Abstract
Background
The role of neural regulation in expression and function of intestinal hexose transporters is unknown.
Aim
To determine the role of intestinal innervation in gene expression and function of the membrane hexose transporters, SGLT1, GLUT2, and GLUT5 in the enterocyte.
Hypothesis
Denervation of the small intestine decreases expression of hexose transporters leading to decreased glucose absorption.
Methods
Six groups of Lewis rats were studied (n=6 each): control, 1 wk after sham laparotomy, 1 and 8 wk after syngeneic (no immune rejection) orthotopic small bowel transplantation (SBT) (SBT1, SBT8) to induce complete extrinsic denervation, and 1 and 8 wk after selective disruption of intrinsic neural continuity to jejunoileum by gut transection and reanastomosis (T/A1, T/A8). All tissue was harvested between 8AM and 10AM. In duodenum, jejunum, and ileum, mucosal mRNA levels were quantitated by real time PCR, protein by Western blotting, and transporter-mediated glucose absorption using the everted sleeve technique.
Results
Across the six groups, relative gene expression of hexose transporter mRNA and protein levels were unchanged and no difference in transporter-mediated glucose uptake was evident in any region. Glucose transporter affinity (Km) and functional transporter levels (Vmax) calculated for duodenum and jejunum showed no difference between the six groups.
Conclusion
Baseline regulation of hexose transporter function is not mediated tonically by intrinsic or extrinsic neural continuity to the jejunoileum.
Keywords: small bowel transplant, hexose transporters, glucose transport
Introduction
The success of small bowel transplantation (SBT), although improving, has been limited (1-3). Even in the absence of immune-mediated graft rejection, intestinal transplantation is complicated by enteric dysfunction characterized by dysmotility, diarrhea, and malabsorptive syndromes which may be due, in large part, to the intestinal denervation associated with SBT (4-12). SBT necessitates complete transection of extrinsic neurons (from the central nervous system) that course through the mesentery and innervate the intestine, as well as a complete disruption of enteric myoneural continuity to the transplanted jejunoileum (intrinsic innervation) (13-15). It is well accepted that gut function is highly dependent on neural modulation of absorption and motility, two key components of normal intestinal function (6,7,16,17). It is not surprising then that intestinal dysfunction is seen in other clinical scenarios of denervation, such as vagotomy or disorders of neural function where disruption of enteric neural input also occurs and results in intestinal dysmotility and malabsorption (18-22). Specifically, the effect that intestinal denervation has on hexose transporter expression and function is not known; such changes in innervation could be a factor in the malabsorptive syndromes associated commonly with SBT.
The current study was designed to determine the role of neural regulation in the expression and function of the primary intestinal hexose transporters of the small bowel enterocyte: sodium-glucose transporter-1 (SGLT1), glucose transporter-2 (GLUT2), and glucose transporter-5 (GLUT5), and how changes in the expression and function of these transporters may contribute to enteric dysfunction after SBT. We hypothesized that intestinal innervation plays a substantive role in regulating hexose transporter expression and function in the intestinal brush border, and therefore, denervation of the gut after SBT will result in decreased expression and ultimately function of these transporters.
Methods
Groups and Procedures
Under the policies established by our Institutional Animal Care and Use Committee in accordance with the National Institutes of Health guidelines for the humane use and care of laboratory animals, we studied 250- to 300-g male Lewis (inbred) rats (Harlan, Indianapolis, IN) between 8AM and 10AM. A set time period of the light/dark cycle was chosen, because gene expression and transport function of the hexose transporters of interest are well-known to vary diurnally (25,26). We studied two groups, each at 1 and 8 wk after operation, with one group undergoing SBT and the other subjected to transection/anastomosis (T/A) of the proximal jejunum and distal ileum. For baseline values, we also studied a group 1 wk after sham celiotomy as well as a group of non-operated (naïve) control rats.
The first group (SBT; n=12) underwent complete disruption of extrinsic innervation and intrinsic neural input to the jejunoileum via orthotopic, jejunoileal isotransplantation as described previously (23). In brief, these syngeneic Lewis rats were anesthetized using 2% inhaled isoflurane induction followed by intraperitoneal injection of 50 mg/kg sodium pentobarbital. The donor jejunoileum was resected with preservation of the portal vein and the superior mesenteric artery (SMA) with a cuff of aorta as vascular conduits. In the recipient, the jejunoileum was resected leaving a short, 3-cm portion of proximal jejunum and distal ileum for anastomosis of the transplanted jejunoileum. Revascularization of the transplanted jejunoileum in the recipient involved implantation of the donor portal vein and SMA into the recipient inferior vena cava and abdominal aorta, respectively, using 10-0 nylon suture material. Intestinal continuity was restored with end-to-end intestinal anastomoses of donor jejunum and ileum to recipient jejunum and ileum using a running 6-0 polypropylene suture. The abdomen was closed in two layers, and the animal was recovered. Animals received a liquid diet for the first 3 postoperative days and resumed solid chow (5001 Rodent Diet, PMI Nutrition International LLC, Brentwood, MO) on postoperative day 4. The animals were given acetaminophen in their drinking water for 1 day preoperatively and 2 days postoperatively. Animals were then studied at 1 wk (SBT1; n=6) and 8 wk (SBT8; n=6).
The second group (T/A; n=12) underwent disruption of intrinsic neural continuity to their native jejunoileum via transection and reanastomosis of the proximal jejunum and terminal ileum. These animals were anesthetized as in the SBT group. Through a midventral celiotomy, the proximal jejunum was transected 3 cm from the ligament of Treitz and reanastomosed in an end-to-end fashion using a running 6-0 polypropylene suture. A similar transection and reanastomosis was then performed in the distal ileum, 10cm proximal to the ileocecal valve. The abdomen was closed in two layers, and the animals were managed similar to the SBT group in that solid food consumption was not resumed until postoperative day 4. These animals were also studied at 1 wk (T/A1: n=6) and 8 wk (T/A8: n=6).
As controls for anesthesia and celiotomy, we used rats 1 wk after a sham laparotomy (sham: n=6). After identical anesthesia and celiotomy, the entire jejunoileum was manipulated manually for 5 min. Postoperatively, the animals were managed in the same way as the experimental groups. Naïve, unoperated rats served as baseline controls (control: n=6). All rats (controls and experimental) were housed in a strictly controlled, 12-h light-dark cycle animal facility and were fed standard rat chow. Feeding patterns were monitored closely by measuring the weight of food consumed at the beginning and the end of each light-dark cycle. Animal weights were recorded at the initial procedure and at harvest. A group of naïve rats were used to assess normal feeding patterns and weight gain in rats over 8 wk.
Tissue Harvest
Because of the known diurnal variation in expression and function of hexose transporters in the rat small intestinal mucosa, all tissue was harvested within one hour of 9AM (24-26). We chose this time point, not to evaluate the effects of denervation on diurnal variation in gene and protein expression, but rather to evaluate the effects of denervation on baseline expression. After anesthetization, the duodenum was cannulated at the pylorus, and the entire small intestine was flushed with chilled (4°C) mammalian Ringer’s solution (in mM: 128 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 20 NaHCO3; pH 7.3-7.4; 290 mOsm) to remove luminal content and decrease activity of intraluminal proteases. The small intestine was then excised rapidly using sharp dissection and placed immediately in chilled (4°C), oxygenated (95% O2/5% CO2) mammalian Ringer’s Solution. The mucosa was scraped bluntly from portions of each intestinal segment using a glass slide into cold, phosphate-buffered saline (PBS). Half of the mucosal scrapings were placed into RNA stabilization buffer (RNALater, Qiagen, Valencia, CA), snap frozen in liquid nitrogen, and stored at -80°C. The remaining mucosal scrapings were placed in cold PBS containing protease inhibitors (Complete, Roche Applied Sciences, Indianapolis, IN) before being snap-frozen in liquid nitrogen and stored at -80°C.
mRNA Measurements
Real-time reverse transcription polymerase chain reaction (RT-PCR) was used to quantitate mRNA levels (27,28). The mucosal samples stored in RNA stabilization buffer were thawed on ice and then homogenized by repeated passing of the solution through a 20-gauge needle. RNA was isolated using the RNeasy Midi kit (Qiagen, Valencia, CA). Samples not to be used immediately were stored at -80°C.
DNase digestion (Invitrogen DNase, amplification grade) was performed on the isolated RNA to remove contaminating DNA. Then, 1.7 μg of the RNA was reverse transcribed into cDNA using the Super Script III kit (Invitrogen, Carlsbad, CA) using random hexamer primers. The resultant cDNA was stored at -20°C until real-time RT-PCR was used to quantitate mRNA levels for SGLT1, GLUT2, GLUT5, and the stably expressed housekeeping gene, glyceraldehyde-6-phosphate dehydrogenase (GAPDH) against which levels were quantitated and normalized. RT-PCR was performed using Taqman® chemistries with primers and fluorescently-labeled probes in assay mixes from Applied Biosystems (Applied Biosystems, San Francisco, CA). The standard procedure from Applied Biosystems of 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min was used. Standard curves from serial dilutions of known copy numbers were used to calculate the number of cDNA copies for each sample. All copy numbers of protein transporters were normalized to copy numbers of GAPDH, allowing estimates of expression per enterocyte. All RT-PCR runs were performed in triplicate.
Protein Measurements (Western blot)
Samples stored in PBS containing protease inhibitors were thawed on ice and placed in RIPA lysis buffer also containing protease inhibitors to prevent protein degradation. These samples were then homogenized using a Kontes Pellet Pestle (Fischer Scientific, Pittsburg, PA). The protein-containing supernatant was then separated by centrifugation at 5000 × g for 15min. Protein concentrations were measured by the bicinchoninic acid method (Pierce, Rockfor, IL). Then, 200ug of protein was separated on a 10% SDS-PAGE gel (Bio-Rad, Hercules, CA); the proteins on the gel were transferred electrically to a PVDF membrane (Millipore, Bedford, MA). The membranes were blocked using 5% milk in tris-buffered saline with TWEEN (TBS-T). To quantitate protein and GAPDH on the same Western Blot, the membranes were cut between GAPDH and the specific proteins of interest: SGLT1 and GLUT2. The cut gels were then incubated overnight at 4°C with primary antibody (SGLT1 [1:3000] and GLUT2 [1:500] antibody from Chemicon International, Temecula, CA; GAPDH [1:500] antibody from US Biological, Swampscott, MA). After incubation with the primary antibody, the membranes were rinsed three times with TBS-T and incubated with a secondary antibody (1:10000) in TBS-T containing 5% milk. Horseradish peroxidase-conjugated, goat anti-rabbit IgG was used for SGLT1 and GLUT2, (Sigma, St. Louis, MO and Upstate, Lake Placid, NY, resp), and horseradish peroxidase-conjugated, goat anti-mouse IgG was used for GAPDH (Sigma, St. Louis, MO). After rinsing, the protein bands were visualized with a colorimetric reaction using Opti-4CN (Bio-Rad, Hercules, CA) substrate. Amplified Opti-4CN substrate kit (Bio-Rad) was used to enhance the SGLT1 and GLUT2 bands. The membranes were scanned, and the Scion Range program (Scion Corp, MA) was used for semi-quantitative measurements of protein levels based on band densitometry. All protein measurements were normalized to levels of GAPDH. Western blots for GLUT5 were unable to be performed because of lack of a reliable antibody to GLUT5 despite multiple attempts to purchase and develop our own antibody.
Glucose Absorption
We measured transporter-mediated glucose absorption using a modified everted sleeve technique as described by Karasov and Diamond (29). The targeted segment of intestine was everted over a steel rod with pre-formed grooves at 1-cm intervals so that the mucosal surface was exposed externally. The segment was secured with two 5-0 silk ties, and the redundant edges were excised leaving a 1-cm everted segment. These intestinal sleeves were kept in chilled (4°C) mammalian Ringers bubbled with 95% O2/5% CO2 until ready for absorption experiments. Prior to measurements of absorption, the tissues were transferred to a 38°C bath, preincubated in 8 ml of mammalian Ringers bubbled with 95% O2/5% CO2 for 5min, and then placed in 8-ml of 38°C mammalian Ringers with iso-osmotic replacement of NaCl using either 1, 20, or 50mM d-glucose. The solution was stirred at 1,200 rpm to mix the “unstirred layer.” Radiolabelled glucose probes (0.5 to 1 μCi of 14C-D-glucose and 1 to 2 μCi of 3H-L-glucose) were included in the test solution to measure the different modes (active, passive) of glucose absorption (see below). After incubation in the glucose solution for 1 min, the tissues were removed, rinsed quickly in 30 ml of chilled mammalian Ringers stirred at 1,200 rpm, and placed in glass scintillation vials. One-half ml of tissue solubilizer (Perkin-Elmer, Boston, MA) was added with the segments kept in a 50°C water bath for 3 h. After complete solubilization, 10 ml of scintillation counting cocktail (Opti-Flour, Perkin-Elmer, Shelton, CT) was added, and probe counts were determined using techniques of dual-marker liquid scintillation counting with a standard quench curve.
Extensive preliminary validation studies using the everted sleeve technique for transporter-mediated glucose absorption were carried out previously in our laboratory using jejunal segments of 300- to 350-g Sprague-Dawley rats (Harlan, Indianapolis, IN) incubated for varying time periods (0.5, 1, 2, 4, or 8min) at six different glucose concentrations (1, 5, 10, 15, 20, and 50 mM) to determine the kinetics of the transporter system and select optimal incubation times and concentrations. Michaelis-Menten plots were made, and Km and Vmax values were calculated using Lineweaver Burke plots and were compared to published values for validation (30,31). Additionally, phlorizin, a known inhibitor of the primary apical glucose transporter, SGLT1, was used at 0.05 mM, 0.1 mM, and 0.2 mM concentrations to antagonize transporter-mediated glucose absorption to further validate the technique (32-34).
Analysis of Data
mRNA and protein levels
To determine relative changes in gene expression of mRNA and protein levels, the measurements of SGLT1, GLUT2, and GLUT5 were normalized to levels of GAPDH, the housekeeping gene. For mRNA, all samples to be compared were subjected to the same reverse transcription run, in duplicate (values meaned), and run simultaneously during the real time RT-PCR. Similarly, the relative expressions of protein for SGLT1 and GLUT2 were compared on the same Western Blot to prevent potential errors in loading; all samples were also run in duplicate (values meaned). Values of mRNA, protein, and glucose uptake were meaned across rats in each group and a grand mean calculated per group.
Glucose absorption
To calculate transporter-mediated glucose uptake, total glucose uptake needed to be corrected for any adherent glucose in the unstirred (non-absorbed) layer and for passive, non-carrier mediated absorption. 3H-L-glucose is not absorbed by transporter-mediated uptake and was thus used to correct for this adherent glucose and passive uptake. Carrier-mediated uptake was measured in duplicate for each segment and calculated as nmol·cm-1·min-1. Uptake was plotted as Lineweaver-Burke plots to allow calculation of Vmax and Km using classic Michaelis-Menten kinetics.
Statistical Analysis
Statistical analysis was performed using JMP software. Kruskal-Wallace was used to make comparisons across study group with Wilcoxon rank sums used for direct comparisons of groups. P values were corrected according to the Bonferoni method, and a corrected p value of ≤ 0.05 was considered significant. All data are reported as the median ± interquartile range or mean ±SE unless otherwise specified; n values are number of rats.
Results
All surviving animals tolerated the experimental procedures. No differences in chow consumption were appreciated over this 8-wk period either (data not shown).
Transporter Expression
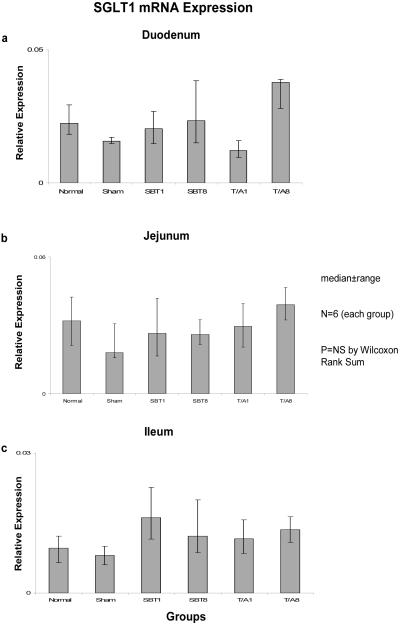
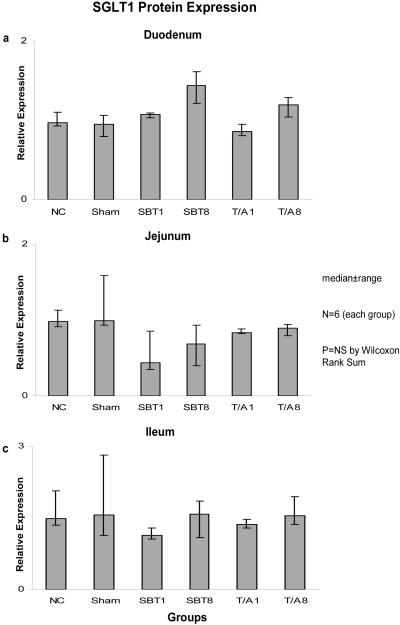
Relative expression of SGLT1 mRNA among the six groups was not different in duodenum (0.02-0.03, p=0.2), jejunum (0.02-0.04, p=0.6), nor ileum (0.01-0.02, p=0.2) (Figure 1). Relative expression of mRNA was less in ileum versus duodenum and jejunum. Similarly, protein expression as measured by semiquantitative Western blotting techniques did not differ among the six groups in duodenum (0.85-1.14, p=0.1), jejunum (0.44-1.00, p=0.1), or ileum (1.11-1.57, p=0.4) (Figure 2).
Figure 1.
Expression of SGLT1 mRNA in (a) duodenum, (b) jejunum, and (c) ileum; the animal groups are as outlined in the text. mRNA is shown as “relative expression” whereby the copy numbers of the transporter transcripts of interest are normalized to the copy numbers of the housekeeping gene GAPDH in attempt to allow an estimate of total cellular expression per enterocyte. NC = naïve control, sham-sham celiotomy; SBT1 and SBT8 = small bowel iso-transplantation at 1 wk and 8 wk postoperatively, resp; and T/A1 and T/A8 = jejunal and ileal transection/reanastomosis at 1 wk and 8 wk postoperatively, resp..
Figure 2.
Expression of SGLT1 protein in (a) duodenum, (b) jejunum, and (c) ileum. SGLT1 protein is shown as “relative expression” whereby the semi-quantitative density measurements from the Western blots are normalized to the density measurements of the housekeeper protein GAPDH.
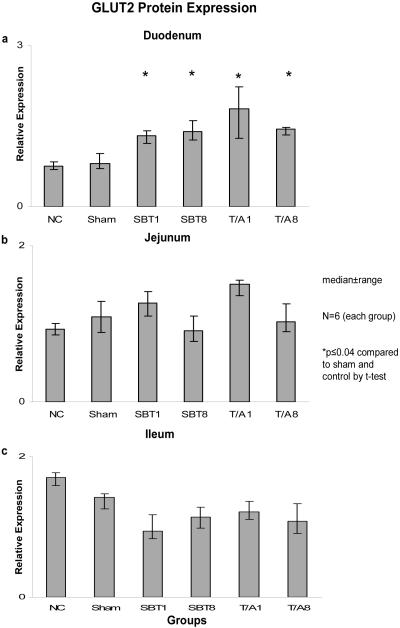
Relative expression of GLUT2 mRNA was also unchanged in duodenum (0.02-0.3, p=0.7), jejunum (0.02-0.03, p=0.6), and ileum (0.001-0.004, p=0.6) (Figure 3). Relative expression of mRNA was less in ileum versus duodenum and jejunum. Protein expression for GLUT2 demonstrated no difference in the jejunum (0.9 - 1.5, p>0.02) nor in the ileum (0.94-1.75, p=0.02). A difference was observed, however, across the duodenum (0.75-1.82, p=0.0004). GLUT2 protein expression was greater in the experimental groups (T/A1, TA/8, SBT1, and SBT8) compared to the sham and controls (p<0.04, Figure 4).
Figure 3.
Expression of GLUT2 mRNA in (a) duodenum, (b) jejunum, and (c) ileum. Relative expression of mRNA is as described in the legend of Figure 1.
Figure 4.
Expression of GLUT2 protein in (a) duodenum, (b) jejunum, and (c) ileum. Relative expression of protein is as described in the legend of Figure 2.
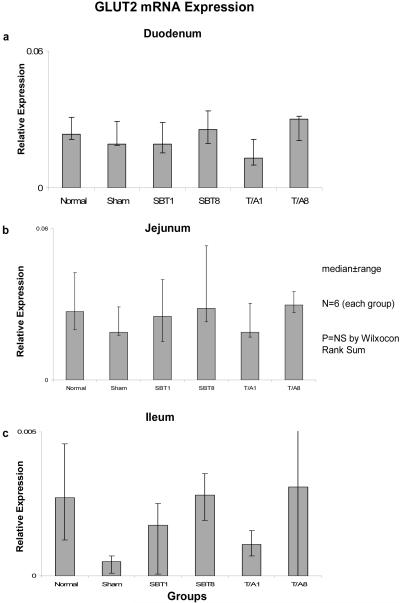
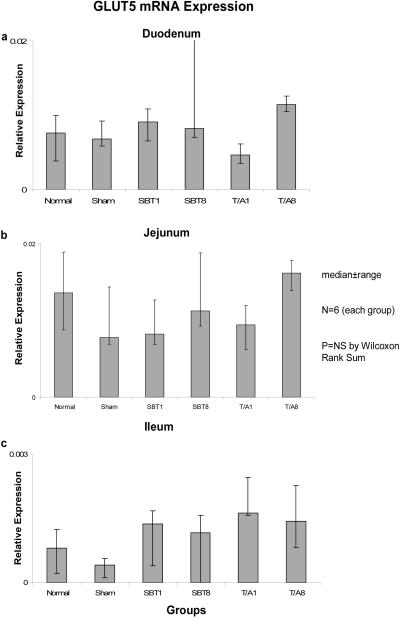
Relative expression of GLUT5 mRNA also remained unchanged in duodenum (0.001-0.004, p=0.3), jejunum (0.01-0.02, p=0.1), and ileum (0.001-0.002, p=0.1) (Figure 5), and relative expression of mRNA was much less (by a factor of 10) in ileum versus duodenum and jejunum.
Figure 5.
Expression of GLUT5 mRNA in (a) duodenum, (b) jejunum, and (c) ileum. Relative expression of mRNA is as described in the legend of Figure 1.
Glucose Uptake
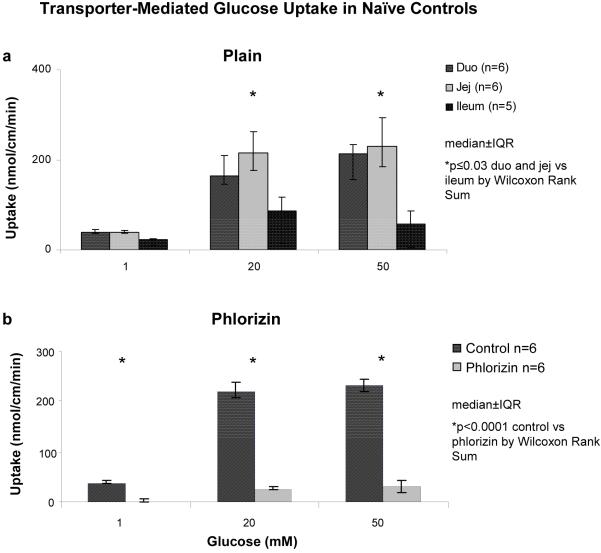
Transporter-mediated glucose uptake demonstrated saturation kinetics over the three substrate concentrations for all six groups in each intestinal segments (Figure 6A). Baseline kinetic values were determined using the naive controls. The Km in duodenum was less than in jejunum (4.5±0.3 vs 6.0±0.3 mM; p=0.002); Vmax was 3.6±0.2 nmol/cm/min in duodenum and 4.6±0.3 nmol/cm/min in jejunum (p=0.002). Total measured transporter-mediated uptake was also greatest in jejunum and duodenum compared to ileum in the controls at saturation levels of substrate (p≤0.03)(Figure 6A). Phlorizin (0.2mM), an SGLT1 inhibitor, resulted in near-complete inhibition of transporter-mediated glucose uptake in duodenum and jejunum (jejunal data shown, Figure 6B) and complete inhibition of transporter-mediated glucose uptake in the ileum compared to transport in the absence of phlorizin (data not shown, p<0.0001). The sham group exhibited comparable findings to the controls, although there was no difference in Km measured between duodenum and jejunum (4.4±0.3 mM vs 4.0±0.5 mM, p=0.06).
Figure 6.
Transporter-mediated glucose uptake in controls at (a) 1, 20, and 50 mM concentrations of d-glucose in all three segments in “plain” uptake solution; (b) effect in jejunum of 0.2 mM phlorizin (SGLT1 inhibitor) in uptake solution.
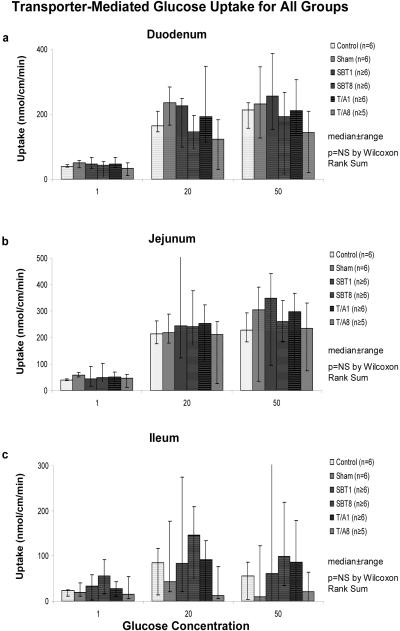
In the T/A1 and T/A8 groups, no change in total transporter-mediated glucose uptake was evident in duodenum, jejunum, or ileum compared to the controls and sham groups (Figure 7). Furthermore, calculated Km and Vmax values for duodenum and jejunum were also unchanged compared to controls. Km did not differ in duodenum and jejunum (T/A1: 4.0±1 vs 5.3±0.5 mM, p=0.1; T/A8: 3.5±0.4 vs 4.6±0.5 mM, p=0.2).
Figure 7.
Transporter-mediated glucose uptake in all groups in (a) duodenum, (b) jejunum, and (c) ileum.
In both SBT1 and SBT8, total transporter-mediated glucose uptake also was unchanged compared to controls (Figure 7). Calculated Km and Vmax values were similar as well. Vmax was also similar in transplanted jejunum compared to native duodenum (SBT1: 5.6±0.7 vs 5.0±0.4 nmol/cm/min, p=0.06; SBT8: 4.6±0.6 vs 3.6±0.4 nmol/cm/min, p=0.15). Furthermore, transplanted jejunal Km was not different from native duodenal Km (SBT1: 5±1 vs 6.2±1 mM; p=0.12 and SBT8: 4.6±1 vs 4.8±1 mM, p=0.15).
Discussion
The exact regulatory mechanisms controlling and modulating hexose transporter expression and function have not been elucidated comprehensively. Because expression and function of the hexose transporters vary diurnally (27,28), we hypothesized that expression and function is mediated predominantly by neural mechanisms and that complete extrinsic intestinal denervation, such as that inherent to SBT (whereby the extrinsic neurons to the gut are transected completely and continuity of the intrinsic, enteric myoneural innervation is disrupted) would cause a decrease in intestinal hexose transporter expression and a subsequent decrease in hexose transport. To further implicate a specific neural pathway (extrinsic versus intrinsic [intramural] neural continuity), we included a group of animals with complete but selective disruption of intrinsic, intestinal myoneural continuity to the jejunoileum from the duodenum proximally and the large intestine distally via transection and reanastomosis of the proximal and distal small intestine; we carefully, however, maintained the extrinsic neural input. We elected to study hexose transporter expression and function at 9AM because this represents baseline expression and function and circumvents confounding factors such as luminal substrates.
There were no differences in relative expression of mRNA for all three of the intestinal hexose transporters (SGLT1, GLUT2, and GLUT5) compared to the housekeeping gene, GAPDH, in native duodenum or in the jejunoileum after intestinal transection/reanastomosis or SBT. These findings were consistent regarding protein expression of SGLT1 and GLUT2 as well. While a minor difference was observed for GLUT2 protein expression only in the duodenum, the fold change was so small that this likely bears no clinical importance; the mild increase was noted in both experimental groups (SBT and T/A) and did not occur in the sham operated group, and thus may represent an effect of intestinal transection. Our study cannot shed further insight into this small change in mRNA expression. Additionally, the findings with SGLT1 correlated with functional uptake studies of glucose using the everted sleeve technique, where transporter-mediated glucose uptake remained unchanged across all six experimental groups for each intestinal segment. Furthermore, kinetic analyses of receptor affinity (Km) and number of functional transporters (Vmax) also did not demonstrate any changes after denervation (intrinsic or extrinsic) of the small intestine. GLUT5 transports primarily fructose, and our functional studies did not assess GLUT5 activity.
Our study also analyzed GLUT5 levels of mRNA. GLUT5 transports fructose which is one of the primary hexoses ingested by rats. Any implications about GLUT5 function are limited, because we have no good means of measuring GLUT5 protein, and we did not assess fructose uptake because this study was designed to address glucose uptake. We can, however, say that GLUT5 mRNA, similar to GLUT2, is expressed to very low levels under baseline conditions in the ileum, most likely because virtually all fructose is absorbed in the proximal gut. This finding of a marked decrease in GLUT5 and GLUT2 mRNA in the ileum differs from the relative expression of mRNA for other mucosal transporters such as SGLT1; in our study, although mRNA for SGLT1 is somewhat less than in the duodenum and jejunum, the relative decrease is much smaller than for GLUT2 or GLUT5. In addition, mRNA for the peptide transporter PEPT1 is expressed in similar relative copy numbers to its expression in the duodenum and jejunum (35), possibly because of more protein reaching the distal small intestine from enterocyte shedding or other processes requiring uptake of protein.
These findings suggest that extrinsic and intrinsic intestinal innervations do not appear to play a major role in regulating baseline gene expression or function of hexose transporters in the jejunoileum. The malabsorptive syndromes and diarrhea seen after SBT and intestinal resections do not appear to be secondary to abnormalities in hexose transporters, either in their expression (mRNA, protein) or their function (glucose uptake). Abnormalities in enteric function may relate solely to changes in motility affecting luminal substrate concentrations or abnormalities in water and electrolyte absorption and secretion secondary to extrinsic denervation, or they may be related to other unidentified mechanisms not related to intestinal denervation (3,5,7,36-39).
A novel mechanism may lie in the results observed for the Km values in the controls. Based on the traditional concept of intestinal hexose transport, SGLT1 is the only available transporter at the apical membrane for glucose transport and the remainder of glucose absorption occurs via solvent drag (40-43). In the controls, however, the calculated Km was significantly greater in the jejunum than in the duodenum, suggesting that there is either a conformational change in SGLT1 expression in the duodenum versus the jejunum possibly by post-translational processing or there is more than one transporter involved in apical glucose absorption. Recent studies have suggested that GLUT2, a facilitated glucose transporter, is trafficked to the apical membrane via signaling through SGLT1 and accounts for the dramatic rapid increase in glucose absorption beyond saturation levels (44-47). This difference in Km (duodenum compared to jejunum) was not seen in the other five groups, which may represent an unremarkable finding, or it may represent an effect on the enterocytes of intestinal denervation and/or postoperative inflammation (because this finding was not observed in the shams either). In this proposed mechanism, expression of mRNA or protein for the hexose transporter is unchanged, but the ratio of functional transporters (SGLT1, GLUT2, or others) that are present at the apical membrane is altered, which suggests a post-translational mechanism. Clearly, the data presented here are insufficient to solidify this model and further investigations into apical, transporter-mediated glucose uptake and regulation of hexose transporter expression and function are indicated.
Conclusion
Intestinal denervation after syngeneic SBT does not affect expression of intestinal hexose transporters or function. The glucose transporter, SGLT1, may have different conformations dependent on its anatomic location along the intestine, or apical transporter-mediated glucose absorption may be mediated by more than one glucose transporter whereby post-translational regulation of hexose transporter function may serve as a neurally mediated pathway for intestinal adaptation.
Acknowledgements
The authors are grateful to Sigurd Lenzen and Christopher Corpe for providing the entire cDNA sequences for GLUT2 and GLUT5, resp., and to Deborah Frank for her assistance in the preparation of the manuscript.
This work was supported in part by a grant from the National Institutes of Health (DK39337 - Dr. Michael G. Sarr, Principle Investigator).
Abbreviations Used in Text
- GAPDH
glyceraldehyde-6-phosphate dehydrogenase
- GLUT2
glucose transporter-2
- GLUT5
glucose tranporter-5
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcription polymerase chain reaction
- SBT
small bowel transplantation
- SBT1
1 wk after small bowel transplantation
- SBT8
8 wk after small bowel transplantation
- SGLT1
sodium-glucose transporter-1
- SMA
superior mesenteric artery
- T/A1
1 wk after transection and reanastomosis
- T/A8
8 wk after transection and reanastomosis
- TBS-T
Tris-buffered saline with toluene
Footnotes
This work was presented in part at the Academic Surgical Congress Phoenix, AZ February 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delegge M, Alsolaiman MM, Bassas S, et al. Short bowel syndrome: parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci. 2007;52:876–892. doi: 10.1007/s10620-006-9416-6. [DOI] [PubMed] [Google Scholar]
- 2.Castillo RO, Zarge R, Cox K, et al. Pediatric intestinal transplantation at Packard Children’s Hospital/Stanford University Medical Center: report of a four-year experience. Transp Proc. 2006;38(6):1716–1717. doi: 10.1016/j.transproceed.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Kasparek MS, Iqbal CW, Sarr MG, et al. Role of VIP and substance P in NANC innervation in the longitudinal smooth muscle of the rat jejunum: influence of extrinsic denervation. J Surg Res. 2007;141:22–30. doi: 10.1016/j.jss.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Rovera G, Furukawa H, Reyes J, et al. The use of clonidine for the treatment of high intestinal output following small bowel transplantation. Transp Proc. 1997;29:1853–1854. doi: 10.1016/s0041-1345(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 5.Sarr MG, Duenes JA, Walters AM. Jejunal and ileal absorptive function after a model of canine jejunoileal autotransplantation. J Surg Res. 1991;51:233–239. doi: 10.1016/0022-4804(91)90100-z. [DOI] [PubMed] [Google Scholar]
- 6.Mikko PP, Pirinen P, Lauronen J, et al. Effects of transection and extrinsic denervation and a model of autotransplantation of the porcine jejunoileum on cholesterol biodynamics. J Ped Surg. 2003;38(11):1585–1590. doi: 10.1016/s0022-3468(03)00569-4. [DOI] [PubMed] [Google Scholar]
- 7.Libsch KD, Duininck TM, Sarr MG. Ileal resection enhances jejunal absorptive adaptation for water and electrolytes to extrinsic denervation: implications for segmental bowel transplantation. J Ped Surg. 38(3):502–507. doi: 10.1053/jpsu.2003.50088. [DOI] [PubMed] [Google Scholar]
- 8.Tsiotos GG, Kendrick ML, Sarr MG, et al. Ileal absorptive adaptation to jejunal resection and extrinsic denervation: implications for living-related small bowel transplantation. J Gastrointest Surg. 2001;5:517–524. doi: 10.1016/s1091-255x(01)80090-1. [DOI] [PubMed] [Google Scholar]
- 9.Nakaa A, Moore BA, Bauer AJ, et al. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut. 2003;52:1278–1285. doi: 10.1136/gut.52.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugitani A, Reynolds JC, Todo S. Immunohistochemical study of enteric nervous system after small bowel transplantation in humans. Digest Dis Sci. 1994;39:2448–2456. doi: 10.1007/BF02087666. [DOI] [PubMed] [Google Scholar]
- 11.Nishizaki K, Nakao K, Noguchi K, et al. Induction of neuronal nitric oxide synthase by sympathetic denervation is mediated via 2-adrenoreceptors in the jejunal myenteric plexus. Brain Res. 2003;965:121–129. doi: 10.1016/s0006-8993(02)04148-3. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto N, Ohyanag H. Effect of transplantation on mucosal disaccharidase activity and tissue peptide in a canine small intestine model. Transplant Proc. 2002;34:1013–1014. doi: 10.1016/s0041-1345(02)02694-5. [DOI] [PubMed] [Google Scholar]
- 13.Goyal RK, Hirano I. The enteric nervous system. N Eng J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 14.Grundy D, Schemann M. Enteric nervous system. Curr Opinion Gastroenterol. 2007;23:121–126. doi: 10.1097/MOG.0b013e3280287a23. [DOI] [PubMed] [Google Scholar]
- 15.Schemann M. Control of gastrointestinal motility by the “gut brain” -the enteric nervous system. J Ped Gastrointest Nutr. 2005;41:S4–S6. doi: 10.1097/01.scs.0000180285.51365.55. [DOI] [PubMed] [Google Scholar]
- 16.Lohmeier TE, Hildebrandt DA, Dwyer TM, et al. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension. 2007;49:373–379. doi: 10.1161/01.HYP.0000253507.56499.bb. [DOI] [PubMed] [Google Scholar]
- 17.Balsiger BM, Ohtani N, Sarr MG, et al. Chronic extrinsic denervation after small bowel transplantation in rat jejunum: effects and adaptation in nitrergic and non-nitrergic neuromuscular inhibitory mechanisms. Surgery. 2001;129:478–489. doi: 10.1067/msy.2001.112070. [DOI] [PubMed] [Google Scholar]
- 18.Houghton SG, Zarroug AE, Sarr MG, et al. The diurnal periodicity of hexose transporter mRNA and protein levels in the rat jejunum: role of vagal innervation. Surgery. 2006;139:542–549. doi: 10.1016/j.surg.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Tavakkolizadeh A, Ramsanahie A, Levitsky LL, et al. Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J Surg Res. 2005;129:73–78. doi: 10.1016/j.jss.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Goto S. Diagnosis and treatment of Hirschsprung’s disease in Japan: an analysis of 1628 patients. Ann Surg. 1984;199(4):400–405. doi: 10.1097/00000658-198404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland-Cunz s, Goppl M, Rauch U, et al. Acquired intestinal aganglionosis after a lytic infection with varicella-zoster virus. J Ped Surg. 2006;41(3):e29–31. doi: 10.1016/j.jpedsurg.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 22.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol. 2004;286:G964–G972. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 23.Murr MM, Miller VM, Sarr MG. Contractile properties of enteric smooth muscle after small bowel transplantation in rats. Am J Surg. 1996;171:212–218. doi: 10.1016/S0002-9610(99)80102-0. [DOI] [PubMed] [Google Scholar]
- 24.Corpe CP, Burant CF. Hexose transporter expression in rat small intestine: effect of diet on diurnal variations. Am J Physiol. 1996;271:G211–G216. doi: 10.1152/ajpgi.1996.271.1.G211. [DOI] [PubMed] [Google Scholar]
- 25.Pan X, Terada T, Okuda M, et al. The diurnal rhythm of the intestinal transporters SGLT1 and PEPT1 is regulated by the feeding conditions in rats. J Nutr. 2004;134:2211–2215. doi: 10.1093/jn/134.9.2211. [DOI] [PubMed] [Google Scholar]
- 26.Houghton SG, Iqbal CW, Duenes JA, Fatima J, Kasparek MS, Sarr MG. Coordinated, diurnal hexose transporter expression in rat small bowel: implications for small bowel resection. Surgery. 2008;143:79–93. doi: 10.1016/j.surg.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butsin SA. Quantification of mRNA using real-time reverse transcriptase PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 28.Houghton SG, Cockerill FR. Real-time PCR: overview and applications. Surgery. 2006;139:1–5. doi: 10.1016/j.surg.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Karasov WH, Diamond JM. A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol. 1983;152:105–116. [Google Scholar]
- 30.Rickenbacher A, Seiler R, Honegger U, et al. Role of beta1-, beta2-, and beta3-adrenoceptors in contractile hypersensitivity in a model of small bowel transplantation. Surgery. 2008;143:94–102. doi: 10.1016/j.surg.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Zheyu C, Lunan Y. Early changes of small intestine function in rats after liver transplantation. Transplant Proc. 2006;38:1564–1568. doi: 10.1016/j.transproceed.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Tomita R, Fujisaki S, Park E, et al. Substance P and vasoactive intestinal peptide in rat small-bowel isografts. Am J Surg. 2005;189:63–70. doi: 10.1016/j.amjsurg.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Beiler HA, Schafer KH, Hagl C, et al. Histologic changes in neuronal innervation of the ileum mucosa after autologic-allotopic ileum mucosa transplantation. Pediatr Surg Int. 2004;20:96–100. doi: 10.1007/s00383-003-1095-1. [DOI] [PubMed] [Google Scholar]
- 34.Lee EA, Weiss SL, Diamond JM. A method for assaying intestinal brush-border sucrase in an in tact intestinal preparation. Proc Natl Acad Sci. 1998;95:2111–2116. doi: 10.1073/pnas.95.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qandeel H, Duenes J, Zheng Y, Sarr M. Expression and function of peptide transporter (PEPT1): diurnal and anatomic variation in rat small intestine. J Surg Res. doi: 10.1016/j.jss.2009.03.052. Abstract. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss SL, Lee EA, Diamond JM. Evolutionary matches of enzyme and transporter capacities to dietary substrate loads in the intestinal brush border. Proc Natl Acad Sci. 1998;95:2117–2121. doi: 10.1073/pnas.95.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oulianova N, Falk S, Berteloot A. Two-step mechanism of phlorizin binding to the SGLT1 protein in the kidney. J Membrane Biol. 2001;179:223–242. doi: 10.1007/s002320010049. [DOI] [PubMed] [Google Scholar]
- 38.Loike JD, Hickman S, Kuang K, et al. Sodium-glucose cotransporters display sodium- and phlorizin-dependent water permeability. Am J Physiol. 1996;40:C1774–C1779. doi: 10.1152/ajpcell.1996.271.5.C1774. [DOI] [PubMed] [Google Scholar]
- 39.Manome SH, Kuriaki K. Effects of insulin, phlorizin and some metabolic inhibitors on the glucose absorption from the small intestine. Arch Internatl Pharmacodyn Ther. 1961;130:187–194. [PubMed] [Google Scholar]
- 40.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membrane Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 41.Drozdowski LA, Thomson ABR. Intestinal sugar transport. World J Gastroenterol. 2006;12(11):1657–1670. doi: 10.3748/wjg.v12.i11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohl P, Saparov SM. Solvent drag across gramicidin channels demonstrated by microelectrodes. Biophysical J. 78:2426–2434. doi: 10.1016/S0006-3495(00)76786-5. 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullen TL, Muller M, Van Bruggen JT. Role of solute drag in intestinal transport. J Gen Physiol. 1985;85:347–363. doi: 10.1085/jgp.85.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helliwell PA, Richardson M, Kellett GL, et al. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000;350:149–154. [PMC free article] [PubMed] [Google Scholar]
- 46.Au A, Gupta A, Cheeseman CI, et al. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350:155–162. [PMC free article] [PubMed] [Google Scholar]