Abstract
Background
Notch1 regulates binary cell fate determination and is critical for angiogenesis and cardiovascular development. However, the pathophysiological role of Notch1 in the postnatal period is not known. We hypothesize that Notch1 signaling in vascular smooth muscle cells (SMC) may contribute to neointimal formation following vascular injury.
Methods and Results
We performed carotid artery ligation in wild-type (WT), control (smCre-Tg), general Notch1 heterozygous deficient (N1+/-), SMC-specific Notch1 heterozygous deficient (smN1+/-), and general Notch3 homozygous deficient (N3-/-) mice. Compared to WT or control mice, N1+/- and smN1+/- mice showed a 70% decrease in neointimal formation following carotid artery ligation. However, neointimal formation was similar between WT and N3-/- mice. Indeed, SMC derived from explanted aortas of either N1+/- or smN1+/- mice showed decreased chemotaxis and proliferation, and increased apoptosis compared to control or N3-/- mice. This correlated with decreased staining of PCNA positive cells and increased staining of cleaved Caspase-3 in the intima of N1+/- or smN1+/- mice. In SMC derived from CHF1/Hey2-/- mice, activation of Notch signaling did not lead to increase SMC proliferation or migration.
Conclusion
These findings indicate that Notch1, rather than Notch3, mediates SMC proliferation and neointimal formation following vascular injury through CHF1/Hey2 and suggest that therapies, which target Notch1/CHF1/Hey2 in SMC, may be beneficial in preventing vascular proliferative diseases.
Keywords: smooth muscle cells, proliferation, vascular injury, signal transduction, arteriosclerosis
Introduction
The Notch receptors are highly conserved membrane-bound receptors, which regulate binary cell fate determination and embryonic development 1. Binding of the Notch receptor ligands, Jagged or Delta-like, leads to enzymatic cleavage and nuclear translocation of the Notch intracellular domain (NICD). NICD binds to and de-represses the transcriptional repressors of the CSL family such as Cp-binding factor 1 (CBF-1), recombination signal sequence-binding protein-Jκ (RBP-Jκ), suppressor of hairless [Su(h)], and Lag-1, leading to transcriptional activation and cell fate determination 2, 3.
Mutation of Notch3 is responsible for the autosomal dominant inherited disorder called CADASIL syndrome, which is characterized by subcortical infarcts and leukoencephalopathy 4. Alternatively, mutation of the Notch ligand, Jagged, leads to Alagille syndrome 5. Interestingly, deletion of Notch3 in mice does not recapitulate CADASIL syndrome suggesting that hypermorphism of Notch3 mutation rather than its deletion may contribute to the observed disorder. In contrast, Notch1-deficient mice exhibit wide spread apoptosis of the CNS, impaired somitogenesis, and defective angiogenesis, and are lethal at embryonic day 9.5-10.5 6. Interestingly, all of these abnormal phenotypes including the cause of lethality of global Notch1-deficient mice are recapitulated in mutant mice with endothelial-specific deletion of Notch1 7, suggesting the critical role of endothelial Notch1 in embryonic development. The precise role of Notch1 in SMC, however, remains unknown.
Although recent in vitro studies have suggested that Notch1 may be important in regulating SMC proliferation 8 and differentiation 9, the pathophysiological correlate of these findings in vivo, especially in the postnatal period, has not been demonstrated. This is due, in part, to the embryonic lethality of Notch1-deficient mice and the lack of availability of mice with tissue-specific deletion of Notch1 in SMC. Accordingly, we have developed the SMC-specific Notch1 heterozygous-deficient mice, which were created by mating conditional mice harboring the Notch1-loxP-targeted allele with the SMC-specific Cre recombinase transgenic (smCre-Tg) mice. These mice, along with Notch1 heterozygous deficient (N1+/-) and Notch3 homozygous deficient (N3-/-) mice, were subjected to vascular injury to determine the role of Notch1 and Notch3 in mediating neointimal formation and vascular remodeling. Here we show that Notch1, rather than Notch3, is critical for SMC proliferation, migration and survival.
Materials and Methods
Animals
Notch1 general heterozygous knockout (Notch1+/- or N1+/-) and Notch3 general homozygous knockout (N3-/-) mice were generated as previously described 6, 10-12. SMC-specific deletion of Notch1 was accomplished by crossing Notch1flox/flox with smCre-Tg mice 7, 13. The background of all mice is C57Bl/6. Wild type (Notch1+/+) mice, smCre-Tg mice, and corresponding littermates served as controls for N1+/- and smN1+/- mice. The Standing Committee on Animal Care at Harvard Medical School approved all animal experimentation protocols.
Isolation and Mouse Endothelial and Smooth Muscle Cells
Endothelial cells (ECs) from hearts and SMCs from aortas were isolated from WT, smCre-Tg, Notch1flox/+, N1+/-, and smN1+/- mice. ECs were isolated using 2-sort antibody coated magnetic beads as described previously 14. The SMCs were obtained using collagenase and elastase digestion of aortas as previously described 15. The ECs were cultured with Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum (FBS), heparin, endothelial cell growth factor, and antibiotics (100 U/mL penicillin G, 100 μg/mL streptomycin sulfate). The SMCs were cultured with DMEM supplemented with 20% FBS, nonessential amino acids (GIBCO), and antibiotics. All ECs and SMCs used were of passages between 2 to 5.
Western Blot Analysis
Cells were incubated in lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mg of leupeptin per liter, and 1 mM phenylmethylsulfonyl fluoride). The cell lysates were separated on SDS-PAGE, transferred to PVDF membrane, and probed with polyclonal antibody for Notch1 (Santa Cruz Biotechnology, sc-6014), Notch3 (Santa Cruz Biotechnology, sc-7424), and actin (Sigma, A2066).
Carotid Artery Ligation
The mouse carotid artery was ligated with a 6-0 silk suture just proximal to the carotid bifurcation as described 16. At 1 and 4 weeks following the procedure, the carotid arteries were obtained and perfusion-fixed with 4% paraformaldehyde. The samples were then embedded in OCT compound and frozen. Five cryosections (each 6 μm thick) at 3 to 4 mm proximal to the ligation site were obtained in each animal. Areas of lumen, intima and media were measured in sections stained with hematoxylin and eosin (HE) stain and analyzed with NIH Image program (National Institutes of Health) as previously described 17. A mean value between 5 sections in each animal was used for analysis.
Immunofluorescence
Immunofluorescence for smooth muscle actin (Sigma, A2547), Notch1 (Santa Cruz Biotechnology, sc-6014), activated Notch1 (NICD, Abcam, ab8925), Notch2 (Rockland, 100-401-406), Notch3 (Santa Cruz Biotechnology, sc-7424), cleaved caspase3 (Cell signaling technology, 9664) and PCNA (Lab Vision, RB-9055) was performed by standard procedures.
SMC Migration and Chemotaxis Assay
Explants of SMC outgrowth from aortas were assessed as described previously 18. Briefly Aortic explants (1×1 mm) from WT, smCre-Tg, N1+/-, and smN1+/- mice were placed with the luminalside down and allowed to attach to the surface of a 24-well plate. The SMC culture medium was changed every 3 days. The number of SMCs migrated from the explants was assessed after 3 and 7 days. In some studies, SMC from wild-type or CHF1 mutant mice were infected with Ad.GFP or Ad.NICD at MOI of 100 for 24 hours. Chemotactic migration was measured using a modified Boyden chamber with gelatin-coated 8-μm-pore polycarbonate filter as previously described 19. Briefly, 5 × 104 SMC from control and Notch mutant mice were suspended in DMEM containing 1% FBS in the upper chamber of a 48-well Boyden chamber apparatus with serum stimuli in the lower chamber for 4 hours. The cells that migrated to the lower sides of the filter membranes were fixed and stained with Diff-Quick stain set (Dade Behring). Experiments were performed at least three times in quadruplicate.
Proliferation Assay
Proliferation assay was performed as previously described 20. Briefly, SMCs (100 μl, 2,500 cells) were cultured on gelatin-coated 96-well plates. In some studies, SMC from wild-type or CHF1 mutant mice were infected with Ad.GFP or Ad.NICD at MOI of 100 for 24 hours. After 72 h of incubation, cells were fixed and stained with 0.1% crystal violet solution. The number of cells was quantified by measurement of absorbance at 590 nm with a microplate reader (SPECTRA Fluor, TECAM). Experiments were performed at least 3 times in triplicate.
Cellular DNA synthesis was assessed by [3H]thymidine uptake as previously shown 21. SMCs were seeded on 1% gelatin-coated 24-well plates at 104 cells/well and incubated in culture medium for 24 h, followed by serum starvation. Cells were incubated in SMC culture medium for 16h. Then 0.5 μCi of [3H] thymidine and 0.5μg of thymidine (Sigma) were added per well. After 6h, cells were washed and fixed (10 min on ice with 5% trichloroacetic acid), the DNA was released by alkaline lysis (0.5 N NaOH), and supernatants were quantified in a beta-counter (Beckmann LS6000SC; Beckman Coulter, Somerset, NJ). Experiments were performed at least 3 times in triplicate.
Assessment of Apoptosis
Annexin V staining was used for assessment of apoptotic cells after 24-hour exposure to 50 μmol/L H2O2 followed by serum starvation 22. After staining treated SMCs (passage 2 to 4) with EGFP-Annexin V solution (1:40; Clontech) and 4', 6'-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma), Annexin V-EGFP-positive cells with nuclear cleavage were evaluated. Random fields were chosen and up to 2000 cells were counted for the analysis 23.
Statistical Analysis
Results were expressed as mean ± SD. The data among groups were compared using either 1-way or 2-way ANOVA followed by Bonferroni's post hoc test for multiple comparisons. A P value of less than 0.05 was taken to be statistically significant.
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Vascular Notch1 Expression in N1+/- and smN1+/- Mice
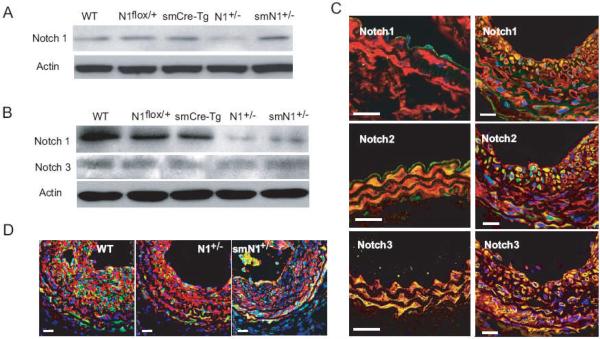
Primary cultures of EC and SMC from WT, smCre-Tg, Notch1flox/+, N1+/-, and smN1+/- mice were obtained. In EC, the Notch1 expression was decreased by half in N1+/- but not smN1+/- mice compared to that of WT or control mice (Figure 1A). However, in SMC, Notch1 expression was decreased by more than half in both N1+/- and smN1+/- mice (Figure 1B). These findings indicate specificity of Cre-mediated excision of Notch1 in SMC, but not in EC, in smN1+/- mice.
Figure 1. Expression of Notch receptors in endothelial and smooth muscle cells.
A. Western blot analysis shows that the expression of total Notch1 in EC isolated from hearts of WT, Notch1flox/+, smCre-Tg, N1+/-, and smN1+/- mice.
B. Western blot analysis shows that the expression of total Notch1 and Notch3 in SMC isolated from aortas of WT, Notch1flox/+, smCre-Tg, N1+/-, and smN1+/- mice.
C. Immunofluorescence staining of Notch1, Notch2, and Notch3 in uninjured carotid artery (left panel) and ligated injured carotid artery (14 days after ligation, right panel) of WT mice. Vessels were co-stained for smooth muscle actin (SMA). SMA is stained red; Notch receptors were stained green; and nuclei were stained blue (DAPI). Yellow indicates presence of both red and green staining. White bar was 20 μm.
D. Immunofluorescence co-staining of smooth muscle actin (SMA) with activated Notch1 (NICD). Red was SMA, green was NICD, and blue was DAPI. White bar was 20 μm.
Expression of Notch1, Notch2, and Notch3 Following Carotid Artery Ligation
Immunofluorescence staining of smooth muscle actin (SMA) and Notch1, Notch2, or Notch3 was performed in intact and ligated carotid artery (14 days after surgery) from WT mice (Figure 1C). In intact carotid artery, Notch1 was expressed mostly in ECs, Notch2 was expressed in both ECs and SMCs, whereas Notch3 was expressed mostly in medial SMCs. After ligation injury, Notch1 was highly expressed in neointimal SMCs, whereas Notch2 and Notch3 were constitutively expressed in medial and neointimal SMCs. These different patterns of expression suggest that Notch1, Notch2 and Notch3 may have distinct roles in SMC function. Indeed, compared to carotid artery from WT mice, activated Notch signaling as determined by staining for Notch intracellular domain (NICD) was substantially reduced in SMC of N1+/- and smN1+/- mice (Figure 1D). Thus, Notch1 and its downstream signaling pathway may be more temporally responsive and critical to SMC proliferation following vascular injury.
SMC Notch1 and Neointimal Formation
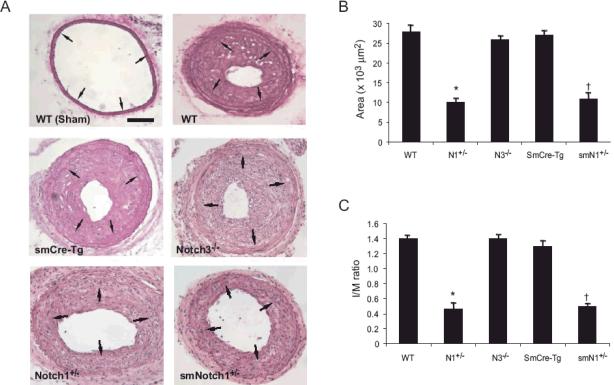
To determine whether Notch1 in SMCs is involved in vascular lesion formation, we performed carotid artery ligation in WT, smCre-Tg, N1+/-, and smN1+/- mice (n=10 in each group) (Figure 2A). There were no differences in hemodynamic parameters such as BP and HR between the groups of mice, either basally or following carotid artery ligation (data not shown). Following carotid artery ligation, WT and smCre-Tg mice exhibited substantial increase in the neointima as measured by the area between the lumen and the internal elastic lamina (2.86 ± 0.14 and 2.77 ± 0.13 × 104 μm2, respectively) (Figures 2B). However, the ligated carotid arteries from N1+/- and smN1+/- mice revealed less neointimal areas compared with those in WT and smCre-Tg mice (0.99 ± 0.10 and 0.98 ± 0.07 × 104 μm2, respectively, P<0.01 for both compared to WT or smCre-Tg). In contrast, neointimal areas of N3-/- mice (2.66 ± 0.18 × 104 μm2) was not different from that of WT (P=NS). The medial areas of the ligated carotid arteries were not different between the groups. Therefore, the intima to media (I/M) ratio was substantially reduced in ligated carotid arteries from N1+/- and smN1+/- mice (0.44 ± 0.05 and 0.45 ± 0.05, respectively) compared to that of WT or smCre-Tg mice (1.36 ± 0.20 and 1.25 ± 0.12, respectively, P<0.01) (Figure 2C). Furthermore, there was no difference in the neointimal areas and I/M ratio between ligated carotid arteries of N1+/- and smN1+/- mice, indicating that Notch1 in intimal SMC, and not in other cell types, is primarily responsible for neointima formation.
Figure 2. Neointimal formation in Notch mutant mice following vascular injury.
Carotid arteries were collected 28 days after the procedure. HE staining was performed on sections from WT, smCre-Tg, N1+/-, N3-/- and smN1+/- mice. Arrows indicate internal elastic lamina.
A. Representative sections are shown. Scale bar, 100 μm.
B. Quantitative morphometric analysis of intimal area in WT, smCre-Tg, N1+/-, N3-/- and smN1+/- mice. n=10 in each group, *; P<0.01, vs. WT; †; P<0.01, vs. smCre-Tg
C. Attenuated intima/media area ratio in Notch1 mutant, but not N3-/- mice compared with control mice. n=10 in each group, *; P<0.01, vs. WT; †; P<0.01, vs. smCre-Tg
Notch1 Mediates SMC Migration
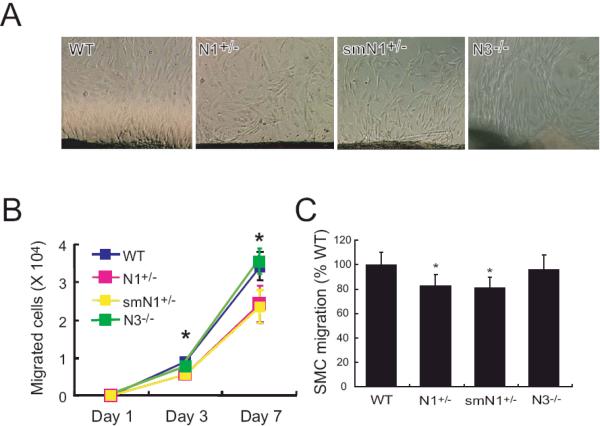
To determine whether the decrease in neointima formation in N1+/- and smN1+/- mice was due to decreased SMC migration and/or proliferation, we first investigated whether Notch1 regulates SMC migration. Using explants of aortas, we found that outgrowth of SMC from aortas of N1+/- and smN1+/- mice were substantially less compared with that of WT or N3-/- mice (WT vs. N1+/-, smN1+/-, and N3-/- mice: 7.9 ± 1.0 vs. 6.2 ± 0.8, 6.5 ± 1.1, and 7.3 ± 1.1 × 103 cells at 3 days, P<0.05; 3.1 ± 0.4 vs. 2.4 ± 0.5, 2.4 ± 0.4, and 3.3 ± 0.33 × 104 cells, at 7 days, P<0.05, n=8 in each group) (Figures 3A and 3B). The SMC outgrowth was similar between aortas of WT and smCre-Tg mice. To determine whether Notch1 could also mediate SMC chemotaxis, we isolated SMC from WT, N1+/-, smN1+/-, and N3-/- mice and determined their chemotactic response to serum in a modified Boyden chamber. Compared to WT or N3-/- mice, the migration of SMC in response to serum was decreased in SMC derived from N1+/- and smN1+/- mice (WT vs. N1+/-, smN1+/-, and N3-/- mice: 100 ± 10% vs. 83 ± 9%, 81 ± 9%, 96 ± 12%, P<0.05, n=12 in each group) (Figure 3C). These findings indicate that Notch1 is critically important for SMC migration in response to serum.
Figure 3. Migration of SMC in Notch mutant mice.
A. Micrographs of SMC migration from aortic explants from WT, N1+/-, smN1+/-, and N3-/- mice.
B. Quantification of SMC migration from aortic explants of WT, N1+/-, smN1+/-, and N3-/- mice. n=8, *; P<0.05
C. SMC chemotaxis in response to serum stimulation (percent of migrated WT SMC). n=12, *; P<0.05, vs. WT
Notch1 Mediates SMC Proliferation
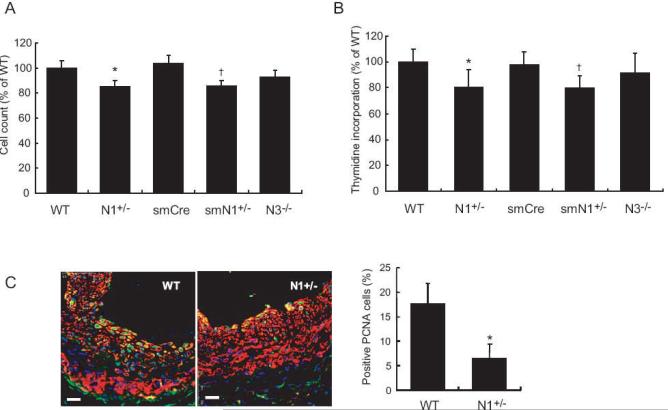
To determine whether Notch1 mediates SMC proliferation, we assessed whether indices of SMC proliferation are altered in SMC from N1+/- and smN1+/- mice. In response to serum, the cell counts for SMC from N1+/- and smN1+/- mice were decreased compared to that of WT or smCre-Tg mice (WT vs. N1+/- mice: 100 ± 6 vs. 85 ± 5%, P<0.05; smCre-Tg vs. smN1+/- mice: 104 ± 6 vs. 86 ± 4%, P<0.05, n=10 in each group) (Figure 4A). The cell counts were similar between WT and N3-/- mice (WT vs. N3-/- mice: 100 ± 6 vs. 93 ± 5%, P=N.S.). This correlated with decreased [3H] thymidine incorporation in SMCs of N1+/- and smN1+/- mice (WT vs. N1+/- mice: 100 ± 10 vs. 81 ± 13%, P<0.05; smCre-Tg vs. smN1+/- mice: 98 ± 10 vs. 80 ± 9%, P<0.05, n=6 in each group) (Figure 4B). The [3H] thymidine incorporation in SMCs were similar between WT and N3-/- mice (WT vs. N3-/- mice: 100 ± 10 vs. 92 ± 15%, P=N.S.). Following carotid artery ligation, increased PCNA staining was observed in the neointima of WT mice (18 ± 4% PCNA positive cells, n=6) (Figures 4C). In contrast, PCNA staining was substantially reduced in the neointima of N1+/- mice (7 ± 3% PCNA positive cells, n=6). The PCNA staining in the media was comparable between WT and N1+/- mice. These findings suggest that SMC Notch1 mediates neointima formation following vascular injury.
Figure 4. Proliferation of SMC in Notch mutant mice.
A. SMC proliferation in response to serum after 3days (percent of WT SMC). n=10, *; P<0.05, vs. WT; †; P<0.05, vs. smCre-Tg
B. [3H]Thymidine incorporation (percent of incorporation of WT SMC). n=6, *; P<0.05, vs. WT; †; P<0.05, vs. smCre-Tg
C. Left panel: Co-staining for SMA (red) and PCNA (green) 14 days after ligation injury in WT and N1+/- mice. White scale bar was 20 μm. Right panel: Quantification of PCNA positive cells in neointima of WT and N1+/- mice. n=6; *; P<0.05, vs. WT
Notch1 Mediates SMC Survival
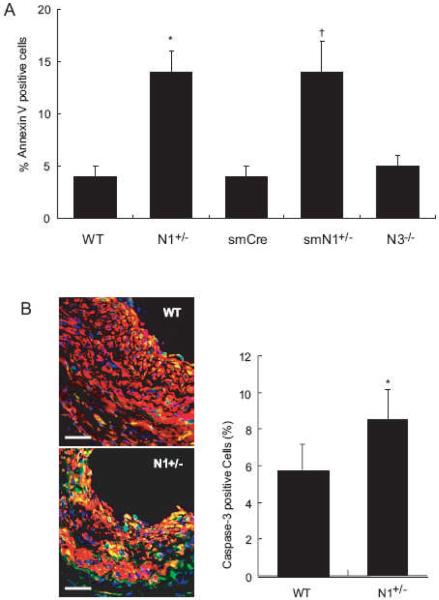
To determine whether haploinsufficiency of Notch1 leads to increase SMC apoptosis, we induced SMC apoptosis with 24-hour exposure to H2O2 (50 μmol/L) followed by immunofluorescence staining with DAPI and Annexin V. Haploinsufficiency of Notch1 in SMCs led to a substantial increase in the percentage of apoptotic nuclei (WT vs. N1+/- mice: 4 ± 1 vs. 14 ± 2%, P<0.01, n=10; smCre-Tg vs. smN1+/- mice: 4 ± 1 vs. 14 ± 3%, P<0.01, n=10) (Figure 5A). The percentage of apoptotic nuclei was comparable between WT and N3-/- mice (WT vs. N3-/- mice: 4 ± 1 vs. 5 ± 1%, P=N.S.). This correlated with increased Caspase-3 cleavage in the neointima of N1+/- mice compare to that of WT mice (WT vs. N1+/- mice 6 ± 1 vs. 9 ± 2%, P<0.05, n=6) (Figures 5B). These findings indicate that the decrease neointimal formation following vascular injury in N1+/- and smN1+/- mice is attributed to both decreased SMC proliferation and survival.
Figure 5. Apoptosis of SMC in Notch mutant mice.
A. H2O2-induced apoptosis of SMC. n=10, *; P<0.01, vs. WT; †; P<0.01, vs. smCre-Tg.
B. Left panel: Co-staining for SMA (red) and cleaved Caspase-3 (green) 14 days after ligation injury in WT and N1+/- mice. Right panel: Quantification of cleaved Caspase-3-positive cells in neointima from WT and N1+/- mice n=6, *; P<0.05, vs. WT
CHF1/Hey2 Mediates Notch Signaling in SMC
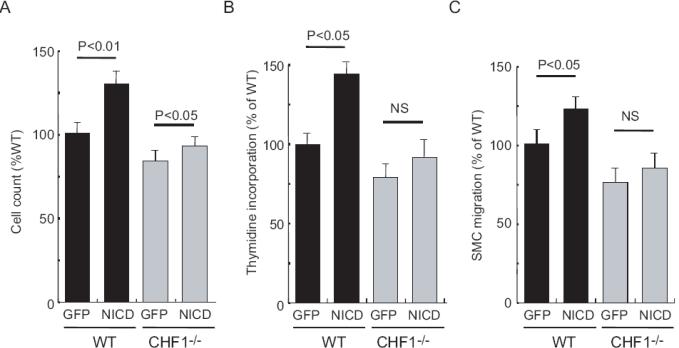
Previous studies show that CHF1/Hey2 regulate neointimal formation and vascular smooth muscle proliferation 24. Because CHF1/Hey2 is the mammalian homologue of the zebrafish gridlock and gridlock lies downstream of Notch 25, we investigated whether CHF1/Hey2 mediates Notch-induced SMC proliferation and migration. Quiescent SMC from WT mice proliferated in response to infection with adenovirus carrying Notch intracellular domain (NICD) (Figures 6A and 6B). However, SMC from CHF1-/- mice did not proliferate in response to Ad.NICD. Furthermore, increased migration was observed with Ad.NICD in SMC from WT, but not CHF1-/- mice (Figure 6C). These findings suggest that CHF1 may be the downstream mediator of Notch-induced SMC proliferation and migration.
Figure 6. CHF1/Hey2 mediates SMC proliferation and migration.
A. Effects of transfection with Ad.GFP or Ad.NICD on SMC proliferation (cell count) from WT or CHF1-/- mice. n=6
B. Effects of transfection with Ad.GFP or Ad.NICD on SMC proliferation (thymidine incorporation) from WT or CHF1-/- mice. n=6
C. Effects of transfection with Ad.GFP or Ad.NICD on SMC migration (% of WT) from WT or CHF1-/- mice. n=6
Discussion
We have shown that Notch1, but not Notch3, in SMC mediates neointimal formation following vascular injury. The mechanism is due, in part, to mediate SMC migration, proliferation, and survival. These findings indicate that Notch1 is an important regulator of SMC function in the postnatal period and suggests that therapy, which modulates Notch1 signaling in SMC, may be beneficial in treating vascular proliferative diseases. However, it should be noted that the role of Notch1 and Notch3 in SMC could also be dependent upon the model of vascular injury. Thus, it remains to be determined whether Notch1 is the sole Notch receptor in SMC mediating the response to vascular injury.
The downstream mediators of Notch signaling in the vasculature are incompletely understood. Previous studies have identified CHF1/Hey2 as a downstream target of Notch and as a regulator of neointimal formation after vascular injury 24. Loss of CHF1/Hey2 attenuates the response of SMC to growth factors and leads to decreased SMC proliferation in vivo and in vitro, through decreased activation of the small GTPase Rac1. To determine whether CHF1/Hey2 functions downstream of Notch in SMCs, we attempted to rescue the proliferation and migration defect in CHF1/Hey2 null SMCs by transfection of the Notch1 intracellular domain. Both proliferation and migration were blocked, indicating that CHF1/Hey2 is an important downstream mediator of Notch1 in SMCs. Identification of additional downstream components remains under investigation.
Notch signaling has been implicated in cell-to-cell communication and the regulation of cell fate in a variety of cell types 1, 2, 26. However, the role of Notch signaling in vascular cells, especially in the postnatal period, is not entirely known. Previous studies suggest that the expression of several Notch components, including Notch1, Notch2, and Notch3, were altered after vascular injury27, 28. Our results showed different expression patterns of Notch1, Notch2 and Notch3 after vascular injury. Neointimal SMC transiently expressed Notch1 after carotid artery ligation, whereas Notch2 and Notch3 expressions were constitutive in both medial and neointimal SMC. These findings suggest that Notch1, Notch2, and Notch3 may have distinct roles in SMC function with Notch1 being more responsive to vascular injury and critical to SMC proliferation.
Because bone marrow derived vascular progenitor cells could also contribute to neointimal formation in the carotid artery ligation model 29, we compared neointimal formation in N1+/- and smN1+/- mice in response to vascular injury. Notch1 expression in bone marrow cells in smN1+/- mice was similar to that of WT mice (data not shown). Furthermore, there was little or no difference in the amount of neointimal formation between global and SMC-specific Notch1 haploinsufficient mice, suggesting that Notch1 in SMC, and not in other cell types including bone marrow-derived progenitor cells, mediates neointimal formation. As a control for potential nonspecific effects of Cre recombinase expression in SMC, we also found no difference in neointimal formation between WT and smCre-Tg mice.
Transfection of Epstein Barr virus encoded gene product, RPMS-1, in SMC leads to attenuation of Notch signaling and decrease proliferation and survival of SMC 8. In contrast, activation of Notch signaling by overexpression of intercellular domain of Notch increases SMC proliferation and survival 8, 30. These results are consistent with our findings that SMC from Notch1 mutant mice exhibit impairment of cellular migration, proliferation, and survival. Indeed, a recent study suggests that SMC growth, migration, and apoptosis by Notch1 and Notch3 signaling are mediated by CBF-1/RBP-Jk 8. These effects of Notch1 may be the underlying mechanism contributing to the observed decrease in neointimal formation in our Notch1 mutant mice. However, the role of Notch signaling in SMC differentiation is somewhat controversial. In vitro studies suggest that augmentation of Notch signaling represses myocardin-induced SMC differentiation 9. In contrast, Notch3 deficient mice were reported to exhibit impairment of maturation and differentiation of SMC in small arteries 31. Thus, the role of Notch signaling in SMC differentiation requires further investigation.
In summary, we have shown that Notch1 in SMC is critical for neointimal formation following vascular injury. Notch1 mediates SMC migration, proliferation, and survival, and may be an important therapeutic target in vascular diseases involving excessive SMC proliferation such as restenosis, arteriosclerosis, and transplant-associated arteriopathy. It remains to be determined what some of the downstream targets of Notch1 are and how they regulate Notch1-mediated SMC function.
Acknowledgments
Funding Source This work was supported by grants from the National Institutes of Health (HL052233, HL080187, DK065992, NS036437). K. Takeshita is a recipient of the Japan Heart Foundation/Bayer-Yakuhin Research Grant Abroad. P. Liu is a recipient of the Scientist Physician Award from the National Health Research Institute in Taiwan.
Footnotes
Conflict of Interest There are no conflicts of interests to report for all of the authors.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 3.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 4.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 5.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 6.Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 7.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM, Cahill PA. Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk dependent pathway. Faseb J. 2004;18:1421–1423. doi: 10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- 9.Proweller A, Pear WS, Parmacek MS. Notch signaling represses myocardin-induced smooth muscle cell differentiation. J Biol Chem. 2005;280:8994–9004. doi: 10.1074/jbc.M413316200. [DOI] [PubMed] [Google Scholar]
- 10.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 11.Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 12.Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139–143. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- 13.Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics. 2002;10:211–215. doi: 10.1152/physiolgenomics.00054.2002. [DOI] [PubMed] [Google Scholar]
- 14.Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr., Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol. 2003;162:1591–1601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, Zou Y, Dietrich H, Wick G, Xu Q. Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation. 1999;100:861–868. doi: 10.1161/01.cir.100.8.861. [DOI] [PubMed] [Google Scholar]
- 16.Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest. 2006;116:334–343. doi: 10.1172/JCI26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 18.Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–2283. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bornfeldt KE, Raines EW, Nakano T, Graves LM, Krebs EG, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marra DE, Simoncini T, Liao JK. Inhibition of vascular smooth muscle cell proliferation by sodium salicylate mediated by upregulation of p21(Waf1) and p27(Kip1) Circulation. 2000;102:2124–2130. doi: 10.1161/01.cir.102.17.2124. [DOI] [PubMed] [Google Scholar]
- 21.Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1) J Biol Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 22.Li WG, Miller FJ, Jr., Brown MR, Chatterjee P, Aylsworth GR, Shao J, Spector AA, Oberley LW, Weintraub NL. Enhanced H(2)O(2)-induced cytotoxicity in “epithelioid” smooth muscle cells: implications for neointimal regression. Arterioscler Thromb Vasc Biol. 2000;20:1473–1479. doi: 10.1161/01.atv.20.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Liao JK. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2004;24:2238–2244. doi: 10.1161/01.ATV.0000147894.22300.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakata Y, Xiang F, Chen Z, Kiriyama Y, Kamei CN, Simon DI, Chin MT. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler Thromb Vasc Biol. 2004;24:2069–2074. doi: 10.1161/01.ATV.0000143936.77094.a4. [DOI] [PubMed] [Google Scholar]
- 25.Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Campos AH, Prince CZ, Mou Y, Pollman MJ. Coordinate Notch3-hairy-related transcription factor pathway regulation in response to arterial injury. Mediator role of platelet-derived growth factor and ERK. J Biol Chem. 2002;277:23165–23171. doi: 10.1074/jbc.M201409200. [DOI] [PubMed] [Google Scholar]
- 28.Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–883. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 30.Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res. 2005;96:567–575. doi: 10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- 31.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]