Preface
Active resolution of acute inflammation is a previously unrecognized interface between innate and adaptive immunity. Once thought to be a passive process, the resolution of inflammation is now shown to involve active biochemical programmes that enable inflamed tissues to return to homeostasis. This Review presents newly uncovered cellular and molecular mechanisms for the resolution of inflammation, revealing key roles for eicosanoids, such as lipoxins, and new families of endogenous chemical mediators, termed resolvins and protectins. These mediators carry antiinflammatory and pro-resolution properties with leukocytes, protect organs and stimulate mucosal antimicrobial defence and clearance. Together, they control local inflammatory responses at multiple levels to stimulate resolution.
Introduction
On challenge of host tissues by microorganisms, tissue injury or surgical trauma, two main routes lead to release of exogenous and endogenous chemical mediators that in turn give rise to the initial cardinal signs of inflammation described by Celsus in the first century1, rubor (redness), calor (heat), tumor (swelling) and dolor (pain) (FIG. 1). Exogenous mediators include microbial-derived peptides that are chemoattractants and recruit neutrophils to the site, where they can phagocytoze microbial invaders and cellular debris. Within neutrophils, newly formed phagosomes fuse with lysosomal granules to become phagolysosomes. These granules contain degradative enzymes and produce reactive oxygen species (ROS) to kill trapped microorganisms or degrade cellular debris. Hence the initial inflammatory response is host protective and its timely resolution ideally self-limited.
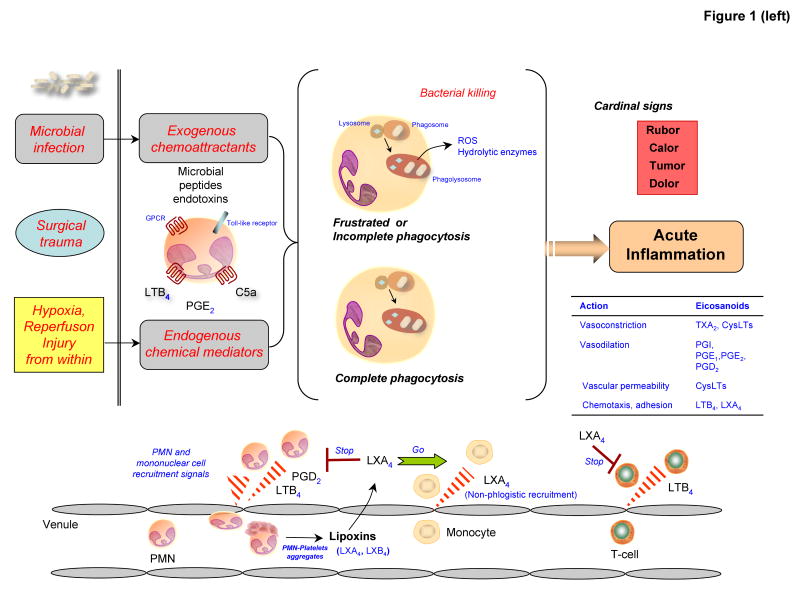
Figure 1. Decision paths in acute inflammation: resolution or chronic inflammation and the roles of endogenous chemical mediators.
(Left) Microbial invasion of the host, injury from outside or the loss of barrier function initiates the release of exogenous chemoattractants, such as microbial peptides or endotoxins, that can activate leukocyte surface receptors, including Toll-like receptors and seven-transmembrane G-protein-coupled receptors. Signalling through these receptors initiates antimicrobial activities and further leukocyte recruitment and the formation and release of endogenous chemical mediators. Microorganisms taken up by phagocytosis activate bacterial killing mechanisms in, for example, neutrophils after complete phagosomal and phagolysosomal vacuole formation, termed complete phagocytosis.
(Left lower) Injury from within as in surgical trauma and ischemia-reperfusion injury also activates the release of endogenous chemical mediators, such as leukotriene B4, prostaglandin E2 and C5a. Both exogenous and endogenous chemoattractant gradients stimulate the recruitment of neutrophils via diapedesis from postcapillary venules, an event that is amplified by the production of local leukotriene B4. During the tissue progression of inflammatory events, intravascular platelet-leukocyte interactions evoke the formation of lipoxin A4 and lipoxin B4, which stop further recruitment of neutrophills and stimulate nonphlogistic monocyte infiltration.
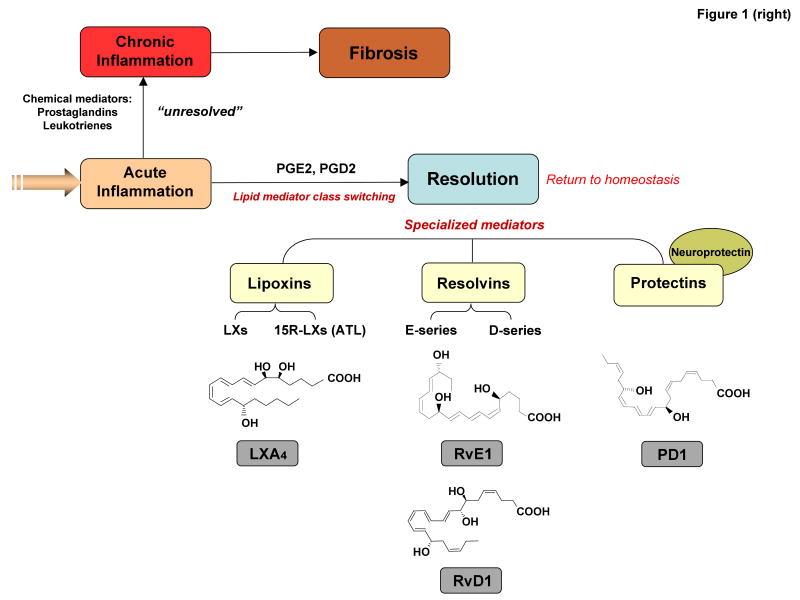
(Right) The outcome of acute inflammation — resolution, chronicity and fibrosis — may be influenced by many factors, such as the nature and intensity of the injury, the location of the injury and the over-responsiveness of the host, which is broadly controlled by both genetic and nutritional elements. The return from inflammation to normal homeostasis (complete resolution) is an actively regulated programme at the tissue level, coined catabasis6. Specific prostaglandins (PGE2 and PGD2) and leukotriene B4 are involved in the initiation and amplification of acute inflammation. Both PGE2 and PGD2 stimulate the switching of arachidonic-acid-derived lipids from LTB4 production to lipoxin A4 production, for example, and then the switching of lipid mediator families to produce anti-inflammatory and pro-resolution lipid mediators, such as E-series and D-series resolvins and protectins. Alternatively, chronic inflammation can result from excessive and/or unresolved inflammatory responses and can lead to chronic disorders. Arachidonic-acid-derived lipid mediators such as pro-inflammatory prostaglandins and leukotrienes can amplify this process. Fibrosis can occur when inflammatory injury causes substantial tissue destruction, connective tissue replacement occurs and results in loss of tissue function.
Sometimes neutrophil granule contents can inadvertently spill into the extracellular milieu before complete engulfment of microorganisms or debris2. This leads to local tissue damage and amplification of acute inflammatory signs within minutes to hours of insult (FIG. 1). This unintentional spilling of granule contents, in particular the release of hydrolytic enzymes from phagolysosomal vacuoles also occurs when phagocytes encounter foreign surfaces that they attempt to ingest but cannot, such as crystals, bacterial biofilms or other slimy surfaces1.
The intentional generation of endogenous chemical mediators, including eicosanoids (i.e. prostaglandin E2, leukotriene B4) and complement components (C5a), and their local release from infiltrating cells, which are physiologic events during host defence, can also lead inadvertently to tissue damage. Endogenous chemical mediators are released during sterile injury as in ischemia-reperfusion injury3. This route of chemical mediator release can be amplified by overt activation and excessive recruitment of neutrophils to the site of injury that in turn aggravates further spilling of noxious granule-contents as well as generation of new mediators at the site, which sustains the cardinal signs of inflammation (FIG. 1).
Acute inflammation has several programmed fates including progression to chronic tissue fibrosis or the ideal outcome of complete resolution1. Challenges are met by infiltrating phagocytic cells of the innate system, primarily neutrophils, that traffic sensing gradients of chemoattractants from postcapillary venules via diapedesis (FIG. 1). These chemoattractants consist of endogenous lipid mediators, such as leukotrienes, and protein mediators, including chemokines and cytokines4, as well as exogenous chemoattractants liberated, for example, from microorganisms. Time-dependent progression of cell infiltration is led by specific leukocyte subtypes, namely, professional phagocytes, neutrophils being the first to enter, followed by monocytes5.
Once initiating noxious materials are removed via phagocytosis, the inflammatory reaction must be resolved to prevent the inflammation from spreading, becoming chronic or causing disease. Resolution of inflammation, or its catabasis6, is the reduction or removal of leukocytes and debris from inflamed sites enabling the return to homeostasis. The resolution of inflammatory leukocytic infiltrates was previously considered to be passive7. Local chemotactic stimuli and gradients, for example, were thought to dissipate or simply “burn out” with time, enabling tissues to drain, repair and return to normal function1. With recent findings reviewed here, it is now apparent that resolution is not merely a passive termination of inflammation, but rather an active biochemical and metabolic process6, 7. The resolution process is rapidly initiated after acute challenges by cellular pathways that actively biosynthesize local specialized dual-acting anti-inflammatory and pro-resolution lipid mediators, such as lipoxins, resolvins and protectins8, 9 (FIG. 1).
In this Review, we provide an update and overview of newly identified mediators that play pivotal roles in resolution and disease models. Unexpectedly, two of these new families, resolvins and protectins (Box 1), are biosynthesized from precursor essential omega-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)9-11.
Box 1
Complete stereochemistries of lipoxins and the main resolvins and protectins have been established. They are as follows;
Resolvin D1: 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid
Resolvin E1: 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid
Neuroprotectin D1/Protectin D1: 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid
Lipoxin A4: 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid
Aspirin-triggered lipoxin A4: 5S,6R,15R-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid
Lipoxin B4: 5S,14R,15S-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid.
Aspirin-triggered Resolvin D1: 7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid
Resolution of inflammation: new concepts, mediators and actions
Resolution was defined in modern molecular terms relatively recently6, 8, 12, 13. Importantly, resolution is now considered to be a distinct process from anti-inflammatory processes5. This is because, in addition to serving as agonists to stop and lower neutrophil infiltration to inflamed tissues, pro-resolution molecules promote uptake and clearance of apoptotic cells as well as microbes by macrophages in inflamed sites14, 15 (FIG. 2), and stimulate antimicrobial activities of mucosal epithelial cells16, 17. Resolution of inflammation is accompanied by an active switch in the mediators that predominate in exudates. Initially mediators are generated, such as classic prostaglandins and leukotrienes, which activate and amplify the cardinal signs of inflammation. Next, prostaglandin E2 and prostaglandin D2 gradually induce via key enzymes in the production of mediators that have both anti-inflammatory and pro-resolution activities, such as the lipoxins8, 14, resolvins and protectins9-11 (FIG. 1). These families of endogenous pro-resolution molecules are not immunosuppressive, but instead function in resolution by activating specific mechanisms to promote homeostasis. In general, pro-resolution molecules stimulate and accelerate resolution via mechanisms at the tissue level that are multi-factorial (FIG. 2). Specific lipoxins and members of the resolvin and protectin families are potent stimuli that selectively stop neutrophil and eosinophil infiltration; stimulate nonphlogistic recruitment of monocytes (that is, without elaborating pro-inflammatory mediators); activate macrophage phagocytosis of microorganisms and apoptotic cells; increase lymphatic removal of phagocytes; and stimulate expression of antimicrobial defence mechanisms5, 10, 16, 17.
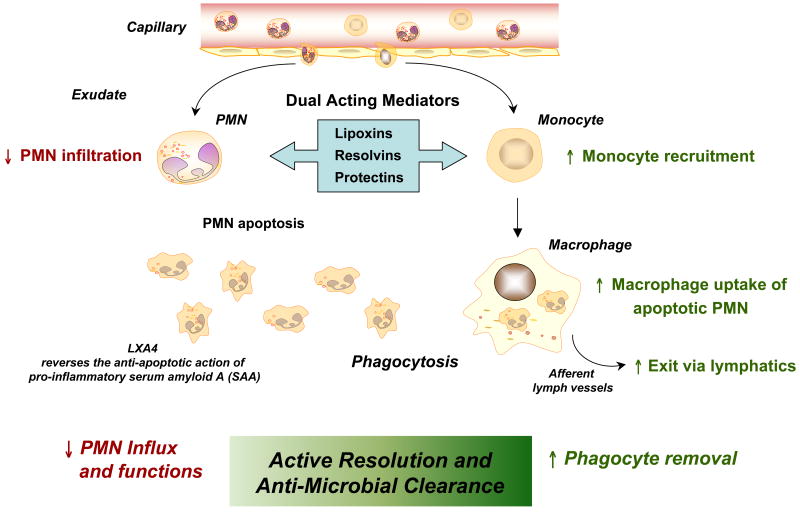
Figure 2. Dual anti-inflammatory and pro-resolution actions of specific lipoxins, resolvins and protectins.
The key histological feature in resolution is the loss of neutrophils from the local inflamed sites. This is a programmed process that is actively regulated at multiple levels: by reducing neutrophil infiltration into the exudates, increasing monocyte recruitment, stimulating macrophage uptake of apoptotic neutrophils, and promoting phagocyte removal via the lymphatics.
Lipid mediators are widely appreciated for their pro-inflammatory activities, such as prostaglandins18 and leukotrienes19. These are rapidly biosynthesized within seconds to minutes of acute challenge by leukocytes from membrane-derived arachidonic acid, using either cyclooxygenase or lipoxygenase. Recent results, however, indicate that as inflammation proceeds neutrophils within confined exudates stop producing chemoattractants, such as leukotriene B4, and begin (within hours) to convert arachidonic acid into lipoxins, that are protective, serving as agonists to actively terminate inflammation and promote resolution7-9. In contained exudates, prostaglandin E2 and prostaglandin D2 are both pro-inflammatory18 in certain tissues and diseases1, 20, 21, and each can promote the switch in local biosynthetic enzyme expression by neutrophils in contained exudates changing their phenotype to pre-resolution8. It is noteworthy that PGE2 was shown to be anti-inflammatory in certain settings when delivered pharmacologically22 to increase cAMP but is not a pro-resolution mediator per se23, while PGE2 together with LTB4 or C5a enhances inflammation24 and is not a direct acting pro-resolution agonist. PGD2 increases intracellular cAMP in specific cell types and can show anti-inflammatory actions25, whereas its non-enzymatic degradation products, i.e., 15-deoxy-delta-PGJ2 and related cyclopentaenones, can enhance resolution by targeting apoptosis mechanisms26 and macrophage clearance via inhibition of NF-κB activation27.
Lipid mediators with dual anti-inflammatory and pro-resolution activities
Lipoxins were the first mediators recognized to have dual anti-inflammatory and pro-resolution activities14, 28. Lipoxins (such as lipoxin A4 and lipoxin B4) are unique structures from arachidonic acid and have potent actions in vivo and in vitro. For in-depth reviews on the biosynthesis, total organic synthesis and actions of lipoxins, aspirin-triggered lipoxins and their stable analogues, see ref. 14. Lipoxins are biosynthesized by the sequential actions of lipoxygenase(s) and other enzymes to produce bioactive trihydroxytetraenes, a structure that is found in all eicosanoids of this class. In human tissues, initial oxygenation of arachidonic acid via 15-lipoxygenase type I followed by 5-lipoxygenase is one route of lipoxin biosynthesis that has been observed in mucosal tissues, such as the respiratory tract, the gastrointestinal tract and oral cavity, via epithelial cell interactions with leukocytes14. This activity is enhanced during inflammation but is also likely to provide a homeostatic mechanism, because mucosal surfaces are continuously exposed to microorganisms in vivo29-31. In mucosa, lipoxins are generated by neutrophils from 15-hydroxyeicosatetraenoic acid (15-HETE) precursor, provided by mucosal epithelial cells29. Another main source of lipoxin biosynthesis in humans is within blood vessels, with initiation of arachidonic acid oxygenation by 5-lipoxygenase in leukocytes and release of the intermediate leukotriene A4, which is converted by platelet lipoxin-synthase activity of the 12-lipoxygenase. This route is exemplified by platelet–leukocyte interactions within vessels or possibly within exudates that form a nidus for transcellular biosynthesis.
In inflamed sites, neutrophils can interact with other neighbouring cells, such as other leukocytes, platelets, endothelial cells, mucosal epithelial cells and fibroblasts, in their immediate vicinity and acquire the ability to produce lipoxins. More than 50% of the leukocyte-derived epoxide intermediate leukotriene A4 is released by cells for processing by platelet 12-lipoxygenase or mucosal 15-lipoxygenase to produce lipoxins32-34. Lipoxins generated by cell–cell interactions and transcellular biosynthesis stop neutrophil diapedesis and recruitment into the tissues (FIG. 1)29-31.
It is now clear that neutrophils change their phenotype to produce different profiles of lipid mediators depending on cells and substrates present in their local environment8, 9. For example, neutrophils in resolving inflammatory exudates switch from leukotrienes to lipoxins and resolvins, whereas those in peripheral blood generate and release leukotriene B4 on activation as one of their main bioactive products8. In this context, local prostaglandin E2 and prostaglandin D2 stimulate the processing of 15-lipoxygenase mRNA in leukocytes to produce functional enzyme for lipoxin production8. Other cell types can acquire the ability to generate lipoxins when exposed to specific cytokines or growth factors32 or when, for example, macrophages engulf apoptotic leukocytes35. These findings are of interest in pro-resolution mechanisms because lipoxin A4 generated by macrophages probably contributes to stimulating their phagocytic activity15, 17 without elaborating pro-inflammatory mediators — namely, the nonphlogistic process.
In addition to the provision of components for lipoxin biosynthesis from host cells, pathogens can contribute. Pseudomonas aeruginosa encodes the first identified secretory lipoxygenase that converts host arachidonic acid to 15-HETE for local lipoxin production36. High levels of lipoxins, greater than those considered to be physiological, are produced by host cells infected by Toxoplasma gondii, which carry their own 15-lipoxygenase37, 38. Thus, 15-lipoxygenase expressed by pathogens may interact with endogenous biosynthetic circuits of the host to generate local “stop signals” at levels that can divert the host immune defence.
Pro-resolution actions
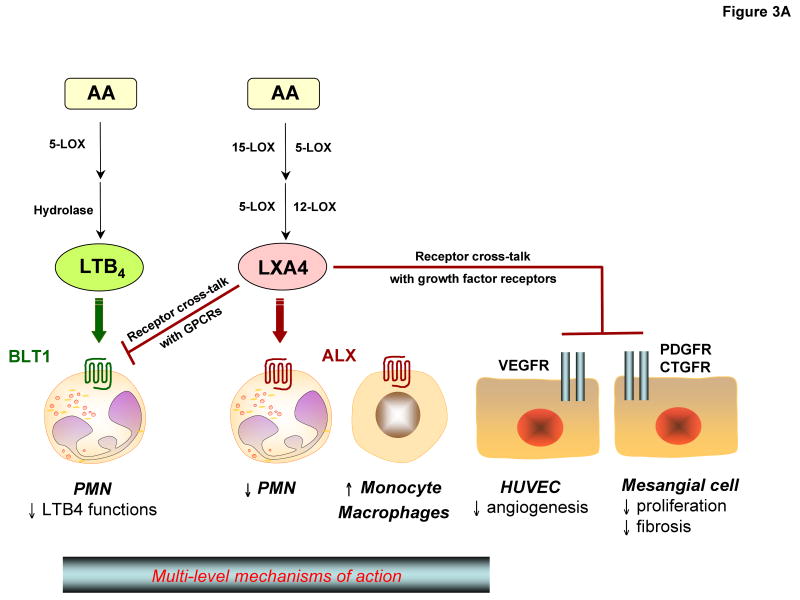
Lipoxin A4 and lipoxin B4 each stop further neutrophil entry into inflamed sites and counter-regulate the main signs of inflammation. They act on many cell types including blood cells, neural and stromal cells39-42 (Table 1). Lipoxin A4 regulates leukocyte responses in vitro and trafficking in vivo by activating its specific receptor, lipoxin A4 receptor (denoted ALX) (FIG. 3A). ALX is a G-protein-coupled receptor (GPCR) expressed by leukocytes with cell-type-specific signalling pathways40. For example, on neutrophils, lipoxin A4–ALX interactions stop migration and do not signal via intracellular Ca2+ mobilization, whereas in monocytes lipoxin A4–ALX interactions stimulate chemotaxis and nonphlogistic responses41. So, unlike classic chemoattractant receptors that mobilize intracellular Ca2+ to evoke chemotaxis, lipoxins instead induce changes in the phosphorylation of proteins of the cytoskeleton that cause cells to arrest 42, 43. In addition to these actions in resolution of inflammation, lipoxin A4 reduces organ fibrosis, acts directly on both vascular and smooth muscle (for reviews, see refs 14, 40) and has a direct action in reducing pain44.
Table 1. Key cellular actions of lipoxins, resolvins and protectins in the innate immune system.
| Mediators | Cell type | Action(s) | |
|---|---|---|---|
| Lipoxin A4 (or aspirin-triggered lipoxin A4) | Whole blood leukocyte | Downregulates CD11b/CD18 expression, prevents shedding of L-selectin and reduces peroxynitrite generation on neutrophils, monocytes and lymphocytes93, 94 | |
| Neutrophil | Stops chemotaxis, adherence and transmigration87 | ||
| Stops neutrophil-epithelial and neutrophil-endothelial cell interactions95 | |||
| Blocks superoxide anion generation96 | |||
| Reduces CD11b/CD18 expression and IP3 formation97 | |||
| Inhibits peroxynitrite generation93 | |||
| Attenuates AP-1, NF-κB accumulation, inhibits IL-8 gene expression93 | |||
| Eosinophil | Stops migration and chemotaxis in vivo, and inhibits eotaxin and IL-5 generation98, 99 | ||
| Monocyte | Stimulates chemotaxis and adhesion to laminin without increase in cytotoxicity41 | ||
| Inhibits peroxynitrite generation93 | |||
| Reduces IL-8 release by cells from asthma patients100 | |||
| Macrophage | Stimulates nonphlogistic phagocytosis of apoptotic neutrophils15, 28, 72 | ||
| T-cell | Inhibits TNF secretion by blocking ERK activation53 | ||
| Upregulates CCR5 expression73 | |||
| Dendritic cell | Blocks IL-12 production37 | ||
| Epithelia | Inhibits TNF-induced IL-8 expression and release in enterocytes101 | ||
| Inhibits Salmonella typhimurium-induced IL-8 in enterocytes102 | |||
| Endothelia | Stimulates protein-kinase-C-dependent prostacyclin formation103 | ||
| Blocks reactive oxygen species generation104 | |||
| Inhibits VEGF-induced endothelial-cell migration105 | |||
| Fibroblast | Inhibits IL-1β-induced IL-6, IL-8 and MMP3 production106 | ||
| Inhibits CTGF-induced proliferation107 | |||
| Hepatocyte | Reduces PPARα and CINC1 expression levels108 | ||
| Mesangial cell | Inhibits leukotriene-D4-induced proliferation109 | ||
| Inhibits CTGF-induced chemokine production110 | |||
| Neural stem cell | Attenuates cell growth111 | ||
| Astrocyte | Inhibits ERK and JNK activation44 | ||
| Resolvin E1 | Neutrophil | Stops transepithelial and transendothelial migration9 | |
| Macrophage | Stimulates nonphlogistic phagocytosis of apoptotic neutrophils15 | ||
| Dendritic cell | Blocks IL-12 production58 | ||
| T-cell | Upregulates CCR5 expression73 | ||
| Resolvin D1 | Microglia | Inhibits IL-1β expression10 | |
| Aspirin-triggered resolvin D1 | Neutrophil | Stops transmigration10, 67 | |
| Protectin D1 | Neutrophil | Upregulates CCR5 expression73 | |
| Macrophage | Stimulates nonphlogistic phagocytosis of apoptotic neutrophils15 | ||
| T cell | Inhibits TNF and IFNγ secretion, promotes apoptosis71 | ||
| Upregulates CCR5 expression73 | |||
| Microglia | Inhibits IL-1β expression11 | ||
| Epithelia | Protects from oxidative-stress-induced apoptosis in retinal pigment epithelia70 | ||
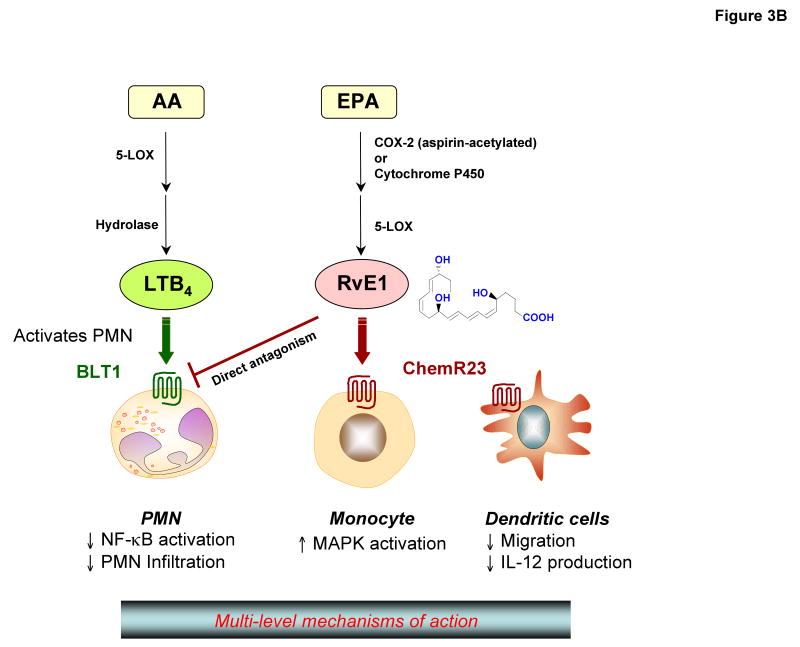
Figure 3. Mechanisms of action of lipoxin A4 and resolvin E1: regulation at multiple levels via GPCRs.
(A) Lipoxin A4: two main “classic” lipoxygenase-mediated pathways of lipoxin generation appear to be used in human cells and tissues (in the vasculature and at mucosal surfaces). The overall action of lipoxin A4in vivo is likely to be attributed to its interactions with G-protein-coupled receptors (GPCRs) and growth-factor receptors. Direct activation of the lipoxin A4 receptor (denoted ALX) by lipoxin A4 has cell-type-specific signalling events that stop neutrophil migration and stimulate monocytes and macrophages. Indirect inhibition, via receptor crosstalk, of other GPCRs (such as leukotriene B4 receptor, denoted BLT1) and growth-factor receptors (such as VEGF on endothelium and PDGF and CTGF on mesangial cells) by lipoxin A4 reduces angiogenesis and mesangial-cell proliferation and fibrosis.
(B) Resolvin E1: Aspirin acetylates cyclooxygenase-2 (COX2) in vascular endothelial cells and generates 18R-hydroperoxyeicosapentaenoic acid (18R-HPEPE), which is further converted via 5-lipoxygenase in leukocytes and additional enzymatic reactions to form resolvin E1. Microbial cytochrome P450 enzymes can also contribute to resolvin E biosynthesis by converting eicosapentaenoic acid (EPA) to 18-HEPE. Resolvin E1 directly interacts with at least two G-protein-coupled receptor (GPCR) systems in a cell-type-specific manner. Resolvin E1 directly activates ChemR23 on mononuclear cells and dendritic cells and directly inhibits the leukotriene B4 receptor, denoted BLT1, on human neutrophils.
Aspirin impinges the endogenous lipoxin-generating system during cell–cell interactions. Inhibition of prostaglandin biosynthesis by aspirin is a well-appreciated mechanism in its anti-thrombotic and anti-inflammatory effect45. Of interest, aspirin triggers endogenous formation of carbon-15 epimeric lipoxins, namely aspirin-triggered lipoxins (ATLs)46. Cells that express cyclooxygenase-2 (COX2), including vascular endothelial cells, epithelial cells, macrophages and neutrophils, are involved in ATL production. Acetylation of COX2 by aspirin blocks its ability to biosynthesize prostaglandins but does not impair its ability to produce ATL precursors. Accordingly, the production of ATLs is elevated in aspirin-treated animals, and importantly in healthy humans taking low doses of aspirin47.
ATLs induce haeme oxygenase 1 (HO1) expression by endothelial cells48, a key system in endogenous anti-inflammation and tissue protection. Mice lacking 15-lipoxygenase type I have an impaired HO1 response. Topical lipoxin A4 in these mice restores HO1 expression and protects from inflammatory challenge49. When triggered by aspirin, lipoxins and 15-epi-lipoxins stimulate nitric oxide by endothelial cells50, blocking leukocyte adhesion to vascular endothelial cells. These mechanisms for lipoxins and 15-epi-lipoxins may be particularly relevant in the local resolution of inflammation. Compelling findings in experimental animal systems demonstrating both anti-inflammatory and pro-resolution actions of lipoxins and ATLs in vivo (Table 2) establish that preventative treatment with a pro-resolution mediator reduces inflammation and disease status15, 51. Therefore, endogenous ATLs (15-epi-lipoxins) offer new and potentially important mechanisms that underlie clinical benefits of aspirin47-50. 15-epi-lipoxin production may be an essential mechanism in other widely used drugs, such as statins, which stimulate endogenous 15-epi-lipoxin A452.
Table 2. Dual anti-inflammatory and pro-resolution actions of lipoxins, resolvins and protectins in complex disease models.
| Mediators | Species/disease model | Action(s) | Refs |
|---|---|---|---|
| Lipoxin A4/ATL | Rabbit/Periodontitis | Reduce neutrophil infiltration; prevent connective tissue and bone loss | 74 |
| Mouse/Peritonitis | stops neutrophil recruitment and lymphatic removal of phagocytes | 6,15 | |
| Mouse/Dorsal air pouch | stops neutrophil recruitment | 112 | |
| Mouse/Derma l inflammation | stops neutrophil recruitment and vascular leakage | 39, 113 | |
| Mouse/Colitis | Attenuate proinflammatory gene expression and reduce severity of colitis. Inhibit weight loss, inflammation and immune dysfunction | 101, 77,30 | |
| Mouse/Asthma | Inhibit airway hyper-responsiveness and pulmonary inflammation | 82 | |
| Mouse/Cystic fibrosis | Decrease neutrophilic inflammation, pulmonary bacterial burden and disease severity | 91 | |
| Mouse/Ischemia-reperfusion | Attenuate hind-limb I/R-induced lung injury | 114 | |
| Detachment of adherent leukocytes in mesenteric I/R | 115 | ||
| Mouse/Cornea | Accelerate cornea re-epithelialization, limit sequelae of thermal injury (i.e. neovascularization, opacity) and promote host defence | 78 | |
| Mouse/Angiogenesis | Reduce angiogenic phenotype: endothelial cell proliferation and migration | 116 | |
| Mouse/Bone-marrow transplant (BMT) | Protect against BMT-induced graft-versus-host diseases | 117 | |
| Murine/Glomerulonep hritis | Reduce leukocyte rolling and adherence Decrease neutrophil recruitment | 118 | |
| Rat/Hyperalgesia | Prolong paw withdraw latency, reducing hyperalgesic index Reduce paw oedema | 44 | |
| Rat/Pleuritis | Shorten the duration of pleural exudation | 98 | |
| Resolvin E1 | Rabbit/Periodontitis | Reduces neutrophil infiltration; prevents connective tissue and bone loss; promotes healing of diseased tissues; regeneration of lost soft tissue and bone | 23, 61 |
| Mouse/Peritonitis | Stops neutrophil recruitment; regulates chemokine/cytokine production | 6, 58 | |
| Promotes lymphatic removal of phagocytes | 15 | ||
| Mouse/Dorsal air pouch | Stops neutrophil recruitment | 9 | |
| Mouse/Retinopathy | Protects against neovascularization | 119 | |
| Mouse/Colitis | Decreases neutrophil recruitment and proinflammatory gene expression; improves survival; reduces weight loss | 62 | |
| Resolvin D1 | Mouse/Peritonitis | Stops neutrophil recruitment | 11, 67 |
| Mouse/Dorsal skin air pouch | Stops neutrophil recruitment | 10, 11 | |
| Mouse/Kidney ischemia-reperfusion | Protects from ischemia-reperfusion-induced kidney damage and loss of function; regulates macrophages | 80 | |
| Mouse/Retinopathy | Protects against neovascularization | 119 | |
| Protectin D1 | Mouse/Peritonitis | Inhibit neutrophil recruitment; regulate chemokine/cytokine | 6, 58 |
| production | 15 | ||
| Promote lymphatic removal of phagocytes | 71 | ||
| Regulate T-cell migration | |||
| Mouse/Asthma | Protect from lung damage, airway inflammation and airway hyperresponsiveness | 84 | |
| Human/Asthma | Protectin D1 is generated in humans and appears to be diminished in asthmatics | 84 | |
| Mouse/Kidney ischemia-reperfusion | Protect from ischemia-reperfusion-induced kidney damage and loss of function; regulate macrophages | 80 | |
| Mouse/Retinopathy | Protect against neovascularization | 119 | |
| Rat/Ischemic stroke | Stop leukocyte infiltration, inhibit NF-κB and cyclooxygenase-2 induction | 66 | |
| Human/Alzheimer's disease | Diminished protectin D1 production in human Alzheimer's disease | 69 |
Of interest, both lipoxins and ATLs directly act on human T cells53 to block tumour-necrosis factor (TNF) secretion. Human peripheral T cells express ALX, and activation of these receptors blocks CD3-specific-antibody-induced activation of extracellular signal-regulated kinase (ERK) required for TNF secretion. Lipoxin B4 blocks phosphorylation of ERK in T cells, as does one of its analogues, 5-(R/S)-methyl lipoxin B4, which also inhibits TNF secretion by activation of lipoxin B4 receptors. Lipoxins provide a link between innate cells involved in resolution of inflammation and cells of the adaptive immune system54.
Resolvins and protectins: new families of mediators in resolution
Literature reports that essential omega-3 PUFAs given at high doses (milligrams to grams daily) have beneficial actions in many inflammatory diseases, cancer and human health in general20, 55, 56. The molecular basis of omega-3 fatty acid action was not established until recently. To identify potential mechanisms that are actively involved in resolution of inflammation, we devised a new lipid mediator lipidomics9, 10 and informatics57 approach, with liquid chromatography-ultraviolet-tandem mass spectrometry-based analyses, to map and profile the appearance and/or loss of mediators in resolving inflammatory exudates. When novel bioactive compounds were encountered, their structures were elucidated and their bioactivity and role(s) confirmed in vivo9-11, 51, 58. These studies uncovered two new families of bioactive mediators, termed resolvins and protectins, biosynthesized from omega-3 essential PUFAs.
Resolvins
The first resolvin was identified in exudates9 collected from inflamed murine dorsal air pouches in the spontaneous resolution phase59 and so-named because it proved to be a potent regulator of resolution. Resolvins are derived from EPA and DHA with two chemically unique structural forms, the E-series and D-series of resolvins, respectively. E-series member resolvin E1 reduces inflammation in vivo and blocks human neutrophil transendothelial migration9. Resolvin E1 can be produced in vitro, recapitulating events in vivo, by treating human vascular endothelial cells in a hypoxic environment with aspirin. These cells convert EPA to 18R-hydroperoxyeicosapentaenoic acid (18R-HPEPE) and release 18R-hydroxyeicosapentaenoic acid (18R-HEPE), which is rapidly transformed by activated human neutrophil 5-lipoxygenase9, 60. Resolvin E1 is produced in healthy individuals and is increased in plasma of individuals taking aspirin and/or EPA58.
Resolvin E2 is the second member of the E-series that reduces zymosan-initiated neutrophil infiltration, thereby displaying potent anti-inflammatory actions60. These EPA-derived E-series resolvins may contribute to beneficial actions attributed earlier to omega-3 PUFA in human diseases, such as skin inflammation, peritonitis9, periodontal disease23, 61 and colitis62 (Table 2). These results illustrate that 5-lipoxygenase in human leukocytes is pivotal for these beneficial effects and is controlled in part by temporal and spatial events in vivo to signal production of either leukotrienes or anti-inflammatory mediators, such as lipoxins and resolvins. Of interest, microbial and mammalian cytochrome P450 enzymes convert EPA into 18-HEPE9, which can be transformed by human neutrophils to resolvin E1 and resolvin E2. Hence, it is likely that microorganisms at inflamed sites or in the gastrointestinal tract can contribute to production of E-series resolvins in humans.
At least two receptors involved in the actions of resolvin E1 are defined (FIG. 3B). The GPCR chemokine-like receptor 1 (CMKLR1, also known as ChemR23) attenuates TNF-stimulated nuclear factor-κB activation in response to resolvin E1 binding, indicating a counter-regulatory action of this ligand–receptor pair58. Counter-regulation of TNF signalling was used to identify this GPCR because TNF is a key mediator in the early steps of acute inflammation9, 58. Recently, a second GPCR that interacts with resolvin E1 was identified, the leukotriene B4 receptor, denoted BLT163. Resolvin E1 interacts in a stereospecific manner with BLT1 on human neutrophils as a receptor antagonist9, attenuating leukotriene-B4-dependent pro-inflammatory signals via BLT163. The anti-inflammatory actions of resolvin E1 are markedly reduced in BLT1-deficient mice. So, to counter-regulate inflammation and promote resolution, resolvin E1 selectively interacts with at least two GPCRs present on different cell types, namely CMKLR1 on mononuclear and dendritic cells, and BLT1 on neutrophils.
DHA is substrate for two groups of resolvins produced by different biosynthetic routes, denoted the 17S and 17R D-series resolvins (FIG. 1), during resolution in inflammatory exudates10, 11. D-series resolvins display potent anti-inflammatory actions10 and are particularly interesting because the brain, synapses and retina are highly enriched in DHA64-66. Endogenous DHA is converted in vivo via lipoxygenase-initiated mechanisms to the 17S-hydroxy-containing series of four resolvins, denoted D1–D410, 11. Each of these potent bioactive resolvins was first isolated in exudates from mice given aspirin and DHA, which led to the identification of several new 17R-hydroxy-containing products isolated from exudates in the resolution phase10. Their ability to stop neutrophil infiltration was used to assess biological function for structural elucidation studies10 and their biosynthesis was reconstructed to establish their potential origins. Results from these studies showed that human recombinant COX2 converts DHA into a 13-hydro(peroxy)-containing product. In the presence of aspirin, oxygenation at carbon-13 switches to the carbon-17 position with an R configuration that is a precursor for potent bioactive aspirin-triggered 17R D-series resolvins, denoted aspirin-triggered (AT)-RvD1-D4. These are produced in exudates and in the brain in response to aspirin treatment10, 11. Resolvin D1 and aspirin-triggered resolvin D1 have both been shown to be potent regulators of human and mouse neutrophils10, 67 (Table 1). In microglial cells, both 17S and 17R D-series resolvins block TNF-induced transcripts for pro-inflammatory cytokine interleukin-1β (IL-1β), which is expressed rapidly in response to neuronal injury11,68. Resolvins control inflammation at many levels, by reducing peritonitis and skin inflammation10, 11, protecting organs from reperfusion injury and neovascularization (Table 2). Thus, D-series resolvins are of interest in the control of inflammation-resolution in host defence and in neural tissues.
Protectins
DHA is converted in resolving exudates to another new family of mediators named protectins. Mediators of this family are distinguished by the presence of a conjugated triene double bond system and their potent bioactivity 10, 11. They are biosynthesized via a lipoxygenase mechanism that converts DHA to a 17S-hydroperoxide-containing intermediate11, which is rapidly converted by human leukocytes into a 16(17)-epoxide that is enzymatically opened in these cells into a 10,17-dihydroxy-containing anti-inflammatory molecule10, 11. This bioactive compound, initially coined 10,17-diHDHA or 10,17S-docosatriene11, is now known as protectin D1 owing to its potent protective activity in inflammatory51 and neural systems documented in studies with N. Bazan and colleagues66, 69, 70. It is termed neuroprotectin D170 when produced by neural tissues; the prefix neuro is added to signify its biosynthetic origin51.
Several 10,17-dihydroxy-containing products are produced in vivo via different biosynthetic routes, the most potent being protectin D1. The other natural protectin D1 isomers have different double-bond configurations and are less potent in dampening neutrophil recruitment and inflammation51. Protectin D1 is stereo-selective and log-orders of magnitude more potent in vivo than its precursor DHA. Protectin D1 is also produced by human peripheral blood mononuclear cells in T helper-2-type conditions in a lipoxygenase-dependent manner via a 16(17)-epoxide intermediate. Protectin D1 blocks T-cell migration in vivo, reduces TNF and interferon-γ secretion, and promotes T-cell apoptosis71.
Agonists of resolution
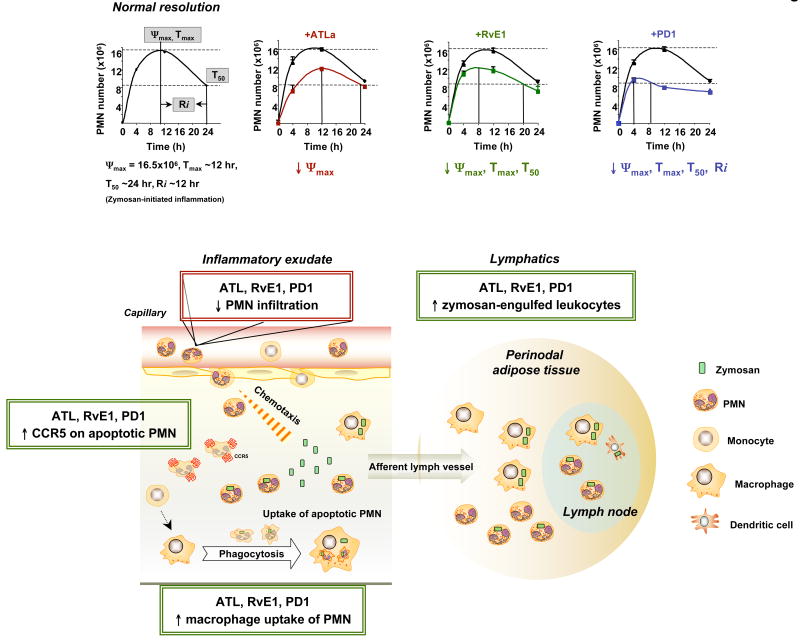
Specific resolvins, protectins and lipoxins stereoselectively stimulate resolution and reduce the magnitude of the inflammatory response in vivo (Tables 1 and 2). The clearance of apoptotic neutrophils by professional phagocytes, such as macrophages, is a cellular hallmark of tissue resolution72, and this can be used to quantify resolution using specific indices6 (FIG. 4). Resolvin E1 initiates resolution, decreasing neutrophils in exudates at earlier times than spontaneous resolution. Protectin D1 shifts the onset of resolution to an earlier time point and in addition shortens the time taken to reduce the number of maximum neutrophils by half as indicated by calculation of the resolution interval (FIG. 4). As mechanisms in their pro-resolution actions, both resolvin E1 and protectin D1 mediate this activity by reducing neutrophil influx and stimulating macrophage ingestion of apoptotic neutrophils, as well as by enhancing the number of phagocytes present in lymph nodes and spleen15. Disruption of biosynthesis of these pro-resolution mediators by either COX2 or lipoxygenase inhibitors gives rise to a “resolution deficit” phenotype, characterized by impaired phagocytic removal, delayed resolution and prolonged inflammation. These findings emphasize a pivotal homeostatic function for lipoxygenase(s) and COX2 pathways in the timely resolution of acute inflammation. More importantly, pro-resolution mediators at lower doses than inhibitors of COX2 and lipoxygenase rescue the deficit in resolution caused by these interventions15.
Figure 4. Resolution indices pinpoint the tissue level mechanism of action of anti-inflammatory-pro-resolution lipid mediators.
(Upper part) The main events in resolution of acute inflammation can be quantified by introducing resolution indices: (i) Magnitude (Ψmax, Tmax) -- The time point (Tmax) when neutrophil numbers reach maximum (Ψmax); (ii) Duration (R50, T50) -- The time point (T50) when the neutrophil numbers reduce to 50% of Ψmax (R50); (iii) Resolution Interval (Ri) --The time interval from the maximum neutrophil point (Ψmax) to the 50% reduction point (R50) [i.e. T50 - Tmax]. For calculating specific resolution indices, see refs. 8 and 18 for details. ATLa lowers the maximal neutrophil numbers (↓Ψmax); RvE1 and PD1, in addition, initiate the resolution at the earlier time (↓Tmax and T50); PD1 further shortens the resolution interval (↓Ri).
(Lower part) The resolution agonists lipoxins, resolvins and protectins promote homeostasis by stimulating exudate cells and surrounding tissues to limit further neutrophil infiltration; increase CC-chemokine receptor 5 (CCR5) expression on apoptotic neutrophils and lymphocytes, which enhances the removal of chemokines and cytokines that adhere to dying leukocytes and are transported out of the tissues and phagocytosed by macrophages (for detailed mechanism, see ref 73); promote macrophage uptake of neutrophils in the exudates and the removal of macrophages carrying such debris; enhance the clearance of zymosan-engulfed leukocytes in lymph nodes and in spleen; stimulate the antimicrobial actions and clearance mechanisms of mucosal epithelial-cell surfaces16, 17.
Resolvin E1 and protectin D1 upregulate CCR5 expression on dying neutrophils 73. CCR5 expression on late apoptotic neutrophils acts as a “terminator” of chemokine signalling, clearing pro-inflammatory ligands CCL3 and CCL5, which bind CCR5 and ferry them along from the inflammatory site. In keeping with a role in stimulating clearance and the return to homeostasis, resolvin E1 selectively induces the expression of CD55, an anti-adhesion molecule apically expressed on mucosal epithelial cells, and thereby promotes CD55-dependent clearance of neutrophils across mucosal surfaces17. Thus, these EPA- and DHA-derived mediators are potent resolution agonists activating cell-type-specific programmes in, for example, neutrophils, macrophages and epithelial cells (Table 1), at multiple levels to accelerate resolution.
Resolution of inflammation in disease models
Uncontrolled inflammation is now appreciated in the pathogenesis of many diseases that were not previously considered classic inflammatory diseases. These include atherosclerosis, cancer, asthma and several neurological disorders, such as Alzheimer's disease and Parkinson's disease. Natural pro-resolution mechanisms involving lipoxins, resolvins and protectins were tested for their ability to promote resolution and control inflammation (Table 2). It is now clear that endogenous anti-inflammation alone is not an identical mechanism of action compared to mediators that possess dual anti-inflammatory and pro-resolution actions5. In this regard, lipoxins, resolvins and protectins have potent multi-level mechanisms of action in disease models and promote resolution in animal models of oral, lung, ocular, kidney, skin and gastrointestinal inflammation as well as in ischaemia-reperfusion injury and angiogenesis (Table 2).
Inflammatory diseases
Periodontal diseases such as gingivitis and periodontitis are leukocyte-driven inflammatory diseases characterized by soft-tissue and osteoclast-mediated bone loss21. As a model of inflammatory diseases, periodontitis has several advantages in that many, if not all, of the tissues involved in inflammatory processes of other organ systems are affected in periodontitis, including the epithelium, connective tissue and bone. There are many noteworthy similarities in the pathogenesis of periodontitis and arthritis. Results from rabbit periodontitis demonstrate an important role for resolution in disease prevention. Overexpression of 15-lipoxygenase-type 1 in transgenic rabbits increases the levels of endogenous lipoxin A4 and protects against periodontitis, as well as reduces atherosclerosis74, 75. In prevention studies using this model, topical treatment with resolvin E1 prevents >95% of alveolar bone destruction. Histological analysis of resolvin-E1-treated rabbits revealed few, if any, neutrophils and little tissue damage. In addition, osteoclasts responsible for bone resorption are reduced in resolvin-E1-treated rabbits61. In established disease, resolvin E1 prevents periodontitis tissue destruction; both soft tissue and bone that were lost during disease were regenerated23.
In humans, the differential actions of resolvin E1 were studied using neutrophils from patients with localized aggressive periodontitis (LAP) and healthy individuals. Resolvin E1 reduces neutrophil superoxide generation in response to TNF or the bacterial surrogate peptide N-formyl-methionyl-leucyl-phenylalanine. Neutrophils from both healthy subjects and LAP patients produced ∼ 80% less superoxide treated with resolvin E1. In comparison, neutrophils from LAP patients do not exhibit inhibition of superoxide production following treatment with lipoxin A4, suggesting a molecular basis for excessive inflammation in these patients.
In murine air pouches, nanogram amounts of resolvin E1 (∼100 nM) reduce leukocyte infiltration by 50-70%, levels comparable to those achieved using microgram amounts of dexamethasone (∼30 μM) or milligrams of aspirin (∼6 mM)9, 58. Similarly, in spontaneously resolving peritonitis induced by the yeast cell-wall component zymosan, both resolvin E1 and protectin D1 activate and accelerate resolution15. Resolvins and protectins reduce neutrophil infiltration and increase nonphlogistic recruitment of monocytes. Notably, resolvin E1, protectin D1 and an ATL analogue each display different kinetics and molecular profiles of action (Tables 1 and 2).
Organ-specific diseases
Lipoxins are produced in the human gut mucosa76, where limit persistent inflammation. As in the oral cavity, this is important given the continuous exposure of this organ to commensal bacteria. Patients with ulcerative colitis exhibit low to absent levels of lipoxin A4 and have lower mucosal 15-lipoxygenase76. Interestingly, in the trinitrobenzene sulphonic acid (TNBS)-induced mouse model of Crohn's disease, oral administration of a lipoxin A4 analogue has potent efficacy in promoting the resolution of colitis77. Resolvin E1 reduces colitis-associated mortality and protects animals from weight loss and shortening of the colonic mucosa62. Histology analysis of treated animals revealed fewer colonic ulcerations and reduced transmural infiltration of neutrophils, monocytes and lymphocytes. Serum levels of TNBS-specific IgG were also decreased by this treatment, suggesting diminished antigen presentation and antibody production. Resolvin-E1-treated mice with colitis have reduced production of the pro-inflammatory cytokines TNF and IL-12 p40 and increased levels of inducible nitric oxide synthase and COX2, whereas the levels of interferon-γ, IL-4 and IL-10 remain essentially unchanged62. Thus, agonists of resolution of inflammation prevent immune-mediated tissue damage and restore tissue homeostasis.
In Alzheimer's disease, soluble amyloid precursor protein-α, which stimulates in vitro proliferation of neural embryonic stem cells, activates neuroprotectin D1 biosynthesis. The hippocampal cornu ammonis region 1, but not the thalamus or occipital lobes, has decreased levels of DHA and neuroprotectin D169. The hippocampus of patients with Alzheimer's disease shows decreased expression of phospholipase A2 and 15-lipoxygenase — key enzymes in neuroprotectin D1 biosynthesis in this tissue hence they produces less of this endogenous protective mediator than healthy tissues66, 69. Neuroprotectin D1 reduces the expression of pro-inflammatory genes and upregulates the expression of anti-apoptotic genes69, 70. These suggest that neuroprotectin D1 promotes brain cell survival by inducing anti-apoptotic and neuroprotective genes69.
Retinal pigment epithelium (RPE) of the eye generates neuroprotectin D1 from endogenous DHA that protects them from oxidative-stress-induced apoptosis by limiting pro-inflammatory gene expression70. Photoreceptor cell integrity depends on the RPE, and loss of integrity is characteristic of retinitis pigmentosa and age-related macular degeneration. RPE cells undergoing oxidative stress generate neuroprotectin D1 and upregulate expression of the anti-apoptotic proteins BCL-2 (B-cell lymphoma 2) and BCL-XL, decreasing the levels of pro-apoptotic proteins BAX (BCL-2-associated X protein) and BAD (BCL-2-antagonist of cell death). In addition, neuroprotectin D1 reduces leukocyte infiltration and pro-inflammatory gene expression in brain ischaemia-reperfusion injury66, 69, 70.
Independent of their neutrophil-directed actions, both lipoxin A4 and protectin D1 also influence wound healing. Mouse cornea generates lipoxin A4 and protectin D178. In corneal thermal injury, topical application of either lipoxin A4 or protectin D1 increases the rate of re-epithelialization by ∼75%. Removal of corneal epithelial cells initiates neutrophil infiltration and increases the levels of the pro-inflammatory chemokine CXCL1 (murine equivalent of human IL-8) produced by the corneal stroma. Local treatment of lipoxin A4 or protectin D1 decreased CXCL1 levels by ∼60%78.
Acute kidney injury is an inflammatory process where repair and regeneration following acute inflammatory events can lead to interstitial fibrosis, scarring and chronic kidney failure with persistent leukocyte infiltration79. Ischemia followed by reperfusion leads to endogenous mobilization and increased levels of DHA in the blood and the production of D-series resolvins and protectins80. When treated with resolvins before bilateral renal ischemia, mouse kidneys were protected from injury; creatinine serum levels were lower in treated mice compared to control mice. Protectin D1 was also protective, and both protectin D1 and resolvins reduce tissue neutrophils and limit the deposition of interstitial collagen, thereby protecting against tissue fibrosis. Resolvin D1 when given after ischemic kidney injury protects mice from acute renal failure; however, this was not the case with protectin D1. These results suggest that D-series resolvins and protectin D1 activate resolution circuits in acute kidney injury80.
Dietary incorporation of DHA is protective against murine liver necroinflammatory injury through increased local production of DHA-derived mediators81. Hepatocytes incubated with DHA or 17-HDHA show less hydrogen-peroxide-induced DNA damage and less cellular oxidative stress. Mice fed a DHA-enriched diet are protected from carbon-tetrachloride-induced necroinflammatory hepatic damage81.
In the lungs, lipoxins generated in murine models of asthma are potent regulators of airway inflammation and hyperresponsiveness82. Lipoxins and their stable analogues reduce pulmonary inflammation by decreasing neutrophil, eosinophil and lymphocyte recruitment and activation. They also block oedema formation and reduce levels of pro-inflammatory mediators IL-5, IL-13, eotaxin, prostanoids and cysteinyl leukotriene82. Consistent with this key counterregulatory role, severe human asthmatics have apparent defects in lipoxin biosynthesis83. Protectin D1 is also diminished in asthmatic subjects and potentially reduces both airway inflammation and hyperreactivity84. Thus, it appears that pro-resolution lipid mediators have roles in both physiologic and pathophysiologic processes in specific tissues.
Concluding remarks and future directions
With the discovery of lipid mediators that possess both anti-inflammatory and pro-resolution activities (dual action mediators), these new families of resolvins and protectins and class of eicosanoids, e.g., lipoxins, constitute a novel genus of pro-resolution mediators. Together, they help open new avenues for treatment and the potential for resolution-based pharmacology and lipidomics-based therapeutics. Since many current and widely used drugs were developed without knowledge of their impact in resolution circuits, some agents, such as selective COX2 inhibitors and certain lipoxygenase inhibitors, have proven to be toxic to the tissue programmes of resolution, delaying the return to homeostasis5, 15, 85, whereas others, such as glucocorticoids86, aspirin87, cyclin-dependent kinase inhibitors88 and statins52, seem to work in concert with endogenous pro-resolution processes. Hence, in the near future resolution-directed therapeutics may involve small molecule mimetics in designer resolution-anti-inflammatories13. COX inhibitors reduce the amplitude of and cardinal signs of inflammation by inhibiting prostanoid biosynthesis. Thus, combining pro-resolution molecules together with LOX or COX pathway antagonists may be a useful strategy to rescue resolution and control excessive inflammation. Also, pro-resolution mediators may have therapeutic potential in settings where sustained inflammation and impaired resolution are components of disease pathophysiology (Table 2).
The relationship between essential fatty acids in nutrition, dietary supplementation and the biosynthesis of resolvins and protectins is an area of active interest. Along these lines, our studies with Kang and colleagues in fat-1 transgenic mice, which overexpress fatty acid desaturase from C. elegans, help to address discrepancies in dietary studies that can arise from individual genetic and feeding variations. The fat-1-transgenic mice produce and store higher levels of EPA and DHA in their tissues and as a result generate increased levels of resolvins and protectins. On provocation, fat-1-transgenic mice show reduced gastrointestinal inflammation89 and less tumor metastasis90. Whether these exciting findings extend to humans will be of interest in further studies.
Lipoxins and resolvins act at multiple tissue and cellular levels with different receptors. Susceptibility to chronic inflammatory disorders may therefore result from uncontrolled resolution circuits that can arise from defects in either receptors and/or their signalling, or in de novo biosynthesis of pro-resolution molecules84, 91. In the case of periodontal disease, which has many of the pathogenic features, including infection, observed in chronic inflammatory disorders in other locations in the body, the stage is now set for proof of principle in humans, because lipoxin A4 and resolvin E1 both reduce inflammation in periodontal disease in rabbits and mice and expedite the return to homeostasis21, 23.
It is worth pointing out that resolvins and protectins are structures that are conserved in evolution, as they are present in fish brain and haematopoietic tissues92. It will be important to learn whether resolution pharmacology leads to new treatments for human disease. For example, will it be possible to treat organ specific inflammatory disorders with different pro-resolution mediators and/or their analog mimetics, with tissue specific precision? Will dietary supplementation with intermediates and/or precursors be beneficial for the local production of pro-resolution mediators? Will knowledge of these new pathways lead to an increased appreciation of potential deleterious effects of excess DHA and EPA intake that could lead to increases in autooxidation of these fatty acids that can damage cells and tissues initiating inflammation? It is likely that the serum levels of EPA and DHA are tightly regulated and that exceeding their physiological boundaries could have a negative impact in human systems. Moreover, since lipoxins, resolvin E1 and protectins act on T cells, dendritic cells and phagocytic cells, communication(s) between the innate and cellular immune systems appears likely to have a molecular link to nutrition. Further evidence is needed to substantiate these points. In relation to essential fatty acids, knowledge of these temporal-spatial signalling pathways and their actions should be harnessed in humans to improve health and reduce disease.
Acknowledgments
We thank Mary H. Small for expert assistance in manuscript preparation. This study was supported in part by National Institutes of Health Grant Nos. GM38765 (C.N.S.), DK074448 (C.N.S.) and P50-DE016191 (C.N.S., T.E.V.D., N.C.). Apologies to our colleagues if their original contributions are missed in the references because of space limitations.
Glossary
- Phagolysosome
An intracellular vesicle that results from the fusion of phagosomes, which enclose extracellular material that has been ingested, and lysosomes, which contain lytic enzymes.
- Diapedesis
Diapedesis is the migration of leukocytes across the endothelium, which occurs by squeezing through the junctions between adjacent endothelial cells. This is the last step in the leukocyte–endothelial-cell adhesion cascade that includes tethering, triggering, tight adhesion and transmigration.
- Lipoxins
A class of eicosanoids that are produced by lipoxygenase-mediated metabolism of arachidonic acid. They are trihydroxytetraene-containing structures with potent biological activities in the resolution of inflammation.
- Resolvins
Lipid mediators that are induced in the resolution-phase following acute inflammation. They are synthesized from the essential omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
- Prostaglandins
Cyclopentane-ring-containing lipids derived from the metabolism of arachidonic acid by the action of cyclooxygenases and downstream synthase enzymes. They have a diverse range of biological activities and a well recognized role in inflammation and pain.
- Leukotrienes
A family of eicosanoids derived from the metabolism of arachidonic acid by the action of leukocyte 5-lipoxygenase and other enzymes. They have a conjugated triene double-bond structure and various pro-inflammatory activities, including leukocyte activation (by leukotriene B4) and bronchoconstriction (by leukotriene C4 and leukotriene D4).
- Protectins
A family of docosahexaenoic acid (DHA)-derived mediators characterized by the presence of a conjugated triene double-bond structure and 22 carbons with six double bonds.
- Eicosanoids
A family of bioactive products that contain 20 (eicos in Greek) carbons. They are biosynthesized from arachidonic acid by the initial activities of either cyclooxygenases (isoforms COX1 or COX2) or lipoxygenases and downstream enzymatic reactions. There are several main classes of eicosanoids prostaglandins, prostacyclins, thromboxanes, leukotrienes and lipoxins.
- Transcellular biosynthesis
The biosynthesis of biologically active mediators that involves two or more cell types, for example, when the necessary enzymes are differentially expressed in two or more cell types. In this example, a donor cell converts a precursor compound (such as arachidonic acid, EPA and DHA) into an intermediate product. The acceptor cell then converts the intermediate product into the final active product. Transcellular biosynthesis therefore provides a means to produce mediators that neither cell type can generate alone.
- G-protein-coupled receptor
(GPCR). One of a large group of receptors that bind a diverse set of molecules, including chemokines, complement components, biologically active amines and neurotransmitters. GPCRs are seven-transmembrane-spanning receptors and are coupled to heterotrimeric, GTP-regulated signalling proteins composed of αβ and βγ subunits.
- Statins
A class of drugs that inhibit the rate-limiting enzyme (3-hydroxy-3-methylglutaryl coenzyme A reductase) in the pathway of cholesterol biosynthesis. These molecules are mainly used as cholesterol-lowering drugs, but they also have immunoregulatory and anti-inflammatory properties.
- Murine dorsal air pouch model
A well-characterized model for studying inflammatory responses. Air pouches are formed by subcutaneous injection of air in the back of a mouse, into which potential inflammatory stimuli, such as tumour-necrosis factor, can be added. Importantly, when the stimulus is titrated, inflammatory reactions undergo spontaneous resolution. These structurally contained compartments have been likened to the inflamed synovium.
- Cytochrome P450 enzymes
A large and diverse superfamily of haemoproteins. Cytochrome P450 enzymes use a plethora of both exogenous and endogenous compounds as substrates. The most common reaction catalysed by cytochrome P450 is a monooxygenase reaction, that is, insertion of one atom of oxygen into an organic substrate while the other oxygen atom is reduced to water.
- Rabbit model of periodontitis
A model of periodontitis in rabbits induced by application of Porphyromonas gingivalis to ligatures tied to second premolars.
- ulcerative colitis
An inflammatory bowel disease characterized by chronic inflammation of the colon.
- Alzheimer's disease
A degenerative neurological disease that is characterized by progressive deterioration of the brain, dementia, and the presence of senile plaques, neurofibrillary tangles and neuropil threads. Disease onset can occur at any age, and women seem to be affected more frequently than men.
- age-related macular degeneration
A medical condition predominantly found in elderly adults in which the centre of the inner lining of the eye, known as the macula area of the retina, suffers thinning, atrophy, and in some cases bleeding, and can result in loss of central vision.
- ischemia-reperfusion injury
An injury in which the tissue first suffers from hypoxia as a result of severely decreased, or completely arrested, blood flow. Restoration of normal blood flow then triggers inflammation, which exacerbates the tissue damage.
- Creatinine
Creatinine is a component of urine and the final product in the metabolism of creatine. An increase in serum concentration is used as a marker of kidney dysfunction.
- Exudate
Biological fluids that filter from the circulatory system into lesions or areas of inflammation. Exudate formation is caused by inflammation, and is characterized by a high content of plasma proteins, cells and cellular debris. Pus is an example of an exudate found in infected wounds that contains bacteria and high concentrations of white blood cells.
Biographies
Prof. Charles N. Serhan. Dr. Serhan's research focuses on the structural elucidation of pathways and bioactive compounds in acute inflammation and its resolution. He is Director of the Center for Experimental Therapeutics and Reperfusion Injury at Brigham and Women's Hospital and is the Simon Gelman Professor of Anaesthesia (Biochemistry and Molecular Pharmacology), Harvard Medical School. He received degrees in Biochemistry, Experimental Pathology and Medical Sciences from NYU, and post-doctoral training in Physiological Chemistry at the Karolinska Institute with Prof. Bengt Samuelsson (1982 Nobel Laureate).
Nan Chiang received her doctoral degree in Medicinal Chemistry and Pharmaceutics, University of Kentucky. She did postdoctoral training in Prof. Charles Serhan's laboratory with an Arthritis Foundation Fellowship, and is currently an Assistant Professor in the Center for Experimental Therapeutics and Reperfusion Injury at Brigham & Women's Hospital and Harvard Medical School.
Thomas E. Van Dyke, D.D.S., Ph.D. is a professor in the Department of Periodontology and Oral Biology at Boston University's Goldman School of Dental Medicine, directs the Clinical Research Center, and is associate director at B.U. School of Medicine General Clinical Research Center.
Footnotes
- Complete resolution of an acute inflammatory response and its return to homeostasis are essential for healthy tissues. Resolution of acute inflammation is an active process, not just passive termination of inflammation.
- Novel families of lipid mediators are generated in inflammatory exudates during resolution phase and they can promote and/or accelerate resolution. These include lipoxins and omega-3 essential fatty acids EPA- and DHA-derived resolvins and (neuro)protectins.
- The pro-resolution lipid mediators (PRM) are agonists of resolution, with novel mechanisms of action at the tissue level; they “stop” PMN and eosinophil infiltration, stimulate non-phlogistic recruitment of monocytes, enhance macrophage phagocytosis of apoptotic PMN, increase lymphatic removal of phagocytes and stimulate mucosal anti-microbial defence.
- Pro-resolution and anti-inflammation are not equivalent; pro-resolution programs stimulate and activate endogenous pathways to terminate inflammation.
- PRM have multi-level protective actions in a wide range of cell types and in complex disease systems.
- PRM promote resolution in oral, lung, ocular, kidney, neural and gastrointestinal inflammation as well as in ischemia-reperfusion and angiogenesis.
References
- 1.Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. Oxford University Press; New York: 2004. [Google Scholar]
- 2.Weissmann G, Smolen JE, Korchak HM. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980;303:27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- 3.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–1138. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]; First consensus report from leading authorities on definitions and mechanisms in resolution.
- 6.Bannenberg GL, et al. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]; First molecular mapping in vivo focusing on the formation and actions of protectins and resolvins using a mediator lipidomics and proteomics systems approach, the first systems approach to resolution.
- 7.Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]; First identification of specialized lipid mediators in the biosynthesis of spontaneous lipid mediators in resolution, which is now known as resolvin E1.
- 10.Serhan CN, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]; First documentation of the resolvins identified in resolving exudates in vivo; complete structural elucidation of the D-series and E-series resolvins and first protectins/neuroprotectins from DHA and their bioactions.
- 11.Hong S, Gronert K, Devchand P, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]; Identification of the DHA-derived anti-inflammatory resolvins and protectins.
- 12.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]; Review emphasizing the resolution terrain as ripe for drug discovery.
- 14.Serhan CN, editor. Special Issue on Lipoxins and Aspirin-Triggered Lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73(34):139–321. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]; This issue features nineteen reviews by experts covering the anti-inflammatory actions of lipoxins, aspirin-triggered lipoxins, and therapeutic potential of stable metabolic analogs. This is a detailed resource for original research and critical review of results on lipoxins and their mechanism of action.
- 15.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canny G, et al. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci USA. 2002;99:3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell EL, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 18.Flower RJ. Prostaglandins, bioassay and inflammation. Br J Pharmacol. 2006;147:S182–S192. doi: 10.1038/sj.bjp.0706506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]; 1982 Nobel Laureate Bengt Samuelsson reviews the biosynthesis and bioactions of leukotrienes.
- 20.Lands WEM, editor. Proceedings of the AOCS Short Course on Polyunsaturated Fatty Acids and Eicosanoids. American Oil Chemists' Society; Champaign, IL: 1987. [Google Scholar]
- 21.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 22.Kunkel SL, Ogawa H, Conran PB, Ward PA, Zurier RB. Suppression of acute and chronic inflammation by orally administered prostaglandins. Arthritis Rheum. 1981;24:1151–1158. doi: 10.1002/art.1780240906. [DOI] [PubMed] [Google Scholar]
- 23.Hasturk H, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]; The demonstration of the ability of resolvin E1 to stimulate bone regeneration.
- 24.Williams TJ, Jose PJ, Wedmore CV, Peck MJ, Forrest MJ. Mechanisms underlying inflammatory edema: the importance of synergism between prostaglandins, leukotrienes, and complement-derived peptides. Adv Prostaglandin Thormboxane Leukot Res. 1983;11:33–37. [PubMed] [Google Scholar]
- 25.Pons F, Williams TJ, Kirk SA, McDonald F, Rossi AG. Pro-inflammatory and anti-inflammatory effects of the stable prostaglandin D2 analogue, ZK 118.182. Eur J Pharmacol. 1994;261:237–247. doi: 10.1016/0014-2999(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 26.Rajakariar R, et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci USA. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haworth O, Buckley CD. Resolving the problem of persistence in the switch from acute to chronic inflammation. Proc Natl Acad Sci USA. 2007;104:20647–20648. doi: 10.1073/pnas.0710633105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godson C, et al. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]; First demonstration that lipoxins stimulate the uptake of apoptotic neutrophils.
- 29.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by IL-13 and IFN-gamma and inhibits TNF-alpha-induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace JL, Fiorucci S. A magic bullet for mucosal protection and aspirin is the trigger! Trends Pharmacol Sci. 2003;24:323–326. doi: 10.1016/S0165-6147(03)00166-4. [DOI] [PubMed] [Google Scholar]; An authoritative review on the importance of triggering of endogenous aspirin-triggered lipoxins.
- 31.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiore S, Serhan CN. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. J Exp Med. 1990;172:1451–7. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano M, Serhan CN. Lipoxin generation by permeabilized human platelets. Biochemistry. 1992;31:8269–8277. doi: 10.1021/bi00150a021. [DOI] [PubMed] [Google Scholar]
- 34.Edenius C, Haeggstrom J, Lindgren JA. Transcellular conversion of endogenous arachidonic acid to lipoxins in mixed human platelet-granulocyte suspensions. Biochem Biophys Res Commun. 1988;157:801–807. doi: 10.1016/s0006-291x(88)80320-6. [DOI] [PubMed] [Google Scholar]
- 35.Freire-de-Lima CG, et al. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 36.Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ. The opportunistic pathogen Pseudomonas aeruginosa carries a novel secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci USA. 2004;101:2135–2139. doi: 10.1073/pnas.0307308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 38.Bannenberg GL, Aliberti J, Hong S, Sher A, Serhan CN. Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis. J Exp Med. 2004;199:515–523. doi: 10.1084/jem.20031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiang N, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 41.Maddox JF, et al. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 42.Gronert K, Colgan SP, Serhan CN. Characterization of human neutrophil and endothelial cell ligand-operated extracellular acidification rate by microphysiometry: impact of reoxygenation. J Pharmacol Exp Ther. 1998;285:252–261. [PubMed] [Google Scholar]
- 43.Patcha V, et al. Differential inside-out activation of beta2-integrins by leukotriene B4 and fMLP in human neutrophils. Exp Cell Res. 2004;300:308–319. doi: 10.1016/j.yexcr.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin stop inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vane JR. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell; Stockholm: 1982. pp. 181–206. [Google Scholar]
- 46.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration of the formation of aspirin-triggered lipid mediators in vivo in humans in a double-blind randomized trial.
- 48.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol Cell Physiol. 2005;289:C557–C563. doi: 10.1152/ajpcell.00045.2005. [DOI] [PubMed] [Google Scholar]; First demonstration that lipoxin A4 stimulates the induction of the HO-1 system.
- 49.Biteman B, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–2266. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- 50.Paul-Clark MJ, van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serhan CN, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 52.Birnbaum Y, et al. Augmentation of myocardial production of 15-epi-lipoxin-A4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]; The discovery that statins stimulate endogenous 15-epi-lipoxin A4 formation, a potential endogenous anti-inflammatory messenger of statin's actions.
- 53.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 54.Kowal-Bielecka O, Kowal K, Distler O, Gay S. Mechanisms of disease: leukotrienes andlipoxins in scleroderma lung disease--insights and potential therapeutic implications. Nat Clin Pract Rheumatol. 2007;3:43–51. doi: 10.1038/ncprheum0375. [DOI] [PubMed] [Google Scholar]
- 55.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 56.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 57.Lu Y, Hong S, Tjonahen E, Serhan CN. Mediator-lipidomics: databases and search algorithms for PUFA-derived mediators. J Lipid Res. 2005;46:790–802. doi: 10.1194/jlr.D400020-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Arita M, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]; Complete stereochemical assignment of natural RvE1 and its total organic synthesis and interactions with GPCR receptors.
- 59.Winyard PG, Willoughby DA, editors. Inflammation Protocols. Humana; Totowa, NJ: 2003. [Google Scholar]
- 60.Tjonahen E, et al. Resolvin E2: Identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Hasturk H, et al. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 62.Arita M, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arita M, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 64.Bazan NG, Birkle DL, Reddy TS. Docosahexaenoic acid (22:6, n-3) is metabolized to lipoxygenase reaction products in the retina. Biochem Biophys Res Commun. 1984;125:741–747. doi: 10.1016/0006-291x(84)90601-6. [DOI] [PubMed] [Google Scholar]
- 65.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]; Authoritative review of the important role of the essential fatty acid DHA in neural systems.
- 66.Marcheselli VL, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]; Identification of NPD1/PD1 in vivo in brain ischemia and its protective role in neural damage.
- 67.Sun YP, et al. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J Biol Chem. 2007;282:9323–34. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 68.Wood PL, editor. Neuroinflammation: Mechanisms and Management. Humana Press; Totowa, NJ: 1998. [Google Scholar]
- 69.Lukiw WJ, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of neuroprotectin D1/protectin D1 in cell survival and its potential deficiency in Alzheimer's disease.
- 70.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ariel A, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell S, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]; In vivo demonstration of the uptake of apoptotic neutrophils by lipoxins.
- 73.Ariel A, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution via modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serhan CN, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 75.Shen J, et al. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mangino MJ, Brounts L, Harms B, Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Oth Lipid Mediat. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Fiorucci S, et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration that the second-generation lipoxin stable analogs are protective in gastrointestinal inflammation disease models.
- 78.Gronert K, et al. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 79.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 80.Duffield JS, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 81.González-Périz A, et al. Docosahexanenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 82.Levy BD, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 83.Levy BD, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levy BD, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilroy DW, et al. Inducible cycloxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 86.Rossi AG, Sawatzky DA, editors. The Resolution of Inflammation. Birkhäuser: Verlag AG, Basel; 2007. [Google Scholar]
- 87.Serhan CN, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 88.Rossi AG, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–64. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 89.Hudert CA, et al. Transgenic mice rich in endogenous n-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xia S, et al. Melanoma growth is reduced in fat-1 transgenic mice: Impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci USA. 2006;103:12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karp CL, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]; Demonstration that lipoxins are anti-inflammatory and protective in a model of cystic fibrosis.
- 92.Hong S, et al. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins -- mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–116. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci USA. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN. Anti-inflammatory actions of lipoxin A4 stable analogs are demonstrable in human whole blood: Modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood. 1999;94:4132–4142. [PubMed] [Google Scholar]
- 95.Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- 96.Levy BD, et al. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a “stop” signaling switch for aspirin-triggered lipoxin A4. FASEB J. 1999;13:903–911. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- 97.Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 98.Bandeira-Melo C, et al. Cutting edge: Lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 block allergen-induced eosinophil trafficking. J Immunol. 2000;164:2267–2271. doi: 10.4049/jimmunol.164.5.2267. [DOI] [PubMed] [Google Scholar]
- 99.Lee TH, et al. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci. 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 100.Bonnans C, et al. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 101.Gewirtz AT, et al. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- 102.Gewirtz AT, et al. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brezinski ME, Gimbrone MA, Jr, Nicolaou KC, Serhan CN. Lipoxins stimulate prostacyclin generation by human endothelial cells. FEBS Letters. 1989;245:167–172. doi: 10.1016/0014-5793(89)80214-5. [DOI] [PubMed] [Google Scholar]
- 104.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- 105.Cezar-de-Mello PF, Nascimento-Silva V, Villela CG, Fierro IM. Aspirin-triggered lipoxin A4 inhibition of VEGF-induced endothelial cell migration involves actin polymerization and focal adhesion assembly. Oncogene. 2006;25:122–129. doi: 10.1038/sj.onc.1209002. [DOI] [PubMed] [Google Scholar]
- 106.Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- 107.Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A4 inhibits proliferation of human lung fibroblasts induced by connective tissue growth factor. Am J Respir Cell Mol Biol. 2006;34:65–72. doi: 10.1165/rcmb.2005-0184OC. [DOI] [PubMed] [Google Scholar]
- 108.Planagumà A, et al. Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor a-mediated CINC-1 release in rat liver cells: novel actions of lipoxin A4 (LXA4) and ASA-triggered 15-epi-LXA4. FASEB J. 2002;16:1937–1939. doi: 10.1096/fj.02-0224fje. [DOI] [PubMed] [Google Scholar]
- 109.McMahon B, et al. Lipoxin A4 antagonizes the mitogenic effects of leukotriene D4 in human renal mesangial cells: Differential activation of MAP kinases through distinct receptors. J Biol Chem. 2000;275:27566–27575. doi: 10.1074/jbc.M001015200. [DOI] [PubMed] [Google Scholar]
- 110.Wu SH, et al. Lipoxin A4 inhibits connective tissue growth factor-induced production of chemokines in rat mesangial cells. Kidney Int. 2006;69:248–256. doi: 10.1038/sj.ki.5000025. [DOI] [PubMed] [Google Scholar]
- 111.Wada K, et al. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- 112.Clish CB, et al. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takano T, et al. Aspirin-triggered 15-epi-lipoxin A4 and LXA4 stable analogs are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiang N, et al. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scalia R, Gefen J, Petasis NA, Serhan CN, Lefer AM. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: Role of P-selectin. Proc Natl Acad Sci USA. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: Aspirin-triggered-15R-lipoxin A4 and lipoxin A4. J Pharmacol Exp Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 117.Devchand PR, et al. A synthetic eicosanoid LX-mimetic unravels host-donor interactions in allogeneic BMT-induced GvHD to reveal an early protective role for host neutrophils. FASEB J. 2005;19:203–210. doi: 10.1096/fj.04-2565com. [DOI] [PubMed] [Google Scholar]
- 118.Munger KA, et al. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci USA. 1999;96:13375–13380. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]