Abstract
Shp2 is a non-receptor protein tyrosine phosphatase containing two Src homology 2 (SH2) domains that is implicated in intracellular signaling events controlling cell proliferation, differentiation and migration. To examine the role of Shp2 in brain development, we created mice with Shp2 selectively deleted in neural stem/progenitor cells. Homozygous mutant mice exhibited early postnatal lethality with defects in neural stem cell self-renewal and neuronal/glial cell fate specification. Here we report a critical role of Shp2 in guiding neuronal cell migration in the cerebellum. In homozygous mutants, we observed reduced and less foliated cerebellum, ectopic presence of external granule cells and mispositioned Purkinje cells, a phenotype very similar to that of mutant mice lacking either SDF-1α or CXCR4. Consistently, Shp2-deficient granule cells failed to migrate toward SDF-1α in an in vitro cell migration assay, and SDF-1α treatment triggered a robust induction of tyrosyl phosphorylation on Shp2. Together, these results suggest that although Shp2 is involved in multiple signaling events during brain development, a prominent role of the phosphatase is to mediate SDF-1α/CXCR4 signal in guiding cerebellar granule cell migration.
INTRODUCTION
The development of cortical structures in mammalian central nervous system (CNS) is characterized by a concerted process of neuronal differentiation, migration and consequent assembly into compact neuronal cell layers (Hatten, 1999; Herrup and Kuemerle, 1997). Whereas the radial glial fibers serve as a scaffold, local environmental cues provide the critical information in orchestrating directed movement of neurons in the developing brain (Hatten, 2002). It has been widely recognized that specific components of extracellular matrices (ECM), cytokines, and chemokines act to coordinate neuronal migration events, and much of our knowledge in this regard has been contributed by phenotypic analyses of classical and gene-targeted mouse mutants with defects in brain development (Gupta et al., 2002; Hatten, 1999). However, relatively little is known about the specific cytoplasmic components linking various neuronal migration pathways and, so far, only fragmented experimental data are available for several protein kinases and scaffold proteins that operate in this process.
Several groups have shown that the chemokine stromal cell-derived factor 1α (SDF-1α) binds to its receptor CXCR4 to control neuronal cell migration in the cerebellum (Ma et al., 1998; Zhu et al., 2002; Zou et al., 1998). The CXCR4-deficient mice die perinatally and exhibit disturbed external germinal layer (EGL), ectopically positioned Purkinje cells, and many chromophilic cell clumps within the cerebellar anlage. Interestingly, mice deficient for either SDF-1α or CXCR4 display an almost identical phenotype in the cerebellum, suggesting an unusual monogamous relationship between a ligand and a receptor in orchestrating cerebellar development (Ma et al., 1998).
Shp2, a Src homology 2 (SH2)-containing protein tyrosine phosphatase, is a widely expressed intracellular enzyme (Lai et al., 2004; Neel et al., 2003). Although Shp2 has been implicated in several signaling pathways, compelling evidence from in vitro and in vivo studies strongly suggest a critical role of Shp2 in control of cell migration during animal development. A targeted deletion of exon 3, encoding 65 amino acids in the SH2-N domain of murine Shp2 (Shp2Δ46-110), results in embryonic lethality in homozygotes, with abnormalities in the patterning, particularly a posterior truncation, of mesodermal structures due to cell migration defect (Saxton et al., 1997). Chimeric animal analysis with homozygous Shp2Δ46-110 mutant embryonic stem (ES) cells identified a Shp2 function in guiding morphogenetic cell movement during gastrulation and also in limb development (Qu et al., 1998; Saxton et al., 2000; Saxton and Pawson, 1999). Consistently, Shp2-deficient mouse embryonic fibroblast (MEF) cells are defective in migration in vitro, through modulation of focal adhesion kinase (Fak) activity and cytoskeletal reorganization (Oh et al., 1999; Saxton and Pawson, 1999; Yu et al., 1998).
In most recent studies, we generated a mutant mouse model with Shp2 selectively deleted in neural stem/progenitor cells (Ke et al., 2007). The conditional Shp2 knockout mice exhibited growth retardation and early postnatal lethality, with multiple defects observed in neuronal migration and differentiation in cerebral and cerebellar cortices, particularly a migration defect of granule cells in the cerebellum. In this communication, we present experimental data suggesting that Shp2 is a critical signal transducer downstream of SDF-1α/CXCR4 in guiding granule cell migration during cerebellar development.
MATERIALS AND METHODS
Animals
Mice were maintained in the animal facility of Burnham Institute for Medical Research in accordance with NIH guidelines and approved by the Institute's animal research committee. Generation of a conditional Shp2 mutant allele (Shp2flox) was reported previously (39). Generation and characterization of Shp2flox/flox :nestin-Cre transgenic mice were described elsewhere (Isaka et al., 1999; Ke et al., 2007).
Reagents and Antibodies
Anti-GFAP monoclonal antibody (G3893) and anti-calbindin monoclonal antibody (C9848) were from Sigma. Rabbit anti-neurofilament M antibody (AB1987) was from Chemicon. Rabbit anti-L1 antibody was a generous gift from Dr. Stallcup (Burnham). Monoclonal antibodies against nestin, TAG-1, and RC2 were from DSHB, University of Iowa. Rabbit anti-SH-PTP2 (Shp2) (C-18) antibody (sc-280) and anti-PCNA antibody (sc-7907) were from Santa Cruz biotechnology. Anti-p27kip1 (AHZ0452) and anti-Cyclin D1 (AHF0102) antibodies were from Biosource. Fluorophore-labelled secondary antibodies were purchased from Molecular Probes. Antibodies to phospho-p44/42 Erk (pThr202/pTyr204, #9101), phospho-SHP-2 (pTyr542, #3751), FAK (#3285), phospho-FAK (pTyr925, #3284), and phospho-FAK (p Tyr576/577, #3281) were purchased from Cell Signaling. Rabbit anti-Erk1/2 antibody was generated in our own laboratory. NRG1-1α, BDNF, NT-4/5, and SDF-1α were purchased from PeproTech Inc.
Immunohistochemistry
Immunochemical staining of brain sections was performed following standard protocols. Paraffin sections were heated in 55°C oven for 30 min, deparaffinized, hydrated in ethanol, washed in DW, immersed in boiled citrate buffer for 30 min, washed in PBS, blocked in PBS with 5% normal goat serum (or normal donkey serum) and 1% Triton X-100, incubated with primary antibody, washed, incubated with secondary antibody from Molecular Probes. After washing, mounted using a Dapi or PI mounting solution (Vector). Envision system, HRP (DAKO) was used as a solution of secondary antibody for DAB staining.
Tissue Culture
The cerebellum was removed from E16.5 and P1 mice, incubated in a solution of papain or trypsin with DNase for 5 min at room temperature, titrated in a solution of DNase using a fire-polished glass pipette. Fibroblasts were removed by incubation in DMEM with 10% FBS for 20 min at 37°C. Then the cells were incubated for 2 hours at 37°C to allow glial cells attachment on culture plates. Attached glial cells were incubated in DMEM with 10% FBS. The medium was replaced with DMEM without FBS overnight before stimulation (NRG: 50 ng/ml or 10 nM). The supernatant containing neurons was incubated for 4 hours at 37 °C so neurons were attached on cover glasses or culture dishes coated with poly-D-lysine or poly-DL-ornithine plus laminin. The medium was replaced with neurobasal medium with B27 (Gibco). Isolated cells were incubated in a 10% O2 and 5% CO2 incubator at 37°C. The neurons were starved in neurobasal medium without B27 for 4 hours before growth factor stimulation (BDNF, 10 ng/ml; SDF-1α, 100 ng/ml).
Granule Cell Axon Extension Assays
Granule cells from P1 mice were cultured with growth factors (BDNF, 10 ng/ml; NT4/5, 10 ng/ml; SDF-1α, 100 ng/ml) for 3 days. The growth factors were added to culture medium every day. Cells were fixed and stained with anti-neurofilament M antibody, and the neurite length was measured under a microscope.
In Vivo Cell Proliferation Assay
5-Bromo-2′-deoxy-uridine (BrdU) labeling and detection kit I (Roche) was used to measure cell proliferation. For cerebellum, BrdU was intraperitoneally injected into P1 mice (20 μl/g BW) under anesthesia. After 1 hour, the brain was removed, fixed in 4% PFA/PBS. Sections at size of 5 μm were cut using the cryostat. Brain sections were post-fixed in 4 % PFA/PBS for 5 min, washed, incubated in 2N HCl for 20 min, washed in PBS, incubated in 0.2% Triton X-100/PBS for 20 min, 0.1 M boric acid for 20 min, washed, and processed following the manufacturer's protocol.
Cerebellar Explant Assay for External Granule Cell Migration
Granule cell migration was measured in vitro as previously described (Zhu et al., 2002), with slight modification. Embryonic brain at E16.5 or 17.5 was taken out and put in L-15 media (Invirtrogen) on ice. The forebrain was isolated, and then the cerebellar meninge was removed from the ventral side. Cerebellum was taken out from the brainstem in L-15 medium under a dissection microscope, and put in L-15 medium on ice until it was chopped. The cerebellum was transferred to the top of the tissue chopper using a top cut 1000 μl pipette tip, and the excess medium was sucked off. The cerebellum was cut sagitally at thickness of 200 μm. The pieces of the chopped cerebellum were washed out in L-15 medium and kept on ice until use. The external granular layer (EGL) was cut out from the pieces in L-15 medium under a dissection microscope using a tip bent tungsten needle which was fined in 0.5 N NaOH electrically, and the rest part was discarded. Then the EGL was cut into small rectangle as the layer side was longer, and were kept on ice until use.
Gel A was made from the mixture of 9 μl of 10 × DMEM, 85 μl of collagen gel (BD Science) diluted 1:1 by 0.1 × DMEM, and 1 μl of 1 N NaOH, and kept on ice. Gel B was made by adding gel A to 60 μl of matrigel (BD Science) and 30 μl of DMEM with 10% FBS and 1% penicillin-streptomycin, and kept on ice. All reagents were kept on ice, and 45 μl of gel A and 5 μl of SDF-1α (100 ng/μl) or PBS (as control solution) was mixed, and then 5 μl of the mixture was put in a 30-mm culture dish. The top was flatted with a pipette tip, and the mixture was left at room temperature for 5 min to be solidified. The prepared EGLs were transferred around the gel using a 200-μl pipette, and the excess medium was sucked off. Gel B (30 μl) was added around the gel, and the EGLs were organized around the gel so that the shorter side faced to the gel in the distance of approximately 200-300 μm using the tungsten needle. After the gel was kept at room temperature for 30 min, the dishes were put in a 5% CO2 incubator for 30 min so that the gel was solidified. Then 2 ml of DMEM with 10% FBS and 1% penicillin-streptomycin was added into the dishes. After 3-day incubation, the medium was aspirated, 1.2 ml of 4% PFA was added to the dishes, and the dishes were incubated at room temperature for 1 hr, washed with PBS 3X for 20 min each, and mounted using Dapi mounting solution (Vector). Pictures were taken and the number of migratory cells on the proximal (P) and distal (D) sides was counted, and the P/D ratios were calculated. Statistical analyses were carried out using Student's t test.
RESULTS
Shp2 Expression in Cerebellum
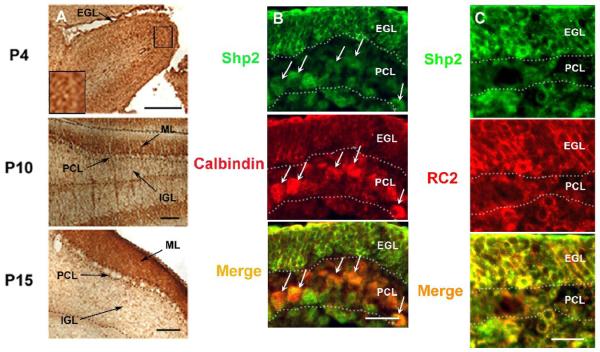
We and others have previously shown that Shp2 is widely expressed in all cell types, as compared to another SH2-containing tyrosine phosphatase Shp1 whose expression is restricted to hematopoietic and epithelial cells (Feng and Pawson, 1994; Neel, 1993). In this study, we examined Shp2 expression profiles in the cerebellum in early postnatal and adult mice. In the early postnatal cerebellum, Shp2 expression was detected at high levels in the cell body of granule cells and fibers of the external granular layer (EGL) at P4 (Figures 1A, B, and C). Strong expression of Shp2 was also recognized in some large cells just below EGL at P4 (the inset of Figure 1A). Next, we tried to identify the cells and fibers by double-staining with calbindin, a marker of Purkinje cells, and RC2, a marker of Bergmann radial glia. While calbindin-positive cells overlapped with Shp2-positive Purkinje cells, there were a number of large Shp2-positive cells below the calbindin-positive Purkinje cells (Figure 1B). RC2-positive cells and fibers overlapped Shp2-positive cells and fibers in most parts (Figure 1C). Therefore, we conclude that the Shp2-positive cells below the Purkinje cells were Bergmann radial glia. At P10, Shp2 expression was recognized in parallel fibers in the molecular layer (ML), and weaker staining was found in the cell body of granule cells in the internal granular layer (IGL) (Figure 1A). At P15, the EGL disappeared and strong staining was recognized on parallel fibers from granule cells in ML (Figure 1A). Shp2 expression was also found in the cytoplasm of Purkinje cells (Figure 1A).
Figure 1. Shp2 expression in cerebellum.
(A)Immunostaining of Shp2 in developing cerebellum. At P4, the staining was in the external granular layer and the strong staining was inside cerebellum. The inset showed a higher magnification of large cells expressing Shp2 strongly, scale bar: 50μm. At P10, strong expression was in the ML and the cytoplasm of Purkinje cells. Scattered staining was also present in the internal granule layer, scale bar: 100 μm. At P15, the staining was mainly in the ML, scale bar: 100 μm; EGL, external granule layer; ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granule layer.
(B) At P4, Shp2 (green) was expressed in calbindin+ Purkinje cells (red), Shp2-positive cells were also present below calbindin+ Purkinje cells, scale bar: 50μm; EGL, external granule layer; PCL, Purkinje cell layer.
(C) At P4, RC2+ Bergmann glia (red) overlapped Shp2+ cells (red) in most parts, scale bar: 50 μm; EGL, external granule layer; PCL, Purkinje cell layer.
Aberrant Granule Cell Migration in Cerebellum
To conditionally delete Shp2 in the brain, we crossed Shp2floxed (Shp2F) mice with nestin-cre transgenic mice, as described previously (Ke et al., 2007). The resultant homozygous mutant mice (Shp2F/F:Cre/+) displayed early postnatal lethality and defects in corticogenesis, and reduced proliferation of progenitor cells in the ventricular zone. In vitro analysis of stem/progenitor cells isolated from E14.5 embryonic brain demonstrated a critical role of Shp2 in mediating basic fibroblast growth factor (bFGF) signals for self-renewing proliferation of neural stem cells (Ke et al., 2007).
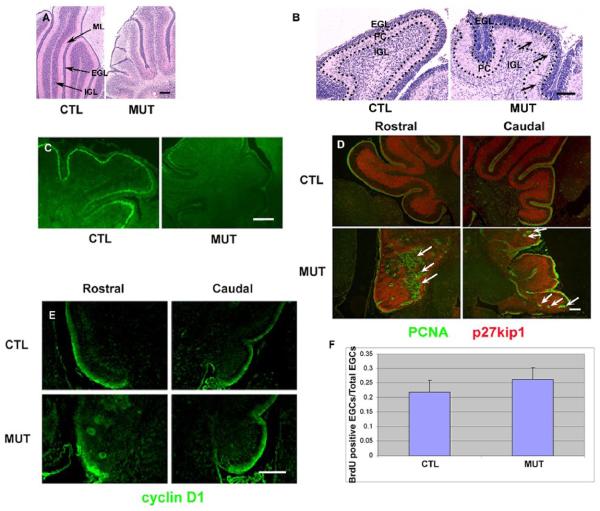
In this study, we focused our attention on examining the consequence of Shp2 deletion in cerebellar development and observed less foliation and substantial decrease in size in mutant cerebellum at P10. The medial-sagittal section analysis of Shp2F/F:Cre brain at P10 indicated that the cerebellar volume was reduced as compared to the controls (Figure 2A), with more mutant external granule cells already migrated inward than the controls at P4 (Figure 2B). One remarkable abnormality in the mutants was the existence of a thinner and disorganized molecular layer, ML (Figure 2A). As evaluated by TUNEL assay, there was no significant difference in apoptotic cell numbers between control and mutant cerebella (data not shown). Thus, the reduced size of mutant cerebella is not due to increased cell apoptosis, but is more likely associated with defects in axon extension and in Bergmann glia formation (see the results below).
Figure 2. Developmental defects in the cerebellum.
(A) H&E staining at P10. Note that the mutant (MUT) lobes were smaller and the mutant cerebellum had a remarkably thinner ML than the control (CTL). Abnormal cell streaks were present in the mutant. Scale bar: 100 μm.
(B) H&E staining at P4. Note that many external granule cells already migrated inward from the EGL and were present within the Purkinje layer in the mutant (MUT) cerebellum (arrows). Scale bar: 25 μm.
(C) TAG-1 expression (green) at P4. TAG-1 was expressed in the inner part of EGL in the control (CTL). Almost no TAG-1 expression was found in the whole mutant cerebellum. Scale bar: 50μm.
(D) The expression of PCNA (green) and p27kip1 (red) at P10. PCNA staining was found in the outer part of EGL, while p27kip1 expression was present in the inner part of EGL and internal granular layer in the control (CTL). Ectopic PCNA staining (arrows) was recognized in the deep inside of the mutant (MUT) cerebellum. Scale bar: 100 μm.
(E) Cyclin D1 expression at P6. The expression was present in the outer part of EGL in the control (CTL). Abnormal rosette-like staining was recognized in the deep inside of the mutant (MUT) cerebellum. Scale bar: 50μm.
(F) BrdU incorporation assay. The ratio was BrdU+ external granular cell number divided by external granular cell number. (n = 3, P > 0.05).
To further examine laminar organization in the cerebellum, we assessed expression of TAG-1, a marker for postmitotic and premigratory granule cells in the inner external granular layer (Furley et al., 1990). The expression intensity decreased in almost the whole folia in the mutants at P4, while clearly maintained in control littermates at this stage (Figure 2C). This result suggests a delay in differentiation of external granule cells in Shp2 mutants. Next, we evaluated PCNA and p27kip1 expression to identify proliferative external granule cells, postmitotic external, and internal granule cells. PCNA is expressed in the outer EGL, related to the S phase in cell cycle, while p27kip1, a cyclin-dependent protein kinase inhibitor, is expressed in the inner EGL and internal granule layer (IGL) (Miyazawa et al., 2000). Surprisingly, PCNA+ granule cells were ectopically and intensively recognized in the cerebellum of mutant mice at P10 (Figure 2D). To verify this observation, expression of cyclin D1, a cell cycle marker, was examined at P6. A similar abnormal staining pattern as PCNA was recognized in the mutants as well (Figure 2E). To investigate whether the PCNA+ cells are simply due to an increase in cell proliferation, BrdU incorporation assay was performed at P1. A similar number of BrdU+ external granule cells was detected in mutants and controls (Figure 2F), suggesting that ectopic presence of external granule cells is not due to increased proliferation, but rather a migration problem, in the mutant cerebellum.
A Critical Role of Shp2 in the SDF-1α/CXCR4 Pathway
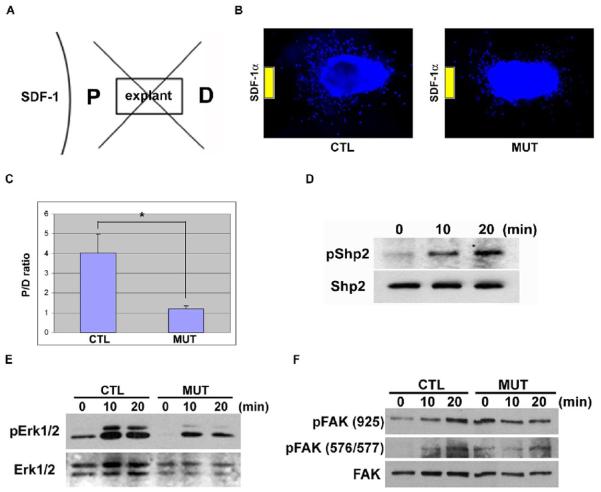
The premature inward migration pattern of external granule cells was very similar to the cerebellar phenotype observed in mice lacking SDF-1α or its receptor CXCR4 (Ma et al., 1998; Zou et al., 1998), suggesting a possibly essential role of Shp2 in the SDF-1α/CXCR4 pathway. It was recently reported that over-expression of Shp2 in primary T lymphocytes enhanced CXCR4-mediated cell migration (Hoff and Brunner-Weinzierl, 2007). We performed cerebellar explant assay for determination of external granule cell migration (Zhu et al., 1999; Zhu et al., 2002). The number of migratory cells was quantitated as described elsewhere (Zhu et al., 1999). Briefly, the area surrounding each explant was divided into four quadrants (Figure 3A). The number of cells in the quadrant proximal (P) to a potential source of guidance (SDF-1α) was compared to that in the distal (D) quadrant. Whereas the control external granule cells extensively migrated toward the gel containing SDF-1α, Shp2-deficient cells exhibited reduced motility toward SDF-1α (Figures 3B). The P/D ratio of cell number was significantly higher in control explants than that in the mutants (Figure 3C).
Figure 3. Deletion of Shp2 inhibits granule cell response to SDF-1α.
(A) A diagram showing the area of quadrants around each explant. P, the proximal quadrant; D, the distal quadrant.
(B) Representative pictures of control and mutant EGL explants. Control cells migrated toward the gel of SDF-1α, while mutant cells exhibited defect in directed movement.
(C) The ratio (P/D) of the cell number migrated into the proximal (P) and distal (D) area to the gel was calculated. The means and SEM of the controls (n = 28) and the mutant (n = 29) were shown, P < 0.01.
(D) p-Shp2 (Tyr542) levels were determined in P0 cerebellar neurons following stimulation of SDF-1α (100 ng/ml) for 0, 10 and 20 min.
(E) p-Erk1/2 signals were evaluated in response to SDF-1α (100 ng/ml) in P1 cerebellar neurons. The signals were quantified by NIH image. Phospho-Erk1/2: C0 (3463); C10 (12538); C20 (10062); M0 (145); M10 (5747); M20 (4146). Erk1/2: C0 (6600); C10 (5080); C20 (4796); M0 (5821); M10 (6642); M20 (5900). The ratios (Phospho-Erk1/2 divided by Erk1/2): C0 (0.52); C10 (2.47); C20 (2.10); M0 (0.02); M10 (0.87); M20 (0.70).
(F) p-FAK (Tyr925) and p-FAK (Tyr576/577) levels were measure after SDF-1α stimulation (100 ng/ml) in P1 cerebellar neurons.
To obtain direct biochemical evidence for Shp2 involvement in SDF-1α/CXCR4 signaling, we examined phosphorylation status of Shp2 on Tyr542 residue following SDF-1α stimulation of cerebellar neurons. A phosphonate at Tyr542 was reported to interact intramolecularly with the N-terminal SH2 domain to relieve basal inhibition of the PTPase (Lu et al., 2001). Treatment of neuronal cells with SDF-1α for 10 or 20 min induced robust phosphorylation of Shp2 on Tyr542, suggesting that Shp2 is indeed a player downstream of SDF-1α/CXCR4 (Figure 3D). The next question is whether Shp2 ablation disrupts SDF-1α-elicited cytoplasmic signaling events downstream of CXCR4. To address this issue, we assessed phospho-Erk signals in control and mutant cerebellar granule cells. As shown in Figure 3E, SDF-1α-stimulated phospho-Erk1/2 levels were attenuated in mutant cerebellar neurons as compared to controls, suggesting a requirement for Shp2 in transmitting/amplifying cytoplasmic signals elicited by SDF-1α.
To decipher the biochemical mechanism by which Shp2 modulates SDF-1α-stimulated granule cell migration, we examined the effect of Shp2 ablation on phosphorylation levels of focal adhesion kinase (FAK), a critical player in control of neuronal cell motility (Li et al., 2004; Liu et al., 2004; Ren et al., 2004). As shown in Figure 3F, SDF-1α induced significant increase of FAK tyrosine phosphorylation on Tyr925 and Tyr576/577, as detected by site-specific anti-phosphotyrosine antibodies. Interestingly, deletion of Shp2 resulted in constitutive tyrosine phosphorylation of FAK on these sites in the absence of SDF-1α stimulation, thereby disrupting the cycling of FAK between unphosphorylated and phosphorylated forms. It is believed that inactivation of FAK is required for re-activation of this kinase in orchestrating the dynamic assembly of signalsomes controlling cell motility (Cohen and Guan, 2005; Yu et al., 1998).
Reduced Axon Extension of Granule Neurons
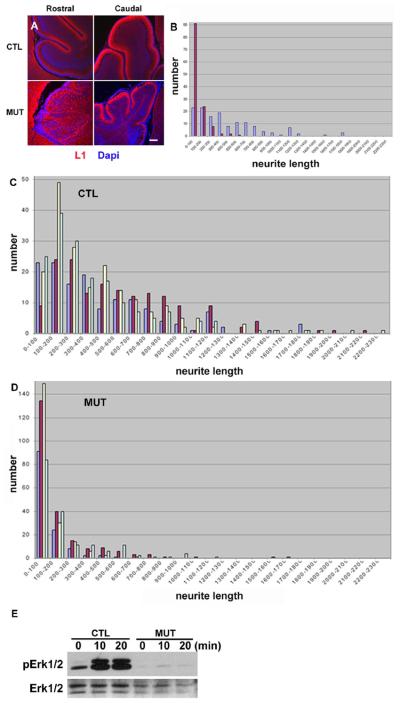
To determine the cellular basis for the thin molecular layer in mutant cerebellum, we examined parallel fibers of granule cells in the ML at P10 using the L1 marker (Stallcup et al., 1985). Proper organization of the ML was disrupted especially in the rostral part, and the L1 expression was detected in granule cell bodies rather than in parallel fibers in the mutants (Figure 4A), suggesting a defect in axonal extension of granule cells. When measured in vitro, the mutant axons were obviously shorter than the control, consistent to the immunohistochemical data (Figures 4B). We further examined granule cell axon extension under the treatment of SDF-1α, BDNF and NT-4/5, as described previously (Arakawa et al., 2003; Gao et al., 1995). While control granule cells extended neurites in response to BDNF, NT-4/5 and SDF-1α, mutant cells displayed much reduced capacity (Figures 4C and 4D). Therefore, the thinner and disorganized molecular layer is evidently contributed by a defect in axonal extension of granule cells. Consistent with the defective axon extension, BDNF-induced phospho-Erk1/2 signals were evidently reduced in Shp2-deficient cerebellar neurons (Figure 4E), similar to the reduction in phospho-Erk levels following SDF-1α stimulation (Figure 3E).
Figure 4. Defects in axon extension of granule cells.
(A)L1 expression at P10. L1 expression (red) was present in the ML in the control (CTL). The section was counterstained with Dapi (Blue). Abnormal L1 staining was recognized in cell bodies of the deep rostral cerebellum in the mutant. The molecular layer stained by L1 was not evident in the mutant rostral cerebellum. L1 expression was absent in a part of the mutant caudal cerebellum. Scale bar: 100 μm.
(B) Granule cells at P1 were cultured for 3 days, fixed, and stained with anti-neurofilament M antibody. The neurite length was measured; Blue, control; Red, mutant.
(C) The control granule cells from P1 were cultured for 3 days with BDNF (10 ng/ml), NT-4/5 (10 ng/ml), or SDF-1α (100 ng/ml), fixed, and stained with anti-neurofilament M antibody. The neurite length was measured. Blue, no stimulation; red, BDNF; yellow, NT-4/5, and light blue, SDF-1α. The distribution of the neurite length sifted to right in response to all stimuli.
(D)The mutant granule cells from P1 were cultured, stimulated with BDNF, NT-4/5 or SDF-1α, and the neurite length was measured as in C.
(E) The p-Erk1/2 levels were measured in response to BDNF (10 ng/ml) in P1 cerebellar neurons.
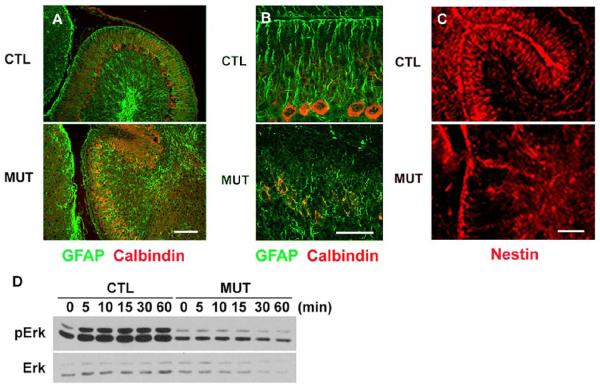
The external granule cells migrate inward and Purkinje cells move upward along the radial glia (2). To explore another possibility that may account for their abnormal migration, we examined formation of radial glia by assessing GFAP expression, a radial glial marker (Hoser et al., 2007; Lordkipanidze and Dunaevsky, 2005; Wiencken-Barger et al., 2007), on cerebellar sections at P6 and P10. The normal direction of radial glia was disrupted in the mutant and the end of the processes did not extend to the pial surface (Figures 5A and B). On the same sections, Purkinje cells were stained with an anti-calbindin antibody. As shown in Figures 5A and B, Purkinje cells formed a monolayer in the control, but they were not properly positioned in the mutant cerebellum, exhibiting multiple layers. We also examined Bergmann glia formation at an earlier stage, P4, using another marker, nestin (Allais et al., 2007; Andrae et al., 2001), and found that the formation of Bergmann glia was disorganized at the early stage in mutant cerebella (Figure 5C).
Figure 5. Abnormal glial development and molecular signaling in the cerebellum.
(A) The Bergmann glia (GFAP, green) were disorganized, and the Purkinje cells (calbindin, red) formed multiple layers in the mutant (MUT) at P6. Scale bar: 100 μm.
(B) A high magnification at P10 similar to (A). Scale bar: 50 μm.
(C) The Bergmann glia (nestin, red) were already disorganized in the early stage at P4 in the mutant (MUT). Scale bar: 50 μm.
(D) The p-Erk1/2 levels were measured following stimulation of neuregulin (10 nM) in the glia of the cerebellum.
It is known that treatment with neuregulin (NRG) potently transforms astrocytes into radial glias (Pinkas-Kramarski et al., 1994; Rio et al., 1997). In addition, previous in vitro experimental data suggested a role of Shp2 downstream of neuregulin (NRG) receptor, a member of the ErbB family (Tanowitz et al., 1999). We examined the effect of Shp2 deletion on NRG signaling. The phospho-Erk1/2 levels were remarkably reduced in mutant glia compared to controls following NRG stimulation (Figure 5D). Together, our results show that Shp2 play critical roles in cerebellar development by modulating signals elicited by SDF-1α and other cytokines. Multiple developmental problems exist in Shp2F/F:nestin-cre cerebellum, with a distinct phenotype of granule cell migration defect indicating a Shp2 function in the SDF-1α/CXCR4 pathway.
DISCUSSION
In this study, we first examined Shp2 expression in developing cerebellum in wild-type mice. The results presented here are largely consistent with previous data published by us and other groups (Feng et al., 1993; Servidei et al., 1998). Shp2 is well known as a widely expressed enzyme in different cell types, in contrast to its close relative Shp1 that is predominantly expressed in hematopoietic, lymphocytic and epithelial cells (Feng and Pawson, 1994). In the central nervous system, high levels of Shp2 expression were detected in actively dividing neural progenitor cells, consistent results obtained by several groups in vitro and in vivo (Gauthier et al., 2007; Ke et al., 2007; Servidei et al., 1998). We report here that Shp2 expression is rich in external granule cells, Purkinje cells, axons of internal granule cells, and Bergmann glia in cerebellum. The minor difference between our data shown here and that of Servidei et al might be explained by different antibodies used by the two groups, Servidei et al used an antibody against the SH2-N domain of Shp2 (Servidei et al., 1998), while our antibody was raised against the C-terminal part of Shp2 protein. The 3-dimensional structure of Shp2 is changed between the catalytically active and inactive forms, which may affect the reactivity with an antibody recognizing the SH2-N domain (9).
Conditional deletion of Shp2 directed by nestin-cre leads to a dramatic phenotype of early postnatal lethality, apparently due to multiple defects in the brain (Ke et al., 2007). This mouse model has allowed for demonstration of Shp2 functions in self-renewing proliferation of neural stem cells and also in neuronal/glial fate specification from progenitor cells (Ke et al., 2007). Reeves' group generated a transgenic mouse line expressing a catalytically inactive mutant of Shp2 under control of a nestin promoter sequence (Aoki et al., 2000). However, the transgenic mice were viable, appeared to develop normally, and did not show any obvious histological abnormalities in the embryonic and adult brain except for increased susceptibility to ischemia-induced brain damage (Aoki et al., 2000). This mild phenotype of transgenic mice suggests that expression of the Shp2 mutant did not efficiently suppress the activities of endogenous Shp2 enzyme.
In examining cerebellar development, we found that PCNA+ granule cells were ectopically located below the EGL, apparently due to premature inward migration of EGL cells. This cerebellar phenotype is remarkably similar to that of both SDF-1α and CXCR4 knockout mice (Ma et al., 1998; Zou et al., 1998), suggesting a severe interference or disruption of the SDF-1α/CXCR4 pathway in the Shp2-deficient brain. To establish and extend this point, we directly measured SDF-1α-induced granule cell motility using cerebellar explants derived in an in vitro cell movement assay (Zhu et al., 2002). Indeed, we detected significantly reduced migration capability of Shp2-deficient granule cells in response to SDF-1α stimulation, as compared to control cells. The phenotype of altered granule cell migration in vivo and in vitro identifies Shp2 as an essential player in transmitting/amplifying signals downstream of CXCR4. In support of the biological role of Shp2 for SDF-1α/CXCR4 signaling, we obtained biochemical evidence that SDF-1α treatment induced robust tyrosine phosphorylation of Shp2 in wild-type granule cells. Deletion of Shp2 resulted in suppression of SDF-1α-induced phospho-Erk signal.
FAK as one of key players in promoting cell migration was phosphorylated on tyrosine in control cells upon SDF-1α stimulation. Shp2 ablation leads to constitutive increase of FAK phosphorylation, and this observation is consistent to our previous data obtained in Shp2-deficient mouse embryonic fibroblast cells (Yu et al., 1998), which strongly suggests a critical role of Shp2 in control of reversible FAK phosphorylation necessary for promotion of cell migration. The action of FAK in promoting cell motility requires its cycling between phosphorylation and dephosphorylation, correlating with its activation, inactivation and reactivation. Therefore, one biochemical mechanism for Shp2 action downstream of SDF-1α/CXCR4 is likely to control the turnover of FAK in guiding cell migration. In mutant mice, p-Erk signals were remarkably reduced after BDNF stimulation.
However, it is evident that deletion of Shp2 affected multiple signaling pathways in cerebellar development. Another interesting phenotype we observed is the disorganization of radial glia in the mutant cerebellum. External granule cells migrate inward and Purkinje cells climb out from ventricular zone partly using Bergmann radial glia in cerebellum (Hatten, 1999). In Shp2 mutants, disorganized radial glia formation may have also contributed to ectopic presence of granule cells and mispositioning of Purkinje cells. To further determine the molecular and cellular basis for the premature granule cell migration phenotype, it will be necessary to create another mouse model in which Shp2 is deleted in granule cells only, by crossing Shp2 floxed mice with Math1-cre transgenic mice. It is also worthwhile to note that the molecular layer is much thinner in mutant than in control cerebella, which might be a major contributing factor to the reduced size of the mutant cerebellum. Consistent with the thin molecular layer, mutant cerebellar internal granule cells exhibited reduced capability of extending their axons in vitro, in response to SDF-1α, BDNF and NT-4/5 stimulation. Thus, Shp2 also participates in axonal outgrowth through modulation of signals triggered by SDF-1α, BDNF and NT-4/5. Consistently, previous experiments suggest that Shp2 acts downstream of TrkB, the receptor of BDNF and NT-4/5 (Bibel and Barde, 2000). Furthermore, an effect of Shp2 deletion on granule cell differentiation as shown by reduced TAG-1 expression may also contribute to the defective granule cell migration phenotype.
In summary, combined genetic and biochemical data support a notion that Shp2 is a key player in relay of SDF-1α/CXCR4 signals in orchestrating granule cell migration during cerebellar development, although this observation does not exclude Shp2 involvement in other signaling events guiding cell movement. Shp2 was also shown to participate in the pathway of CXCR4-mediated chemotaxis and chemoinvasion in breast cancer cells (Fernandis et al., 2004). Shp2 was implicated in differentiation and apoptosis of primary T lymphocytes (Hoff and Brunner-Weinzierl, 2007). On a separate project, we have obtained experimental results suggesting a role of Shp2 in mediating SDF-1 signals in B lymphocytes (our unpublished data). Thus, the Shp2 function in mediating SDF-1/CXCR4-guided cell migration pathway appears to be conserved in a variety of cell types in mammals. Furthermore, phenotypic analysis of mutant embryos with homozygous deletion of exon 3 in Ptpn11/Shp2 identified a severe defect in mesodermal cell migration, resulting in a posterior truncation (Saxton et al., 1997). Generation of chimeric animals by injecting exon 3−/− embryonic stem cells into wild-type embryos revealed a role of Shp2 in morphogenic movement at gastrulation, supporting a notion that Shp2 is required for a chemotactic response to signals initiated by fibroblast growth factors (Saxton and Pawson, 1999). Together, these results establish a role of Shp2 in modulation of intracellular signals guiding cell motility in mammals.
Acknowledgements
This work was supported by NIH grant R01DK73945 to G.S.F. We thank Drs. B. Ranscht, K. Murai and W.B. Stallcup for valuable suggestions, Dr. P. Baran in the Scripps Research Institute for critical reading, Dr. Jong de Castro for kindly providing reagents, and Feng lab members for helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allais A, Burel D, Isaac ER, Gray SL, Basille M, Ravni A, Sherwood NM, Vaudry H, Gonzalez BJ. Altered cerebellar development in mice lacking pituitary adenylate cyclase-activating polypeptide. Eur J Neurosci. 2007;25:2604–18. doi: 10.1111/j.1460-9568.2007.05535.x. [DOI] [PubMed] [Google Scholar]
- Andrae J, Hansson I, Afink GB, Nister M. Platelet-derived growth factor receptor-alpha in ventricular zone cells and in developing neurons. Mol Cell Neurosci. 2001;17:1001–13. doi: 10.1006/mcne.2001.0989. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Huang Z, Thomas SS, Bhide PG, Huang I, Moskowitz MA, Reeves SA. Increased susceptibility to ischemia-induced brain damage in transgenic mice overexpressing a dominant negative form of SHP2. Faseb J. 2000;14:1965–73. doi: 10.1096/fj.00-0105com. [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol. 2003;161:381–91. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–37. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–43. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- Feng GS, Hui CC, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–11. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- Feng GS, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–8. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–67. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–70. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gao WQ, Zheng JL, Karihaloo M. Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J Neurosci. 1995;15:2656–67. doi: 10.1523/JNEUROSCI.15-04-02656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–62. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3:342–55. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–39. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–3. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- Herrup K, Kuemerle B. The compartmentalization of the cerebellum. Annu Rev Neurosci. 1997;20:61–90. doi: 10.1146/annurev.neuro.20.1.61. [DOI] [PubMed] [Google Scholar]
- Hoff H, Brunner-Weinzierl MC. The tyrosine phosphatase SHP-2 regulates differentiation and apoptosis of individual primary T lymphocytes. Eur J Immunol. 2007;37:1072–86. doi: 10.1002/eji.200636240. [DOI] [PubMed] [Google Scholar]
- Hoser M, Baader SL, Bosl MR, Ihmer A, Wegner M, Sock E. Prolonged glial expression of Sox4 in the CNS leads to architectural cerebellar defects and ataxia. J Neurosci. 2007;27:5495–505. doi: 10.1523/JNEUROSCI.1384-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka F, Ishibashi M, Taki W, Hashimoto N, Nakanishi S, Kageyama R. Ectopic expression of the bHLH gene Math1 disturbs neural development. Eur J Neurosci. 1999;11:2582–8. doi: 10.1046/j.1460-9568.1999.00699.x. [DOI] [PubMed] [Google Scholar]
- Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng GS. Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol Cell Biol. 2007;27:6706–17. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai LA, Zhao C, Zhang EE, Feng GS. The Shp-2 tyrosine phosphatase. In: Arino J, Alexander D, editors. Protein phosphatases. Vol. 5. Berlin Heidelberg; Springer-Verlag: 2004. pp. 275–299. [Google Scholar]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–21. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–32. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordkipanidze T, Dunaevsky A. Purkinje cell dendrites grow in alignment with Bergmann glia. Glia. 2005;51:229–34. doi: 10.1002/glia.20200. [DOI] [PubMed] [Google Scholar]
- Lu W, Gong D, Bar-Sagi D, Cole PA. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol Cell. 2001;8:759–69. doi: 10.1016/s1097-2765(01)00369-0. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K, Himi T, Garcia V, Yamagishi H, Sato S, Ishizaki Y. A role for p27/Kip1 in the control of cerebellar granule cell precursor proliferation. J Neurosci. 2000;20:5756–63. doi: 10.1523/JNEUROSCI.20-15-05756.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel BG. Structure and function of SH2-domain containing tyrosine phosphatases. Sem. Cell. Biol. 1993;4:419–32. doi: 10.1006/scel.1993.1050. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–93. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Oh ES, Gu H, Saxton TM, Timms JF, Hausdorff S, Frevert EU, Kahn BB, Pawson T, Neel BG, Thomas SM. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol. 1999;19:3205–15. doi: 10.1128/mcb.19.4.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Eilam R, Spiegler O, Lavi S, Liu N, Chang D, Wen D, Schwartz M, Yarden Y. Brain neurons and glial cells express Neu differentiation factor/heregulin: a survival factor for astrocytes. Proc Natl Acad Sci U S A. 1994;91:9387–91. doi: 10.1073/pnas.91.20.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu CK, Yu WM, Azzarelli B, Cooper S, Broxmeyer HE, Feng GS. Biased suppression of hematopoiesis and multiple developmental defects in chimeric mice containing Shp-2 mutant cells. Mol Cell Biol. 1998;18:6075–82. doi: 10.1128/mcb.18.10.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, Song HJ, Mei L, Xiong WC. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7:1204–12. doi: 10.1038/nn1330. [DOI] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- Saxton TM, Ciruna BG, Holmyard D, Kulkarni S, Harpal K, Rossant J, Pawson T. The SH2 tyrosine phosphatase shp2 is required for mammalian limb development. Nat Genet. 2000;24:420–3. doi: 10.1038/74279. [DOI] [PubMed] [Google Scholar]
- Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–64. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton TM, Pawson T. Morphogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proc. Natl. Acad. Sci. USA. 1999;96:3790–5. doi: 10.1073/pnas.96.7.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servidei T, Bhide PG, Huang Z, Moskowitz MA, Harsh G, Reeves SA. The protein tyrosine phosphatase SHP-2 is expressed in glial and neuronal progenitor cells, postmitotic neurons and reactive astrocytes. Neuroscience. 1998;82:529–43. doi: 10.1016/s0306-4522(97)00292-3. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley LL, Levine JM. Antibody against nerve growth factor-inducible large external (NILE) glycoprotein labels nerve fiber tracts in the developing rat nervous system. J Neurosci. 1985;5:1090–101. doi: 10.1523/JNEUROSCI.05-04-01090.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, Si J, Yu DH, Feng GS, Mei L. Regulation of neuregulin-mediated acetylcholine receptor synthesis by protein tyrosine phosphatase SHP2. J Neurosci. 1999;19:9426–35. doi: 10.1523/JNEUROSCI.19-21-09426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. A role for Connexin43 during neurodevelopment. Glia. 2007;55:675–86. doi: 10.1002/glia.20484. [DOI] [PubMed] [Google Scholar]
- Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. Protein tyrosine phosphatase Shp-2 regulates cell spreading, migration and focal adhesion. J. Biol. Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li H, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–85. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–20. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]