Abstract
Taxanes (paclitaxel and docetaxel) are currently used to treat ovarian, breast, lung, and head and neck cancers. Despite its clinical success taxane-based treatment could be significantly improved by identifying those patients whose tumors are more likely to present a clinical response. In this mini-review we discuss the accumulating evidence indicating that the breast and ovarian cancer susceptibility gene product BRCA1 mediates cellular response to taxanes. We review data from in vitro, animal, and clinical studies, and discuss them in context of response to therapy. We argue that levels of BRCA1 in tumors may provide a predictive marker for the response to treatment with taxanes. In addition, the study of the role of BRCA1 in the mechanism of action of taxanes might reveal alternative approaches to avoid resistance.
Keywords: Taxol, taxane, BRCA1, cancer, biomarker, microtubule, tubulin, chemotherapy
Isolation and characterization of Taxol
Isolation of Taxol from the bark of the pacific yew tree, Taxus brevifolia, permanently changed the map of cancer treatment and research. Enthusiasm surrounding this finding ran high in both chemical and biological circles as the taxane ring proved to be a novel structure with anti-cancer properties (Fig. (1))[1]. Because of Taxol’s wide spectrum of anti-tumor activity, the need to understand how this compound worked became a priority. It was not until the early 1970’s when Susan Horwitz and colleagues reported that Taxol inhibited cell division of exponentially growing HeLa cells at low concentrations with no secondary effects in nucleic acid metabolism or protein synthesis [2]. Using in vitro microtubule assembly assays, they showed that, contrary to previous plant-derived compounds, such as colchicines which inhibit microtubule assembly, Taxol promoted and stabilized microtubule assembly (rendering cells into late G2/M blockage)[2]. Taxol was also shown to be effective in blocking cell replication in mouse fibroblasts and inhibiting 3T3 fibroblasts migration, indicating that the Taxol-microtubule interaction could have an impact in several morphological and physiological processes critical for cell survival, migration, and replication [3].
Fig. 1.
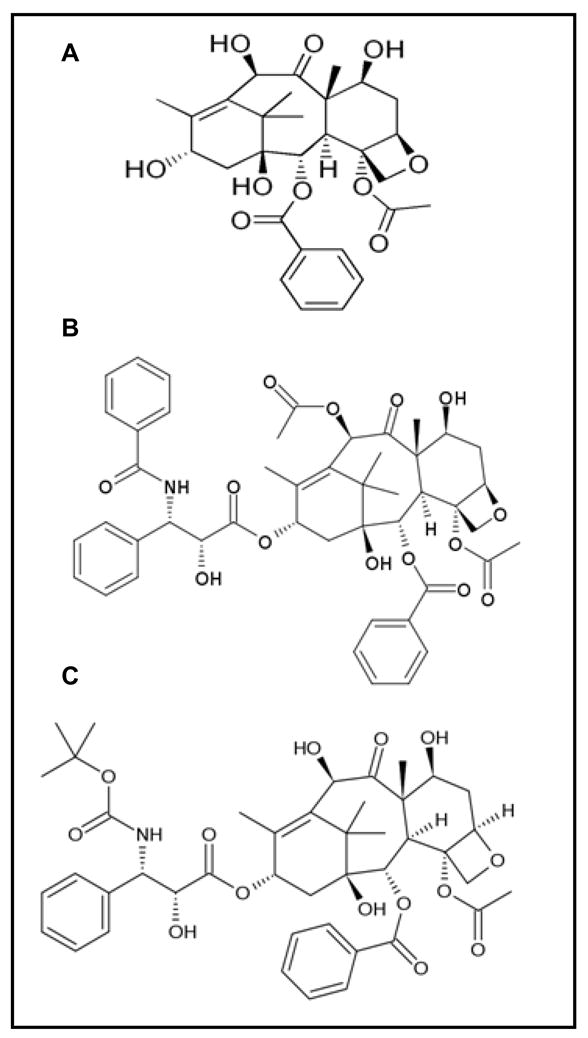
Structural formulas for A (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)- 12b-(Acetyloxy)-12-(benzoyloxy)- 1,2a,3,4,4a,6,9,10,11,12,12a,12b- dodecahydro-4,6,9,11-tetrahydroxy- 4a,8,13,13-tetramethyl-7,11-methano- 5H-cyclodeca(3,4)benz(1,2-b) oxet-5-one, 10-deacetylbaccatin (10-DAB), B (2α,4α,5β,7β,10β,13α)-4, 10-bis(acetyloxy)-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5, 20-epoxytax-11-en-2-yl benzoate, Taxol (paclitaxel) and C (2R,3S)-N-carboxy-3-phenylisoserine, N-tert-butyl ester, 13-ester with 5, 20-epoxy-1, 2, 4, 7, 10, 13-hexahydroxytax-11-en-9-one 4-acetate 2-benzoate, trihydrate, Taxotere (docetaxel).
Microtubules, considered to be the main component of the cellular skeleton, are composed of heterodimers of α and β-tubulin which are ~40% homologous. An abundance of isotype forms for both α and β co-exist in the cell, which undergo several post-translational modifications [4]. Each subunit has a guanine tri-phosphate (GTP) nucleotide binding site and hydrolysis only occurs on GTP bound to the β-subunit during microtubule assembly [4]. The precise mechanism by which Taxol interacts with microtubules was characterized in detail using photoaffinity Taxol analogues. This approach mapped the region to the β-tubulin subunit in which Taxol binding occurs [5–7]. The predicted region was later demonstrated to be in agreement with the crystal structure of the α,β-tubulin dimer at 3.7 Å resolution [8]. This was further corroborated by functional studies that demonstrated Taxol-resistant human ovarian cancer cells and Chinese hamster ovarian cells had β-tubulin mutations effectively compromising Taxol driven polymerization [9,10].
Clinical Success of Taxanes
Taxanes have been well incorporated in adjuvant and neoadjuvant chemotherapeutic drug regimens given to patients with operable or metastatic breast cancer [11]. Several phase III trials have addressed the effects of using either paclitaxel or docetaxel (Fig. (1)) in combination with typical first line treatment protocols such as 5-fluorouracil (5-FU), doxorubicin, and cyclophosphamide. In cases of paclitaxel treatment used in adjuvant therapy, significant increases in disease-free survival (DFS) were recorded for up to six years post treatment [12–14]. Docetaxel given in combination with doxorubicin and cyclophosphamide as adjuvant therapy showed marked decreases in DFS as compared to 5-FU/doxorubicin/cyclophosphamide in the Breast Cancer International Research Group phase III study [15]. However, using docetaxel as a neoadjuvant to surgery following treatment with doxorubicin/cyclophosphamide showed improved clinical and pathological complete response rates, as well as increased overall clinical response rates [16]. A similar result was also observed in a University of Aberdeen phase II trial that looked at neoadjuvant treatment of locally advanced breast cancer with anthracycline therapy in combination with docetaxel; significant results were accomplished establishing a basis for using docetaxel as standard neoadjuvant therapy [17].
Retrospective evaluation of HER2 status in patients was undertaken by The Cancer and Leukemia Group B (CALGB) trial, and their findings indicated increased benefits from treatment when patients were found to have HER2-positive tumor cells versus HER2-negative patients [18]. Further, specific molecular subtyping of HER2 has proven beneficial to predicting efficacy of treatment as shown in CALGB 9344 and CALGB 9741 trials [18]. Inasmuch as molecular subtyping of breast cancer cells by their HER2 or Estrogen Receptor (ER) status may provide useful diagnostic data to the clinician, the challenge to identify additional predictors of treatment response presents the next phase in realizing the goal of personalized medicine.
The use of taxanes in several additional types of cancers has also been studied, namely non-small cell lung cancer (NSCLC), ovarian cancer, and head and neck squamous cell carcinoma (HNSCC). Phase II studies have examined the use of docetaxel in combination with gemcitabine as a second line treatment in NSCLC with promising results [19]. In a randomized, open-label, phase III trial comparing treatment with the standard first line platinum containing regimen (paclitaxel plus carboplatin) versus a platinum-free experimental regimen (triplet of vinorelbine, gemcitabine, and docetaxel), researchers found treatment efficacy to be equivalent with unique toxicity profiles [20]. No superiority over either treatment was recorded in this study thus allowing for greater options to patients [20,21]. Taxane-based chemotherapy has also become the first line treatment in ovarian cancer [22]. Studies have shown a marked improvement in progression-free survival rates of patients treated with a combination of taxane (paclitaxel or docetaxel) and cisplatin over cisplatin/cyclophosphamide [23,24]. These types of combination therapy approaches have become the cornerstone of treating cisplatin-resistant ovarian cancer [25].
The treatment of HNSCC with docetaxel as induction chemotherapy has also been established in clinical trials. Combinations of docetaxel and cisplatin have been shown to exhibit upwards of fifty-three percent overall response rates in these patients [26]. More recently a randomized phase III trial compared docetaxel, cisplatin, and 5-fluorouracil (5-FU) versus cisplatin and 5-FU alone (both groups receiving follow-up chemoradiotherapy). The results showed significantly higher overall response rates in the taxane comprising treatment group versus cisplatin and 5-FU alone (72% versus 64%, respectively)[27]. Taken together, these data serve to illustrate the establishment of taxanes in treatment of many types of cancers as a standard chemotherapeutic option.
Although we continue to learn a great deal about taxane response in past and present clinical trials, continuous improvement in determining drug efficacy either as a single agent or as an adjuvant therapy exists. Chemoresistance determinants [11], new drug formulations and the search of derivatives with better therapeutic index [28] are good examples of areas in which improvements could be achieved. In addition, identifying markers in tumors prior to treatment could help researchers and clinicians in designing future clinical trials with higher predicted response rates. For example, it has been demonstrated that inhibition of Aurora Kinase A in conjunction with paclitaxel treatment synergistically enhanced apoptosis induction in HNSCC cells and xenografts [29]. Based on the current literature, we propose that the breast and ovarian cancer susceptibility gene 1 (BRCA1) protein could be a potential predictive marker for taxane treatment. Furthermore, understanding the role of BRCA1 in taxane response could aid in streamlining the clinical approach to improved chemotherapeutic therapy.
BRCA1
BRCA1 [OMIM #113705] was mapped to chromosome 17q21 and isolated by positional cloning in 1994 as a breast and ovarian cancer susceptibility gene [30–33]. BRCA1 germline mutations have been attributed to a considerable increase in the risk of developing breast (56–80% versus 11% in the general population) and ovarian cancer (15–60% versus 1.4–2.5%) with early onset of the disease [33]. BRCA1 is rarely mutated in sporadic breast cancers but epigenetic inactivation of BRCA1 has been documented in high grade sporadic tumors suggesting that it also plays a role in non-familial cases [34,35]. The gene encodes a nuclear phosphoprotein that plays a role in a number of different biological processes such as DNA damage response, cell cycle control, and regulation of transcription, but it is not yet clear which molecular function(s) are major contributors to the gene’s tumor suppressive activity (reviewed in refs. [36–40]).
BRCA1 and Resistance to Microtubule-Disrupting Drugs
Cells lacking BRCA1 are prone to apoptosis and are more sensitive to DNA damaging agents [41–42]. Conversely, a series of experiments have suggested that low levels of BRCA1 in cell lines correlate with resistance to taxanes and vinca alkaloids (Table 1)[42–47]. While this proved true for breast cancer cell lines, it was not observed in one ovarian cancer cell line [48] and no clear sensitivity differences were found in lymphocytes from BRCA1 mutation carriers [49]. Thus, cell culture experiments suggest that BRCA1 is required for sensitivity to microtubule poisons but this may vary with gene dosage as well as with cell type.
Table 1.
Sensitivity to microtubule-interfering drugs in cell lines
| Cells | BRCA1 status | IC50 | Drug [exposure time] & assay used |
|---|---|---|---|
| Comparison across breast cancer cell lines | |||
| HCC1937 | ERneg, one deleted allele and one C- terminally truncated allele. | >2μM | Paclitaxel [48h] & MTT (Tassone et al. 2003; Ref. 45) |
| MCF-7 | ERpos, hemizygous for wild type BRCA1 | 0.1–0.2 μM | |
| MDA-MB231 | Homozygous for wt BRCA1 | 10–20 nM | |
| Comparison across isogenic breast cancer cell lines | |||
| HCCBR116 | HCC1937 transfected with wt BRCA1 | 7.73 nM | Paclitaxel [72h] & MTT (Gilmore et al. 2004; ref. 43). |
| HCCEV1 | HCC1937 transfected with empty vector | 6.21 μM | |
| MBR62-bcl2 | MCF-7 derivative stably transfected with tet-inducible wt BRCA1 (induced) | 7.7 nM | Paclitaxel [72h] & cell counting (Quinn et al. 2003; ref. 42). |
| MBR62-bcl2 | MCF-7 derivative stably transfected with tet-inducible wt BRCA1 (non-induced) | 96.4 pM | |
| T47D | T47D (BRCA1+/+ and ERpos) transfected with a control siRNA | 2.2 μM | Paclitaxel [72h] & cell counting (Quinn et al. 2003; ref. 42). |
| T47D | T47D transfected with a BRCA1 siRNA | >0.1 mM | |
| HCCBR116 | HCC1937 transfected with wt BRCA1 | 7.7 nM | Paclitaxel [72h] & cell counting (Quinn et al. 2003; ref. 42). |
| HCCEV1 | HCC1937 transfected with empty vector | 6.2 μM | |
| HCCBR116 | 1.9 nM | Vinorelbine [72h] & cell counting (Quinn et al. 2003; ref. 42). | |
| HCCEV1 | 17 μM | ||
| HCCBR18 | HCC1937 transfected with wt BRCA1 | 0.3 nM | Paclitaxel [72h] & cell counting (Quinn et al. 2003; ref. 42). |
| HCCEV2 | HCC1937 transfected with empty vector | 1.6 μM | |
| HCCBR mix | HCC1937 transfected with wt BRCA1 | 1.5 nM | Paclitaxel [72h] & cell counting (Quinn et al. 2003; ref. 42). |
| HCCEV3 | HCC1937 transfected with empty vector | 10.7 μM | |
| MCF-7 | MCF-7 transfected with a control siRNA | 36 nM* | Paclitaxel [72h] & WST-1 cleavage (Chabalier et al. 2006; ref. 46). *IC25 shown. |
| MCF-7 | MCF-7 transfected with a BRCA1 siRNA | 4.1 nM* | |
The evidence highlighting the role of BRCA1 in taxane sensitivity is not limited to studies with cell lines. In a K14cre;Brca1F/F;p53F/F mouse (targeted deletion of Brca1 and p53 in the mammary gland) spontaneous tumors became invariably resistant to docetaxel but not to cisplatin [50]. Acquired resistance in this case might be due to increased drug elimination via upregulated transport proteins. But even if this is the case, one can imagine that by targeting tumors that are more sensitive (high BRCA1 levels) will preclude the accumulation of genetic or epigenetic changes needed to develop resistance. A different mouse model in which ovarian explants from K5-TVA;Brca1lox/lox;p53lox/lox (targeted deletion of Brca1 and p53 combined with the expression of the avian receptor TVA)[51] are cotransduced ex vivo with Cre and Myc, and injected subcutaneously in nude mice illustrate once again potential cell type differences. When treated in vitro with paclitaxel, Brca1−/− or Brca1+/+ cell lines showed comparable sensitivity [52].
The Mechanism of BRCA1 Response
Although the mechanism by which cells require BRCA1 to respond to taxanes is largely unknown, three modes of action have been proposed and none of them are mutually exclusive. The first mechanism is a differential apoptotic response; the second confers a requirement in spindle-assembly checkpoint; and the third provides a role in centrosome-mediated microtubule stability.
It has been proposed that resistance of BRCA1-deficient cells to taxanes is correlated to a defective apoptotic response but it has not yet been formally demonstrated [42]. The induction of ectopic BRCA1 expression led to an increased sensitivity to paclitaxel in a derivative of the human mammary carcinoma cell line MDA435 when compared to a non-induced control. Treatment triggered cell cycle arrest in G2/M phase and a dramatic increase in apoptosis, with concomitant induction at the transcriptional level of GADD45 by BRCA1 [44]. Interestingly, treatment of A375 and DLD1 cells (melanoma and colon cancer, respectively) with paclitaxel and/or docetaxel also causes transcriptional induction of GADD45 (DeLigio and Zorio, unpublished data).
A rybozyme-based strategy to inhibit expression of BRCA1 in breast epithelial HBL100 cells caused decreased sensitivity to mitotic-spindle poisons Taxol and vincristine [47]. Upon treatment with Taxol there was a strong increase in JNK phosphorylation and subsequent apoptosis in BRCA1-expressing versus non-expressing cells. This observation was consistent with previous data showing that BRCA1 can enhance apoptosis through a pathway involving H-Ras, MEKK4, JNK, and activation of caspases 8 and 9 [53].
By stabilizing microtubules, paclitaxel disrupts mitotic spindle assembly and triggers the spindle checkpoint [54]. BRCA1 participation in spindle assembly checkpoint signaling is confirmed by the experiments that show targeted knockdown of BRCA1 in MCF7 cells increases resistance to paclitaxel. MCF7 cells exhibited premature sister chromatid separation after treatment with the drug suggesting that the role of BRCA1 in the response to paclitaxel may need spindle assembly checkpoint signaling [46]. This hypothesis is supported by the fact that BRCA1 is important for transcriptional regulation of spindle assembly checkpoint proteins BUBR1 [46] and MAD2 [55]. These proteins (BUBR1, MAD2, and BRCA1) have a critical role in mitotic microtubule organization and spindle pole assembly in Xenopus egg extracts and cultured mammalian HeLa cells. The BRCA1/BARD1 heterodimer controls efficient transport to the spindle poles of microtubule associated protein TPX2 [56]. This function was partially dependent upon BRCA1/BARD1 ubiquitin ligase activity. Among the phenotypes observed in BRCA1-depleted cells were compromised mitotic exit, chromosome segregation defects, and micronucleus formation [56]. Defects in the spindle checkpoint have been associated with resistance to taxanes [57,58]. Thus, it is conceivable that BRCA1 also modulates taxane sensitivity through its effects on the spindle checkpoint. A completely inactive checkpoint seems to be lethal, while a weakened (signal produced but not sustained) checkpoint leads to chromosome instability [59]. Absent or low levels of BRCA1 may act as a hypomorphic mutation and allow for a defective or attenuated spindle assembly checkpoint.
In addition to its role in the spindle-assembly checkpoint, BRCA1 is also involved in the regulation of centrosome function [60]. The hypophosphorylated form of BRCA1 has been found associated with centrosomes during mitosis [61]. BRCA1 (amino acids 504–803) interacts with γ-tubulin, and over expression of this fragment causes accumulation of mitotic cells with multiple centrosomes and abnormal spindles; which in turn interferes with cell growth and induces apoptosis in COS7 cells [62].
Transient inhibition of BRCA1 in cells derived from mammary tissues also leads to amplification and fragmentation of centrosomes [63]. Important activity relationships have been divulged in vitro regarding ubiquitination of centrosomal proteins by BRCA1/BARD1. Specifically, this protein complex monoubiquitinates γ-tubulin at lysine residues 48 and 344. Individually mutating γ-tubulin to abolish BRCA1 ubiquitination at these residues has shown BRCA1 controls both centrosome duplication and its microtubule nucleation properties in different stages of the cell cycle [64].
BRCA1 as a Marker for Clinical Outcome
Despite the evidence from in vitro studies showing a trend towards cells that have no BRCA1 (or express lower BRCA1 levels) being more resistant to taxanes, a positive correlation in clinical studies would prove noteworthy. In the few studies published so far focusing on breast, ovarian, and most recently, lung cancer, a case for this relationship is starting to emerge.
Absence of BRCA1 expression in primary tumors of metastatic breast cancer patients who were treated with taxanes was identified as an independent predictor of shorter time to progression, although there was no clear correlation with clinical tumor response [65]. On the other hand, a study in primary breast cancers failed to see a correlation between BRCA1 levels (grouped as either high or low expressers) and resistance to docetaxel [66]. However, in a study comparing BRCA1 germ-line mutation carriers and non-carriers, response rates to neoadjuvant docetaxel treatment in the carrier group was limited while non-carriers showed a high number of complete or partial responses [67].
Patients with familial ovarian cancers (which included carriers and non-carriers of the 5382insC founder truncating mutation in BRCA1) responded less favorably to treatment with paclitaxel and cisplatin or carboplatin than patients with sporadic tumors [68]. In addition, a recent study in sporadic ovarian cancer patients provided evidence that BRCA1 mRNA expression levels can be used as a predictive marker of survival. The overall median survival for high BRCA1 expressing patients was increased after taxane-containing chemotherapy [69].
A detailed review of the clinical studies described in the previous paragraphs reveal important limitations in design and methodology. They all have small sample sizes which may lack sufficient power to detect effects. Many do not provide patient genotyping information and in many cases treatment combined several other classes of chemotherapy drugs with taxanes. More importantly, they vary widely in how BRCA1 status was determined (e.g. quantitative RT-PCR or immunohistochemistry) and how differences in levels are accounted for (e.g. continuous or discrete). Nevertheless, those limitations are more likely to underestimate differences. Thus, the correlative clinical results, combined with the in vitro data, provide a solid starting point for the hypothesis the BRCA1 can be used as a marker of clinical outcome after treatment with taxanes.
Low BRCA1 protein expression, due to promoter methylation, was shown to be a common feature in NSCLC samples [70]. Researchers found that patients subjected to neoadjuvant therapy with gemcitabine/cisplatin whose tumors expressed low levels of BRCA1 mRNA had a better outcome than those expressing high levels [71]. In conflict to these results, one study of NSCLC patients treated with gemcitabine/cisplatin or epirubicin/gemcitabine did not show any predictive value using comparisons of BRCA1 levels in the tumors as measured by immunohistochemistry [72]. However, analysis of mRNA expression levels in metastatic malignant effusions from NSCLC patients revealed BRCA1 expression level as positively correlated to docetaxel sensitivity [73]. Finally, overexpression of BRCA1 mRNA was strongly associated with poor survival (Hazard Ratio: 1.98; 95% confidence interval 1.11-6) in chemonaive NSCLC patients [74]. Thus, although these studies also suffer from limitations (e.g. using mRNA measurements without also analyzing protein levels), they provide preliminary evidence supporting the role of BRCA1 status as a marker for outcome in lung cancer. In light of this evidence, the Spanish Lung Cancer Group has initiated a BRCA1 Expression Customization (BREC) study to test the usefulness of BRCA1 in current and future customized therapy of NSCLC [75]. This exploratory evaluation attempts to expose BRCA1 as a potential genetic marker given the hypothesis that low BRCA1 levels correlate with increased sensitivity to DNA damaging agents such as cisplatin. The results of the BREC study regarding time to progression of disease and BRCA1 levels will be highly anticipated in that its implications reach toward a targeted and efficient approach to chemotherapy with taxanes and/or DNA damaging agents in NSCLC.
Conclusion
BRCA1 plays an important role in the cell’s response to chemotherapy. In the past few years several lines of evidence have indicated that the status of BRCA1 protein influences the ability of cells to respond to agents that cause DNA damage. Recently, data has emerged suggesting that the status of BRCA1 may also influence response to agents that do not cause direct DNA damage, such as microtubule inhibitors. If this role of BRCA1 is confirmed, BRCA1 may represent an ideal biomarker with the ability to predict response to a wide array of agents currently used in cancer therapy.
Acknowledgments
Work in the Monteiro Lab is supported by a DoD predoctoral fellowship BC083181 (A.V.), NIH award CA92309, a grant from the Florida Breast Cancer Coalition Foundation, and pilot funds from Lung SPORE P50-CA119997. The authors acknowledge Drs. Robert Holton and Mike Edler for critical review of this manuscript.
ABBREVIATIONS
- 5-FU
5-fluorouracil
- 10-DAB
10-deacetylbaccatin
- BARD1
BRCA1 associated RING domain 1
- BMS
Bristol-Meyers Squibb
- BRCA1
breast and ovarian cancer susceptibility gene 1
- DFS
disease-free survival
- ER
estrogen receptor
- GTP
guanine tri-phosphate
- HER2
human epidermal growth factor receptor 2
- HNSCC
head and neck squamous cell carcinoma
- JNK
Jun N-terminal kinase
- NSCLC
non-small cell lung cancer
- RT-PCR
reverse transcriptase polymerase chain reaction
Footnotes
Conflicts of interest
No potential conflicts of interest were disclosed by the authors.
Reference List
- 1.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 3.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- 5.Rao S, Krauss NE, Heerding JM, Swindell CS, Ringel I, Orr GA, Horwitz SB. 3′-(p-azidobenzamido)taxol photolabels the N-terminal 31 amino acids of beta-tubulin. J Biol Chem. 1994;269(5):3132–3134. [PubMed] [Google Scholar]
- 6.Rao S, Orr GA, Chaudhary AG, Kingston DG, Horwitz SB. Characterization of the taxol binding site on the microtubule. 2-(m-Azidobenzoyl)taxol photolabels a peptide (amino acids 217–231) of beta-tubulin. J Biol Chem. 1995;270(35):20235–20238. doi: 10.1074/jbc.270.35.20235. [DOI] [PubMed] [Google Scholar]
- 7.Rao S, He L, Chakravarty S, Ojima I, Orr GA, Horwitz SB. Characterization of the Taxol binding site on the microtubule. Identification of Arg(282) in beta-tubulin as the site of photoincorporation of a 7-benzophenone analogue of Taxol. J Biol Chem. 1999;274(53):37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 8.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391(6663):199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 9.Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, Poruchynsky MS. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272(27):17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A beta-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274(34):23875–23882. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- 11.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785(2):96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E, III, Schilsky RL, Wood WC, Muss HB, Norton L. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 13.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 14.Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, Wickerham DL, Yothers G, Soran A, Wolmark N. Paclitaxel After Doxorubicin Plus Cyclophosphamide As Adjuvant Chemotherapy for Node-Positive Breast Cancer: Results From NSABP B-28. J Clin Oncol. 2005;23(16):3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 15.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E, Guevin R, Howell A, Fornander T, Hainsworth J, Coleman R, Vinholes J, Modiano M, Pinter T, Tang SC, Colwell B, Prady C, Provencher L, Walde D, Rodriguez-Lescure A, Hugh J, Loret C, Rupin M, Blitz S, Jacobs P, Murawsky M, Riva A, Vogel C. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 16.Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, Ah-See AK, Eremin O, Walker LG, Sarkar TK, Eggleton SP, Ogston KN. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20(6):1456–1466. doi: 10.1200/JCO.2002.20.6.1456. [DOI] [PubMed] [Google Scholar]
- 18.Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, Broadwater G, Goldstein LJ, Martino S, Ingle JN, Henderson IC, Norton L, Winer EP, Hudis CA, Ellis MJ, Berry DA the Cancer and Leukemia Group. HER2 and Response to Paclitaxel in Node-Positive Breast Cancer. N Engl J Med. 2007;357(15):1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 19.Huang CH, Millenson MM, Sherman EJ, Borghaei H, Mintzer DM, Cohen RB, Staddon AP, Seldomridge J, Treat OJ, Tuttle H, Ruth KJ, Langer CJ. Promising survival in patients with recurrent non-small cell lung cancer treated with docetaxel and gemcitabine in combination as second-line therapy. J Thorac Oncol. 2008;3(9):1032–1038. doi: 10.1097/JTO.0b013e31818307c2. [DOI] [PubMed] [Google Scholar]
- 20.Kubota K, Kawahara M, Ogawara M, Nishiwaki Y, Komuta K, Minato K, Fujita Y, Teramukai S, Fukushima M, Furuse K. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol. 2008;9(12):1135–1142. doi: 10.1016/S1470-2045(08)70261-4. [DOI] [PubMed] [Google Scholar]
- 21.Barlesi F. Lung cancer: moving forward with tailored strategies. Lancet Oncol. 2008;9(12):1116–1117. doi: 10.1016/S1470-2045(08)70290-0. [DOI] [PubMed] [Google Scholar]
- 22.Markman M. Pharmaceutical management of ovarian cancer: current status. Drugs. 2008;68(6):771–789. doi: 10.2165/00003495-200868060-00004. [DOI] [PubMed] [Google Scholar]
- 23.Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, Stuart G, Kaye S, Vergote I, Blom R, Grimshaw R, Atkinson RJ, Swenerton KD, Trope C, Nardi M, Kaern J, Tumolo S, Timmers P, Roy JA, Lhoas F, Lindvall B, Bacon M, Birt A, Andersen JE, Zee B, Paul J, Baron B, Pecorelli S. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92(9):699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 24.Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, Parkin D, Paul J, Hay A, Kaye SB. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96(22):1682–1691. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 25.Stordal B, Pavlakis N, Davey R. A systematic review of platinum and taxane resistance from bench to clinic: an inverse relationship. Cancer Treat Rev. 2007;33(8):688–703. doi: 10.1016/j.ctrv.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Schoffski P, Catimel G, Planting AS, Droz JP, Verweij J, Schrijvers D, Gras L, Schrijvers A, Wanders J, Hanauske AR. Docetaxel and cisplatin: an active regimen in patients with locally advanced, recurrent or metastatic squamous cell carcinoma of the head and neck. Results of a phase II study of the EORTC Early Clinical Studies Group. Ann Oncol. 1999;10(1):119–122. doi: 10.1023/a:1008360323986. [DOI] [PubMed] [Google Scholar]
- 27.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio RC, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM, Jr, Haddad RI. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 28.Ferlini C, Gallo D, Scambia G. New taxanes in development. Expert Opin Investig Drugs. 2008;17(3):335–347. doi: 10.1517/13543784.17.3.335. [DOI] [PubMed] [Google Scholar]
- 29.Mazumdar A, Henderson YC, El-Naggar AK, Sen S, Clayman GL. Aurora kinase A inhibition and paclitaxel as targeted combination therapy for head and neck squamous cell carcinoma. Head Neck. 2008 doi: 10.1002/hed.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 31.Narod SA, Feunteun J, Lynch HT, Watson P, Conway T, Lynch J, Lenoir GM. Familial breast-ovarian cancer locus on chromosome 17q12–q23. Lancet. 1991;338(8759):82–83. doi: 10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- 32.Vogelstein B, Kinzler KW. Has the breast cancer gene been found? . Cell. 1994;79(1):1–3. doi: 10.1016/0092-8674(94)90393-x. [DOI] [PubMed] [Google Scholar]
- 33.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 34.Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Bennett LM, Haugen-Strano A, Swensen J, Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- 35.Wilson CA, Ramos L, Villasenor MR, Anders KH, Press MF, Clarke K, Karlan B, Chen JJ, Scully R, Livingston D, Zuch RH, Kanter MH, Cohen S, Calzone FJ, Slamon DJ. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21(2):236–240. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 36.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 37.Scully R, Livingston D. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature. 2000;408:429–432. doi: 10.1038/35044000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkitaraman AR. Cancer Susceptibility and the Functions of BRCA1 and BRCA2. Cell. 2002;108(2):171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 39.Scully R, Xie A, Nagaraju G. Molecular Functions of BRCA1 in the DNA Damage Response. Cancer Biol Ther. 2004;3(6):521–527. doi: 10.4161/cbt.3.6.842. [DOI] [PubMed] [Google Scholar]
- 40.Lane TF. BRCA1 and transcription. Cancer Biol Ther. 2004;3(6):528–533. doi: 10.4161/cbt.3.6.843. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96(22):1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- 42.Quinn JE, Kennedy RD, Mullan PB, Gilmore PM, Carty M, Johnston PG, Harkin DP. BRCA1 Functions as a Differential Modulator of Chemotherapy-induced Apoptosis. Cancer Res. 2003;63(19):6221–6228. [PubMed] [Google Scholar]
- 43.Gilmore PM, McCabe N, Quinn JE, Kennedy RD, Gorski JJ, Andrews HN, McWilliams S, Carty M, Mullan PB, Duprex WP, Liu ET, Johnston PG, Harkin DP. BRCA1 Interacts with and Is Required for Paclitaxel-Induced Activation of Mitogen-Activated Protein Kinase Kinase Kinase 3. Cancer Res. 2004;64(12):4148–4154. doi: 10.1158/0008-5472.CAN-03-4080. [DOI] [PubMed] [Google Scholar]
- 44.Mullan PB, Quinn JE, Gilmore PM, McWilliams S, Andrews H, Gervin C, McCabe N, McKenna S, White P, Song YH, Maheswaran S, Liu E, Haber DA, Johnston PG, Harkin DP. BRCA1 and GADD45 mediated G2/M cell cycle arrest in response to antimicrotubule agents. Oncogene. 2001;20(43):6123–6131. doi: 10.1038/sj.onc.1204712. [DOI] [PubMed] [Google Scholar]
- 45.Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, Goel A, Barbieri V, Costanzo F, Boland CR, Venuta S. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88(8):1285–1291. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chabalier C, Lamare C, Racca C, Privat M, Valette A, Larminat F. BRCA1 Downregulation Leads to Premature Inactivation of Spindle Checkpoint and Confers Paclitaxel Resistance. Cell Cycle. 2006;5(9):1001–1007. doi: 10.4161/cc.5.9.2726. [DOI] [PubMed] [Google Scholar]
- 47.Lafarge S, Sylvain V, Ferrara M, Bignon YJ. Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene. 2001;20(45):6597–6606. doi: 10.1038/sj.onc.1204812. [DOI] [PubMed] [Google Scholar]
- 48.Zhou C, Smith JL, Liu J. Role of BRCA1 in cellular resistance to paclitaxel and ionizing radiation in an ovarian cancer cell line carrying a defective BRCA1. Oncogene. 2003;22(16):2396–2404. doi: 10.1038/sj.onc.1206319. [DOI] [PubMed] [Google Scholar]
- 49.Trenz K, Lugowski S, Jahrsdorfer U, Jainta S, Vogel W, Speit G. Enhanced sensitivity of peripheral blood lymphocytes from women carrying a BRCA1 mutation towards the mutagenic effects of various cytostatics. Mutat Res. 2003;544(2–3):279–288. doi: 10.1016/j.mrrev.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Rottenberg S, Nygren AOH, Pajic M, van Leeuwen FWB, van der Heijden I, van de Wetering K, Liu X, de Visser KE, Gilhuijs KG, van Tellingen O, Schouten JP, Jonkers J, Borst P. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. PNAS. 2007:0702955104. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1(1):53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xing D, Orsulic S. A Mouse Model for the Molecular Characterization of Brca1-Associated Ovarian Carcinoma. Cancer Res. 2006;66(18):8949–8953. doi: 10.1158/0008-5472.CAN-06-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thangaraju M, Kaufmann SH, Couch FJ. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J Biol Chem. 2000;275(43):33487–33496. doi: 10.1074/jbc.M005824200. [DOI] [PubMed] [Google Scholar]
- 54.Wassmann K, Benezra R. Mitotic checkpoints: from yeast to cancer. Curr Opin Genet Dev. 2001;11(1):83–90. doi: 10.1016/s0959-437x(00)00161-1. [DOI] [PubMed] [Google Scholar]
- 55.Wang RH, Yu H, Deng CX. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. PNAS. 2004;101(49):17108–17113. doi: 10.1073/pnas.0407585101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joukov V, Groen AC, Prokhorova T, Gerson R, White E, Rodriguez A, Walter JC, Livingston DM. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127(3):539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 57.Sudo T, Nitta M, Saya H, Ueno NT. Dependence of Paclitaxel Sensitivity on a Functional Spindle Assembly Checkpoint. Cancer Res. 2004;64(7):2502–2508. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- 58.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3(1):51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 59.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8(1):7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Pujana MA, Han JD, Starita LM, Stevens KN, Tewari M, Ahn JS, Rennert G, Moreno V, Kirchhoff T, Gold B, Assmann V, Elshamy WM, Rual JF, Levine D, Rozek LS, Gelman RS, Gunsalus KC, Greenberg RA, Sobhian B, Bertin N, Venkatesan K, yivi-Guedehoussou N, Sole X, Hernandez P, Lazaro C, Nathanson KL, Weber BL, Cusick ME, Hill DE, Offit K, Livingston DM, Gruber SB, Parvin JD, Vidal M. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat Genet. 2007;39(11):1338–1349. doi: 10.1038/ng.2007.2. [DOI] [PubMed] [Google Scholar]
- 61.Hsu LC, White RL. BRCA1 is associated with the centrosome during mitosis. Proc Natl Acad Sci U S A. 1998;95(22):12983–12988. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu LC, Doan TP, White RL. Identification of a gamma-tubulin-binding domain in BRCA1. Cancer Res. 2001;61(21):7713–7718. [PubMed] [Google Scholar]
- 63.Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP, Gygi SP, Parvin JD. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol Cell Biol. 2004;24(19):8457–8466. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD. Centrosomal Microtubule Nucleation Activity Is Inhibited by BRCA1-Dependent Ubiquitination. Mol Cell Biol. 2005;25(19):8656–8668. doi: 10.1128/MCB.25.19.8656-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurebayashi J, Yamamoto Y, Kurosumi M, Okubo S, Nomura T, Tanaka K, Sonoo H. Loss of BRCA1 expression may predict shorter time-to-progression in metastatic breast cancer patients treated with taxanes. Anticancer Res. 2006 ;26(1B):695–701. [PubMed] [Google Scholar]
- 66.Kim SJ, Miyoshi Y, Taguchi T, Tamaki Y, Nakamura H, Yodoi J, Kato K, Noguchi S. High Thioredoxin Expression Is Associated with Resistance to Docetaxel in Primary Breast Cancer. Clin Cancer Res. 2005;11(23):8425–8430. doi: 10.1158/1078-0432.CCR-05-0449. [DOI] [PubMed] [Google Scholar]
- 67.Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Narod SA, Lubinski J. Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat. 2008;108(2):289–296. doi: 10.1007/s10549-007-9600-1. [DOI] [PubMed] [Google Scholar]
- 68.Wojciechowska-Lacka A, Markowska J, Skasko E, Kruczek A, Steffen J. Frequent disease progression and early recurrence in patients with familial ovarian cancer primarily treated with paclitaxel and cis- or carboplatin (preliminary report) Eur J Gynaecol Oncol. 2003;24(1):21–24. [PubMed] [Google Scholar]
- 69.Quinn JE, James CR, Stewart GE, Mulligan JM, White P, Chang GKF, Mullan PB, Johnston PG, Wilson RH, Harkin DP. BRCA1 mRNA Expression Levels Predict for Overall Survival in Ovarian Cancer after Chemotherapy. Clin Cancer Res. 2007;13(24):7413–7420. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- 70.Lee MN, Tseng RC, Hsu HS, Chen JY, Tzao C, Ho WL, Wang YC. Epigenetic Inactivation of the Chromosomal Stability Control Genes BRCA1, BRCA2, and XRCC5 in Non-Small Cell Lung Cancer. Clin Cancer Res. 2007;13(3):832–838. doi: 10.1158/1078-0432.CCR-05-2694. [DOI] [PubMed] [Google Scholar]
- 71.Taron M, Rosell R, Felip E, Mendez P, Souglakos J, Ronco MS, Queralt C, Majo J, Sanchez JM, Sanchez JJ, Maestre J. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet. 2004;13(20):2443–2449. doi: 10.1093/hmg/ddh260. [DOI] [PubMed] [Google Scholar]
- 72.Wachters FM, Wong LSM, Timens W, Kampinga HH, Groen HJM. ERCC1, hRad51, and BRCA1 protein expression in relation to tumour response and survival of stage III/IV NSCLC patients treated with chemotherapy. Lung Cancer. 2005;50(2):211–219. doi: 10.1016/j.lungcan.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Wei J, Qian X, Yin H, Zhao Y, Yu L, Wang T, Liu B. ERCC1 and BRCA1 mRNA expression levels in metastatic malignant effusions is associated with chemosensitivity to cisplatin and/or docetaxel. BMC Cancer. 2008 ;8(1):97. doi: 10.1186/1471-2407-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A, Rzyman W, Puma F, Kobierska-Gulida G, Farabi R, Jassem J. BRCA1: A Novel Prognostic Factor in Resected Non-Small-Cell Lung Cancer. PLoS ONE. 2007;2(11):e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reguart N, Cardona AF, Carrasco E, Gomez P, Taron M, Rosell R. BRCA1: a new genomic marker for non-small-cell lung cancer. Clin Lung Cancer. 2008;9(6):331–339. doi: 10.3816/CLC.2008.n.048. [DOI] [PubMed] [Google Scholar]


