectomized rats. Brain Res Bull. 1979;4:203–205. doi: 10.1016/0361-9230(79)90282-x. [DOI] [PubMed] [Google Scholar]
- 27.Wong M, Eaton MJ, Moss RL. Electrophysiological actions of luteinizing hormone-releasing hormone: intracellular studies in the rat hippocampal slice preparation. Synapse. 1990;5:65–70. doi: 10.1002/syn.890050106. [DOI] [PubMed] [Google Scholar]
- 28.Romanelli RG, Barni T, Maggi M, Luconi M, Failli P, Pezzatini A, Pelo E, Torricelli F, Crescioli C, Ferruzzi P, Salerno R, Marini M, Rotella CM, Vannelli GB. Expression and function of gonadotropin-releasing hormone (GnRH) receptor in human olfactory GnRH-secreting neurons: an autocrine GnRH loop underlies neuronal migration. J Biol Chem. 2004;279:117–126. doi: 10.1074/jbc.M307955200. [DOI] [PubMed] [Google Scholar]
- 29.Fan NC, Peng C, Krisinger J, Leung PC. The human gonadotropin-releasing hormone receptor gene: complete structure including multiple promoters, transcription initiation sites, and polyadenylation signals. Mol Cell Endocrinol. 1995;107:R1–R8. doi: 10.1016/0303-7207(94)03460-b. [DOI] [PubMed] [Google Scholar]
- 30.Yeung CM, An BS, Cheng CK, Chow BK, Leung PC. Expression and transcriptional regulation of the GnRH receptor gene in human neuronal cells. Mol Hum Reprod. 2005;11:837–842. doi: 10.1093/molehr/gah241. [DOI] [PubMed] [Google Scholar]
- 31.Wilson AC, Salamat MH, RJ, Roche K, Karande A, Meethal SV, Terasawa E, Bowen RL, Atwood CS. Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone espression. J Endocrinol. 2006;191:651–663. doi: 10.1677/joe.1.07047. [DOI] [PubMed] [Google Scholar]
- 32.Ishiwata T, Hasegawa H, Yasumatsu M, Akano F, Yazawa T, Otokawa M, Aihara Y. The role of preoptic area and anterior hypothalamus and median raphe nucleus on thermoregulatory system in freely moving rats. Neurosci Lett. 2001;306:126–128. doi: 10.1016/s0304-3940(01)01865-1. [DOI] [PubMed] [Google Scholar]
- 33.Srividya R, Mallick HN, Kumar VM. The changes in thermal preference, sleep-wakefulness, body temperature and locomotor activity in the rats with medial septal lesion. Behav Brain Res. 2005;164:147–155. doi: 10.1016/j.bbr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Caraty A, Skinner DC. Progesterone priming is essential for the full expression of the positive feedback effect of estradiol in inducing the preovulatory GnRH surge in the ewe. Endocrinology. 1999;140:165–170. doi: 10.1210/endo.140.1.6444. [DOI] [PubMed] [Google Scholar]
- 35.Rossmanith WG. Gonadotropin secretion during aging in women: review article. Exp Gerontol. 1995;30:369–381. doi: 10.1016/0531-5565(94)00030-7. [DOI] [PubMed] [Google Scholar]
- 36.Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- 37.Macaulay AS, Hahn GL, Clark DH, Sisson DV. Comparison of calf housing types and tympanic temperature rhythms in Holstein calves. J Dairy Sci. 1995;78:856–862. doi: 10.3168/jds.S0022-0302(95)76698-X. [DOI] [PubMed] [Google Scholar]
- 38.Long NC, Otterness I, Kunkel SL, Vander AJ, Kluger MJ. Roles of interleukin 1 beta and tumor necrosis factor in lipopolysaccharide fever in rats. Am J Physiol. 1990;259:R724–R728. doi: 10.1152/ajpregu.1990.259.4.R724. [DOI] [PubMed] [Google Scholar]
- 39.Davidson J, Milton AS, Rotondo D. A study of the pyrogenic actions of interleukin-1 alpha and interleukin-1 beta: interactions with a steroidal and a non-steroidal anti-inflammatory agent. Br J Pharmacol. 1990;100:542–546. doi: 10.1111/j.1476-5381.1990.tb15843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans NP, Dahl GE, Glover BH, Karsch FJ. Central regulation of pulsatile GnRH secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology. 1994;134:1806–1811. doi: 10.1210/endo.134.4.8137746. [DOI] [PubMed] [Google Scholar]
- 41.Caraty A, Skinner DC. Gonadotropin-releasing hormone in third ventricular cerebrospinal fluid: endogenous distribution and exogenous uptake. Endocrinology. 2008;149:5227–5234. doi: 10.1210/en.2007-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skinner DC, Caraty A, Malpaux B, Evans NP. Simultaneous measurement of gonadotropin-releasing hormone in the third ventricular cerebrospinal fluid and hypophyseal portal blood of the ewe. Endocrinology. 1997;138:4699–4704. doi: 10.1210/endo.138.11.5494. [DOI] [PubMed] [Google Scholar]
- 43.Skinner DC, Malpaux B, Delaleu B, Caraty A. Luteinizing hormone (LH)-releasing hormone in third ventricular cerebrospinal fluid of the ewe: correlation with LH pulses and the LH surge. Endocrinology. 1995;136:3230–3237. doi: 10.1210/endo.136.8.7628356. [DOI] [PubMed] [Google Scholar]
- 44.Merchenthaler I, Funkhouser JM, Carver JM, Lundeen SG, Ghosh K, Winneker RC. The effect of estrogens and antiestrogens in a rat model for hot flush. Maturitas. 1998;30:307–316. doi: 10.1016/s0378-5122(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 45.Shuto H, Yamauchi A, Ikeda M, Sohda Y, Koga A, Tominaga K, Egawa T, Kataoka Y. Forced exercise-induced flushing of tail skin in ovariectomized mice, as a new experimental model of menopausal hot flushes. J Pharmacol Sci. 2005;98:323–326. doi: 10.1254/jphs.sc0050046. [DOI] [PubMed] [Google Scholar]
- 46.Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biol Reprod. 2003;68:10–18. doi: 10.1095/biolreprod.102.005215. [DOI] [PubMed] [Google Scholar]
- 47.Jelinek J, Kappen A, Schonbaum E, Lomax P. A primate model of human postmenopausal hot flushes. J Clin Endocrinol Metab. 1984;59:1224–1228. doi: 10.1210/jcem-59-6-1224. [DOI] [PubMed] [Google Scholar]
- 48.Turner AS. The sheep as a model for osteoporosis in humans. Vet J. 2002;163:232–239. doi: 10.1053/tvjl.2001.0642. [DOI] [PubMed] [Google Scholar]
- 49.Lomax P, Bajorek JG, Chesarek W, Tataryn IV. Thermoregulatory effects of luteinizing hormone releasing hormone in the rat; Thermoregulatory Mechanisms and Their Therapeutic Implications 4th Int Symp on the Pharmacology of Thermoregulation; Oxford. 1979. pp. 208–211. [Google Scholar]
- 50.Hosono T, Yanase-Fujiwara M, Zhang YH, Xiao-ming C, Fukuda Y, Asaki Y, Yamaji K, Kanosue K. Effect of gonadotropin releasing hormone on thermoregulatory vasomotor activity in ovariectomized female rats. Brain Res. 1997;754:88–94. doi: 10.1016/s0006-8993(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 51.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 52.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]