Abstract
Macrophages are critical cells in mediating the pathology of neurodegenerative disorders and enhancement of neuronal outward potassium (K+) current has implicated in neuronal apoptosis. To understand how activated macrophages induce neuronal dysfunction and injury, we studied the effects of lipopolysaccharide (LPS)-stimulated human monocytes-derived macrophage (MDM) on neuronal outward delayed rectifier K+ current (IK) and resultant change on neuronal viability in primary rat hippocampal neuronal culture. Bath application of LPS-stimulated MDM-conditioned media (MCM) enhanced neuronal IK in a concentration-dependent manner, while non-stimulated MCM failed to alter neuronal IK. The enhancement of neuronal IK was repeated in a macrophage-neuronal co-culture system. The link of stimulated MCM (MCM(+))-associated enhancement of IK to MCM(+)-induced neuronal injury, as detected by PI/DAPI (propidium iodide/4′,6-diamidino-2-phenylindol) staining and MTT assay, was demonstrated by experimental results showing that addition of IK blocker tetraethylammonium to the culture protected hippocampal neurons from MCM(+)-associated challenge. Further investigation revealed elevated levels of Kv 1.3 and Kv 1.5 channel expression in hippocampal neurons after addition of MCM(+) to the culture. These results suggest that during brain inflammation macrophages, through their capacity of releasing bioactive molecules, induce neuronal injury by enhancing neuronal IK and that modulation of Kv channels is a new approach to neuroprotection.
Keywords: Voltage-gated K+ channels, Hippocampus, Neuronal culture, Neurodegeneration
Introduction
Individuals with progressive human immunodeficiency virus type 1 (HIV-1) disease often suffer from cognitive, behavior, and neurological deficiency known as HIV-1-associated neurocognitive disorders (HAND) (Antinori et al. 2007). Neuropathological features linked to this disease include brain macrophage infiltration, formation of multinucleated giant cells, astrogliosis, and neuronal dropout (Masliah et al. 1992; Michaels et al. 1988b; Price et al. 1988). Despite more than two decades of investigation, the neuropathogenic mechanisms for HAND are not well understood. It has been shown that, when followed prospectively, HIV-1-infected patients who developed neuropsychiatric decline before death exhibited an increased number of macrophages in their brain on subsequent autoptical neuropathologic evaluation (Glass et al. 1995). It has also been shown that the distribution of damaged neurons is closely associated with markers of macrophage activation, especially within the subcortical deep gray structures (Adle-Biassette et al. 1999). This suggests that soluble factors released from HIV-1-infected and activated macrophages may serve as the source of neurotoxicity. Indeed, macrophages can be activated by HIV-1 infection (Kaul et al. 2001), bacterial endotoxin lipopolysaccaride (LPS), or by immune stimulation in response to soluble factors released from HIV-1-infected cells (Lipton and Gendelman 1995). Studies of HAND neuropathology and animal models of HIV-1-associated neuronal injury indicate that soluble factors, such as cytokines and viral proteins, are the primary cause of neuronal injury (Kaul et al. 2001; Xiong et al. 2000). It is now generally believed that the pathogenesis of HAND involves viral infection and immune activation of brain macrophages and microglia, and the resultant release of diffusible viral and cellular toxins, leading to neuronal and astrocytic dysfunction and eventual cell death. However, not all HAND patients show profound neuronal loss (Seilhean et al. 1993), and improvements in cognitive function are apparent after highly active anti-retroviral therapy (Antinori et al. 2007). This suggests that cognitive decline may result more from neuronal dysfunction than from cell loss.
Accumulating evidence indicates that neuronal voltage-gated potassium (Kv) channels play an important role in memory processes (Giese et al. 2001; Giese et al. 1998; Solntseva et al. 2003) and are affected in acquired neuronal channelopathies in HAND (Gelman et al. 2004). It has been demonstrated on different model systems that K+ currents decrease during learning and that Kv channel antagonists improve learning and memory (Giese et al. 2001; Solntseva et al. 2003). Intracerebroventricular injection of K+ channel openers provokes amnesia in animal behavior tests and administration of K+ channel blockers, for instance tetraethylammonium (TEA) and charybdotoxin, prevents K+ channel opener-induced amnesia (Ghelardini et al. 1998). Animal mutants with K+ channel dysfunction exhibit deficits in learning and memory (Giese et al. 1998). Nevertheless, Kv channel dysfunction plays a crucial role in memory deficit (Ghelardini et al. 1998; Solntseva et al. 2003). We hypothesize that HIV-1-infected macrophages alter neuronal Kv channel activity through direct macrophage-neuron contact, and/or by paracrine amplification of macrophage secretion of toxic molecules or both, leading to neuronal dysfunction. To test this hypothesis, we studied the effects of human monocyte-derived macrophages (MDM)-conditioned media (MCM), recovered from LPS-stimulated MDM, on outward delayed rectifier K+ current (IK) in primary hippocampal neuronal culture prepared from embryonic Sprague-Dawley rats. Our results showed that LPS-stimulated MCM enhanced neuronal IK, and the enhancement of IK contributes to MDM-associated neuronal death.
Materials and Methods
All reagents, unless otherwise indicated, were purchased from Sigma-Aldrich (St. Louis, MO).
Human monocyte culture and MCM collection
Human monocytes were recovered from peripheral blood mononuclear cells of HIV, and hepatitis B virus seronegative donors after leukopheresis and purified by counter-current centrifugal elutriation. Cells were obtained under a protocol approved by University of Nebraska Institutional Review Board. Monocytes were cultured in DMEM supplemented with 10% heat-inactivated human serum, L-glutamine (2mM), gentamicin (50 μg/ml), ciprofloxacin (10 μg/ml), and macrophage colony-stimulating factor, allowing them to differentiate into macrophages in vitro. The purity of MDM was confirmed by MAC1 immunocytochemistry. MAC1 was expressed in >95% of cells. After 7 days in culture (37°C, 5% CO2), MDM were incubated with/without with LPS (1μg /ml) for 2 h. To obtain a “guaranteed” activation effect, LPS was used as a model molecule to stimulate MDM instead of some physiological means (e.g. CD40 ligand or IL-1β, etc). The culture media was then removed, and Neurobasal media (Invitrogen, Carlsbad, CA, Serum free) was placed onto MDM for 24 h prior to collection. The MCM recovered from LPS-stimulated MDM (MCM(+)) and non-stimulated MDM (MCM(-)) were stored in aliquots at -80 C° until use. On the day of the experiment, MCM were thawed, diluted, and added to neuronal culture through bath perfusion.
Primary hippocampal neuronal culture
Hippocampal neuronal cultures were prepared from rat embryos using the methods described previously (Flavin et al. 1997). Briefly, Sprague-Dawley rats with 18-19 days gestation were anesthetized with Isoflurane, and embryonic pups were surgically removed and decapitated. Hippocampi were harvested under sterile conditions. The hippocampal tissue was enzymatically dissociated in 0.125% trypsin II. Isolated neural cells were placed in poly-D-lysine-coated 35 mm plastic culture dishes containing 2 ml of medium to a culture surface cell density of 5 × 105/ml (400-500/mm2). The culture was maintained in neurobasal medium supplemented with B27 (2%, Invitrogen), glutamine (0.5mM), and penicillin/streptomycin (100U) for at least 7-10 days before being used in experiments. All animal-use procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of University of Nebraska Medical Center (IACUC # 00-062-07).
MDM and hippocampal neuronal co-culture
After 7-10 days in culture, hippocampal neurons were co-cultured with human MDM (with or without LPS stimulation) for 24 h prior to electrophysiological study. The MDM were collected via centrifugation (1500rpm/min for 5 min), and then re-suspended in neurobasal medium, counted, and added to neuronal culture at a concentration of 1×105 cells/ml. The ratio of MDM to neural cells was 1:5.
Confocal imaging of neuronal Kv channels
After exposure to MCM(+) or MCM(-) for 24 h, neuronal cultures were immunocytochemically stained for Kv 1.3 and Kv 1.5 antigens with anti-Kv1.3 and anti-Kv1.5 monoclonal antibodies (Almonade Lab, Israel). Specifically, neuronal cultures treated with MCM(+) or MCM(-) were washed with PBS and fixed in 4% paraformaldehyde (in PBS) for 15 min, then incubated in blocking buffer (10% goat serum in PBS) for 30min at room temperature, followed by incubation with Anti-Kv1.3 and Anti-Kv1.5 antibodies at 4°C overnight. Kv channel expression was visualized with Alexa Fluor-488 (green)- and Alexa Fluor-594 (red)-conjugated secondary antibodies (Invitrogen). Laser-scanning images were obtained using a Nikon Swept-field laser confocal microscope (Nikon Instruments, Melville, NY).
Examination of hippocampal neural cell viability
Hippocampal neural cell viability was determined by two approaches: 1) counting the number of cells and 2) MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] optical density (OD) assay. MCM were added to hippocampal neuronal culture (final concentration was 1:30 dilution) for 24 h, and then cell injury was assessed by staining with a membrane-impermeable DNA-binding dye propidium iodide (PI, Molecular Probes, Eugene, OR) and counterstaining with membrane-permeable 4′′,6-diamidino-2-phenylindol (DAPI). More specifically, after incubation with neurobasal medium containing PI (1 μg/ml) for 15 min, cells were washed three times with PBS, then fixed with 4% paraformaldehyde (in PBS) for 20 min at room temperature. Cells were then permeabilized with 0.2% Triton X-100 (in PBS) for 5 min on ice and washed twice with PBS containing DAPI (0.1μg/ml). Subsequently, under fluorescent microscopy, red (PI) and blue (DAPI) fluorescent images were captured in order to determine the number of injured cells and the total number of cells, respectively. Five different visual fields per culture dish were evaluated.
Cell viability was also analyzed by MTT assay. After cell culture was treated with MCM(+), TEA+MCM(+) or TEA for 24h, a solution of MTT-PBS (5 mg/ml) was added to the neurobasal medium in a 1:10 ratio and mixed gently. The MTT was removed 3-4 h later, and the cells were solubilized with dimethyl sulfoxide. The OD values of were measured with a spectrophotometer at 570 nm (Kinetic Microplate Reader).
Electrophysiology
Whole-cell voltage-clamp was performed on hippocampal neuronal culture in 35-mm tissue culture dishes on the stage of an inverted Nikon microscope using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Patch electrodes, made from borosilicate glass micropipettes, had tip resistance of 4.0-7.5 Mω. The electrodes were advanced towards cells by a Burleigh micromanipulator (EXFO, Canada). After establishment of the whole-cell patch configuration, the cells were allowed to stabilize for 3-5 min before tests. Whole-cell outward currents were induced by voltage steps from the holding potential of -60mV to -40mV in the first step, and then stepped to +60mV in increments of 10mV. Junction potentials were corrected, and the cell capacitance was compensated (∼70%) in most cells. Current signals were filtered at 1 kHz and digitized at 5 kHz using a Digidata 1320A digitizer (Molecular Devices). The current traces were displayed and recorded in a Dell computer using pCLAMP 8.1 data acquisition/analysis system (Molecular Devices). The pipette solution contained (in mM): 108 K2HPO4, 9 HEPES, 9 EGTA, and 2.5 MgCl2 buffered to pH 7.4 with KOH. To promote the stability of the recordings, 14 mM creatine phosphate (Tris salt), 1 mM Mg-ATP, and 0.3 mM Tris-GTP were included in the pipette solution. The extracellular solution contained (in mM): 150 NaCl, 4 KCl, 2 MgCl2, 2 CoCl2, 10 HEPES, 5 4-aminopyridine (4-AP) and 10 glucose, buffered to pH 7.4 with NaOH. All experiments were done at room temperature (22-23°C). During experiments, the neuronal cultures were continuously perfused with oxygenated (95% O2, 5% CO2) extracellular solution at a constant flow rate of 2ml/min. The neuronal cells were identified by their triangular-shaped morphology and their firing of action potentials in response to a depolarizing current injection. MCM and/or chemical reagents were applied through bath perfusion. Data was analyzed by Clampfit 8.1 and graphed using Origin 7.5 (OriginLab, Northampton, MA). For each set of experiments, the steady-state outward currents generated by a voltage step from -60mV to +60mV were measured and analyzed. The current amplitude recorded in a cell under control (before treatment) was treated as 100%, and the current amplitudes recorded during and after treatment were expressed as percentage of control. All data were expressed as mean ± S.D. unless otherwise indicated. Statistical analyses were performed by one-way ANOVA analysis or by Student t tests. A minimum p value of 0.05 was estimated as the significance level for all tests.
Results
Enhancement of neuronal IK by MCM (+)
Enhancement of outward K+ current has been implicated in neuronal death(Yu et al. 1997), and MCM collected from activated macrophages has been shown to induce neuronal injury (Pulliam et al. 1991). To examine whether MCM alters outward K+ currents, we studied effects of MCM on IK in primary hippocampal neuronal cultures using whole-cell patching techniques. Tetrodotoxin (TTX, 0.3 μM) and 4-AP (5mM) were added to the extracellular solution to block voltage-gated Na+ current and transient A-type K+ current (IA). Utilizing the voltage protocol described in the methods section, we successfully recorded whole-cell outward currents in hippocampal neuronal cultures and the recorded outward currents were significantly blocked by TEA, a Kv channel antagonist, demonstrating the expression of IK in primary hippocampal neuronal cultures (data not shown).
After confirmation of expression of IK, we tested the effect of MCM on IK in cultured hippocampal neurons. Bath application of MCM(+) enhanced IK in a concentration-dependent manner. When diluted at 1:3000 and 1:300, the MCM(+) had no significant effects on IK. However, MCM(+) significantly enhanced IK (p<0.05, n=16, Fig. 1) at a dilution of 1:30 (this dilution was used for all other experiments unless otherwise indicated). The MCM(+)-induced enhancement of IK was reversed 10-15 min after the start of washout. In contrast, MCM(-) had no apparent effect on IK at 1:30 dilution (n=16, Fig. 1). These results suggest that the activated MDM release soluble factors activating neuronal Kv channels. The activation of neuronal Kv channel by MCM(+) was further confirmed by experimental results showing that the MCM(+)-induced enhancement of IK was blocked by the addition of TEA, a Kv channel antagonist, to the extracellular solution (Fig. 2).
Figure 1.
MCM(+)-mediated enhancement of neuronal IK. Panel A shows representative current traces recorded from a hippocampal neuron during control (ctrl) and bath application of MCM(+) at different concentrations as indicated at the top of the current traces. The voltage protocol employed to induce outward current is shown in the far right of Panel A (not proportional to the time scale shown in current traces). Panel B is a summary bar graph illustrating MCM(+) significantly enhanced neuronal IK, whereas MCM(-) failed to produce such an enhancement. All current traces were recorded in the presence of 0.3μM TTX, 5mM 4-AP, and 10 μM nifedipine ( the same in other figures showing current traces). * p<0.05 vs control, n=16.
Figure 2.
Blockade of MCM(+)-induced enhancement of neuronal IK by TEA, a Kv channel antagonist. Panel A shows outward current traces recorded from a hippocampal neuron during control, bath application of MCM(+) or TEA + MCM(+). Note MCM(+) significantly enhanced neuronal IK, and this enhancement was blocked by TEA. Panel B illustrates the I-V relationship of outward currents recorded in response to command voltage steps. Each I-V curve is an average of outward currents recorded from 10 neurons. * p<0.05 vs TEA + MCM(+).
During brain inflammation, monocytes migrate into the brain and differentiate into tissue macrophages. To investigate whether macrophages could influence neuronal Kv channel activity when they co-localize with neuronal cells in brain inflammation sites, we analyzed the IK recorded from hippocampal neurons co-cultured with LPS-stmulated human MDM (MDM(+)) or non-stimulated human MDM (MDM(-)). A mild or a robust increase of IK was observed in neurons co-cultured with MDM(-) or MDM(+), respectively. The magnitude of IK recorded in neurons co-cultured with MDM(-) was 135.5±20.3% of control (n=12, Fig. 3), compared with IK recorded in control neurons (without MDM in culture dish, n=15), the difference was statistically significant (p<0.05). In contrast, the magnitude of IK recorded in neurons co-cultured with MDM(+) was 165.4 ± 25.5% of control (n=12, Fig. 3), which is significantly (p<0.05) higher when compared with the magnitude of IK recorded in neurons co-cultured with MDM(-). The data obtained in co-culture studies are in full agreement with the results observed in MCM studies, indicating that macrophages enhance neuronal IK current.
Figure 3.
MDM-mediated enhancement of neuronal IK in a MDM-neuronal co-culture system. Panel A are current traces recorded from three different hippocampal neurons: control, co-cultured with MDM(-), and co-cultured with MDM(+). MDM(+) induced a robust enhancement of neuronal IK. MDM(-) also produced an enhancement of neuronal IK. The average IK magnitude recorded from hippocampal neurons co-cultured with MDM(+) (n=12), or MDM(-) (n=12) are shown in Panel B. * p<0.05, MDM(-) vs control; # p<0.05, MDM(+) vs MDM(-).
Amelioration of MCM(+)-induced neuronal injury by a Kv channel antagonist
In addition to their neurotrophic role, macrophages induce neuronal injury through neurotoxin secretions. To understand whether MCM(+) enhancement of IK is associated with neuronal injury, we examined protective effects of the Kv channel blocker TEA on MCM(+)-induced neuronal injury in primary rat hippocampal neuronal cultures. Neuronal cultures were treated with MCM(+), TEA, or MCM(+) and TEA for 24 h. Then, the cell viability was assessed using combined PI and DAPI staining or MTT assay. Studies using PI/DAPI staining showed that addition of MCM(+) to the culture media produced a significant (∼25%) reduction of cell survival and that this MCM(+)-associated reduction in cell viability was reversed by TEA (Fig. 4). MTT assay revealed an approximately 35% reduction on cell viability, which was also blocked by TEA (Fig. 4). TEA, however, itself did not affect cellular survival when applied alone (Fig. 4).
Figure 4.
Amelioration of MCM(+)-induced cytotoxicity in hippocampal neuronal cultures by TEA. Nuclear morphology and membrane integrity of hippocampal neurons were evaluated by fluorescent dyes PI (red) and DAPI (blue). Panel A shows PI/DAPI staining in four different experimental conditions: untreated (control), treated with MCM(+), TEA+MCM(+), or TEA alone. Incubation of hippocampal neuronal cultures with MCM(+) produced neuronal injury as stained in red, and addition of TEA to culture media ameliorated neuronal injury induced by MCM(+) as illustrated by reduced number of PI-stained cells. Panel B is a summary of the results from Panel A. Survival rate was calculated by counting the numbers of cells from five different visual fields in each dish containing cultured neurons stained by PI/DAPI (n=36 visual fields). Panel C shows MCM(+)-associated neuronal injury, which was assayed using MTT assay in triplicate. Note that the MCM(+)-induced neuronal injury was blocked by TEA. *p<0.05 MCM(+) vs control, #p<0.05 TEA+MCM(+) vs MCM(+).
MCM(+) enhanced Kv1.3 and Kv1.5 channel expression in hippocampal neurons
Since our electrophysiological data showed that MCM(+) markedly increase IK in hippocampal neuronal cultures, we want to know whether this increase is relative to changes in levels of Kv 1.3 and/or Kv 1.5 channel expression. MCM(+) or MCM(-) were added (1:30 dilution) to 7-10 day old neuronal cultures for 24 h, and these neuronal cells were then marked with anti-Kv 1.3 or anti-Kv 1.5 antibody and secondary antibody. Confocal microscopy revealed that MCM(+), but not MCM(-), significantly increased the levels of Kv 1.3 and Kv 1.5 channel expression as evidenced by strong fluorescence intensity for Kv 1.3 and Kv 1.5 labeling in neurons treated with MCM(+) when compared with untreated (control) neurons (Fig. 5). In contrast, no significant change of Kv 1.3 and Kv 1.5 channel expression levels was found in neuronal culture treated with MCM(-) (Fig. 5).
Figure 5.

MCM(+) enhanced Kv1.3 and Kv1.5 channel expression in cultured hippocampal neurons. The neuronal cultures were treated with MCM(+) or MCM(-) for 24h and then the expression levels of Kv1.3 and Kv1.5 were detected by anti-Kv1.3 and Kv 1.5 antibodies. MCM(+), but not MCM(-), significantly increased expression of Kv1.3 and Kv 1.5 channels in hippocampal neurons. These results were reproduced in three batches of neuronal cultures.
Active factors underlying MCM(+)-induced enhancement of neuronal IK current
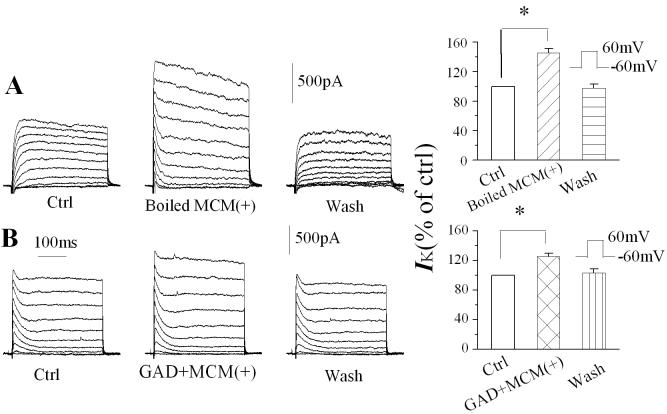
Activated macrophages release a number of bioactive molecules such as cytokines and amino acids. To identify the active molecules involved in the MCM(+)-associated enhancement of IK, we first boiled the MCM(+) for 15 min to denature the proteins secreted by macrophages. Bath application of the boiled MCM(+) at a dilution of 1:30 did not abolish its augmentation of IK (Fig. 6A), suggesting that macrophage-secreted proteins may not be the active molecules in enhancing neuronal IK. Studies have shown that N-methyl-D-aspartate (NMDA) receptors are coupled with Kv channels (Mulholland et al. 2008) and activated macrophages release glutamate (Jiang et al. 2001). To examine if MCM(+) increases IK through glutamate on NMDA receptors, we incubated MCM(+) with glutamate decarboxylase (GAD) at 37°C for 3 h and found that GAD failed to diminish the effects of MCM(+) on IK (Fig. 6B). GAD activity was confirmed by experiments showing that incubation of GAD with glutamate did abolish glutamate-mediated inward current (data not shown). These results suggest that the active molecules were neither proteins nor glutamate, although both are known to be present in the MCM(+). Further identification and characterization of active factors are needed to address this issue.
Figure 6.
Heating (boiling) or glutamate decarboxylase (GAD)-treatment failed to abolish MCM(+)-induced enhancement of neuronal IK. Representative current traces were recorded from two different hippocampal neurons before, during, and after bath application (wash) of boiled (Panel A) or GAD-treated (Panel B) MCMs. The bar graphs summarize the effects of boiled (Panel A, n=8) and GAD-treated (Panel B, n=8) MCM(+) on neuronal IK. Note that both heating (denature proteins) and GAD-treatment (degradation of glutamate) failed to diminish MCM(+) enhancement of neuronal IK. * p<0.05 compared with control.
Discussion
As a mediator for HIV-1 entry into the brain, a target and reservoir for productive and latent HIV-1 infection, and a source of neurotoxic substances, macrophages play an important role in the pathogenesis of HAND. Early studies of HIV-associated neuropathology discovered a prominent monocyte/macrophage brain infiltration (Price et al. 1988). Phenotypic labeling studies demonstrated that cells of monocyte/macrophage lineage were the major contributors to excess cellularity in patients with HIV encephalitis (Michaels et al. 1988a). Correlations have been noted between HAND and the accumulation of macrophages in the central nervous system (Budka 1986; Glass et al. 1995). It is widely accepted that activated macrophages secrete a variety of bioactive substances such as cytokines, leading to neuronal dysfunction and death in many pathophysiological circumstances (Bukrinsky et al. 1995; Gelbard et al. 1994; Genis et al. 1992; Kaul et al. 2001; Toggas et al. 1994; Xiong et al. 2000). The mechanisms underlying macrophage-associated neuropathogenesis are not fully understood. In this study, we demonstrated that the soluble factors secreted by activated macrophages caused neuronal injury by enhancing neuronal IK current and increasing neuronal Kv 1.3 and Kv 1.5 channel expression.
Kv channels play an important role in regulating neuronal excitability and activity. The functions of Kv channels can be modulated by a number of factors, including membrane potential, redox potential, post-translational modification, and a plethora of organic molecules and peptides (Birnbaum et al. 2004; Hille 2001). Kv channel functions can also be modulated by other bioactive molecules such as proinflammatory cytokines. Such modulations of Kv channel functions could lead to an altered excitability resulting in neuronal dysfunction and ultimately neuronal injury. Our experimental results showed that bath application of MCM(+) enhanced neuronal IK current, which was blocked by TEA, in a concentration dependent manner. In contrast, application of MCM(-) failed to increase neuronal IK, suggesting that the activated macrophages release soluble factors enhancing neuronal IK. The enhancement of neuronal IK was reproduced when neurons were co-cultured with stimulated macrophages. It is noteworthy to point out that the non-stimulated macrophages also showed some augmentative effects on neuronal IK while MCM(-) had no significant effects. This discrepancy may be the consequence of heterologous immune activation of human macrophages by rat hippocampal neurons in our co-culture system.
Biological significance of MCM(+)-induced enhancement of neuronal IK was examined in hippocampal neuronal cultures. Using PI/DAPI staining and MTT assay, we found that MCM(+) produced significant neuronal injury, which was blocked by the Kv channel blocker TEA. Our results, which are in full agreement with previous studies (Yu et al. 1998; Yu et al. 1999; Yu et al. 1997), demonstrate that enhancement of IK current was associated with neuronal death. This suggests that during brain immune and inflammatory processes, activated macrophages release soluble factors resulting in neuronal injury by enhancing neuronal IK.
It has been shown that Kv1.1 — Kv1.6 are present in the adult brain including the hippocampus (Grosse et al. 2000). We reasoned that activated macrophages may enhance neuronal IK by either increasing Kv channel open probability or enhancing channel expression on neuronal membrane, or both. Our results showed that MCM(+) enhanced Kv 1.3 and Kv 1.5 expression on the hippocampal neurons. This MCM(+)-associated increase of Kv 1.3 and Kv 1.5 expression may serve a basis for the MCM(+)-induced enhancement of IK. Whether MCM(+) increases Kv channel open probability is a direction for future research.
In an effort to identify potential active component(s) causing enhancement of neuronal IK and resultant neuronal injury, we boiled the MCM(+) or incubated MCM(+) with GAD. Neither heating nor GAD-incubation eliminated the enhancement effect of MCM(+) on neuronal IK, suggesting that the enhancement of IK may not be produced by factors like proteins or glutamate, which may be present in MCM(+) (Kaul et al. 2001; Jiang et al. 2001). Further investigation is needed to identify specific factor(s) inducing the enhancement of neuronal IK and resultant neuronal injury.
The Kv channels play a crucial role in the generation of electrical activity of neurons. They repolarize action potentials (APs), set interspike intervals, modulate the resting membrane potential and AP duration of neurons, and stabilize the membrane potential of excitable cells and non-excitable cells. It is known that excitability can be altered by neuromodulators and/or other bioactive substances. For example, the ability of neurons to fire APs and the shape of AP waveforms are mainly regulated by K+ channels. Thus, K+ channels directly influence neuronal excitability. This K+ channel-associated change in neuronal excitability may cause neuronal dysfunction. It has been proposed that a change in the number or in the pattern of APs leads to encoding information (Rieke et al. 1997). This indicates that the change of K+ channel activity may alter information processing and functions such as learning and memory. Recent genetic targeting studies indicate that Kv channels are of a great importance in the memory process (Giese et al. 2001; Giese et al. 1998; Solntseva et al. 2003). It has been demonstrated on different model systems that K+ current decreases during learning. The antagonists of Kv channels were found to improve learning and memory (Andreani et al. 2000). The alteration or dysfunction of Kv channels is believed to be an important link in the mechanisms of memory disturbance (Solntseva et al. 2003). Our data demonstrate that activated macrophages enhance neuronal IK and induce neuronal injury. Such macrophage-induced and Kv channel-associated neuronal injury may contribute to neurocognitive deficits seen in HIV-1-associated neurodegenerative disorders by which macrophages play a crucial role in the pathogenesis.
In summary, the experimental data provide in vitro evidence that activated macrophages increase neuronal Kv1.3 and Kv1.5 channel expression and enhance neuronal IK leading to neuronal injury. Identification of Kv channels as the targets for macrophage-induced neuronal injury may open new avenues for therapeutic modalities for a number of neurodegenerative disorders through which the infiltrated macrophages and resident microglia play a critical role in the pathogenesis.
ACKNOWLEDGEMENTS
The authors thank Ms. Robin Taylor, Mr. Benjamin Reiner and Ms. Anna Brynskikh for their critical reading of the manuscript. The authors extend special thanks to Ms. Julie Ditter, Ms. Robin Taylor and Ms. Johna Belling for their excellent administrative supports and to two anonymous reviewers for their critical criticisms and helpful comments. This work was supported by NIH grant 2 R01 NS041862 to H.X.
References
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25(2):123–33. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Andreani A, Leoni A, Locatelli A, Morigi R, Rambaldi M, Pietra C, Villetti G. 4-Aminopyridine derivatives with antiamnesic activity. Eur J Med Chem. 2000;35(1):77–82. doi: 10.1016/s0223-5234(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84(3):803–33. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Budka H. Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS) Acta Neuropathol (Berl) 1986;69(3-4):253–8. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M, Notte HSLM, Schmidtmayerova H, Dubrovsky L, Flanagan CR, Mullins ME, Lipton SA, Gendelman HE. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin MP, Coughlin K, Ho LT. Soluble macrophage factors trigger apoptosis in cultured hippocampal neurons. Neuroscience. 1997;80(2):437–48. doi: 10.1016/s0306-4522(97)00078-x. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Nottet HS, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi YB, Zhang D. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68(7):4628–35. doi: 10.1128/jvi.68.7.4628-4635.1994. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Schuenke KW, Keherly MJ, Holzer C, 3rd, Richey FJ, Lahart CJ. Acquired neuronal channelopathies in HIV-associated dementia. J Neuroimmunol. 2004;157(1-2):111–9. doi: 10.1016/j.jneuroim.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176(6):1703–18. doi: 10.1084/jem.176.6.1703. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Bartolini A. Influence of potassium channel modulators on cognitive processes in mice. Br J Pharmacol. 1998;123(6):1079–84. doi: 10.1038/sj.bjp.0701709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Peters M, Vernon J. Modulation of excitability as a learning and memory mechanism: a molecular genetic perspective. Physiol Behav. 2001;73(5):803–10. doi: 10.1016/s0031-9384(01)00517-0. [DOI] [PubMed] [Google Scholar]
- Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learn Mem. 1998;5(4-5):257–73. [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38(5):755–62. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Grosse G, Draguhn A, Hohne L, Tapp R, Veh RW, Ahnert-Hilger G. Expression of Kv1 potassium channels in mouse hippocampal primary cultures: development and activity-dependent regulation. J Neurosci. 2000;20(5):1869–82. doi: 10.1523/JNEUROSCI.20-05-01869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels in Excitable Membranes. Sinauer Associates; Sunderland, MA: 2001. [Google Scholar]
- Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001;117(1-2):97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome [see comments] N Engl J Med. 1995;332(14):934–40. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Michaels J, Price RW, Rosenblum MK. Microglia in the giant cell encephalitis of acquired immune deficiency syndrome: proliferation, infection and fusion. Acta Neuropathol (Berl) 1988a;76(4):373–9. doi: 10.1007/BF00686974. [DOI] [PubMed] [Google Scholar]
- Michaels J, Sharer LR, Epstein LG. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988b;1(1):71–104. [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28(35):8801–9. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew BJ, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Herndier BG, Tang NM, McGrath MS. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991;87(2):503–12. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Warland D, de Ruyter van Steveninck R, Bialek W. Spikes: exploring the neural code. MIT Press; cambridge, MA: 1997. [Google Scholar]
- Seilhean D, Duyckaerts C, Vazeux R, Bolgert F, Brunet P, Katlama C, Gentilini M, Hauw JJ. HIV-1-associated cognitive/motor complex: absence of neuronal loss in the cerebral neocortex [see comments] Neurology. 1993;43(8):1492–9. doi: 10.1212/wnl.43.8.1492. [DOI] [PubMed] [Google Scholar]
- Solntseva EI, Skrebitskii VG. [Memory and potassium channels] Usp Fiziol Nauk. 2003;34(4):16–25. [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice [see comments] Nature. 1994;367(6459):188–93. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Xiong H, Zeng YC, Lewis T, Zheng J, Persidsky Y, Gendelman HE. HIV-1 infected mononuclear phagocyte secretory products affect neuronal physiology leading to cellular demise: relevance for HIV-1-associated dementia. J Neurovirol. 2000;6(Suppl 1):S14–23. [PubMed] [Google Scholar]
- Yu SP, Farhangrazi ZS, Ying HS, Yeh CH, Choi DW. Enhancement of outward potassium current may participate in beta- amyloid peptide-induced cortical neuronal death. Neurobiol Dis. 1998;5(2):81–8. doi: 10.1006/nbdi.1998.0186. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Gottron F, Wang X, Grabb MC, Choi DW. Role of the outward delayed rectifier K+ current in ceramide-induced caspase activation and apoptosis in cultured cortical neurons. J Neurochem. 1999;73(3):933–41. doi: 10.1046/j.1471-4159.1999.0730933.x. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278(5335):114–7. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]