Abstract
Human adipose stem cells were cultured in smooth muscle inductive media and seeded into synthetic bladder composites to tissue engineer bladder smooth muscle. 85:15 poly-lactic-glycolic acid bladder dome composites were cast using an electropulled microfiber luminal surface combined with an outer porous sponge. Cell seeded bladders expressed smooth muscle actin, myosin heavy chain, calponinin, and caldesmon via RT-PCR and immunoflourescence. Nude rats (n=45) underwent removal of half their bladder and repair using: (i) augmentation with the adipose stem cell seeded composites, (ii) augmentation with a matched acellular composite, or (iii) suture closure. Animals were followed for 12 weeks post-implantation and bladders were explanted serially. Results showed that bladder capacity and compliance were maintained in the cell seeded group throughout the 12 weeks, but deteriorated in the acellular scaffold group sequentially with time. Control animals repaired with sutures regained their baseline bladder capacities by week 12, demonstrating a long term limitation of this model. Histological analysis of explanted materials demonstrated viable adipose stem cells and increasing smooth muscle mass in the cell seeded scaffolds with time. Tissue bath stimulation demonstrated smooth muscle contraction of the seeded implants but not the acellular implants after 12 weeks in vivo. Our study demonstrates the feasibility and short term physical properties of bladder tissue engineered from adipose stem cells.
Keywords: stem cell, smooth muscle cell, urinary tract, organ culture, bladder tissue engineering
INTRODUCTION
Surgical repair of the urinary bladder using tissue augmentation is often required in the management of bladders damaged by malignancies, trauma, spinal cord injuries, and congenital pediatric disorders such as spina bifida and bladder exstrophy. The surgery is traditionally performed using a pedicle flap of intestine to reconstruct a urinary reservoir. These reconstructive procedures are technically difficult and associated with numerous sequela including secondary malignancies, urinary calculi, intestinal adhesions, and chronic infections. With advancements in the fields of materials science and tissue engineering, interest has grown in using biomaterials synthetically designed for urinary storage to circumvent the problems above. Bladder biomaterials have been used alone, and with cell-seeding, for these purposes.
Experiments examining cell-seeded, ‘tissue-engineered’, bladder materials have been underway since 1992 and traditionally involved surgical harvest of a portion of a patient’s bladder to obtain a primary culture of bladder smooth muscle and urothelial cells. [1] These cell cultures were expanded in the laboratory for 6 to 8 weeks to achieve a cell mass capable of impregnating or seeding biodegradable tissue molds designed to act as scaffolds and infrastructure for the bladder cells. The reconstituted bladder cells seeded into the bladder scaffold formed the basis of an in vitro tissue engineered bladder. [2] Implantation of these and similar cellular engineered constructs into the bladders of rats, dogs, pigs, and humans were performed with promising results over the last several years. [3-6] The clinical utility of extrapolating these models to malignant and pathological bladders has been limited to date, due in part to the lack of healthy and accessible bladder tissue within the patient population in need of bladder replacement. Even within non-malignant bladders, investigators have found transmission of neuropathic cells from neuropathic bladders into the tissue engineered bladder. [7-9] Tissue engineering critics have also pointed out that the traditional bladder cell harvest procedures are surgically invasive, and the prolonged cell expansion times are expensive, subject to contamination, and unrealistic for routine clinical use.
Alternative sources of healthy and abundant bladder tissue are desired for optimal bladder engineering and bladder regeneration. Skeletal muscle cells, bone marrow stromal cells, embryonic, and parthenogenetic stem cells are all currently under investigation as potential future alternatives, however these modalities are in their infancy. For the purpose of this study, we turned to a stem cell source in the form of adipose stem cells (ASCs), and we attempted to tissue engineer bladder smooth muscle using ASCs processed from human lipoaspirate.
Adipose tissue is akin to muscle fibers and bone marrow stroma, in that it is derived from embryonic mesodermal origins and contains a supportive stroma of regenerative pluripotent progenitor cells. Within adipose tissue, we refer to those pluripotent cells as adipose stem cells (ASCs) in accordance to the consensus set forth by the Second Annual International Fat and Applied Technology meeting. [10] Clonal expansions derived from ASCs demonstrate multipotency and self-renewing capacity analogous to mesenchymal bone marrow cells.[11, 12] ASCs differentiate into myogenic, adipogenic, osteogenic, chrondrogenic, and neurogenic lineages in vitro when cultured in lineage specific conditions. [13] However, unlike bone marrow stem cells, which are difficult to isolate and relatively scarce, ASCs are tremendously abundant and easily accessible. The frequency of ASCs in an aspirate of adipose tissue is approximately 3%, as determined on the basis of adherent colony forming unit cells. In comparison, the frequency of similar cells in bone marrow aspirates is three orders of magnitude less. [11, 14, 15]
The multipotentiality of ASCs, combined with their vast availability, ease of procurement, and ability to differentiate into functional and contractile smooth muscle makes them an attractive source of smooth muscle for bladder tissue engineering. To investigate this hypothesis, we attempted to use ASCs as a cell source to tissue engineer urinary bladder muscle. We created a novel synthetic three dimensional bladder mold, impregnated it with smooth muscle cells derived from ASCs, and evaluated the tissue engineered bladder material in vitro, in vivo in a rat surgical model, and ex-vivo in isometric tissue baths.
MATERIALS AND METHODS
Isolation and Culture of Adipose Stem Cells (ASCs)
Human adipose stem cells (ASCs) were obtained from liposuction procedures under local anesthesia (HSPC#98-08 011-02) as previously described. [15] Raw lipoaspirate was washed with phosphate-buffered saline (PBS), digested with 0.075% collagenase (Sigma, St Louis, MO) and centrifuged. The cell pellet was suspended in 160mM NH4Cl for 10 min, filtered to 100μm, and incubated at 37°C/ 5% CO2 in non-inductive media consisting of DMEM (Mediatech, Herndon, VA), 10% Fetal Bovine Serum (FBS; HyClone, Logan, UT), and 1% antimicrobial solution (Penicillin G/Amphoteracin B/Streptomycin; Mediatech, Herndon, VA). Cell populations that demonstrated clonal differentiation into osteogenic, chondrogenic, and adipogenic lineages were considered adipose stem cells. [13]
Scaffold Construction
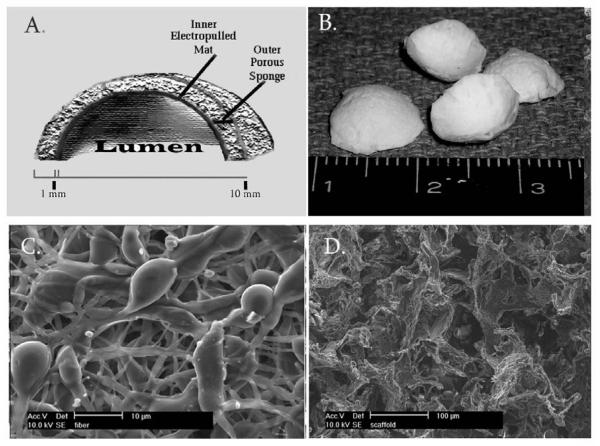
85:15 Poly(D,L-lactic-co-glycolic acid) was purchased (PLGA; latide:glycolide 85:15; viscosity 0.65 dL/g; Birmingham Polymers, Birmingham, AL) for preparation of the bladder scaffolds. The poly-lactic-glycolic acid (PLGA) microfiber mats that comprised the scaffold lumen were prepared using electropulling as previously described. [16] Briefly, PLGA was dissolved in chloroform (15% w/w; viscosity ∼150 cp) and pulled through a 25 gauge needle (BD, Franklin Lakes, NJ) using 28 kV DC. An additional electrode, located below the needle electrode and 15 cm away from the ground collector electrode, had a floating potential and functioned as second collector, and the PLGA solution was electropulled from the floating electrode toward the ground collector. The PLGA microfibers were collected on the distal electrode to form a fiber mat 1mm thick. The fiber mat was freeze dried at 100 mTorr and -110°C (VirTis, Warminster, PA) for 24 hours, then vacuum sealed until use. The outer PLGA sponge portion of the scaffold was fabricated from 85:15 PLGA by solvent casting and particulate leaching. PLGA (20% w/w) was dissolved in a 67/33 (w/w) mixture of chloroform/ methanol. Sucrose particles (diameter range= 100 – 200 μm) were added to create a 95% v/v sucrose:PLGA paste (95% porosity) . The paste was cast and shaped in Teflon rings (dia= 10mm; height= 1mm) and lypholized for 12 hours. Sucrose was leached from the casts in sterile distilled water. The luminal microfiber mats were fused to the outer porous sponges and shaped into a dome to create the final scaffold. To do so, the PLGA sponge was set on top of an equally shaped piece of microfiber mat and both were placed inside of a 5 liter chloroform vapor chamber (8mL 100% chloroform; 5mTorr) for 30 minutes under their own weight. After removal from the vapor chamber, they were shaped by gently placing them into a dome-shaped Teflon mold (inner dia= 10mm; concavity= 3mm) such that the PLGA mat formed the concave (luminal) surface of the final structure (FIG 1) and freeze dried for 24 hrs at 100 mTorr and -110°C. The final construct was disinfected in 3% hydrogen peroxide solution × 3 min, washed in sterile water, and stored in sterile PBS for up to 14 days until ready for clinical or analytical use. Scanning electron micrography(SEM) was used to assess the structural features of the scaffolds.
Figure 1. Construction of the three dimensional synthetic bladder composite.
A) Schematic and B) gross micrograph of the 3-dimensional bladder composite. C) PLGA electropulled microfibers comprising the luminal layer D) PLGA porous sponge was used as the outer layer.
Smooth muscle tissue engineering
Undifferentiated ASCs at passages 3 through 5 were differentiated into smooth muscle cells (SM-ASCs) by incubation in smooth muscle inductive media (SMIM) consisting of Medium MCDB 131, 1% FBS, 100 U/ml heparin for 6 weeks at 37°C/ 5% as previously described.[17] The media was changed every 5 days. Cell splitting was not required. ASCs in non-inductive media were used as controls. SM-ASCs were labeled with 1:200 dialkylcarbocyanine (Vybrant DiI; Molecular Probes, Eugene, OR) for 20min the day prior to scaffold seeding. For scaffold seeding, SM-ASCs were trypsinized, suspended in SMIM (1×106 cells/ 50uL), and pipette directly onto PLGA bladder scaffold (1×106 cells/ scaffold). Tissue engineered constructs were incubated at 37°C/ 5% CO2 in SMIM for 14 days. Unseeded PLGA scaffolds and scaffolds seeded with undifferentiated ASC were prepared identically as controls.
Assessment of SM-ASC viability and differentiation on PLGA scaffolds
SM-ASC density within the cell-seeded scaffolds was assessed using an epiflourescence inverted microscope filtered for DiI. Scaffolds were stained for live and dead cells with 4μM calcein acetoxymethyl and 2μM ethidium homodimer-1 (Invitrogen, Carlsbad, California). Total RNA was isolated from cell-seeded constructs at 14 days in vitro. RNA was lysed by rinsing the scaffolds directly in RNA lysis buffer (ß-mercaptoethanol/RLT; RNeasy Kit, Qiagen, CA). Two-step RT-PCR was performed using 1 μg of total RNA with reverse transcription × 1 hour at 42°C and cDNA amplification over 35 cycles (94°C × 30s, 53°C ×1 min, and 72°C × 1min) with 1 μM of 5′and 3′primer. The following primers were used: αSMA: (5′)ACCCACAATGTC-CCCATCTA, (3′)TGATCCACA-TCTGCTGGAAG, 595bp; MHC: (5′)GGACGACCTGGTTGTTGATT, (3′)GTAGCTGCTTGATGGCTTCC, 656bp; Calponin: (5′) ATGTCCTCTGC-TCACTTCA, (3′)TTTCCGCTCCTGCTTCTCT, 453bp; Caldesmon: (5′)AGATTGAAAGGCGAAGAGCA, (3′)TTCAAGCCAGCAGTTTCCTT, 397bp; SM22: (5′)ATGGCCAACAAGGGTCC, (3′)CTTCAAAGAGGTCAACAG, 349 bp; Smoothelin: (5′)ATGGCGGACGAGGCCTTAG, (3′)CCTCAATCTCCTGAGCCC, 358bp. Reaction products were analyzed by electrophoresis of 10μl aliquots in 1.5% agarose with ethidium bromide staining.
Real time quantitative PCR analysis was performed for human αSMA, calponin and SM22 message RNA using an ABI 7000 Prism Sequence detection system with 900nM of upper and lower primer, 250nM Taqman probe, 50ng cDNA and 1× PCR master mix (Promega, Wisconsin), amplified × 40 cycles with degradation at 95°C ×15s and annealing and elongation at 60°C for 60s. Probes were synthesized by BioSource (Camarillo,CA) with a 5′FAM and a 3′TAMRA quencher. Reactions were run in triplicate. Standard curves were prepared from 1:5 standard dilutions of purified GAPDH cDNA. Relative copy numbers of mRNA were calculated from using automated software analysis (ABI Prism 7000) based on exponential amplification cycles. Smooth muscle mRNA copy numbers were divided by GAPDH mRNA copy numbers and reported as the ratio of marker to GAPDH. The following real time PCR primers and probes were used: αSMA: (5′)GACAGCTACGTGGGTGACGAA, (3′)GATGCCATGTTCTATCGGGTACT, (probe)FAM-CACAGAGCAAAAGAGGAATCCTGACCCTG-TAMRA; Calponin: (5′)CCTGCCTACGGGCTGTCA, (3′)CTCCCGCTGGTGGTCATACTT, (probe)FAM-CCGAGGTTAAG NUAACAAGC-TGGCCCATAMRA; SM22: (5′)GGGTCCTTCCTATGGCATGAG, (3′)CTCCTCCAGCTCCTCGTCATACT, (probe)-FAM-CGCGAAGTGCAGTCCAA-AATCGAGAA-TAMRA. CAACCTGAGGGAGCGGTACTT; MHC (5′)CAACCTGAGGGAGCGGTACTT, (3′)GAGTAGATGGGCAGGTGTTTATAGG, (probe) CAGGGCTAATATATACGTACTCTGGCCTCTTCTGC-TAMRA.
Bladder Augmentation Model
Animal studies were performed in strict accordance to Animal Research Committee guidelines at UCLA. Adult female Rnu athymic rats (NIH-Foxn1rnu, Charles River, Wilmington, MA) weighing 200-250 grams were used. For partial cystectomy and bladder augmentation, rats were anesthetized with 2% isoflurane continuous flow. A 1cm supra-pubic incision exposed the urinary bladder. Partial cystectomy was performed by removing the dome of the bladder to create a 50% total bladder defect. Transected bladder was augmented with tissue-engineered SM-ASC seeded scaffolds (n=15) or acellular unseeded scaffolds (n=15) using interrupted 6-0 prolene sutures at the 12-, 3-, 6-, and 9-O’clock positions. Absorbable 5-0 polydioxanone (PDS) suture was run circumferentially around the anastamosis to provide a water tight 2nd closure. Omentum was loosely draped over the bladder dome. For partial cystectomy, (n=15 rats), the defect was closed with running 5-0 PDS. Post-operative care consisted of food, water, enrofloxacin 10mg/day × 5 days (Bayer, Germany), and carboprofen 0.1mg/kg / day ×3 days (Pfizer, NY). Deceased animals were replaced with identically treated back-up animals to maintain the total number of animals in each treatment arm at 15 animals.
Bladder cystometry (urodynamics)
Rats were anesthetized with intraperitoneal ketamine (70mg/Kg body weight). Bladder was emptied by manual abdominal pressure. A sterile 2Fr transurethral polyurethane catheter (Edwards Lifesciences, Germany) was advanced retrograde into the bladder and connected to a pressure transducer (Duet Logic, Medtronic, Denmark) in line with an infusion pump (Harvard Apparatus, Holliston, MA). Saline was infused at 100μL/ min and continuous pressure/flow measurements recorded (Duet Logic, Medtronic, Denmark). Bladder capacity was defined as the volume of infused saline infused to trigger the first leakage of urine. Bladder compliance was computed from the equation: ΔV/ΔP where Δ P (cm H20) was threshold pressure (pressure which triggered voiding) minus resting bladder pressure. ΔV was the maximal bladder capacity (μL).
Isometric Studies
Rats (n=3 per group/ time point) were sacrificed at 3 days, and 2, 4, 8 and 12 weeks after surgery using pentobarbital injection. Bladder and urethra were removed en-bloc and sectioned through the sagital midline. One half was preserved in 10% formalin fixative for histology. The second half was placed in oxygenated Kreb’s solution at 4°C until tissue bath analysis. The cell seeded and unseeded bladder molds were surgically excised from the native bladders and cut into an 8 × 3 mm longitudinal tissue strip. The scaffold strip was hung between 2 vertical mounted platinum rods attached to an isometric force displacement transducer (Radnoti Glass Technology, Monrovia, California). The scaffold strip was suspended in modified Krebs solution (134 mM NaCl, 3.4 mM KCl, 2.8 mM CaCl2, 1.3 mM Potassium phosphate monobasic, 16 mM NaHCO3, 0.6M MgSO4 and 7.7 mM Glucose) at a pH of 7.4, in a 25mL jacket at 37 °C, 95% O2. A 1.0 gm preload was applied. Tissue was equilibrated for 30 minutes. 80μM KCl was used to depolarize the tissue. Dose-response cholinergic contraction was assessed with carbachol at a dosage range from 1 × 10-6 to 1 × 10-4 M. Atropine 10-4 M was used as competitive antagonist. Data acquisition software (Biopac Systems, Goleta, CA) recorded continuous force displacements. Post procedure, the wet specimens were weighed and placed in formalin fixative for histological analysis.
Histology, immunofluorescence and microscopy
In vitro synthetic materials requiring analysis were air dried and immunostained in culture wells without use of fixation or embedding. In vivo bladder specimens were surgically explanted as described above, fixed in 10% formalin, saturated in 30% sucrose, and embedded in OCT (Sakatura, Japan). Standard hematoxylin and eosin and mason’s trichrome staining was performed on all slides. Immunofluorescence staining used 1:100 dilution of primary antibody and 1:200 fluorescein (FITC) conjugated goat anti-mouse and goat anti-rabbit secondary antibodies (Invitrogen; Carlsbad, California). Nuclei were counterstained with DAPI (Vectashield, Burlingame, CA). Primary antibodies used were: α-smooth muscle actin (αSMA, mouse monoclonal, Dako, CA); smooth muscle myosin heavy chain (MHC, rabbit polyclonal, Biomedical Technologies, CA); CD-11a,b (CD-11, mouse monoclonal, Dako), nerve protein gene product 9.5, (PGP 9.5, mouse monoclonal, RDI, MA). Immunofluorescence was performed using an epifluorescence microscope (Carl Zeiss, Oberkochen, Germany) filtered for excitation/emission at: DAPI- 360/460nm, DiI- 546/590 nm, FITC- 495/519 nm. Image analysis (Spot v4.3, Diagnostic Instruments, Inc., Sterling Heights, MI) was performed.
Statistical Analysis
Bladder volumes and pressures were recorded as continuous integers. Isometric contraction was recorded as maximal contraction (grams) per individual tissue and normalized to total tissue weight (g/100mg). Tissues that were unresponsive were recorded as 0gm contraction and were included in calculations of group means. Statistical differences between treatment groups were calculated with a Kruskal-Wallis Test (Nonparametric ANOVA) and statistics software (Graph Pad Prism, CA).
RESULTS
Synthetic Bladder Mold Construction
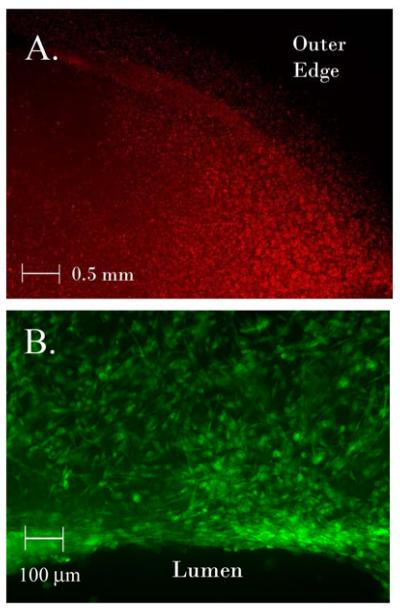
The synthetic PLGA bladder composites had a final diameter of 10mm, concavity of 3mm, and thickness of 0.8 mm (FIG 1a,b). On scanning electron microscopy (SEM), the median diameter of the individual microfiber was 1.0 micron with a range of 0.5 - 10 microns (FIG 1c). The sponge pore size was 150μm with a range 50-250μm (Fig 1d). The synthetic PLGA bladder molds remained stable during incubation in Dulbecco’s Modified Eagle Media (DMEM) and smooth muscle inductive media (SMIM) for 14 days at 37 °C without microscopic or structural changes. The constructs were non-toxic to ASC and SM-ASC cells confirmed by live/dead staining (Fig 3b).
Figure 3. Cell seeding of in vitro constructs.
A) PLGA cell seeded bladder composite 14 days in vitro seeded with SM-ASCs labeled with DiI (red, 40x). B) Luminal edge of SM-ASC seeded scaffold 14 days in vitro stained with calcein AM (green) to demonstrate viable cells (100x).
ASC Differentiation into Smooth Muscle
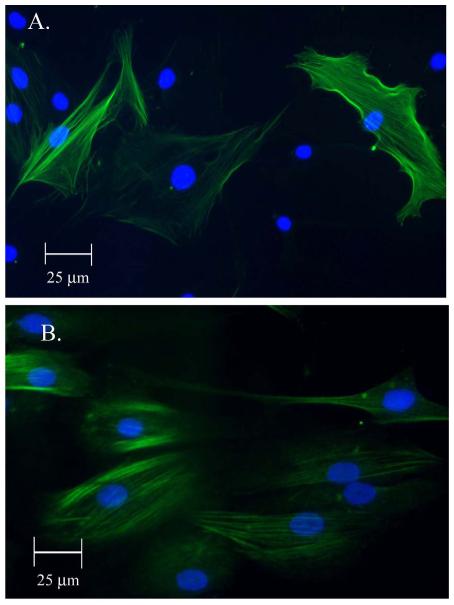
ASC cultures were successfully obtained from human adults undergoing liposuction. 300mL of lipoaspirate yielded 1×108 ASCs. The ASC population doubling time was 60 hours. Smooth muscle induction media (SMIM) induced differentiation of the ASCs into a smooth muscle phenotype (SM-ASC) in which the SM-ASCs demonstrated expression of smooth muscle specific proteins including: α-smooth muscle actin (αSMA), caldesmon, and myosin heavy chain (MHC) (FIG 2).
Figure 2. Differentiation of ASCs into SM-ASCs.
A) Myosin heavy chain (MHC) expression in SM-ASCs after 6 weeks incubation in SMIM (FITC conjugated; nucleus DAPI, 400x). B) Caldesmon expression (FITC, 400x)
Cell-seeding, Viability, and Differentiation of SM-ASCs within the Scaffold
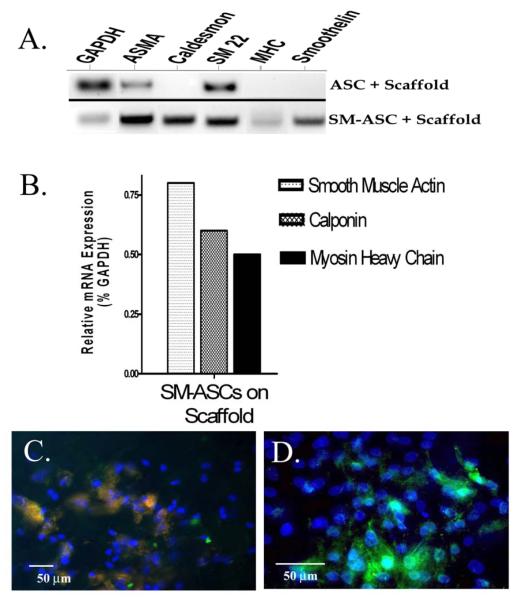
SM-ASCs adhered to the PLGA bladder molds and dispersed evenly throughout the synthetic construct with 50- 80% confluence based on light microscopy analysis (FIG 3a). Confluence and cell viability were unchanged after 14 days of incubation of the tissue engineered grafts in SMIM using a calcein acetoxymethyl/ ethidium ‘live/dead’ assay (FIG 3b). Smooth muscle phenotype was maintained by the SM-ASCs in the seeded scaffolds after 2 weeks in vitro in SMIM. RT-PCR demonstrated mRNA signal expression of αSMA, caldesmon, SM22, MHC, and smoothelin from the SM-ASC seeded constructs (Fig 4a). MHC and smoothelin mRNA, both of which are specific to smooth muscle [18] were specific to the SM-ASC constructs and absent in the undifferentiated ASC constructs. (Fig 4a). αSMA, calponin, and MHC signal expression were upregulated in the SM-ASC grafts using real-time PCR to calculate mRNA copy numbers relative to GAPDH (Fig 4b). Transcriptional messages were verified at the translational level, and SM-ASCs adherent to the PLGA scaffolds immunostained positive for αSMA, calponin, caldesmon, and MHC (Fig 4c,d) up to 14 days in vitro. The acellular PLGA composites lacked any cellular or smooth muscle expression in vitro.
Figure 4. Smooth muscle expression from SM-ASC seeded bladder constructs.
A) RT-PCR and B) real-time RT-PCR of smooth muscle markers extracted from an SM-ASC seeded construct compared to undifferentiated ASCs on a scaffold. C. Caldesmon and D) MHC expression in an SM-ASC seeded scaffold (FITC conjugated, nucleus counterstained DAPI, 200x and 400x respectively).
Rat Bladder Augmentation
45 nude rats underwent a partial cystectomy and repair. 15 of 15 rats (100%) in the suture closure group survived the surgery, while 13 (86%) and 11 (73%) animals in the acellular composite and SM-ASC tissue engineered groups survived respectively. The causes of death in the tissue engineered animals were: urine leak in 3 rats (20%) and anesthesia overdose in one rat (7%). The cause of death in the acellular augmented control animals was urine leak in 2 rats (13%). All deaths occurred within the first 3 post-operative days, and animal survival increased with surgeon experience, such that all of the deceased animals occurred within the first 20 surgeries.
Histology
On gross histological analysis , the SM-ASC cell seeded and the acellular construct showed rapid biodegradation and incorporation into native tissue by 8-12 weeks in vivo (Fig 5). Bladder calculi of various size were present in 3 of 15 suture repaired bladders (20%), 6 of 15 bladders repaired with SM-ASC seeded composites (40%), and 7 of 15 bladders repaired with the acellular composite (47%). The explanted SM-ASC engineered composites and the acellular control composites were analyzed at serial time points for histological changes.
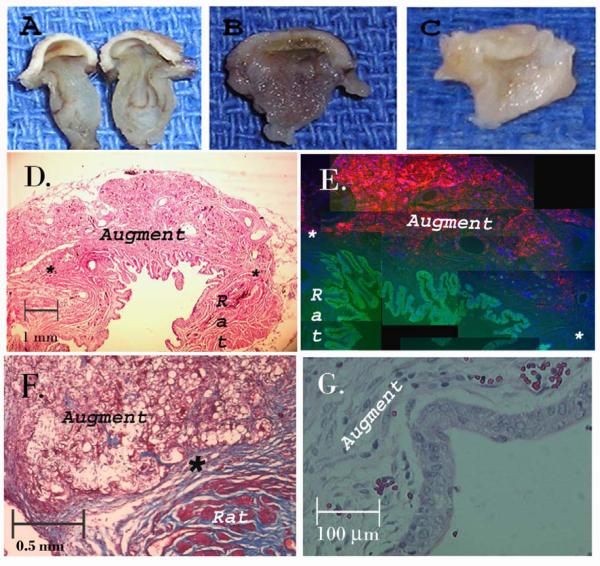
Figure 5. Histology of cell seeded constructs after bladder augment.
A — C) Gross appearance of tissue engineered implant after 3 days, 4 weeks, and 12 weeks respectively. D) Cross section of cell seeded augment at 8 weeks, anastamosis marked with an asterix, H & E, 10x. E) Same specimen under fluorescent microscope with SM-ASCs red (DiI), nuclei blue (Dapi), and urothelium green (FITC), reconstructed from 100x. F) Higher power demonstrating the anastamosis at 4 weeks (Mason’s Trichrome, 50x) and G) early formation of urothelium by 2 weeks, 200x.
2 weeks
After 2 weeks in vivo, both the cell-seeded and the acellular composites demonstrated a lining of urothelial cells covering the luminal surface of the scaffolds (Fig 5g) The urothelium was most prominent at the edges of the anastamosis and thinnest in the center of the implant. The PLGA fibers and sponge remained grossly and microscopically visible in both implant groups. DiI labeled SM-ASCs were visible and nested in the scaffold pores of the cell seeded composites.
4 weeks
Both the SM-ASC and acellular constructs explanted after 4 weeks in vivo had organized urothelium covering the entire lumen (Fig 6a). There was scant smooth muscle infiltration (Fig 6b) along the edges and luminal surfaces of both construct groups without any noticeable histological differences between the 2 groups. The centers of the both grafts lacked organized smooth muscle.
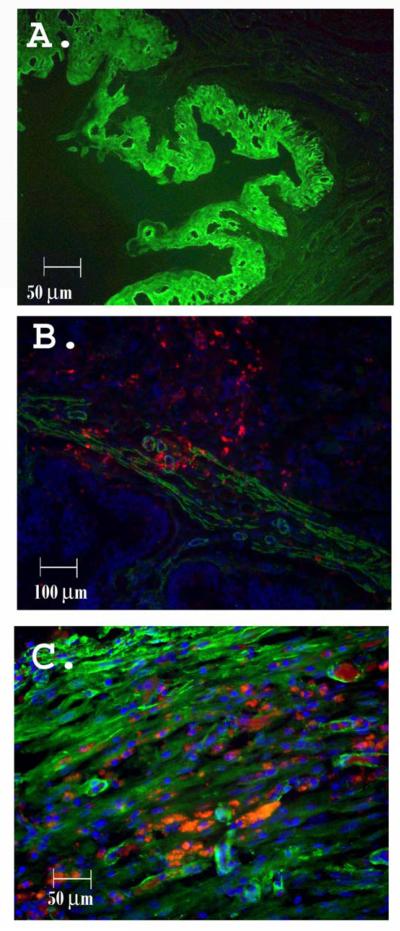
Figure 6. Urothelium and smooth muscle in cell-seeded graft in vivo.
A) Graft urothelium showing absence of DiI after 4 weeks in vivo (FITC; 200x) B) Early growth of smooth muscle bundles expressing MHC in an SM-ASC seeded (red) composite at 4 100x), C) Seeded SM-ASCs (red) after 12 weeks in vivo counterstained for smooth muscle myosin heavy chain (green). Areas with both marker appear yellow on image overlay (200x).
8 weeks
After 8 weeks in vivo, large blood vessels were visible along the scaffold serosa of both treatment groups, and capillaries containing red blood cells were visible within the center of both constructs. The SM-ASCs remained visible in the cell-seeded constructs and they now demonstrated directional orientation and smooth muscle marker expression (Fig 6c). The amount of smooth muscle visible in the core of the SM-ASC seeded scaffolds was greater than that of the acellular scaffolds (Fig 7c,d). The acellular scaffolds had a greater degree of collagen deposition on mason’s trichrome staining (Fig 7e,f).
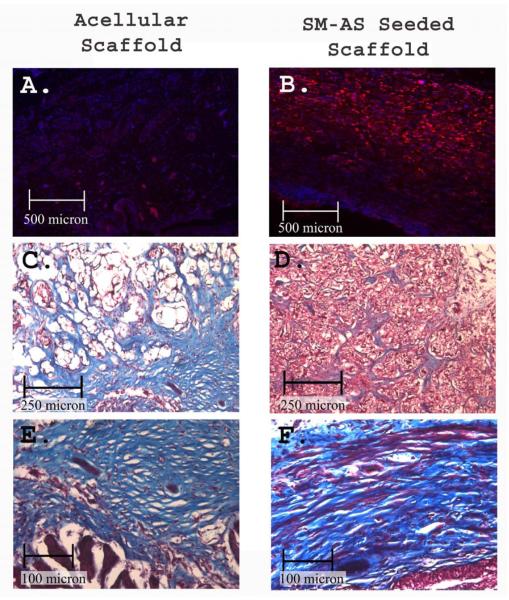
Figure 7. Comparison of SM-ASC and acellular grafts.
A, B) DiI emitting SM-ASCs (red) are present only in the cell seeded scaffolds, nuclei counterstained with DAPI, 50x. C, D) Graft cores demonstrating cellular and collagen deposition in the micropores of the sponge, Mason’s Trichrome, 100x. E, F) Luminal electropulled surface showing smooth muscle and collagen deposition above a urothelial lining, 200x.
12 weeks
At 12 weeks in vivo, the SM-ASCs in the cell-seeded composites were elongated and oriented in a longitudinal direction with smooth muscle bundles present The SM-ASCs demonstrated MHC, actin, calponin, and caldesmon protein expression using immunofluorescence staining. Markers for inflammation including CD-11 were absent in the cell seeded and unseeded constructs (not shown).
Physiologic Bladder Testing
Cystometric bladder measurements, which consisted of continuous filling pressure and volume measurements in the rat bladders, were recorded in all the animals without complication; the results are summarized in Figure 8. The mean rat bladder capacity prior to surgery was 1.2 ± 0.2 mL and the mean bladder compliance was 73 ± 22 μL/ cm H20. Partial cystectomy and suture closure (control) resulted in a decreased bladder capacity of 0.7 ± 0.4 mL and worsened compliance (34 ± 15 μL/ cm H20) two weeks after surgery. By 8 and 12 weeks the ‘suture closed’ control rats regained their natural bladder capacity and compliance, demonstrating the natural healing and long term limitations of rat bladder model.
Figure 8. Physiologic bladder testing.

A) Pressure/ volume curves of pre-operative and 2 week post-operative rat bladders. B) Animals with tissue engineered bladder augments maintained superior bladder volume and C) compliance over 12 weeks.
The bladders repaired with the SM-ASC seeded augments improved their bladder capacity (1.6 ± 0.2mL at 2 weeks; p= 0.02) and compliance (92 ± 69 μL/ cm H20 at 2 weeks; p= 0.01) by the 2-week test point. This improvement was maintained for all 12 weeks following implantation (Fig 8 b,c) The final capacity of the SM-ASC tissue augmented bladders was 1.5 ± 0.8 mL and the compliance was 142± 151 μL/ cm H20. In the acellular augment group, final bladder capacity was 1.08 ± 0.8 mL and compliance was 56 ± 14 μL/ cm H20 by post-operative week 12. There was no significant statistical difference between the bladder capacities of the SM-ASC augmented and the acellular augmented rats throughout the 12 weeks. However, only the SM-ASC augmented bladders maintained normal compliance throughout the study; the acellular augments had worsening compliance at each progressive time point (FIG 8c).
Isometric (organ bath) Tissue Studies
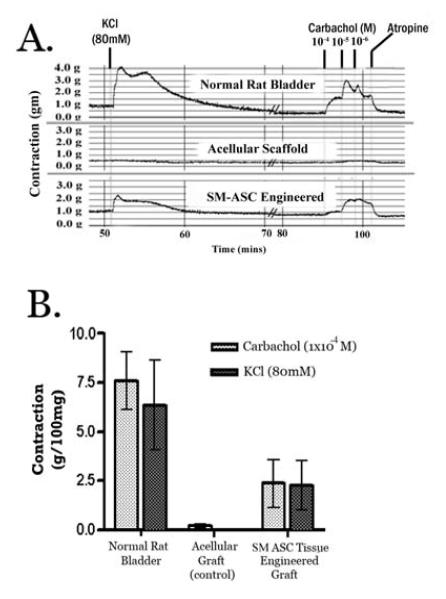
Tissue strips harvested from each experimental and control animal were analyzed for smooth muscle contractility in an oxygenated Kreb’s solution organ bath. Same sized tissue strips, as determined by wet tissue weight, were used in all the organ baths. The mean ± SD weights of the tissues used in the organ baths were: Cell seeded composite= 34.8 ± 1.9mg, acellular composite = 36.2 ±1.3mg, and suture closure = 33.1 ± 2.1mg. Tissue strips obtained from the suture closed bladders had contraction and relaxation profiles identical to normal rat bladder tissue. Stimulation using 1×10-4M carbachol generated a maximal contractile force of 2.8 ± 0.9g/100mg in this group (Fig 9). On the contrary, the acellular PLGA scaffolds showed no response (average contraction 0.07 g/100mg ± 0.13, range =0 – 0.2) to the carbachol and atropine stimulation. The cell seeded composites demonstrated very mild KCl and carbachol induced contractions (10-4 M carbachol mean= 2.5 ± 0.4g/100mg, range = 0 to 4.0g/100mg; Fig 9) after 12 weeks in vivo, which were reversible with atropine. There was no response to KCl or carbachol from cell seeded constructs harvested after 0, 4, or 8 weeks.
Figure 9. Pharmacologic smooth muscle contraction.
A) Carbachol (1 × 10-6 M to 1 × 10-4 M) and KCl (80mM) induced contractions in native rat bladder (partial cystectomy group) and in augmented bladder explants from acellular and cell-seeded bladder constructs. B) Mean tissue contraction (± SEM) of the experimental and control bladder grafts after 12 weeks in vivo.
DISCUSSION
Tissue engineering therapies utilizing smooth muscle regeneration may provide alternative treatments for diseases of smooth muscle pathology such as bladder dysfunction, urinary incontinence, vascular, and cardiovascular diseases. A major obstacle to date has been finding a reliable source of healthy smooth muscle cells that can be safely harvested with minimal manipulation. We examined the possibility of using adipose stem cells as a source of functional smooth muscle. Adipose stem cells have the capacity to differentiate into adipogenic, osteogenic, chondrogenic, myogenic, neurogenic, and cardiomyogenic lineages. [15, 19-21] Unlike bone marrow stem cells (BMCs), ASCs can be obtained in relatively large quantities with minimal morbidity. ASCs are phenotypically similar to BMCs but lack expression of CD106, among other markers, suggesting that they are a truly distinct population of cells from mesenchymal origin. [15] They have been shown to be stable in long-term culture, and can differentiate both phenotypically and functionally into smooth muscle. [17] We previously demonstrated that human ASCs can survive in the lower urinary tract of the nude animal for up to 12 weeks.[22]
In this study, ASCs were used to generate large populations of smooth muscle cells (SM-ASCs) to seed synthetic bladder composites. Smooth muscle phenotypes of the SM-ASCs were verified at the transcriptional and translational level by demonstrating smooth muscle markers including α-smooth muscle actin (αSMA), SM22, calponin, and smooth muscle myosin heavy chain (MHC) within the differentiated SM-ASCs. αSMA is an early marker of smooth muscle differentiation, but, it is not specific and is expressed by a variety of contractile cells including bone marrow stem cells. [23] MHC and smoothelin are specific only to contractile smooth muscle and they are considered markers of advanced smooth muscle differentiation.[18, 24] The finding that our SM-ASCs expressed MHC (FIG 2) suggests that they were both well differentiated and cytologically capable of potential contraction/relaxation. [18]
PLGA was chosen as the polymer for the bladder scaffold for its proven cell affinity and known biocompatibility. [25] PLGA offers clinical advantages over commercially available organic scaffold materials such as small intestinal submucosa, since PLGA can be manufactured on large scales, shaped into neo-organs, and chemically manipulated to control strength and elasticity. [2, 16, 26]. The bladder construct generated for this study was designed in an effort to replicate the cytoskeleton of the native rat bladder. The luminal inner surface of the construct was made from thin layer of electropulled PLGA microfibers tightly woven to provide a cell scaffold that was malleable, soft, non-porous, leak-proof to keep the urine from permeating the graft, and strong enough to hold solid sutures without tearing. A thicker, 95% porous PLGA sponge was added to the outer surface of the composite to allow for greater SM-ASC and host cell seeding surface area and to facilitate external neovascular and nutrient penetration into the core of the graft.
Our SM-ASCs maintained their smooth muscle markers in vitro and in vivo within our synthetic scaffolds, but our study required 6 weeks of pre-differentiation of the ASCs prior to scaffold seeding. In the future, incorporation of impregnated and controlled release smooth muscle differentiation agents such as SMIM or other growth factors into the scaffold core could allow for immediate seeding of the ASCs onto the scaffold at the time of reconstructive surgery, bypassing the in vitro differentiation step used in the current study. [23, 27, 28]
The ability of smooth muscle cells to contract/relax in response to a physiological stimulus has been considered to be the main proof that myocytes are in a differentiated state. [18, 23, 29] We used isometric tissue baths to evaluate the ability of SM-ASC engineered and acellular bladder composites to respond to basic pharmacologic agents. After 12 weeks in vivo, only the SM-ASC engineered, but not the acellular composites, showed very weak dose-dependent contraction in response to the muscarinic agonist carbachol, suggesting the presence of some degree of functional smooth muscle in our SM-ASC augments. The contractile response was inhibited by atropine, and was not seen at any time periods prior to 12 weeks. We hypothesize that the contractility was only observed after 12 weeks in vivo because that was the time required for the PLGA to completely dissolve as well as for the smooth muscle to gain significant mass affect within our augment materials.
An additional benefit of the SM-ASCs in our tissue engineered bladder grafts was demonstrated by the results of our in vivo bladder capacity and compliance studies. Bladder augmentation with the acellular and SM-ASC seeded composites generated similar initial bladder capacities, suggesting an adequate ability of both materials to initially withstand augmentation surgery. However, the unseeded materials had a non statistical reduction in capacity over time, while the cell seeded materials maintained and even improved their capacity over the 12 week study duration. These differences were not significant at the for a p value < 0.05. More dramatically, the animals augmented with the SM-ASC engineered composites maintained their compliance for all 12 weeks of the study, while the animals augmented with the acellular grafts demonstrated worsening compliance over time. Bladder compliance represents the ability of the bladder to expand while maintaining a safe low bladder pressure. Poorly compliant bladders do not stretch and require high pressures to expand. As and they fill under high bladder pressures, these bladders can cause kidney damage.
The discrepancy between the long term capacity and compliance fo the tissue engineered and the acellular control bladders suggests the possibility of an anti-fibrotic benefit provided by the presence of the SM-ASCs. We hypothesize that the seeding effect of the SM-ASCs to cover and coat the fibers and pores of our scaffolds may have provided an anti-fibrotic effect by decreasing the raw surface area of the scaffold biomaterial for host fibroblasts to implant, as well as by providing the cellular basis for the deposition of far more elastic and compliant smooth muscle cells. In line with this hypothesis, the histological analysis of the explanted specimens suggested that the SM-ASC composites had less collagen and greater smooth muscle mass than the acellular composites (Fig 7). These findings are not novel, as other authors have previously reported similar anti-fibrotic benefits from cell seeded biomaterials compared to acellular biomaterials. [1, 3, 4, 30]
Many investigators have reported models of tissue engineered bladder which used dual-cell seeding of urothelial cells and smooth muscle on the serosal surface of the tissue engineered scaffolds. [1] Our model used only SM-ASCs throughout the scaffold and our results were consistent with others studies demonstrating that host urothelium rapidly regenerates. [31] While it remains to be tested whether the pluripotent nature of the ASCs can provide precursors to regenerate urothelium, it is unlikely given the mesenchymal origin of the ASC and their propensity to differentiate into mesodermal structures such as adipose and smooth muscle. Despite not seeding our augment grafts with a distinct urothelial cell line, our histological analyses demonstrated that the host urothelial cells rapidly regrew along the luminal surface of the implanted bladder composites by 2-4 weeks. The urothelial cells likely originated from the host urothelium based on the complete absence of any DiI immunoflourescence in the neo-urothelial layer (Fig 6). These results are consistent with previously reported studies which demonstrated rapid luminal coverage of synthetic and smooth muscle bladder composites with host urothelial cells. [32] Some investigators believe that stem cells may be advantageous towards facilitating urothelial coverage of cell-seeded composites compared to acellular composites [3, 30], but we did not observe any noticeable difference between the SM-ASC treatment and acellular control composites in our study in this regard.
We chose the nude rat animal model to implant our tissue engineered grafts in order to minimize host versus graft antigenicity against the implanted human SM-ASCs. While the rat is sub-optimal for studying larger tissue defects such as those required to augment human bladders, we deemed it a logical starting model since it provided an ethical, well-replicated, and in-expensive animal model for demonstrating the feasibility of an ASC derived neo-organ. The model did unfortunately provide two known drawbacks, one in the form of bladder calculi; the other limitation being host self-regeneration. [32, 33] The bladder calculi were observed in 20%, 40%, and 47% of the animals in the suture closure, acellular, and SM-ASC engineered arms respectively. The formation of urinary calculi is a common reaction to foreign bodies in the urinary tract, as the foreign materials create a nidus for minerals to precipitate out of the urine, and urinary stasis and infection from the surgery further facilitates crystallization and stone formation. Interestingly, the suture closed bladders had the lowest stone rate, presumably due to the fact that they had the least amount of foreign material in their bladder. It is also possible that they had the lowest amount of urinary stasis since they lacked a synthetic, non-muscular, bladder dome that could interfere with complete bladder emptying. The 7% benefit seen in the stone rates of the SM-ASC composites over the acellular composites was insignificant.
We observed that the non-augmented rat bladder had significant self-regenerative abilities, such that our control animals that underwent bladder resection without graft implantation regained their native bladder capacities and normal bladder function by 3 months after surgery. It is possible that the functional and contractile benefits of our SM-ASC seeded augments came from host regenerated smooth muscle rather than SM-ASCs directly. The ingrowth of native bladder smooth muscle into acellular bladder composites is well documented, [9] and it was observed in our acellular grafts as well. However, fluorescent tracking of the ASCs was performed in all of the experiments, and the SM-ASC composites had identifiable fluorescent labeled cells within the composite at all explanted time points in the study (Figs. 5, 6, 7). The DiI label was often incorporated into larger bundles of smooth muscle that were largely unlabeled, as is seen in Figure 6. Molecular studies are needed to identify the origins of these de-novo smooth muscle bundles in the tissue engineered grafts, but we hypothesize that the smooth muscle observed within our SM-ASC engineered composites is a combination of host ingrowth- as we know occurs from our controls, and ASC origins- as suggested by the persistent DiI fluorescent label seen in some of the smooth muscle. Follow-up studies to determine the in vivo smooth muscle origins and differentiation mechanisms including bladder cell co-culture and trans-well experiments are underway to determine the role of the local smooth muscle environment on inducing and maintaining smooth muscle differentiation from the ASCs. While there is much more to be understood about the differentiation potential of ASCs and ASC derived tissue grafts, the abundance and availability of ASCs make them an attractive cell source for tissue engineering applications in the lower urinary tract. Similar applications for cardiac tissue, bone, and cartilage are underway and are encouraging.
CONCLUSION
Adipose stem cells can be differentiated into smooth muscle and seeded onto three dimensional bladder scaffolds while maintaining expression of smooth muscle molecular markers. The bladder composites were created with electropulled absorbable nanofibers and a porous polymer sponge compiled to create a 3-d composite resembling the natural cytoskeleton of the bladder. ASCs were able to maintain their smooth muscle differentiation and viability in vitro and in vivo within bioengineered bladder composites. The tissue engineered bladder composites exhibited ex vivo contractility in organ baths, and had superior compliance, smooth muscle mass, and contractility compared to identical acellular composites that lacked ASCs. The vast availability of ASCs, combined with their ease of procurement and ability to differentiate into contractile smooth muscle, make ASCs a competitive non-embryonic alternative for regeneration of the bladder and other smooth muscle tissues.
Acknowledgments
FUNDING This work was funded in part by National Institute of Child Health and Human Development Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Grant 5-K12-HD01400, and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK067198-01.
Footnotes
COMPETING INTERESTS: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Atala A, Freeman MR, Vacanti JP, J. S, A.B. R. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J Urol. 1993;150(2):608–612. doi: 10.1016/s0022-5347(17)35561-1. [DOI] [PubMed] [Google Scholar]
- 2.Atala A. Tissue engineering for the replacement of organ function in the genitourinary system. American J Transplant. 2004;4(Suppl 6):58–73. doi: 10.1111/j.1600-6135.2004.0346.x. [DOI] [PubMed] [Google Scholar]
- 3.Drewa T, Sir J, Czajkowski R, Wozniak A. Scaffold seeded with cells is essential in urothelium regeneration and tissue remodeling in vivo after bladder augmentation using in vitro engineered graft. Transplant Proc. 2006;38(1):133–135. doi: 10.1016/j.transproceed.2005.11.086. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Kropp BP, Lin HK, Cowan R, Cheng EY. Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng. 2004;10(12):181–187. doi: 10.1089/107632704322791835. [DOI] [PubMed] [Google Scholar]
- 5.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17(2):149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 6.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 7.Lin HK, Cowan R, Moore P, Zhang Y, Yang Q, Peterson JA, Jr., et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol. 2004 Mar;171(3):1348–1352. doi: 10.1097/01.ju.0000108800.47594.8b. [DOI] [PubMed] [Google Scholar]
- 8.Lai JY, Yoon CY, Yoo JJ, Wulf T, Atala A. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J Urol. 2002;168(4 Pt 2):1853–1857. doi: 10.1097/01.ju.0000030040.76258.5a. discussion 1858. [DOI] [PubMed] [Google Scholar]
- 9.Akbal, Lee, Packer, Davis, Rink, Kaefer Bladder Augmentation With Acellular Dermal Biomatrix in a Diseased Animal Model. The Journal of Urology. 2006;176(4):1706. doi: 10.1016/j.juro.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 10.Rubin JP, Zuk P, Gimble JM, March K. Second Annual International Fat and Applied Technology; Pittsburg, Pennsylvania. Oct 5th, 2004.2004. [Google Scholar]
- 11.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multilineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26(6):664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuk PA. Stem cell research has only just begun. Science. 2001;293(5528):211–212. [PubMed] [Google Scholar]
- 15.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 16.Pattison MA, Wurster S, Webster TJ, Haberstroh KM. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials. 2005;26(15):2491–2500. doi: 10.1016/j.biomaterials.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. PNAS. 2006;103(32):12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol. 1997;17(4):665–671. doi: 10.1161/01.atv.17.4.665. [DOI] [PubMed] [Google Scholar]
- 19.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290(2):763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 20.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 21.Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294(2):371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 22.Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA. Rodriguez LV. Processed Lipoaspirate Cells for Tissue Engineering of the Lower Urinary Tract: Implications for the Treatment of Stress Urinary Incontinence and Bladder Reconstruction. J Urology. 2005;174(5):2041–2045. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]
- 23.Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002;278(1):72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- 24.Miano J. Mammalian smooth muscle differentiation: origins, markers and transcriptional control. Results and Problems in Cell Differentiation. 2002;38:39–59. doi: 10.1007/978-3-540-45686-5_2. [DOI] [PubMed] [Google Scholar]
- 25.Pariente JL, Kim BS, Atala A. In vitro biocompatibility assessment of naturally derived and synthetic biomaterials using normal human urothelial cells. J Biomed Mater Res. 2001;55(1):33–39. doi: 10.1002/1097-4636(200104)55:1<33::aid-jbm50>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Baker SC, Rohman G, Southgate J, Cameron NR. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials. 2009;30(7):1321–1328. doi: 10.1016/j.biomaterials.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Chun SY, Lim GJ, Kwon TG, Kwak EK, Kim BW, Atala A, et al. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials. 2007;28(29):4251–4256. doi: 10.1016/j.biomaterials.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21(24):2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 29.Oishi K, Ogawa Y, Gamoh S, Uchida MK. Contractile responses of smooth muscle cells differentiated from rat neural stem cells. J Physiol. 2002;540(1):139–152. doi: 10.1113/jphysiol.2001.013278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung SY, Krivorov NP, Rausei V, Thomas L, Frantzen M, Landsittel D, et al. Bladder reconstitution with bone marrow derived stem cells seeded on small intestinal submucosa improves morphological and molecular composition. J Urol. 2005;174(1):353–359. doi: 10.1097/01.ju.0000161592.00434.c1. [DOI] [PubMed] [Google Scholar]
- 31.Staack AHS, Baskin LS, Cunha GR. Molecular, cellular and developmental biology of urothelium as a basis of bladder regeneration. Differentiation. 2005;73(4):121–133. doi: 10.1111/j.1432-0436.2005.00014.x. [DOI] [PubMed] [Google Scholar]
- 32.Piechota HJ, Gleason CA, Dahms SE, Dahiya R, Nunes LS, Lue TF, et al. Bladder acellular matrix graft: in vivo functional properties of the regenerated rat bladder. Urol Res. 1999;27(3):206–213. doi: 10.1007/s002400050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obara T, Matsuura S, Narita S, Satoh S, Tsuchiya N, Habuchi T. Bladder acellular matrix grafting regenerates urinary bladder in the spinal cord injury rat. Urology. 2006;68(4):892–897. doi: 10.1016/j.urology.2006.04.030. [DOI] [PubMed] [Google Scholar]