Scope and Insidious Nature of Sleep-Related Arrhythmia Risk
Sleep is generally considered to be a protected period, when the cardiovascular system benefits from the restorative influences of the sleeping brain. However, the dynamics of cardiovascular control during sleep states can tax the capacity of the diseased coronary circulation and myocardium with surges in sleep-state related autonomic activity and disruptions in airway function and central nervous system regulation. In this regard, sleep may constitute an autonomic stress test for the heart.
The scope of sleep-related risk for atrial and ventricular arrhythmias is substantial. The major subgroups susceptible to adverse influences of surges in autonomic activity during sleep are those with ischemic heart disease, heart failure, and channelopathies (Table 1).1 It is significant that 20% of myocardial infarctions and 15% of sudden deaths occur at night in the United States.2 Most atrial arrhythmias in patients younger than 61 years of age have nocturnal onset.3 The young are not immune to risk, as sudden infant death syndrome (SIDS) claims 2500 lives in the United States annually.4 Cardiovascular risk is compounded by comorbid factors, most notably apnea, which affects an estimated 4–9% of the general population5 and is considerably more prevalent among obese individuals.6 The more common form is obstructive sleep apnea (OSA), with partial or complete collapse of the pharynx. Half of heart failure patients experience either OSA or central sleep apnea (CSA) with central nervous system-mediated periodic breathing, commonly referred to as Cheyne-Stokes respiration. Such cardiorespiratory disturbances profoundly alter autonomic nervous system activity and increase risk of arrhythmia, hypertension, and myocardial infarction.
Table 1.
Patient Groups at Potentially Increased Risk for Nocturnal Cardiac Events
| Condition (U.S. Patients/Year) | Possible Mechanism |
|---|---|
| Angina, MI, arrhythmias, ischemia, or cardiac arrest at night (20% of MIs (~250,000 cases/yr) and 15% of sudden deaths (~48,750 cases/yr) occur between midnight and 6:00 am.) | The nocturnal pattern suggests a sleep state–dependent autonomic trigger or respiratory distress. |
| Unstable angina | Nondemand ischemia and angina peak between midnight and 6:00 am. |
| Acute MI (1.5 million) | Disturbances in sleep, respiration, and autonomic balance may be factors in nocturnal arrhythmogenesis. Nocturnal onset of MI is more frequent in older and sicker patients and carries a higher risk of congestive heart failure. |
| Heart failure (5.3 million) | Sleep-related breathing disorders are pronounced in the setting of heart failure and may contribute to its progression and to mortality risk. |
| Spousal or family report of highly irregular breathing, excessive snoring, or apnea in patients with coronary disease (15 million U.S. patients with apnea) | Patients with hypertension or atrial or ventricular arrhythmias should be screened for the presence of sleep apnea. |
| Long QT syndrome | The profound cycle-length changes associated with sleep may trigger pause-dependent torsades de pointes in these patients. |
| Near-miss or siblings of victims of SIDS (2500 total SIDS deaths annually in the U.S. or 1 death per 2000 live births) | SIDS commonly occurs during sleep with characteristic cardiorespiratory symptoms. |
| Brugada syndrome in Western populations; Asians with warning signs of SUNDS | SUNDS is a sleep-related phenomenon in which night terrors may play a role. It is genetically related to the Brugada syndrome. |
| Atrial fibrillation (2.2 million) | Twenty-nine percent of episodes occur between midnight and 6:00 am. Respiratory and autonomic mechanisms are suspected. |
Adapted from: Verrier and Mittleman1
Key: MI = myocardial infarction; SIDS = sudden infant death syndrome; SUNDS = sudden unexplained nocturnal death syndrome.
It is surprising, as recently underscored by Malhotra and Loscalzo,7 that the significance of cardiovascular risk during sleep may not be duly recognized within the cardiology community. The reasons are unclear but in part relate to the complex nature of cardiorespiratory interactions during sleep and to lack of monitoring technology suitable for use during the normal flow of clinical evaluation.
The goals of the present review are to discuss briefly the main scientific underpinnings of the link between sleep and cardiac arrhythmias, to review clinical evidence and current understanding of the impact of sleep on atrial and ventricular arrhythmogenesis, and to consider recent developments that can improve opportunities for evaluation of risk within existing diagnostic platforms for 24-hour ECG monitoring, particularly streamlined technology for concurrent monitoring of sleep state, electrocardiogram, oxygen desaturation, and respiration.
Sleep-State Control of Cardiovascular Function
Preserving circulatory homeostasis during sleep requires coordination of control over two complex systems: the respiratory, sustaining essential oxygen exchange, and the cardiovascular, providing blood transport. The dynamics of respiration and heart rhythm vary greatly between sleep states. The difficult balancing act of regulating two motor systems, one that supplies somatic musculature (i.e., diaphragmatic, intercostal, abdominal, and upper airway musculature) and the other involving regulation of autonomic pathways to the heart and vasculature, is a remarkable task during sleep. The challenge is compounded among individuals with diseased respiratory or cardiovascular systems, such as those with apnea and/or heart failure, and in infants, whose control systems are underdeveloped. Mechanisms underlying sleep-state control of cardiovascular function have been discussed in detail.8
Non-rapid eye movement (nonREM) sleep, the initial stage, occupies approximately 80% of sleep time. It is a period of lessened metabolic demands, with relative autonomic stability, when vagus nerve activity is dominant, baroreceptor gain is high, and sympathetic nerve activity is stable, with input to the cardiovascular system reduced by more than half from wakefulness to stage 4 of nonREM sleep.9 A near sinusoidal modulation of heart rate variation results from coupling of respiratory activity and cardiorespiratory centers in the brain to establish normal respiratory sinus arrhythmia, which is generally indicative of cardiac health. Heart rates accelerate briefly during inspiration to accommodate increased venous return and to provide increased cardiac output followed by progressive slowing in rate during expiration. The absence of this intrinsic variability in heart rate, which can be monitored by heart rate variability analysis, has been associated with cardiac pathology and advancing age, with the common denominator of loss of normal vagus nerve function.10
Increased vagus nerve activity provokes bradycardias, and reduced sympathetic vasomotor tone results in hypotension. During transitions from nonREM to rapid eye movement (REM) sleep, bursts of vagus nerve activity may result in pauses in heart rhythm and frank asystole. Thus, in general, the autonomic stability of nonREM sleep, with relative hypotension, bradycardia, and reduced cardiac output and systemic vascular resistance, provides a salutary neurohumoral background during which the heart has an opportunity for metabolic restoration.11
REM sleep is initiated at 90-minute intervals, when the brain’s increased excitability can disrupt cardiorespiratory homeostasis. Major surges in cardiac sympathetic nerve activity are concentrated in short, irregular periods, reach levels higher than during wakefulness, and trigger intermittent striking surges in blood pressure and heart rate, with marked episodes of tachycardia and bradycardia.9 Cardiac efferent vagus nerve tone is generally suppressed, and baroreceptor gain is reduced.12 Breathing patterns are also highly irregular and can provoke oxygen desaturation, particularly in patients with pulmonary or cardiac disease.
Ventricular Arrhythmias
Ventricular arrhythmias are generally suppressed during sleep in parallel with the nocturnal trough in incidence of myocardial infarction, sudden cardiac death, implantable cardioverter-defibrillator discharge, and myocardial ischemic events.13,14 However, sleep is not an entirely protected period, as 15% of sudden cardiac deaths occur at night2 or 48,750 cases annually in the United States alone. Moreover, the nighttime distribution of these events is nonuniform (Fig. 1),2 suggesting physiologic triggering.
Fig. 1.
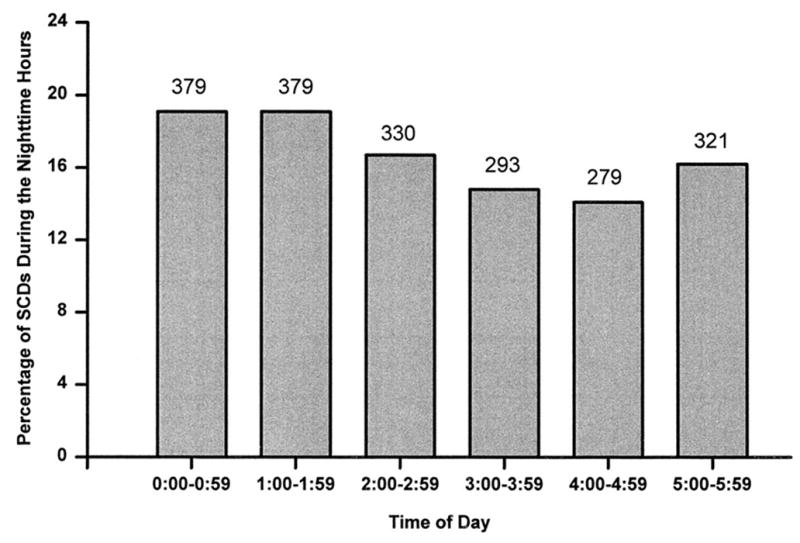
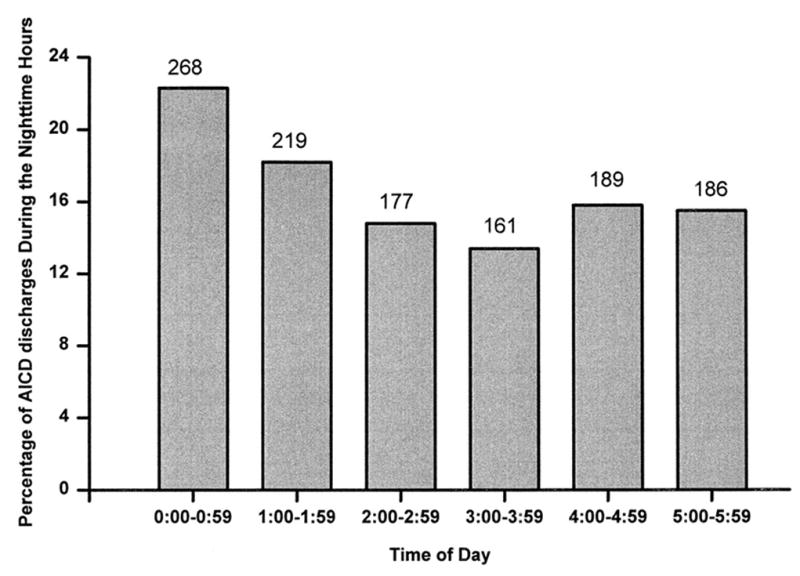
Panel A: Hourly incidence of sudden cardiac death onset between midnight and 5:59 a.m. from 12 studies enrolling 1981 patients.2 Panel B: Hourly incidence of automatic implantable cardioverter defibrillator (ICD) discharge between midnight and 5:59 a.m. from 7 studies enrolling 1197 patients. The number of discharges observed each hour is indicated above each bar.
REM-sleep related surges in sympathetic nerve activity have been implicated in nocturnal ventricular arrhythmias and myocardial ischemia in patients with cardiovascular disease.15 The specific mechanisms of REM-induced cardiac events include direct effects on electrophysiologic stability or indirect effects of heart rate and arterial blood pressure accelerations, which may promote intraarterial platelet aggregation or disrupt plaques to release proarrhythmic constituents. As metabolic demand outstrips supply, neural activity may also trigger myocardial ischemia and/or arrhythmias, particularly in patients with endothelial dysfunction, coronary obstructive disease, or vasospasm.
The nocturnal decline in sympathetic nerve activity typical of healthy individuals is altered in patients with coronary artery disease,14,16 myocardial infarction,10,17 and diabetes, suggesting a decrease in vagus nerve activity during sleep and unopposed cardiac sympathetic nerve activity in these patients, a state conducive to ventricular tachycardia and fibrillation. Changes in cardiac substrate and mechanical function due to disease, infarction, or ageing can also amplify nocturnal cardiac electrical instability. After major surgery, the simultaneous occurrence of hypoxemia and tachycardia during sleep may promote myocardial ischemia. Frequent or complex arrhythmias typify hypertensive patients who do not experience the nocturnal trough in blood pressure.18 REM-related nocturnal arrhythmogenesis may have a significant affective component, as REM sleep dreams may be vivid, bizarre, and emotionally intense, and generate anger and fear, emotions that have been linked in wakefulness to onset of myocardial infarction and sudden death.19
In some cases, arrhythmia frequency may be enhanced during nonREM sleep, when latent slow rhythms are exposed by the generalized reduction in heart rate after withdrawal of overdrive suppression. Also, relative hypotension during sleep may exacerbate impaired coronary perfusion as a result of lowered blood pressure gradients in stenosed vessels.
A marked nocturnal peak in sudden death has been observed in cardiac disease patients with obstructive or central sleep apnea.20 This respiratory dysfunction affects 15 million Americans.21,22 Apneic episodes and oxygen desaturation are highly conducive to nocturnal ischemia, bradyarrhythmia and tachyarrhythmias in patients with coronary artery disease,23,24 with heart failure,25,26 or in the subacute phase after myocardial infarction (Fig. 2).27 Apneas are closely associated in time with subsequent onset of nonsustained ventricular tachycardia (Fig. 3).28,29 The postulated mechanism is the accompanying surge in arterial blood pressure and sympathetic nerve activity,9,30,31 which levels during waking and achieve 249–299% above baseline (Fig. 4).31 Episodic or chronic oxygen desaturation may contribute to the association of apnea with arrhythmias, as it constitutes an independent marker of ventricular arrhythmia risk.21,22,32 Importantly, patients with sleep apnea experience elevated of sympathetic nerve activity and blood pressure even during wakefulness.
Fig. 2.
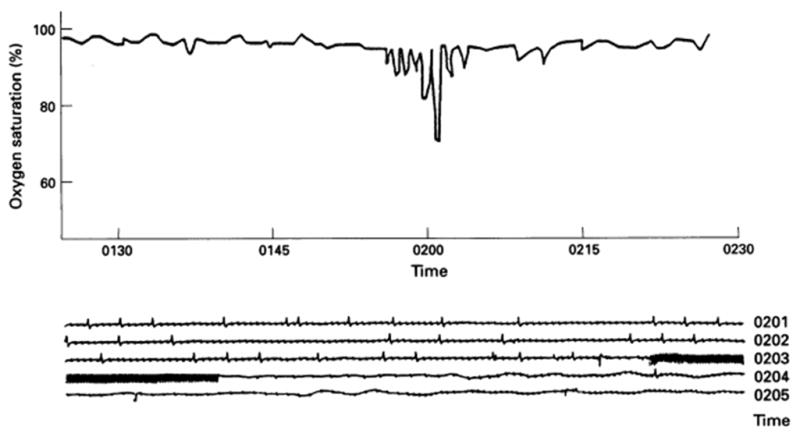
Importance of monitoring nocturnal oxygen saturation in patients who have sustained a myocardial infarction.27 Nonsustained ventricular tachycardia (lower panel) and hypoxemia measured by pulse oximetry (upper panel) occurred simultaneously on the third night after infarction. The patient died on the following day of cardiogenic shock. Reprinted with permission from the British Medical Journal Publishing Group Ltd and the British Cardiovascular Society.
Fig. 3.
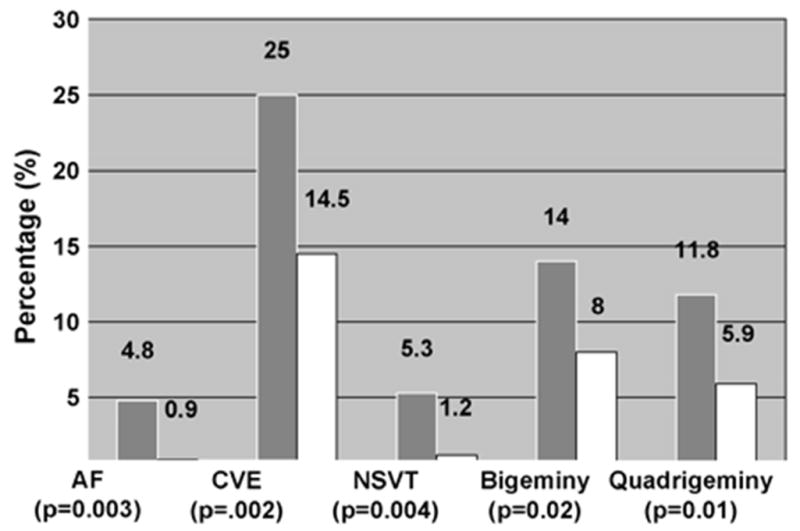
Arrhythmia prevalence (%) according to sleep-disordered breathing (SDB) status.28 Shaded bars, SDB; open bars, non-SDB. AF, atrial fibrillation; CVE, complex ventricular ectopy; NSVT, nonsustained ventricular tachycardia. N = 228 with SDB; N = 338 without SDB. Reprinted with permission from the American Thoracic Society.
Fig. 4.
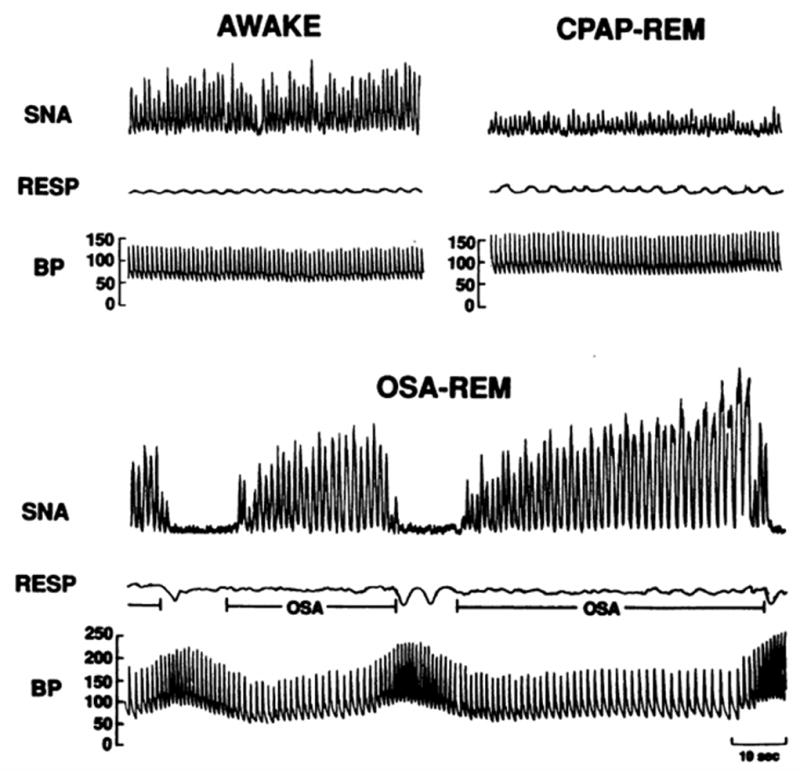
Recordings of sympathetic nerve activity (SNA), respiration (RESP), and intraarterial blood pressure (BP) in the same subject when awake, with obstructive sleep apnea during rapid eye movement (REM) sleep, and with elimination of obstructive apnea by continuous positive airway pressure (CPAP) therapy during REM sleep.31 SNA was very high during wakefulness but increased even further secondary to obstructive apnea during REM. BP increased from 130/65 mmHg when awake to 256/110 mmHg at the end of apnea. Elimination of apneas by CPAP resulted in decreased nerve activity and prevented BP surges during REM sleep. Reprinted with permission from The American Society for Clinical Investigation.
Sleep apnea also potentiates the risk for cardiovascular events33–35 including nocturnal myocardial infarction36 and death from fatal myocardial infarction33 in patients with cardiovascular disease. Increased incidence of myocardial infarction in patients with sleep apnea35 may contribute to ventricular arrhythmias and sudden death32 due to ventricular remodeling and myocardial scar. Resistance-vessel endothelium-dependent vasodilation is impaired in patients with sleep apnea,37 a potential factor in the development of hypertension and heart failure.
Antiarrhythmic therapy should address the electrically unstable myocardial substrate, as for daytime arrhythmias. Nocturnal beta-adrenergic receptor blockade therapy may prove helpful in cases with marked surges in sympathetic nerve activity, with careful attention to avoiding medications that disrupt sleep. Antihypertensive pharmacologic therapy may exacerbate the hypotensive effect of nonREM sleep and introduce the risk of transient ischemia in patients with stenotic lesions in the heart or brain, with potential for myocardial infarction.11 Thus, caution with respect to dosing is necessary. In patients with obstructive sleep apnea, weight control is therapeutic where warranted, and continuous positive airway pressure (CPAP) lessens arrhythmia risk33,34,38–40 and hypertension.41
Post–Myocardial Infarction Patients
During the first weeks after myocardial infarction, sleep is significantly disturbed17 and nocturnal oxygen desaturation, especially in patients with impaired left ventricular function, may be generalized or episodic and directly provoke tachycardia, ventricular premature beats, and ST-segment changes.25,27,42 Both the duration and number of nighttime ischemic events are increased,16 consonant with increased cardiac sympathetic nerve activity or decreased parasympathetic nerve activity,10 particularly in patients with residual myocardial ischemia. Nocturnal levels of norepinephrine are increased and nocturnal secretion of melatonin, an endogenous hormone that suppresses sympathetic nerve activity, is impaired. These symptoms lessen across the first six months; following that period, ventricular tachycardia during sleep is relatively rare.
Heart Failure
An estimated 20% of sudden deaths in heart failure patients occurs at night.43 At least half of patients with congestive heart failure experience disturbed nighttime breathing in the form of either obstructive or central sleep apnea. Although the prevalence of OSA is comparable between men (38%) and women (31%) with heart failure, CSA is uncommon in women.21,22 The specific pathophysiologic mechanisms that exacerbate heart failure differ between the apnea types. Whereas OSA is characterized by mechanical influences due to airway obstruction, CSA has a critical central nervous system component and distinct periodic Cheyne-Stokes breathing.
These sleep-related nocturnal breathing disorders set in motion a cascade of arrhythmogenic factors44 and underlie an overall increase in mortality.5,45–47 Among the most important effects of central and obstructive sleep apnea are nocturnal oxygen desaturations,44 elevated sympathetic tone,21,22 ventricular stretch, remodeling of cardiac chambers, and left ventricular diastolic dysfunction. In patients with systolic heart failure, central sleep apnea, severe right ventricular systolic dysfunction and low diastolic blood pressure are associated with increased mortality risks (Fig. 5).48 The apnea-hypopnea index is a powerful independent predictor of poor prognoses in clinically stable congestive heart failure patients.49 Clearly, the underlying pathophysiologic mechanisms of the two forms of apnea are complex and are critical in diagnosis and selection of therapy, as discussed in recent reviews.5,21,22,26
Fig. 5.
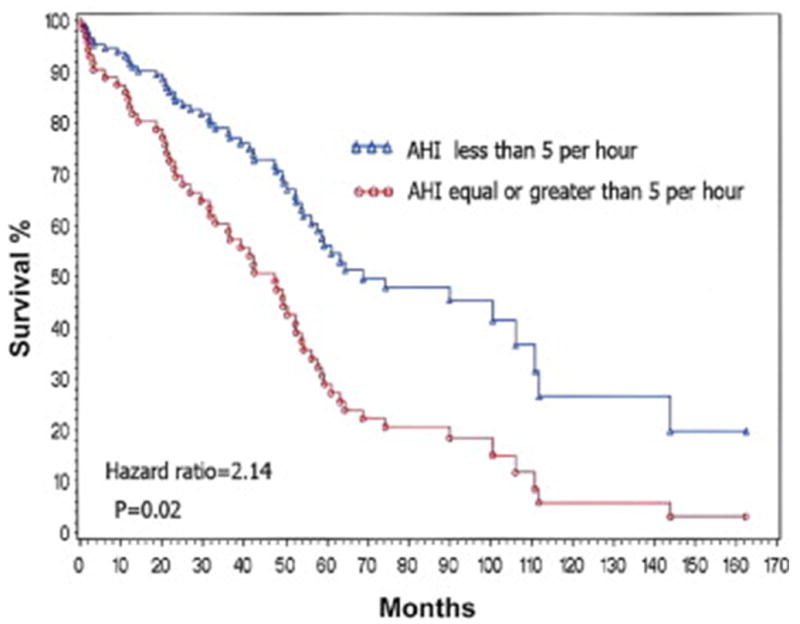
Survival of heart failure patients with or without central sleep apnea (CSA) after accounting for all other confounders.48 AHI = apnea-hypopnea index. Reprinted with permission from American College of Cardiology.
Detection of Arrhythmia Vulnerability in Heart Failure
Although arrhythmias are generally present in heart failure, specific methods for quantifying risk for malignant ventricular arrhythmias have been lacking. Recently, ambulatory ECG-based T-wave alternans, a beat-to-beat fluctuation in the amplitude and shape of the T wave,50 has been shown to be a marker of risk for life-threatening tachyarrhythmias in patients with left ventricular dysfunction.51,52 A recent sizeable prospective study of patients with left ventricular dysfunction demonstrated that ambulatory ECG-based T-wave alternans can identify patients’ one-year risk for cardiovascular mortality (primary endpoint) and sudden cardiac death (secondary endpoint) with odds ratios of 17.1 and 22.6, respectively (Fig. 6).52 An example of elevated T-wave alternans during nighttime ambulatory ECG recording from a hospitalized heart failure patient is provided (Fig. 7).51 Preliminary evidence of a correlation between the severity of sleep apnea, indicated by oxygen desaturation and apnea-hypopnea index, with T-wave alternans magnitude in patients with congestive heart failure has also been reported.53
Fig. 6.
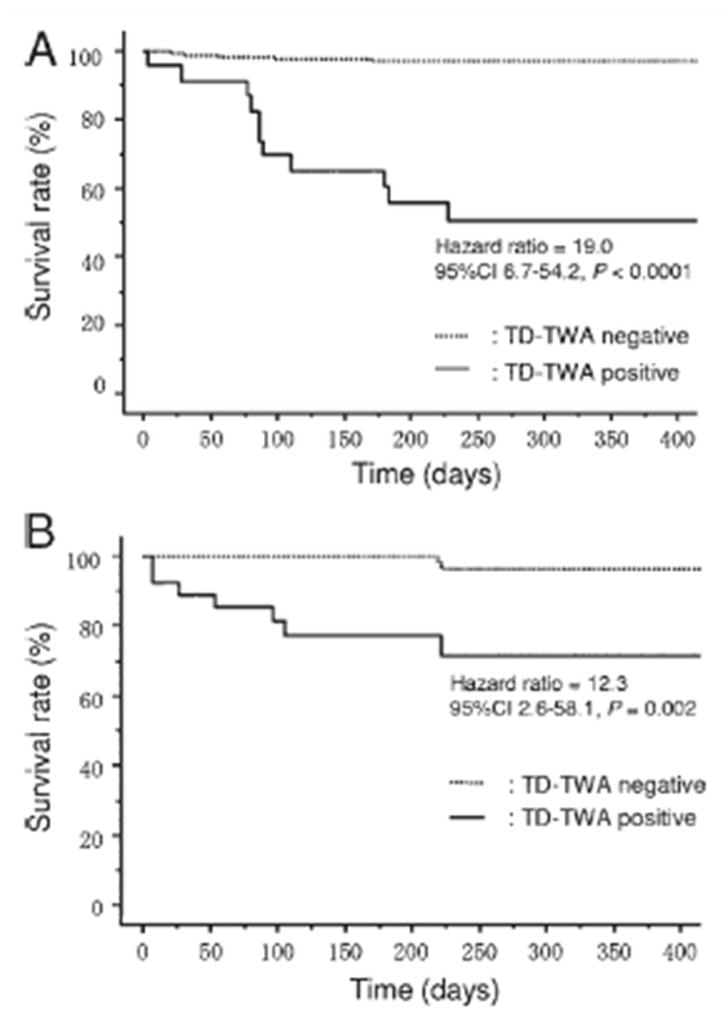
Freedom from cardiac mortality based on modified moving average analysis of T-wave alternans (TD-TWA) from 24-hour ambulatory ECGs in ischemic (A) and nonischemic (B) study subgroups.52 Reprinted with permission from Heart Rhythm Society.
Fig. 7.
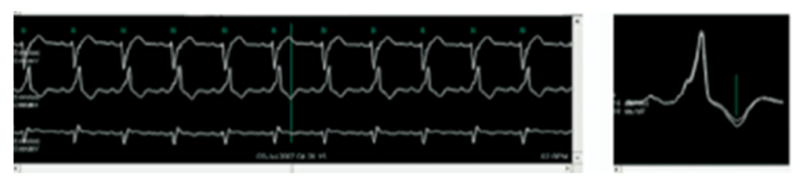
Representative rhythm strip (left) and QRS-aligned superimposed modified moving average waveforms (right) for the maximum TWA (≥65μV) in lead V3 from a heart failure patient enrolled in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS).51 Note the lack of separation between the superimposed beats in the isoelectric PQRS complex, indicating the low level of noise. The ABAB separation is concentrated within the JT segment, as observed experimentally and in other clinical studies. Reprinted from permission from John Wiley & Sons.
Contemporary Therapeutic Approaches
Apnea treatment with CPAP, supplemental oxygen, medications, or mechanical breathing-assist devices can improve exercise tolerance54 and lessen heart failure symptoms.25,55 Its efficacy as an adjunct for arrhythmia prevention or management in patients with obstructive or central sleep apnea is controversial.21,22,26,32 Cardiac resynchronization therapy may reduce central sleep apnea and improve in sleep quality,56 as it promotes reverse remodeling with antiarrhythmic potential.57 Atrial overdrive pacing does not reduce the number of episodes of central or obstructive sleep apnea.58 The role of ICD implantation for primary prevention of sudden death in patients with left ventricular dysfunction requires further investigation.
Nocturnal Asystole and QT Interval Changes
Benign asystoles are not uncommon during sleep in normal individuals who are young or physically fit, such as athletes and heavy laborers. Sinus pauses <2 seconds, prolonged atrioventricular (AV) conduction, Wenckebach AV block, and bradycardia are attributed to effects of increased parasympathetic activity on AV node conduction. Periods of sinus arrest of up to nine seconds during REM sleep in young adults with apparently normal cardiac function have been reported and attributed to exaggerated, if not abnormally elevated, vagal tone.59 However, in patients with coronary atherosclerosis and damaged endothelium, the acetylcholine released by surges in vagus nerve activity could provoke vasoconstriction due to impaired release of endothelium-derived relaxing factor.60
Nocturnal prolonged cycle lengths and asystolic events can facilitate the occurrence of early afterdepolarizations and set the stage for ventricular arrhythmias including torsades de pointes in patients who are predisposed to this arrhythmia. Sleep-related cycle-length prolongation may also be a risk factor in patients treated with agents with class III antiarrhythmic effects or with diuretics, which lower potassium. Thus, determining the presence or absence of nocturnal heart rate pauses is important in treating individuals for whom class III antiarrhythmic drugs (potassium channel blockers) are the primary option.
Nocturnal heart rate pauses may also be particularly arrhythmogenic in subsets of patients with the long QT syndrome, specifically LQTS2 and LQTS3; the latter have mutations on the sodium channel, voltage-gated, type V, alpha gene (SCN5A).61 The lethal arrhythmias in these syndrome subtypes occur almost exclusively at rest or during sleep. (See section on Sudden Infant Death Syndrome, later). The effect of sleep and nocturnal apneas in the short QT syndrome has not been sufficiently investigated.
Reports vary of associations of apnea with nocturnal sinus pauses,28 heart block,24 or ventricular asystole or bradyarrhythmias. However, apnea treatment where indicated has been associated with reversal of sinus arrest and atrioventricular conduction block.24,62
Atrial Fibrillation
Atrial fibrillation has serious consequences in terms of increased morbidity and mortality63 and afflicts 2.2 million people in the U.S. and 4.5 million in the European Union. Nocturnal peaks in onset of atrial tachyarrhythmias, paroxysmal atrial fibrillation, and a higher average nocturnal incidence of atrial fibrillation have been reported.64 Atrial fibrillation during sleep may be evident in a rise in heart rate (Fig. 8).65
Fig. 8.
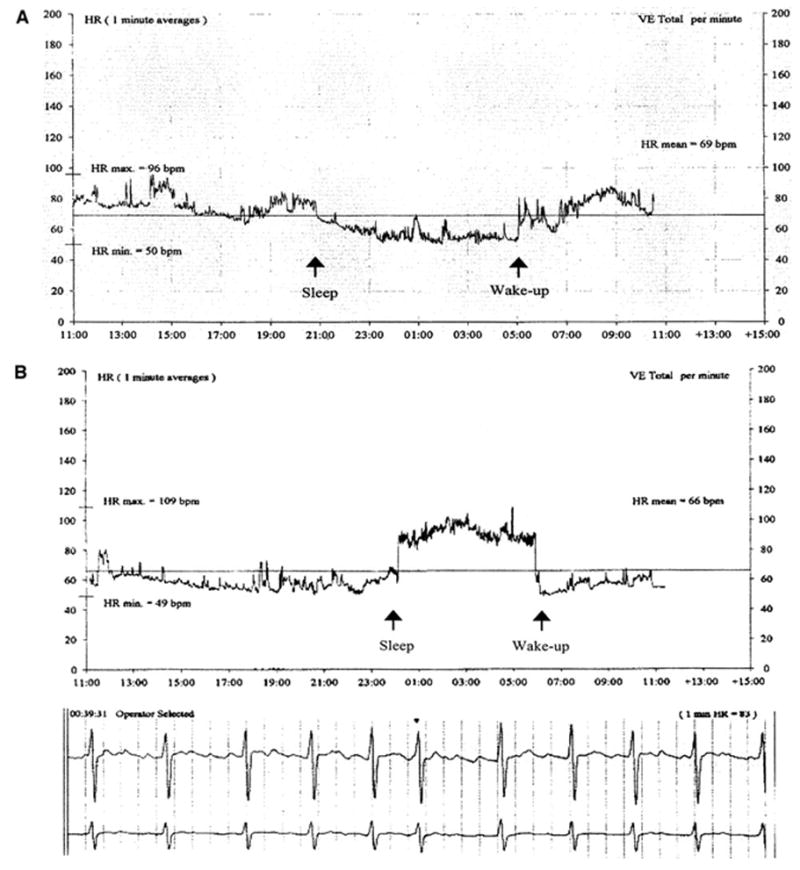
Heart rate trend from an ambulatory ECG (AECG) recording showing a normal circadian rhythm with a sleep-induced decrease in heart rate (panel A) as compared with a nocturnal increase in heart rate caused by paroxysmal atrial fibrillation at the onset of sleep and a drop in heart rate after awakening due to spontaneous conversion to sinus rhythm (Panel B).65 The ECG (below) documents atrial fibrillation during the sleep period.
It is likely that 10–25% of the arrhythmias are facilitated by vagal influences. Nocturnal atrial fibrillation is provoked during periods of intense vagus nerve activity, as indicated by heart rate variability studies66 and by the presence of bradycardia in individuals with structurally normal hearts. These arrhythmias are termed “vagally mediated atrial fibrillation.” Enhanced adrenergic activity may interact in a complex manner with changes in vagal tone to affect atrial refractoriness and dispersion of repolarization and alter intra-atrial conduction, thus increasing the propensity to develop this arrhythmia. The high level of vagus nerve tone maintained during slow wave sleep has the capacity to exacerbate atrial fibrillation in patients whose atria are particularly prone to the arrhythmogenic influence of acetylcholine.
Risks of atrial fibrillation and its recurrence after cardioversion are more than doubled if breathing during sleep is disordered (Fig. 9).20,28,67–69 Incidence of atrial fibrillation in apnea patients is strongly predicted by obesity, by nocturnal oxygen desaturation in subjects <65 years old, and by heart failure in older subjects.6,67 Multiple mechanisms appear to be operative. Apnea provokes nocturnal hypoxemia, sympathetic nerve activity, and hemodynamic stress through surges in blood pressure that distend and remodel atrial chambers and can activate stretch receptors and alter diastolic function.70
Fig. 9.
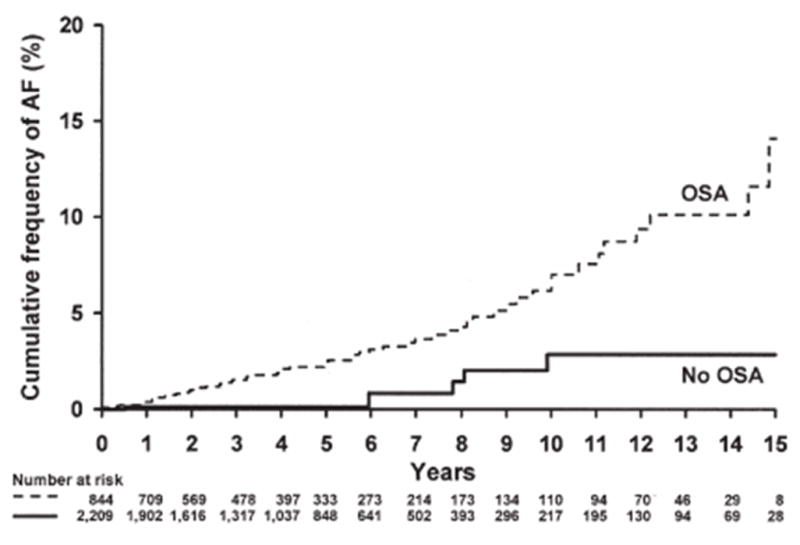
Incidence of AF based on presence or absence of OSA.67 Cumulative frequency curves for incident atrial fibrillation (AF) for subjects <65 years of age with and without obstructive sleep apnea (OSA) during an average 4.7 years of follow-up (P = 0.002). Reprinted with permission from American College of Cardiology.
At present there is no clear evidence for differentiating medical treatment for nighttime as compared with daytime atrial fibrillation. However, individuals with nocturnal onset of atrial fibrillation should be monitored for the presence of sleep-disordered breathing, which can be effectively treated by CPAP.71
Sudden Infant Death Syndrome
Sudden infant death syndrome (SIDS) is the leading cause of mortality in infants between one week and one year of age and typically occurs during sleep.4 The syndrome is diagnosed by exclusion criteria to include all causes that “remain unexplained after a thorough case investigation, including performance of a complete autopsy, examination of the death scene, and review of the clinical history.” The toll of SIDS is approximately one death per 2000 live births or 2500 total SIDS deaths in the United States annually and may be attributable to a variety of etiologies that challenge the developing cardiorespiratory system. Hypotension and bradycardia72 attend the fatal event in SIDS victims, suggesting a deficit in the normal reflex coordination of heart rate, arterial blood pressure, and respiration during sleep. This failure to respond to cardiorespiratory challenges during sleep has been attributed to a binding deficit in the arcuate nucleus of SIDS infants,73 as muscarinic cholinergic activity in this structure at the ventricular medullary surface is postulated to be involved in cardiorespiratory control. Absence of normal breathing pauses, diminished breathing variation, as well as reductions in respiration-induced heart rate variation characterize infants who later succumb to SIDS and may provide clues regarding derangements underlying the syndrome. Heart rates in infants who later died of SIDS are generally higher and exhibit a reduced range, suggesting altered autonomic control.74 Autonomic instability has also been documented in nonREM sleep in infants with aborted SIDS events.75
Repolarization abnormalities have been observed among SIDS victims that typify infants and children with the long QT syndrome genotype linked to chromosome 3 (LQT3). Evidence from a 19-year, prospective, multicenter observational study of 34,442 infants determined that significant prolongation (35 msec or more) of the QT interval characterized the 24 (0.07%) infants who died of SIDS within the first year of life.76 These results suggest that some SIDS cases may be attributed to a genetic defect that produces a developmental abnormality in cardiac sympathetic innervation and alters repolarization to increase the risk of ventricular arrhythmia. Mutations in the sodium channel gene SCN5A are the most common causes of long QT syndrome and are responsible for the arrhythmias and reduced heart rates. The genetic locus of the defect and the length of the QT interval are independent predictors of risk, suggesting opportunities for assessing risk for SIDS through molecular screening as well as noninvasive ECG monitoring.77,78 T-wave alternans has been reported in infants who became SIDS victims79 or were successfully treated.80
A number of straightforward opportunities for intervention are indicated, including placing infants in a supine (face-up) position for sleeping81 and avoidance of maternal smoking during gestation and passive smoking during the child’s infancy.82 Prospective studies are required to examine the potential of sodium channel blockade or cardiac pacing in treating infants diagnosed with the long QT3 syndrome.78 Beta-blockade is the current treatment of choice76,80 and diminishes T-wave alternans magnitude.80
The Brugada Syndrome and Sudden Unexplained Nocturnal Death
The striking phenomenon of sudden death during sleep due to ventricular arrhythmia has been reported in Western adults diagnosed with the Brugada syndrome, which strikes men almost exclusively, and in young, apparently healthy Southeast Asian men with the sudden unexplained nocturnal death syndrome (SUNDS). The latter syndrome is named lai-tai (“sleep death”) in Laos, pokkuri (“sudden and unexpected death”) in Japan, and bangungut (“to rise and moan in sleep”) in the Philippines. These syndromes probably represent the same disorder, which is characterized by right precordial ST segment elevation.83,84
The Brugada syndrome is considered responsible for 4–12% of all sudden cardiac deaths and for approximately 20% of deaths in patients with structurally normal hearts.83 A single sodium channel mutation in the SCN5A gene, QT interval prolongation, and Brugada-like electrocardiogram characterizes 20% of Brugada patients; other mutations are suspected. Genetic defects in the sodium channel are also associated with progressive conduction system disease attended by bradycardia.
Autopsies of SUNDS cases have established that cardiovascular disease is absent, but, in some instances, that conduction pathways are developmentally abnormal.84–86 Vagal tone is lower in SUNDS survivors compared with healthy individuals, particularly at night.87 Currently, implantation of cardioverter-defibrillators appears to be the most effective approach in patients with Brugada syndrome83 or SUNDS.84
Sleep-Disrupting Effects of Cardiac Medications
Several widely employed cardiac medications have the potential to disrupt sleep, including antihypertensive agents and beta-blockers, which reduce sudden death risk but cross the blood–brain barrier.1 The lipophilic beta-blockers propranolol and metoprolol increase the total number of awakenings and total wakefulness compared with placebo and with the nonlipophilic atenolol and may provoke nightmares. Penetration of the blood–brain barrier occurs with prolonged therapy, when these distinctions may become less apparent. The mechanism of sleep disruption by beta-blocking agents may be their well-known tendency to deplete endogenous melatonin, a key sleep-regulating hormone that modulates sympathetic nerve activity. Sleep disturbance has also been documented in conjunction with the widely antiarrhythmic agent amiodarone. Neurologic side effects were attributed to amiodarone in 20–40% of patients.
Conclusions and Implications
The occurrence of serious ventricular and supraventricular arrhythmias during sleep in individuals with heart disease is an important problem in contemporary cardiology. The subtle nature of cardiovascular regulation during sleep belies the intensity of autonomic activity, which can challenge the diseased coronary circulation and myocardium.
Identification of risk and underlying pathophysiology on an individual patient basis remains a major challenge, requiring prospective trials that employ streamlined risk-assessment tools suitable for use in the routine flow of clinical evaluation. Recent developments in ambulatory ECG-based technology for assessment of autonomic tone by heart rate turbulence88 and of cardiac electrical instability by T-wave alternans50–53 may be useful. Given the important role of respiratory factors in sleep-related arrhythmogenesis, future studies should also incorporate simultaneous measurement of ventilation and oxygen saturation to advance our understanding of causal links. Arrhythmia vulnerability is often compounded by disordered nighttime breathing, but whether sleep apnea treatment is capable of reducing risk for atrial and fibrillation, myocardial infarction, and sudden death has not been established definitively and deserves intense investigation. Ultimately, multiparameter assessment could significantly improve diagnosis and therapy and reduce sleep-related arrhythmogenesis and cardiovascular death.
Acknowledgments
Funding sources
Supported by R21 HL085720 from NIH and by Center for Integration of Medicine and Innovative Technology (CIMIT).
Footnotes
Disclosures
Dr. Richard L. Verrier is co-inventor of a patent for T-wave alternans measurement by the Modified Moving Average method, which was assigned to Beth Israel Deaconess Medical Center and licensed to GE Healthcare. He has received research equipment and honoraria from GE Healthcare and grant support from Medtronic, Inc.
Dr. Mark E. Josephson declares no conflicts of interest related to this subject.
References
- 1.Verrier RL, Mittleman MA. Sleep-related cardiac risk. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia: WB Saunders; 2005. pp. 1161–1170. [Google Scholar]
- 2.Lavery CE, Mittleman MA, Cohen MC, Muller JE, Verrier RL. Nonuniform nighttime distribution of acute cardiac events: A possible effect of sleep states. Circulation. 1997;5:3321–3327. doi: 10.1161/01.cir.96.10.3321. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T, Murakawa Y, Hayami N, Sezaki K, Inoue M, Fukui E, Omata M. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol. 1998;82:1364–1367. doi: 10.1016/s0002-9149(98)00642-0. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics: Deaths: Final data for 2001. National Vital Statistics Report. 2003:52. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr52/nvsr52_03.pdf. [PubMed]
- 5.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Parise H, Levy D, D’Agostino RB, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra A, Loscalzo J. Sleep and cardiovascular disease: An overview. Progr Cardiovasc Dis. 2009;51:279–284. doi: 10.1016/j.pcad.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verrier RL, Harper RM, Hobson JA. Central and autonomic mechanisms regulating cardiovascular function. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia: WB Saunders; 2005. pp. 192–202. [Google Scholar]
- 9.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 10.Vanoli E, Adamson PB, Ba-Lin, Pinna GD, Lazzara R, Orr WC. Heart rate variability during specific sleep stages: A comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91:1918–1922. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 11.Mancia G. Autonomic modulation of the cardiovascular system during sleep. N Engl J Med. 1993;328:347–349. doi: 10.1056/NEJM199302043280511. [DOI] [PubMed] [Google Scholar]
- 12.Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: A quantitative method of assessing baroreflex sensitivity. Circ Res. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- 13.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Andrews TC, Fenton T, Toyosaki N, Glasser SP, Young PM, MacCallum G, Gibson RS, Shook TL, Stone PH for the Angina and Silent Ischemia Study Group (ASIS) Subsets of ambulatory myocardial ischemia based on heart rate activity: Circadian distribution and response to anti-ischemic medication. Circulation. 1993;98:92–100. doi: 10.1161/01.cir.88.1.92. [DOI] [PubMed] [Google Scholar]
- 15.Nowlin JB, Troyer WG, Jr, Collins WS, Silverman G, Nichols CR, McIntosh HD, Estes EH, Bogdonoff MD. The association of nocturnal angina pectoris with dreaming. Ann Intern Med. 1965;63:1040–1046. doi: 10.7326/0003-4819-63-6-1040. [DOI] [PubMed] [Google Scholar]
- 16.Patel DJ, Knight CJ, Holdright DR, Mulcahy D, Clarke D, Wright C, Purcell H, Fox KM. Pathophysiology of transient myocardial ischemia in acute coronary syndromes: Characterization by continuous ST-segment monitoring. Circulation. 1997;95:1185–1192. doi: 10.1161/01.cir.95.5.1185. [DOI] [PubMed] [Google Scholar]
- 17.Broughton R, Baron R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol. 1978;45:348–360. doi: 10.1016/0013-4694(78)90187-6. [DOI] [PubMed] [Google Scholar]
- 18.Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Zampi I, Battistelli M, Gattobigio R, Sacchi N, Porcellati C. Association between persistent pressure overload and ventricular arrhythmias in essential hypertension. Hypertension. 1996;28:284–289. doi: 10.1161/01.hyp.28.2.284. [DOI] [PubMed] [Google Scholar]
- 19.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger: Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 20.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 21.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: Obstructive sleep apnea. Circulation. 2003;107:1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 22.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: Central sleep apnea. Circulation. 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 23.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 24.Koehler U, Fus E, Grimm W, Pankow W, Schafer H, Stammnitz A, Peter JH. Heart block in patients with obstructive sleep apnoea: pathogenetic factors and effects of treatment. Eur Respir J. 1998;11:434–439. doi: 10.1183/09031936.98.11020434. [DOI] [PubMed] [Google Scholar]
- 25.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 26.Javaheri S. Sleep dysfunction in heart failure. Current Treatment Options in Neurology. 2008;10:323–335. doi: 10.1007/s11940-008-0035-8. [DOI] [PubMed] [Google Scholar]
- 27.Galatius-Jensen S, Hansen J, Rasmussen V, Bildsoe J, Therboe M, Rosenberg J. Nocturnal hypoxemia after myocardial infarction: Association with nocturnal myocardial ischaemia and arrhythmias. Br Heart J. 1994;72:23–30. doi: 10.1136/hrt.72.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Association of nocturnal arrhythmias with sleep-disordered breathing. The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monahan K, Mehra R, Storfer-Isser A, Shahar E, Mittleman MA, Rottman J, Sanders M, Quan SF, Redline S. Temporal relationship between respiratory events and arrhythmia In sleep-disordered breathing: The Sleep Heart Health Study [abstract] Heart Rhythm. 2008:S151. [Google Scholar]
- 30.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11(6 Pt 2):608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- 31.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gami AS, Somers VK. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J Cardiovasc Electrophysiol. 2008;19:997–1003. doi: 10.1111/j.1540-8167.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 33.Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–86. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 34.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 35.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–264. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 36.Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez-Jimenez F, Somers VK. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 38.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 39.Milleron O, Pilliere R, Foucher A, de Roquefeuil F, Aegerter P, Jondeau G, Raffestin BG, Dubourg O. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–397. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 41.Kapa S, Sert Kuniyoshi FH, Somers VK. Sleep apnea and hypertension: interactions and implications for management. Hypertension. 2008;51:605–608. doi: 10.1161/HYPERTENSIONAHA.106.076190. [DOI] [PubMed] [Google Scholar]
- 42.Cripps T, Rocker G, Stradling J. Nocturnal hypoxia and arrhythmias in patients with impaired left ventricular function. Br Heart J. 1992;68:382–386. doi: 10.1136/hrt.68.10.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carson PA, O’Connor CM, Miller AB, Anderson S, Belkin R, Neuberg GW, Wertheimer JH, Frid D, Cropp A, Packer M. Circadian rhythm and sudden death in heart failure: Results from prospective randomized amlodipine survival trial. J Am Coll Cardiol. 2000;36:541–546. doi: 10.1016/s0735-1097(00)00728-2. [DOI] [PubMed] [Google Scholar]
- 44.Davies SW, John LM, Wedzicha JA, Lipkin DP. Overnight studies in severe chronic left heart failure: arrhythmias and oxygen desaturation. Br Heart J. 1991;65:77–83. doi: 10.1136/hrt.65.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo MA, Stevenson WG, Moser DK, Trelease RB, Harper RM. Patterns of beat-to-beat heart rate variability in advanced heart failure. Am Heart J. 1992;123:704–710. doi: 10.1016/0002-8703(92)90510-3. [DOI] [PubMed] [Google Scholar]
- 46.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL, Ruttanaumpawan P, Tomlinson G, Bradley TD. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 48.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 49.Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107:727–732. doi: 10.1161/01.cir.0000049641.11675.ee. [DOI] [PubMed] [Google Scholar]
- 50.Verrier RL, Kumar K, Nearing BD. Basis for sudden cardiac death prediction by T-wave alternans from an integrative physiology perspective. Heart Rhythm. 2009;6:416–422. doi: 10.1016/j.hrthm.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein PK, Sanghavi D, Domitrovich PP, Mackey RA, Deedwania P. Ambulatory ECG-based T-wave alternans predicts sudden cardiac death in high-risk post-MI patients with left ventricular dysfunction in the EPHESUS study. J Cardiovasc Electrophysiol. 2008;19:1037–1042. doi: 10.1111/j.1540-8167.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 52.Sakaki K, Ikeda T, Miwa Y, Miyakoshi M, Atsuko A, Tsukada T, Ishiguro H, Mera H, Yusu S, Yoshino H. Time-domain T-wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: a prospective study. Heart Rhythm. 2009;6:332–337. doi: 10.1016/j.hrthm.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Takasugi N, Nishigaki K, Kubota T, Takeyama T, Aoyama T, Kawasaki M, Takemura G, Minatoguchi S. Correlation of nocturnal T-wave alternans with the severity of sleep apnea in patients with congestive heart failure [abstract] Eur Heart J. 2008;29(suppl):168. [Google Scholar]
- 54.Andreas S, Clemens C, Sandholzer H, Figulla HR, Kreuzer H. Improvement of exercise capacity with treatment of Cheyne-Stokes respiration in patients with congestive heart failure. J Am Coll Cardiol. 1996;27:1486–1490. doi: 10.1016/0735-1097(96)00024-1. [DOI] [PubMed] [Google Scholar]
- 55.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60:781–785. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha AM, Skobel EC, Breithardt OA, Norra C, Markus KU, Breuer C, Hanrath P, Stellbrink C. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 57.Di Biase L, Gasparini M, Lunati M, Santini M, Landolina M, Boriani G, Curnis A, Bocchiardo M, Vincenti A, Denaro A, Valsecchi S, Natale A, Padeletti L. Antiarrhythmic effect of reverse ventricular remodeling induced by cardiac resynchronization therapy: the InSync ICD (Implantable Cardioverter-Defibrillator) Italian Registry. J Am Coll Cardiol. 2008;52:1442–1449. doi: 10.1016/j.jacc.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 58.Pepin JL, Defaye P, Garrigue S, Poezevara Y, Levy P. Overdrive atrial pacing does not improve obstructive sleep apnoea syndrome. Eur Respir J. 2005;25:343–347. doi: 10.1183/09031936.05.00132703. [DOI] [PubMed] [Google Scholar]
- 59.Guilleminault C, Pool P, Motta J, Gillis AM. Sinus arrest during REM sleep in young adults. N Engl J Med. 1984;311:1006–1010. doi: 10.1056/NEJM198410183111602. [DOI] [PubMed] [Google Scholar]
- 60.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: Gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 62.Becker H, Brandenburg U, Peter JH, Von Wichert P. Reversal of sinus arrest and atrioventricular conduction block in patients with sleep apnea during nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:215–218. doi: 10.1164/ajrccm.151.1.7812557. [DOI] [PubMed] [Google Scholar]
- 63.Josephson ME. Atrial flutter and fibrillation. In: Josephson ME, editor. Clinical Cardiac Electrophysiology Techniques and Interpretations. Philadelphia: Lea & Febiger; 2002. [Google Scholar]
- 64.Mitchell AR, Spurrell PA, Sulke N. Circadian variation of arrhythmia onset patterns in patients with persistent atrial fibrillation. Am Heart J. 2003;146:902–907. doi: 10.1016/S0002-8703(03)00405-8. [DOI] [PubMed] [Google Scholar]
- 65.Singh J, Mela T, Ruskin J. Images in cardiovascular medicine. Sleep (vagal)-induced atrial fibrillation. Circulation. 2004;110:e32–33. doi: 10.1161/01.CIR.0000136810.16068.10. [DOI] [PubMed] [Google Scholar]
- 66.Bettoni M, Zimmerman M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 67.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 68.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 69.Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–1669. doi: 10.1093/eurheartj/ehn214. [DOI] [PubMed] [Google Scholar]
- 70.Otto ME, Belohlavek M, Romero-Corral A, Gami AS, Gilman G, Svatikova A, Amin RS, Lopez-Jimenez F, Khandheria BK, Somers VK. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298–1302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 71.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 72.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:43–49. [PubMed] [Google Scholar]
- 73.Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- 74.Schechtman VL, Harper RK, Harper RM. Aberrant temporal patterning of slow-wave sleep in siblings of SIDS victims. Electroencephalogr Clin Neurophysiol. 1995;94:95–102. doi: 10.1016/0013-4694(94)00263-k. [DOI] [PubMed] [Google Scholar]
- 75.Pincus SM, Cummins TR, Haddad GG. Heart rate control in normal and aborted-SIDS infants. Am J Physiol. 1993;264:R638–R646. doi: 10.1152/ajpregu.1993.264.3.R638. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz PJ, Stramba-Badiale M, Segantini A, Austoni P, Bosi G, Giorgetti R, Grancini F, Marni ED, Perticone F, Rosti D, Salice P. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 77.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz PJ, Crotti L. Ion channel diseases in children: manifestations and management. Curr Opin Cardiol. 2008;23:184–191. doi: 10.1097/HCO.0b013e3282fcc2e3. [DOI] [PubMed] [Google Scholar]
- 79.Weintraub RG, Gow RM, Wilkinson JL. The congenital long QT syndromes in childhood. J Am Coll Cardiol. 1990;16:674–680. doi: 10.1016/0735-1097(90)90359-w. [DOI] [PubMed] [Google Scholar]
- 80.Mache CJ, Beitzke A, Haidvogl M, Gamillscheg A, Suppan C, Stein JI. Perinatal manifestations of idiopathic long QT syndrome. Pediatr Cardiol. 1996;17:118–121. doi: 10.1007/BF02505096. [DOI] [PubMed] [Google Scholar]
- 81.Klonoff-Cohen HS, Edelstein SL. A case-control study of routine and death scene sleep position and sudden infant death syndrome in Southern California. JAMA. 1995;273:790–794. [PubMed] [Google Scholar]
- 82.Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, Srinivasan IP, Kaegi D, Chang JC, Wiley KJ. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA. 1995;273:795–798. doi: 10.1001/jama.1995.03520340051035. [DOI] [PubMed] [Google Scholar]
- 83.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 84.Nademanee K, Veerakul G, Mower M, Likittanasombat K, Krittayapong R, Bhuripanyo K, Sitthisook S, Chaothawee L, Lai MY, Azen SP. Defibrillator Versus Beta-Blockers For Unexplained Death in Thailand (DEBUT): A randomized clinical trial. Circulation. 2003;107:2221–2226. doi: 10.1161/01.CIR.0000066319.56234.C8. [DOI] [PubMed] [Google Scholar]
- 85.Wichter T, Matheja P, Eckardt L, Kies P, Schafers K, Schulze-Bahr E, Haverkamp W, Borggrefe M, Schober O, Breithardt G, Schafers M. Cardiac autonomic dysfunction in Brugada syndrome. Circulation. 2002;105:702–706. doi: 10.1161/hc0602.103677. [DOI] [PubMed] [Google Scholar]
- 86.Kies P, Wichter T, Schafers M, Paul M, Schafers KP, Eckardt L, Stegger L, Schulze-Bahr E, Rimoldi O, Breithardt G, Schober O, Camici PG. Abnormal myocardial presynaptic norepinephrine recycling in patients with Brugada syndrome. Circulation. 2004;110:3017–3022. doi: 10.1161/01.CIR.0000146920.35020.44. [DOI] [PubMed] [Google Scholar]
- 87.Krittayaphong R, Veerakul G, Bhuripanyo K, Jirasirirojanakorn K, Nademanee K. Heart rate variability in patients with sudden unexpected cardiac arrest in Thailand. Am J Cardiol. 2003;91:77–81. doi: 10.1016/s0002-9149(02)03003-5. [DOI] [PubMed] [Google Scholar]
- 88.Bauer A, Malik M, Schmidt G, Barthel P, Bonnemeier H, Cygankiewicz I, Guzik P, Lombardi F, Muller A, Oto A, Schneider R, Watanabe M, Wichterle D, Zareba W. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol. 2008;52:1353–1365. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]


