Abstract
Human papillomavirus (HPV) type 16 infects the epithelial layer of cervical mucosa and is causally associated with the generation of cervical cancer. Langerhans cells (LC) are the resident antigen-presenting cells at the site of infection and therefore are responsible for initiating an immune response against HPV16. On the contrary, LC exposed to HPV16 do not induce a specific T cell immune response, which leads to the immune evasion of HPV16. Demonstrating that Toll-like receptor 7 (TLR7) and TLR8 are expressed on human LC, we hypothesized that imidazoquinolines would activate LC exposed to HPV16, leading to the induction of an HPV16-specific cell-mediated immune response. Surprisingly both phenotypic and functional hallmarks of activation are not observed when LC are exposed to HPV16 virus-like particles (VLP) and treated with imiquimod (TLR7 agonist). However, we found that LC are activated by 3M-002 (TLR8 agonist) and resiquimod (TLR8/7 agonist). LC exposed to HPV16 VLP and subsequently treated with 3M-002 or resiquimod highly up-regulate surface activation markers, secrete pro-inflammatory cytokines and chemokines, induce CCL21-directed migration, and initiate an HPV16-specific CD8+ T cell response. These data strongly indicate that 3M-002 and resiquimod are promising therapeutics for treatment of HPV-infections and HPV-induced cervical lesions.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
Keywords: Human, Dendritic Cells, Viral, Cell Activation, Antigen Presentation
Introduction
High-risk human papillomaviruses (HPV) are causally linked to the generation of cervical cancer (1, 2). Cervical cancer is the second most common cancer among women worldwide, killing approximately a quarter of a million women each year (3). Merck has developed the first prophylatic HPV vaccine, Gardasil. It has been demonstrated in phase III clinical trials to be 100% effective, in preventing high-grade cervical lesions associated with HPV16 and HPV18 (4, 5). Despite the success of Gardasil, it has been predicted that it will take decades to detect a quantifiable effect on cervical cancer rates in the population (6, 7). Additionally, Gardasil will not aid in treating the hundreds of millions of women that are currently infected with high-risk HPV. The majority of women infected with HPV clear the virus, however the average time for clearance is close to a year (8, 9). Conversely about 15% of women that have high-risk HPV infections cannot initiate an effective immune response against HPV and persistence of high-risk HPV infection is a major risk factor in the development of cervical cancer (10, 11). The slow clearance rate and lack of an effective immune response indicates that HPV is escaping immune detection (12). Thus, therapeutic treatments are necessary to treat existing high-risk HPV infections.
High-risk HPV infects the epidermal layer of the mucosa where Langerhans cells (LC) are the primary APC. The principal functions of APC are recognition, internalization, processing, transport, and presentation of antigens to unprimed T cells in the lymph node (LN) (13-17). Since LC are the only APC that HPV will come into contact with during an infection they are responsible for initiating a cell-mediated immune response against HPV. However, we previously demonstrated that human LC do not initiate a specific anti-HPV16 CD8+ T cell response after exposure to chimeric HPV16L1L2-E7 virus-like particles (HPV16 cVLP) (18, 19). Additionally, LC exposed to HPV16L1L2 virus-like particles (HPV16 VLP) appear to have a tolerizing phenotype, cross-presenting HPV peptides on MHC molecules in the absence of surface markers important for T cell co-stimulation and migration, including CD80, CD86 and CCR7, and without secretion of pro-inflammatory cytokines. The molecular mechanism mediating this immune escape process is the activation of PI3K in LC (18-20). As a result, HPV can evade the immune system leading to the delay or absence of viral clearance.
As we demonstrate here, LC express TLR7 and TLR8 thus a potential therapy of HPV16 induced lesions would be to activate HPV16 infected LC using synthetic imidazoquinolines (imiquimod, resiquimod, 3M-002 and 3M-031). Imidazoquinolines are TLR7 and/or TLR8 agonists and therefore are potent innate immune modulators (Table 1, (21)). TLR7 and TLR8 are localized to endosomal membranes and naturally recognize ssRNA (21, 22). Once TLR7 and/or TLR8 are engaged, NF-κB and other transcription factors are activated, leading to the transcription of immune response related genes, including cytokine, chemokine, co-stimulatory marker, and adhesion molecule genes (21, 23, 24). Moreover, imidazoquinolines demonstrate antiviral and antitumor activity through cytokines and chemokines, such as TNF-α, IL-6, IL-8, IL-12, and interferon-inducible protein-10 (IP-10), produced by dendritic cells (DC) and macrophages (21, 25-29).
Table I.
Synthetic imidazoquinolines and the respective receptor(s) they bind and act through.
| Imidazoquinoline | Agonist Receptor(s) |
|---|---|
| 3M-006 | Inactive Analog (TLR7/8) |
| 3M-002 | TLR8 |
| Imiquimod | TLR7 |
| Resiquimod | TLR8/7 |
| 3M-031 | TLR7/8 |
Confirming that TLR7 and TLR8 are expressed on LC we hypothesized that synthetic imidazoquinolines would activate LC previously exposed to HPV16, leading to the induction of an HPV16 specific immune response. Our results indicate that select imidazoquinolines, TLR8 dominant agonists, are promising therapeutic drugs that could potentially be used as a treatment for HPV infections and HPV-induced cervical lesions by inducing an anti-HPV specific cell-mediated immune response via the activation of HPV infected LC.
Materials and Methods
Antibodies and Agonists
The antibodies recognizing conformational HPV16 L1 epitopes (H16.V5, H16.E70) or linear HPV16 L1 epitopes (Camvir-1, H16.D9, H16.H5) were gifts from Neil Christensen (Penn State, Hershey, PA), except Camvir-1, which was purchased from BD Biosciences (San Jose, CA). Polyclonal serum (DK44214) recognizing HPV16 L2 was a gift from John Schiller (National Institutes of Health, Bethesda, MD). The antibodies to human CD197 (CCR7)-PE; CD1a-PE, CD80-FITC; CD86-FITC; HLA-DR, DQ, DP-FITC; HLA-A, B, C-FITC; isotype controls; biotinylated anti-rabbit IgG; streptavidin-PE; and streptavidin-HRP were purchased from BD Biosciences. The antibody to human CD207 (langerin) was purchased from Immunotech (Marseille, France) while the anti-human E-cadherin antibody was purchased from Millipore (Temecula, CA). Anti-human TLR7 and anti-human TLR8-PE were purchased from Abcam (Cambridge, MA). Goat anti-rabbit-HRP was purchased from Biosource (Carlsbad, CA). Anti-human IFN-γ and biotinylated anti-human IFN-γ antibodies were purchased from Mabtech (Cincinnati, OH). TLR7, 8, and 7/8 agonists [3M-006, 3M-002, 3M-005, 3M-007, 3M-031] were gifts from 3M Pharmaceuticals (St. Paul, MN).
Donor material
PBL were obtained by leukapheresis from healthy donors. Leukocytes were purified using Lymphocyte Separation Media (Mediatech, Inc, Herndon, Virgina) by gradient centrifugation, cryopreserved, and stored in liquid nitrogen. HPV serology analysis of all donors showed negative results. All studies using human samples were approved by the USC's IRB and informed consent was obtained from all donors.
DC and LC generation
Frozen PBL were thawed, washed once with RPMI1640, containing 2mM Glutamax (GIBCO, Carlsbad, CA), 10 mM sodium pyruvate (GIBCO), 10mM non-essential amino acids (GIBCO), 100 μg/ml Kanamycin (Sigma-Aldrich, St.Louis, MO) and 10% FBS (Omega Scientific, Tarzana, CA) (complete media). For DC, plastic adherent cells were selected by plating 2 × 108 cells in a 175 cm2 tissue culture flask for 2 h at 37°C. Non-adherent cells were washed away and the remaining cells were cultured for 7 days in complete media containing 1000 U/ml rGM-CSF (Berlex, Seattle, WA) and 1000 U/ml rIL-4 (Biosource) of which 100% was replenished on day 3 and 50% was replenished on day 6. For LC, adherent cells were cultured for 7 days in complete media containing 1000 U/ml rGM-CSF, 1000 U/ml rIL-4, and 10 ng/ml rTGF-β1 (Biosource) of which 100% was replenished on day 3, 50% of rGM-CSF and rIL-4 was replenished on day 6 while 100% of rTGF-β1 was replenished on day 3 and 6.
Virus-Like Particles
HPV16L1L2 VLP and HPV16L1L2-E7 cVLP were produced in insect cells and purified by sucrose and cesium chloride ultra-centrifugation as described (30, 31). Western blot analysis confirmed the presence of L1, L2, and in case of chimeric particles, the E7 protein. To test for intact particles, VLP were subjected to an ELISA, using antibodies that recognize conformationally-dependent L1 surface epitopes or linear epitopes, and transmission electron microscopy. An E-toxate kit (Sigma-Aldrich) was used to quantitate endotoxin and levels in the preparations were found to be less than 0.06 endotoxin units/ml. This level as well as Baculovirus DNA used in VLP production procedure do not activate APC (18).
Imidazoquinoline activation assay
DC and LC were harvested and washed twice with PBS. DC were left untreated or treated with 30μM 3M-006, 5μM 3M-002, 30μM imiquimod, 30μM resiquimod, 5μM 3M-031, or with 10μg LPS (Escherichia coli 026:B6) (Sigma-Aldrich). The cells were incubated for 1h at 37 °C, mixed occasionally, and finally placed at 37°C for 24h in complete media containing 1000 U/ml rGM-CSF. LC were left untreated or exposed to HPV16 VLP at a concentration of 10μg/106 cells. The cells were incubated for 1h at 37°C, mixed occasionally, and placed at 37°C for 24h in complete media containing 1000 U/ml rGM-CSF. Next, the cells were left untreated or treated with 30μM 3M-006, 5μM 3M-002, 30μM imiquimod, 30μM resiquimod, 5μM 3M-031 or with 10μg LPS and incubated for an additional 24h at 37°C. DC and LC were harvested, washed, and analyzed by flow cytometry for the expression and surface markers. Additionally, untreated LC and LC exposed to HPV16 VLP were also analyzed for the expression of TLR7 and TLR8.
Cytokine and chemokine analysis
Supernatants were collected from LC stimulated in the imidazoquinolines activation assay and submitted to the Beckman Center for Immune Monitoring Core at the University of Southern California for cytokine and chemokine analysis. The assays were completed using Human Cytokine LINCOplex Kits (LINCO Research, St. Charles, MO) and the Bio-Plex Suspension Array System (BIO-RAD, Hercules, CA).
Migration assay
Chemokine directed migration of LC were carried out using 24-well Transwell plates with 5μm-pore-size polycarbonate filters (Corning Costar, Cambridge, MA). Briefly, 600μl of medium was added to the lower chamber containing either 250 ng/ml CCL21 (R&D Systems, Minneapolis, MN) or complete media alone, as a control for spontaneous migration. We added 2 × 105 untreated LC, LPS stimulated LC, HPV16 VLP exposed LC, or HPV16 VLP exposed LC treated with each of the imidazoquinolines, using the same concentrations as stated in the imidazoquinoline activation assay, to the upper chambers. The plates were incubated for 3 hrs at 37°C. Cells that migrated to the lower chamber were counted, and migration was calculated as the ratio of cells that migrated with/without CCL21.
In vitro immunization assay
In vitro immunizations assays were performed as described (18, 32). Briefly, LC were left untreated or exposed to 10μg HPV16 cVLP for 1h at 37°C in PBS. Subsequently, the cells were incubated for 4h in complete media supplemented with 1000U/ml of rGM-CSF at 37°C. Then cells were treated with or without each of the imidazoquinolines and incubated for 20h at 37°C. As a control for epitope presentation, imidazoquinoline treated LC were pulsed with an HLA-A2 restricted HPV16-E7 peptide (aa 86-93) (33). LC were irradiated (25 Gy) and mixed with autologous CD8+ T Cells, isolated from PBL by positive selection using a MACS MulitSort CD8+ isolation kit (Miltenyi Biotech, Auburn, CA). Day 7 and 14 re-stimulations were done with LC treated as indicated above. For this the medium was supplemented with IL-2 at 50 U/ml at 48h and 96h after re-stimulation. After 28 days cells were pooled and tested for IFN-γ production by ELISPOT as a measurement of HPV16-E7 specific CD8+ T cell responses. Then, 96-well multiscreen-hemagglutinin plates (Millipore, Bedford, MA) were coated with 10μg/ml anti-human IFN-γ in PBS overnight, washed with PBS/0.5% Tween-20, and blocked for 4h with complete medium at 37°C, 5%CO2. 2.5 × 105 cells/well were incubated in the presence or absence of HPV16-E7 peptide aa 86-93 for 18 h at 37°C. The wells were washed 6 times with PBS/0.5% Tween-20 and plates were incubated for 1h with streptavidin-HRP conjugate diluted in PBS/0.5% BSA solution. Individual spots were counted after staining with 3-amino-9-ethyl-carbazole (AEC) substrate (Sigma-Aldrich). Spots were counted using the video-imaging KS ELISPOT analysis system (Zeiss, Thornwood, NY).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism. Statistical analyses of the DC activation assay and ELISPOT assay were conducted using a two-tailed, t-test, as compared to the negative control. Statistical significance of the LC activation assay, cytokine and chemokine analysis, and migration assay were determined by a one-way ANOVA and Tukey's Multiple Comparison Test as compared to the negative controls.
Results
Characterization of LC
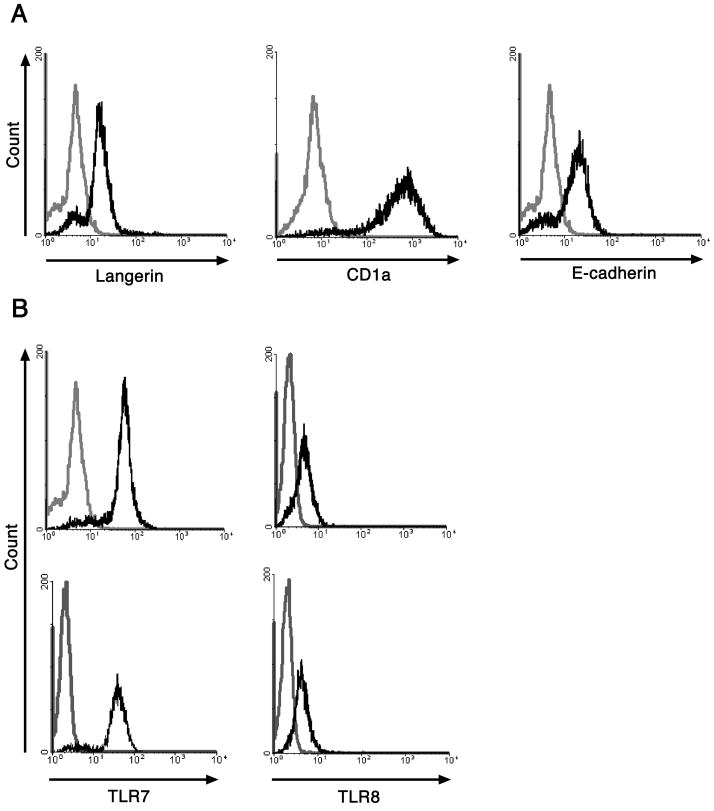
In this study we are examining TLR7 and/or TLR8 agonists as a means to initiate the activation of HPV16 infected LC, thereby inducing an effective cell-mediated immune response against HPV16. To verify the purity of the LC used in this study we assessed by flow cytometry the presence of surface markers commonly used to identify LC: langerin, CD1a, and E-cadherin. Our results show that LC generated from human monocytes are a pure population and express LC associated surface markers, therefore they are phenotypically equivalent to LC found in the epidermis (Fig. 1a). We also analyzed the expression of both TLR7 and TLR8 in immature LC and HPV16 VLP exposed LC by flow cytometry. Our results clearly demonstrate that TLR7 and TLR8 are expressed at similar levels in immature LC and LC exposed to HPV16 VLP (Fig. 1b).
Figure 1. Characterization of monocyte-derived LC.
A. Monocyte-derived LC were stained with either anti-langerin, anti-CD1a, anti-E-cadherin (black histograms) or isotype matched negative controls (grey histograms). The cells were analyzed by flow cytometry. LC generated from monocytes express langerin, CD1a, and E-cadherin. B, Monocyte-derived LC were left untreated or exposed to HPV16 VLP and then permeabilized, fixed, and stained with either anti-TLR7, anti-TLR8 antibodies (black histograms) or isotype matched negative controls (grey histograms). The cells were analyzed by flow cytometry. Immature LC and LC exposed to HPV16 VLP express similar levels of TLR7 and TLR8. One representative experiment of three is shown.
3M-002 and resiquimod up-regulate surface markers, MHC class I, MHC class II, CD80 and CD86, on LC
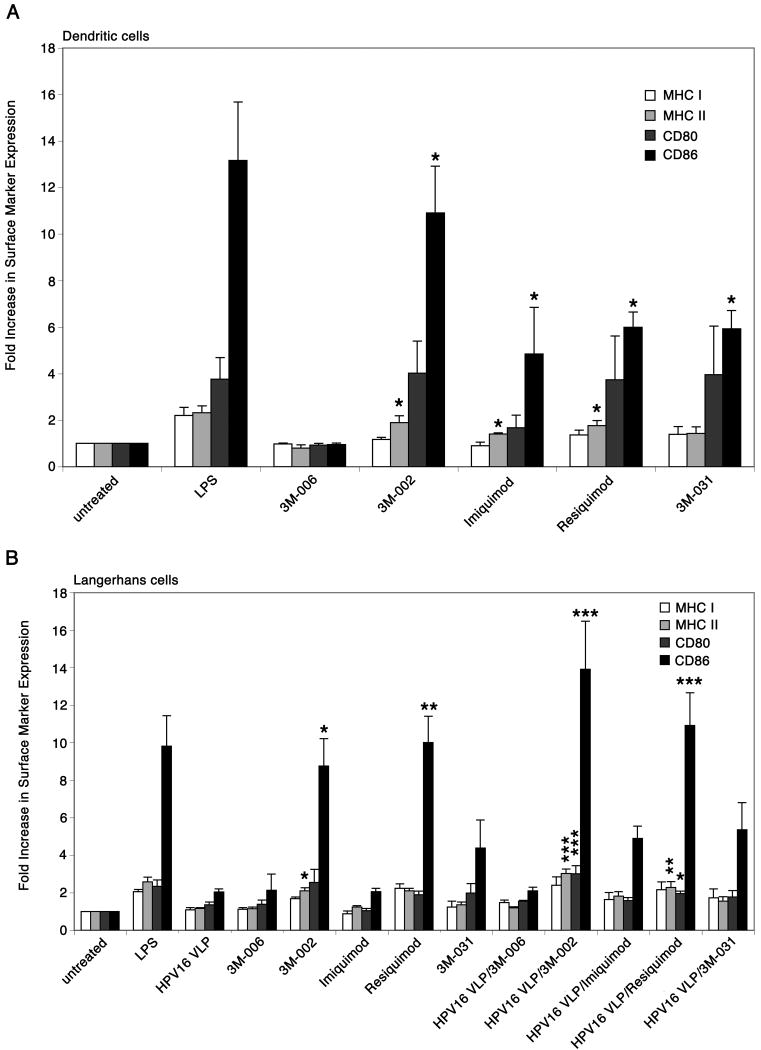
Knowing that TLR7 and TLR8 are expressed in immature LC and LC exposed to HPV16 VLP we sought to determine if selected synthetic imidazoquinolines phenotypically activate LC exposed to HPV16 VLP. We assessed phenotypic activation by the expression of surface markers, MHC class I, MHC class II, CD80 and CD86, on LC that have previously encountered HPV16 VLP and have been treated with each of the imidazoquinolines. DC, which are highly potent professional APC that reside within the dermis, were used as a positive control test for the activity and to determine the optimal concentration of each imidazoquinoline because it has been well established that DC are activated by imidazoquinoline compounds (26, 34, 35). As expected, DC treated with 3M-002, imiquimod, resiquimod, and 3M-031 induced the up-regulation of surface markers, most notably MHC class II and CD86, relative to untreated or 3M-006 treated DC (Fig. 2a). 3M-006 is an inactive small molecule TLR7/8 analog that is produced in a similar manner as the other imidazoquinolines and used as a negative control. The optimal concentration for each imidazoquinoline to activate APC was determined by assessing a range of concentrations (0.1μM-60μM) for each agonist. The concentration of each agonist that resulted in the maximum expression of surface makers on DC, as determined by flow cytometry analysis, was used as the optimal concentration (data not shown).
Figure 2. Differential expression of surface markers on DC and LC stimulated with imidazoquinolines.
A, DC were left untreated, treated with LPS, or treated with each of the imidazoquinolines. The cells were analyzed by flow cytometry for the expression of MHC class I and II molecules, CD80, and CD86. Surface markers are up-regulated when treated with 3M-002, imiquimod, resiquimod, and 3M-031. These data are represented by fold increase in surface marker expression, which are based on mean fluorescence intensity. The mean ± SEM of four separate experiments is presented (*P < .05). B, LC were left untreated, stimulated with LPS, exposed to HPV16 VLP, treated with each of the imidazoquinolines, or exposed to HPV16 VLP and subsequently treated with each of the imidazoquinolines. After the final incubation the cells were analyzed by flow cytometry for the expression of MHC class I and II molecules, CD80, and CD86. 3M-002 and resiquimod induced the up-regulation of surface markers on LC and LC exposed to HPV16 VLP. These data are represented by fold increase in surface marker expression, which are based on mean fluorescence intensity. The mean ± SEM of four separate experiments is presented (*P < .05, **P < .01, ***P< .001).
Since we confirmed the agonists are active and knowing the optimal concentrations needed to activate APC we investigated if each agonist has the ability to reverse the phenotype of LC exposed to HPV16 VLP. LC were left untreated, stimulated with LPS, exposed to HPV16 VLP, treated with each of the imidazoquinolines, or exposed to HPV16 VLP and subsequently treated with each of the imidazoquinolines. Each population of cells was harvested after the final incubation and analyzed by flow cytometry for the expression of surface markers. Consistent with our previously reported data (18), LC exposed to HPV16 VLP did not increase the expression of surface markers when compared to untreated LC and 3M-006 treated LC (Fig. 2b). LC treated with either 3M-002 or resiquimod significantly induced the up-regulation of surface marker, as seen with the positive control, LPS stimulation. Surprisingly, imiquimod and 3M-031 treated LC induced only a minor up-regulation of surface markers above that of the negative controls, untreated LC, LC exposed to HPV16 VLP and 3M-006 treated LC (Fig. 2b). It should be noted that imiquimod could not be used at any higher dose because it was found to be toxic to the cells at two fold higher concentrations than used in our assays. Consequently, when LC were exposed to HPV16 VLP and subsequently treated with each of the imidazoquinolines, only 3M-002 and resiquimod significantly induced the up-regulation of the surface markers, while imiquimod and 3M-031 moderately increased the expression of surface markers on LC exposed to HPV16 VLP, relative to the negative controls (Fig. 2b). Of note, it appears that TLR7 and TLR8 agonists induced a slightly greater up-regulation of surface markers on LC that have previously been exposed to HPV16 VLP than on untreated LC, however these differences in expression are not statistically significant. Thus, these phenotypic data begin to suggest that imidazoquinolines have different effects on DC and LC. Specifically, 3M-002 and resiquimod appear to be far more potent agonists for LC than imiquimod and 3M-031.
Differential production of cytokines and chemokines from LC stimulated with imidazoquinolines
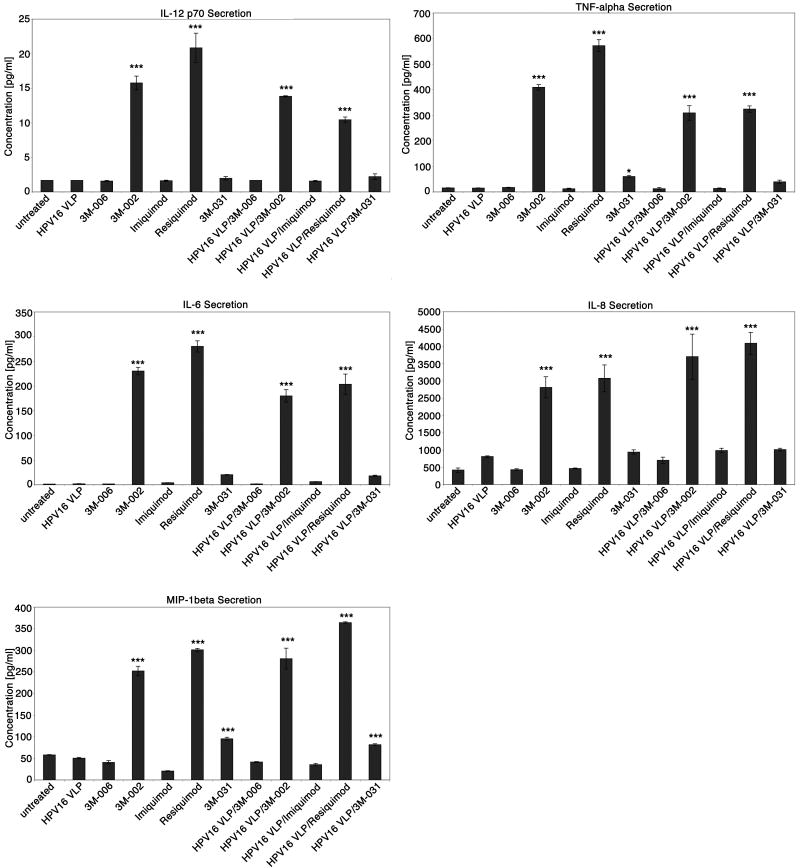
Imidazoquinolines stimulate both an innate and an adaptive immune response. The innate immune response induced by imidazoquinolines drives the adaptive immune response into a Th1 cell-mediated response via the local cytokine and chemokine milieu generated primarily by activated macrophages and DC. Thus, we wanted to determine if selected imidazoquinolines could stimulate LC exposed to HPV16 VLP to produce a pro-inflammatory cytokine and chemokine profile similar to the cytokine milieu known to be generated by imidazoquinoline activated DC. Cytokines and chemokines produced by untreated LC, LC exposed to HPV16 VLP, LC treated with each of the imidazoquinoline compounds, and LC exposed to HPV16 VLP and treated with the imidazoquinoline compounds were evaluated. Supernatant from each treatment was collected and analyzed using a human cytokine LINCOplex assay. IL-12 p70, TNF-α, IL-6, IL-8, and MIP-1β concentrations were statistically significantly elevated when LC were stimulated with 3M-002, resiquimod, or when LC were exposed to HPV16 VLP and then stimulated with either 3M-002 or resiquimod in comparison to the negative controls, untreated LC, LC exposed to HPV16 VLP, 3M-006 treated LC, and LC exposed to HPV16 VLP and treated with 3M-006 (Fig. 3). LC treated with 3M-031 or LC exposed to HPV16 VLP and subsequently stimulated with 3M-031 only slightly induced the production of these cytokines and chemokines above that of the negative controls (Fig. 3). IP-10, MCP-1 and RANTES (CCL5) were also found to be highly secreted by LC treated with 3M-002, resiquimod, or 3M-031 and LC exposed to HPV16 VLP and then stimulated with either 3M-002, resiquimod, or 3M-031 (data not shown). Markedly, imiquimod stimulated LC and LC exposed to HPV16 VLP and subsequently treated with imiquimod secreted comparable amounts of TNF-α, IL-12 p70, IL-6, IL-8, MIP-1β (Fig. 3), IP-10, RANTES or MCP-1 (data not shown) as that observed in the negative controls. The cytokine and chemokine analyses demonstrate that 3M-002 and resiquimod are more efficient activators of HPV16 VLP exposed LC in comparison to 3M-031 and imiquimod. The cytokine and chemokine profiles produced by both 3M-002 and resiquimod activated LC are similar to that of imidazoquinoline stimulated DC (26, 34). Thus, like DC, LC activated by either 3M-002 or resiquimod likely induce a Th1 cell mediated response via the production of cytokines and chemokines.
Figure 3. 3M-002 and resiquimod highly induce the secretion of Th1 associated cytokines and chemokines by LC previously incubated with or without HPV16 VLP.
Supernatants collected from untreated LC, LC exposed to HPV16 VLP, LC treated with each of the imidaziquinolines, or LC exposed to HPV16 VLP and then treated with imidaziquinolines were analyzed in triplicate for the presence of cytokines and chemokines. Cytokine and chemokine levels were quantified using a human cytokine LINCOplex assay. These data are expressed as the mean concentration with error bars representing the SD (*P < .05, ***P< .001). The experiment was repeated three times and yielded similar results.
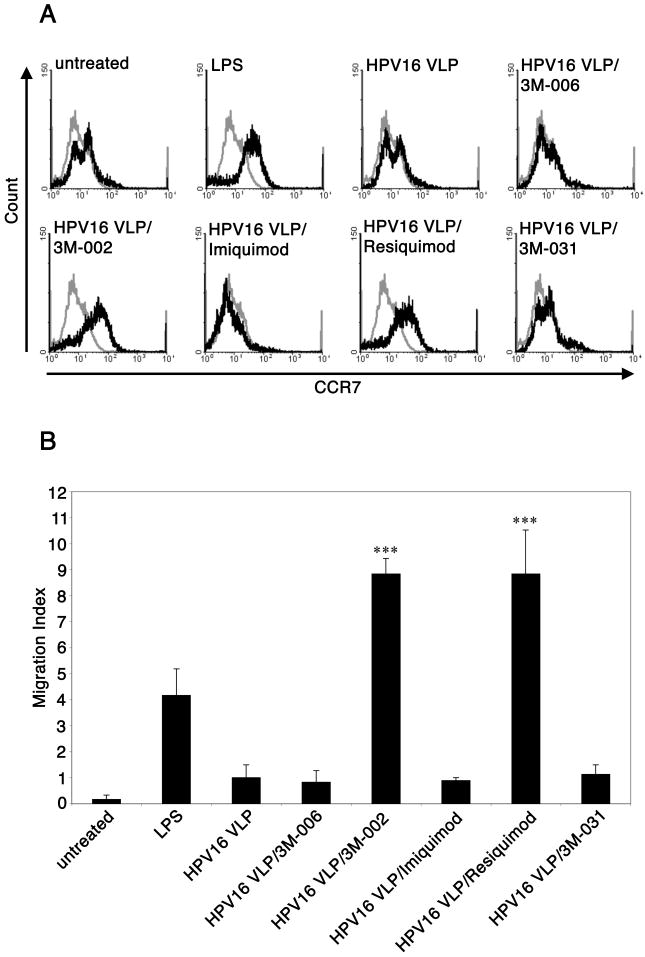
3M-002 and resiquimod induce the up-regulation of CCR7 and migration of LC exposed to HPV16 VLP towards CCL21
In addition to the up-regulation of surface markers and the secretion of pro-inflammatory cytokines and chemokines, another hallmark of LC activation is the up-regulation of CCR7 and the migration out of peripheral tissues towards draining LN. CCR7 mediates the migration of LC to T cell zones of the draining LN by binding to either secondary lymphoid tissue chemokine (SLC/CCL21)3 or MIP-3β (CCL19). Therefore, we investigated whether the imidazoquinoline compounds can induce the up-regulation of CCR7 and CCL21-directed migration of LC exposed to HPV16 VLP. Untreated LC, LPS stimulated LC, LC exposed to HPV16 VLP, and LC exposed to HPV16 VLP and subsequently treated with each of the imidazoquinolines were analyzed for the expression of CCR7 by flow cytometry. LC exposed to HPV16 VLP stimulated with either 3M-002 or resiquimod induced the up-regulation of CCR7 similar to the positive control, LPS-treated LC (Fig. 4a). In contrast, imiquimod and 3M-031 did not induce the expression of CCR7 on LC previously exposed to HPV16 VLP (Fig. 4a). Next, we examined whether the expression of CCR7 functionally corresponded to enhanced migration of LC towards CCL21 by a transwell migration assay. We observed that 3M-002 and resiquimod significantly induced the migration of LC exposed to HPV16 VLP towards CCL21, as seen similarly in the positive control, while imiquimod and 3M-031 did not enhance CCL21-directed migration of LC exposed to HPV16 VLP (Fig. 4b). Taken together, these experiments demonstrate that 3M-002 and resiquimod are providing LC exposed to HPV16 VLP with a potent stimulus to acquire the potential to migrate effectively in response towards a LN derived chemokine, CCL21.
Figure 4. 3M-002 and resiquimod induce the up-regulation of CCR7 and migration of LC exposed to HPV16 VLP towards CCL21.
LC were left untreated, stimulated by LPS, exposed to HPV16 VLP, or exposed to HPV16 VLP and subsequently treated with each of the imidaziquinolines. After the final incubation LC were either, A, harvested and analyzed for the expression of CCR7 (black line) by flow cytometry (grey line is the isotype control antibody), or B, used in a migration assay. The mean ± SEM of three separate experiments is presented (***P< .001).
Induction of an epitope-specific CD8+ T cell response by LC exposed to HPV16 cVLP and stimulated with either 3M-002 or resiquimod
Thus far we have demonstrated that 3M-002 and resiquimod can effectively activate LC previously exposed to HPV16 VLP, unlike imiquimod and 3M-031, so we next sought to determine if LC exposed to HPV16 VLP and stimulated with each of the imidazoquinolines could induce an HPV16-specific, MHC class I-restricted T cell response by performing in vitro immunization assays. HPV16 cVLP were used in these experiments because they contain a well-characterized human HLA-A*0201-restricted epitope (E7 peptide aa 86-93, TLGIVCPI) recognized by human CD8+ T cells (33). Human DC, but not LC, have been shown to initiate epitope-specific responses to this peptide when exposed to the HPV16 cVLP (18, 32). Thus, HPV16 cVLP were used to determine whether the imidazoquinoline compounds are capable of stimulating LC exposed to HPV16 VLP to initiate an epitope-specific immune response against the HPV16 E786-93 peptide.
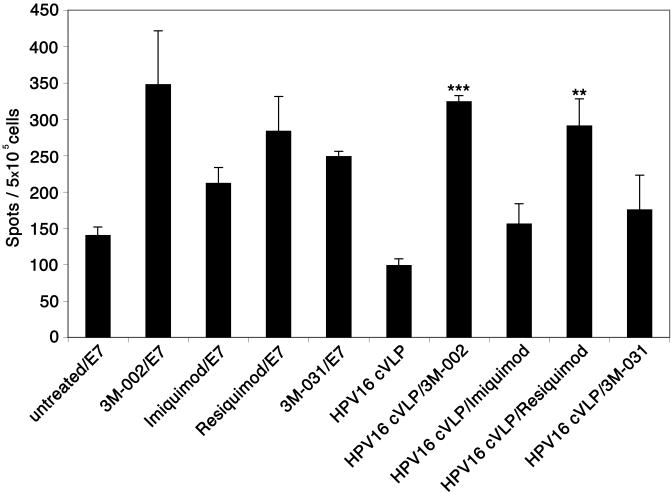
In the experiments presented here, LC generated from HLA-A*0201 positive monocytes were exposed to HPV16 cVLP and treated with each of the imidazoquinolines. We then incubated the cells with autologous naïve CD8+ T cells and the cultures were stimulated twice with their respective treated LC. As control treatments, LC were treated with each of the imidaziquinolines and pulsed with the HPV16-E7 derived HLA-A*0201 restricted CTL epitope (E786-93). Seven days after the last re-stimulation, the cells from each culture were collected and analyzed for a specific CD8+ T cell response to the HLA-A*0201-restricted HPV16-E786-93 peptide by an INF-γ ELISPOT. Of major impact, LC exposed to HPV16 cVLP and stimulated with either 3M-002 or resiquimod initiated a statistically significant HPV16 epitope-specific response when compared to untreated LC and LC exposed to HPV16 cVLP, while LC exposed to HPV16 cVLP and stimulated with either imiquimod or 3M-031 did not induce a significant HPV16 epitope specific immune response (Fig. 5). Collectively, these experiments demonstrate that both 3M-002 and resiquimod effectively induce LC activation and have the ability to initiate an HPV16 specific cell-mediated immune response through the activation of LC.
Figure 5. 3M-002 and resiquimod induce an HPV16 epitope-specific CD8+ T cell immune response through the activation of LC exposed to HPV16 cVLP.
LC were incubated with media alone or with HPV16 cVLP and each of the imidazoquinolines. In control experiments, LC were treated with each of the imidazoquinolines and pulsed with an HPV16-E7 derived HLA-A* 0201 restricted CTL epitope. The treated LC were incubated with autologous CD8+ lymphocytes and re-stimulated twice. Responder cells were analyzed in triplicate for IFN-γ production in an ELISPOT assay against the E786-93 peptide. The number of spots in each well was counted and averaged. These data are expressed as the mean ± SEM (*P < .05 and **P< .01). The experiment was repeated three times using two independent HLA-A* 0201 positive donors and yielded similar results.
Discussion
In this study, we investigated synthetic imidazoquinolines as potential activators of LC previously exposed to HPV16 VLP, which could lead to further exploration of specific imidazoquinolines as therapeutic compounds for treating existing HPV16-induced cervical lesions. Our data clearly demonstrate that 3M-002 and resiquimod can induce the phenotypic maturation of naïve LC and LC previously exposed to HPV16 VLP via the up-regulation of surface markers (MHC class I, MHC class II, CD80 and CD86). Moreover, 3M-002 and resiquimod induce functional activation of LC exposed to HPV16 VLP as demonstrated by the production of Th1 associated cytokines and chemokines, CCL21-directed migration, and the induction of an HPV16-specific CD8+ T cell response. However, imiquimod does not phenotyically or functionally activate LC while 3M-031 partially induces the activation of LC. Collectively, our data strongly suggest that 3M-002 and resiquimod can reverse the phenotype and function of LC exposed to HPV16, unlike imiquimod and 3M-031. Therefore, our results support exploring 3M-002 and resiquimod as therapeutic small-molecule compounds for treating HPV infections and HPV-induced cervical lesions.
The findings presented here are based upon a model system that mimics the interaction between HPV and LC in the human epidermis. Due to limitations of working with human material, the study was not conducted using human LC isolated from mucosal epidermal sheets. The mere process of isolating human LC from epidermal sheets induces the maturation of LC (36), thus making it difficult to carry out our study since activation status is an endpoint. However, monocyte-derived LC are an appropriate alternative model, because they express MHC class II molecules, Langerin, E-cadherin, CD1a, and Birbeck granules (18), which classically define human LC located in the epidermis (37). Recently the status of LC as the only APC in the epithelium that express langerin was challenged. It was reported that dermal langerin+ DC exist in mice and may play a role in the immunosurveillance of the skin (38, 39). However, Klechevsky et al. demonstrated that while two different subsets exist of human dermal DC, neither of these subsets express langerin, highlighting a difference in human and murine APC populations located in the epithelium (36). Moreover, this study was carried out using VLP, which have been developed as an alternative to HPV virions for immunological analysis. This is because the life cycle of HPV is dependent on the differentiation of cells in the squamous epithelium making it very difficult to produce large quantities of HPV virions in vitro. Thus, due to the facts that human LC are the only APC at the site of infection, that monocyte-derived LC have been shown to be phenotypically equivalent to human epidermal LC, and that VLP are an accepted alternative to purified virions for immunological analysis of HPV, this study uses the most appropriate model to critically examine the interaction of HPV and human LC.
It should be noted that imiquimod is a FDA approved drug (Aldara) to treat external anogenital warts (condyloma accuminatum) caused by low-risk HPV infection (HPV 6 and 11). More recently, imiquimod has been shown to be successful in treating high-risk HPV induced vulvar intraepithelium neoplasia (VIN) (40, 41). However, imiquimod has yet to be reported as an effective therapeutic treatment for HPV-induced cervical intraepithelium neoplasia (CIN). The reason why there is a difference in response initiated by imiquimod against different types of HPV induced lesions (genital warts, VIN lesions and CIN lesions) is unclear. This disparity in response could be due to the difference in cellular composition and structure of the external genitalia and the cervix. Considering we demonstrate that imiquimod does not activate LC, an effective immune response against anogenital warts and VIN lesions is likely due to the activation of APC other than LC, such as DC and macrophages.
The effects of synthetic imidazoquinolines on LC had not been well studied until now. Previously, it was shown that imiquimod and resiquimod do not phenotypically but functionally activate LC (42, 43). Past studies assessed phenotypic activation of LC by the expression of surface markers. The results from these studies are in accordance with our results for imiquimod, however, we found that resiquimod does phenotypically activate LC. The reason for this discrepancy between the present and past studies, concerning the effects of resiquimod on LC, could be explained because Burns et al. examined phenotypic activation 6h after LC were treated with resiquimod, while we assessed the maturation of LC 24h post treatment with resiquimod. Furthermore, the functional activation of LC was examined in the previous studies in multiple ways, one of which was by the level of messenger RNA (mRNA) encoding pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-12 p40. The results from these studies showed that imiquimod and resiquimod enhanced the transcription of the genes for these specific cytokines. We also assessed cytokine levels as a means of evaluating functional activation, however, we did so at the more relevant level of protein production. We observed pro-inflammatory cytokine and chemokine secretion by LC treated with either 3M-002 or resiquimod, and to a modest extent with 3M-031, however we did not observe this with imiquimod. Our results are an improvement on previous reports because we assayed for a different end product, namely protein, and mRNA transcripts do not always translate to protein expression. Additionally, our results are consistent with recent findings showing that TLR8 agonists are more effective than TLR7 agonists at inducing pro-inflammatory cytokines and chemokines by monocyte-derived DC (GM-CSF/IL-4/TGF-β) (23).
During activation LC migrate out of the epidermal tissue to draining LN where they activate naïve T cells, thereby inducing a cell-mediated immune response. Previous data has demonstrated that LC exposed to HPV16 VLP cannot up-regulate CCR7, migrate, or induce an HPV16-specific CD8+ T cell response (18, 20). To explore the effects of synthetic imidazoquinolines on the migration of LC exposed to HPV16 VLP we assessed the expression of CCR7 and the ability of LC previously exposed to HPV16 VLP to migrate towards CCL21. Our results clearly show CCR7 is up-regulated on LC exposed to HPV16 VLP that are treated with either 3M-002 or resiquimod, but not when treated with imiquimod or 3M-031. Furthermore, we demonstrate that the expression of CCR7 correlates to the migratory ability of LC exposed to HPV16 VLP. Our data illustrate that only 3M-002 and resiquimod treated LC previously exposed to HPV16 VLP are able to migrate in response to CCL21. However, in a contrasting study it was shown that imiquimod functionally activates LC by demonstrating that imiquimod induces the migration of LC, yet the study was performed using a mouse model and it was not confirmed that the migrating LC were effective in inducing an epitope specific adaptive immune response (43). Nevertheless, we sought to determine if LC exposed to HPV16 VLP that are treated with imidazoquinolines have the ability to induce an HPV16 epitope specific CD8+ T cell response. Our results show that 3M-002 and resiquimod can effectively overcome the phenotype and function of LC exposed to HPV16 VLP and can induce an HPV16-specific CD8+ T cells response, which is critical in mediating the clearance of HPV16 infections and HPV16-induced cervical lesions. In addition to our findings, Burns et al. investigated the functional activation of LC after treatment with either imiquimod or resiquimod by assessing the allostimulatory capacity of the treated LC. They found that imiquimod only modestly induced T cell proliferation in an allogenic MLR assay while resiquimod highly increased the allostimulatory capacity of LC (42). Their results from this functional assay are in line with our functional data, which is further support that resiquimod is more potent than imiquimod in activating LC.
Collectively, our findings imply that strong TLR8 agonists, such as 3M-002 and resiquimod, are more effective in inducing LC activation and overcoming the tolerizing-like phenotype and function of LC exposed to HPV16 VLP, in comparison to TLR7 agonists, such as imiquimod. It has been shown that TLR7 and TLR8 agonists differ in their target cell selectivity (23). Notably, resiquimod and 3M-031 are both TLR7 and TLR8 agonists, however, resiquimod is much more effective in activating LC. This may occur because the agonists differ in their target cell selectivity and preferentially activate one TLR over the other; resiquimod is known to preferentially act through TLR8 (21), while it has yet to be reported which receptor 3M-031 preferentially acts through. This explanation is plausible considering that functional differences have been observed between TLR7 and TLR8 (23, 44). It was demonstrated that TLR7 activation primarily leads to the production of IFN-α and IFN-regulated cytokines, which is similar to TLR9 activation, while TLR8 is functionally associated with the production of pro-inflammatory cytokines, such as TNF- α (23). One explanation for the functional distinction between TLR7 and TLR8 is the difference in the signal transduction pathways initiated by each of the receptors. TLR8-mediated activation of NF-κB and JNK are dependent on MEK kinase 3 (MEKK3) (45), while TLR7–mediated activation of NF-κB is transforming growth factor–β-activated kinase 1 (TAK-1) dependent (46). Bruton tyrosine kinase (Btk) has also been shown to directly interact with the intracellular domain of TLR8 and plays an important role in the signal transduction of TLR8, yet Btk has not been demonstrated to be associated with TLR7 (47, 48). Alternatively, another explanation of our findings may be that TLR8 is inhibiting TLR7 function. In HEK293 cells it was demonstrated that the co-expression of TLR8 and TLR7 results in inhibition of TLR7 to respond to its agonist (44). Therefore, TLR8 may inhibit LC from responding to agonists that preferentially bind TLR7, which explains why TLR8 dominant agonists (such as 3M-002 and resiquimod) are more effective than TLR7 dominant agonists (such as imiquimod and potentially 3M-031) in activating LC and in driving a strong cell-mediated immune response.
Since LC are critical in controlling the induction of an immune response in the epithelium and they are targeted by HPV16 to escape immune detection, LC are highly attractive targets for immunotherapy of HPV16-induced cervical lesions. In addition, LC have recently been shown to be able to directly kill cervical epithelial cells that express HPV16 E6 and E7, thereby generating a source of antigen that could be processed and presented by APC to T cells. LC cytotoxicity is mediated in part by TRAIL expression, which can be up-regulated by the presence of IFN-γ (49). Furthermore, it has been demonstrated that TLR7/8 stimulated DC-like cells have cytotoxic activity, which is mediated by the expression of TRAIL and the secretion of perforin and granzyme B (50). Thus it is conceivable that TLR8 agonists stimulate LC not only to induce an HPV-specific Th1 mediated cellular immune response but may also enhance LC cytotoxicity towards HPV16-infected epithelial cells, further augmenting antiviral and anti-neoplastic activity. In conclusion, TLR8 agonists, 3M-002 and resiquimod, are promising therapeutic compounds for the treatment of HPV infections and HPV induced cervical lesions.
Acknowledgments
Cytokine and chemokine analyses were performed at the Beckman Center for Immune Monitoring Core at USC. W. Martin Kast holds the Walter A. Richter Cancer Research Chair.
Abbreviations
- HPV
human papillomavirus
- LC
Langerhans cell
- LN
lymph node
- SLC
secondary lymphoid tissue chemokine
- cVLP
chimeric virus-like particles
- VLP
virus-like particles
- DC
dendritic cell
- IP-10
interferon-inducible protein-10
Footnotes
These studies were supported by the National Institutes of Health (NIH) grant RO1 CA 74397 and a Whittier Foundation grant (to W.M.K.), a NIH training grant T32 AI07078 (to L.M.F.), and a NIH training grant T32 GM0607587 (to A.B.R.).
Disclosures: The authors declare no conflict of interest or financial interest.
References
- 1.Walboomers JM, Jacobs V, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;182:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical cancer lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 5.The FUTURE II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 6.Dasbach EJ, Elbasha EH, Insinga RP. Mathematical models for predicting the epidemiologic and economic impact of vaccination against human papillomavirus infection and disease. Epidemiol Rev. 2006;28:88–100. doi: 10.1093/epirev/mxj006. [DOI] [PubMed] [Google Scholar]
- 7.Ryding J, French KM, Naucler P, Barnabas RV, Garnett GP, Dillner J. Seroepidemiology as basis for design of a human papillomavirus vaccination program. Vaccine. 2008;26:5263–5268. doi: 10.1016/j.vaccine.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AR, Harris R, Sedjo RL, Baldwin S, Roe D, Papenfuss MR, Abrahamsen M, Inserra P, Olvera S, Hatch K. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: the young women's health study. J Infect Dis. 2002;186:462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 9.Woodman CB, Collins S, Winter H, Balley A, Ellis J, Prior P, Yates M, Rollason TP, Young LS. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;9:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 10.Stanley MA, Pett MR, Coleman N. HPV: from infection to cancer. Biochem Soc Trans. 2007;35:1456–1460. doi: 10.1042/BST0351456. [DOI] [PubMed] [Google Scholar]
- 11.Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL, Franco EL. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–3114. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 12.Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomavirus to escape the host immune response. Curr Cancer Drug Targets. 2007;7:79–89. doi: 10.2174/156800907780006869. [DOI] [PubMed] [Google Scholar]
- 13.Larsen CP, Steinman RM, Witer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larrengina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, Falo LD., Jr Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 15.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell DA, Nair SK, Gilboa E. Dendritic cell/macrophage precursors capture exogenous antigen for MHC class I presentation by dendritic cells. Eur J Immunol. 1998;28:1923–1933. doi: 10.1002/(SICI)1521-4141(199806)28:06<1923::AID-IMMU1923>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 18.Fausch SC, Da Silva DM, Rudolf MP, Kast WM. Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J Immunol. 2002;169:3242–3249. doi: 10.4049/jimmunol.169.6.3242. [DOI] [PubMed] [Google Scholar]
- 19.Fausch SC, Da Silva DM, Kast WM. Differential uptake and cross-presentation of human papillomavirus virus-like particles by dendritic cells and Langerhans cells. Cancer Res. 2003;63:3478–3482. [PubMed] [Google Scholar]
- 20.Fausch SC, Fahey LM, Da Silva DM, Kast WM. HPV can escape immune recognition through Langerhans cell PI3-Kinase activation. J Immunol. 2005;174:7172–7178. doi: 10.4049/jimmunol.174.11.7172. [DOI] [PubMed] [Google Scholar]
- 21.Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 22.Barton GM. Viral recognition by Toll-like receptors. Semin Immunol. 2007;19:33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 25.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 26.Sauder DN. Imiquimod; modes of action. Br J Dermatol. 2003;149:5–8. doi: 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- 27.Wagner TL, Horton VL, Carlson GL, Myhre PE, Gibson SJ, Imbertson LM, Tomai MA. Induction of cytokines in Cynomolgus monkeys by the immune response modifiers, imiquimod, S27609, and S-28463. Cytokine. 1997;9:837–845. doi: 10.1006/cyto.1997.0239. [DOI] [PubMed] [Google Scholar]
- 28.Weeks CE, Gibson SJ. Induction of interferon and other cytokines by imiquimod and its hydroxylated metabolite R-842 in human blood cells in vitro. J Interferon Cytokine Res. 1994;14:81–85. doi: 10.1089/jir.1994.14.81. [DOI] [PubMed] [Google Scholar]
- 29.Sidky YA, Borden EC, Weeks CE, Reiter MJ, Hatcher JF, Bryan GT. Inhibition of murine tumor growth by an interferon inducing imidazoquinolinamine. Cancer Res. 1992;52:3528–3533. [PubMed] [Google Scholar]
- 30.Greenstone HL, Nieland JD, de Visser KE, De Bruijn ML, Kirnbauer R, Roden RB, Lowy DR, Kast WM, Schiller JT. Chimeric papillomavirus virus-like particles elicit antitumor immunity against E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirnbauer R, Taub J, Greenstone H, Roden R, Durst M, Gissmann L, Lowy DR, Schiller JT. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–6939. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolf M, Fausch SC, Da Silva DM, Kast WM. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J Immunol. 2001;166:5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 33.Ressing ME, Sette A, Brandt RM, Ruppert J, Wentworth PA, Hartman M, Grey HM, Oseroff C, Melief CJ, Kast WM. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 34.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 35.Philbin VJ, Levy O. Immunostimulatory activity of Toll-like receptor 8 agonists towards human leucocytes: basic mechanisms and translational opportunities. Biochem Soc Trans. 2007;35:1485–1491. doi: 10.1042/BST0351485. [DOI] [PubMed] [Google Scholar]
- 36.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 38.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Seters M, van Beurden M, ten Kate FJW, Beckmann I, Ewing PC, Eijkemans MJ, Kagie MJ, Meijer CJM, Aaronson NK, KleinJan A, Heijmans-Antonissen C, Zijlstra FJ, Burger MPM, Helmerhorst TJM. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–1473. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 41.van Poelgeest MIE, van Seters M, van Beurden M, Kwappenberg KMC, Heijmans-Antonissen C, Drijfhout JW, Melief CJM, Kenter GG, Helmerhorst TJM, Offringa R, van der Burg SH. Detection of human papillomavirus (HPV) 16-specific CD4+ T cell immunity of patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin Cancer Res. 2005;11:5273–5280. doi: 10.1158/1078-0432.CCR-05-0616. [DOI] [PubMed] [Google Scholar]
- 42.Burns RP, Jr, Ferbel B, Tomai M, Miller R, Gaspari AA. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans' cells. Clin Immunol. 2000;94:13–23. doi: 10.1006/clim.1999.4804. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA, Miller RL, Sauder DN. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114:135–141. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Shao Y, Bennett TA, Shankar RA, Wightman PD, Reddy LG. The functional effects of physical interactions among toll-like Receptors 7, 8, and 9. J Biol Chem. 2006;281:37427–37434. doi: 10.1074/jbc.M605311200. [DOI] [PubMed] [Google Scholar]
- 45.Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J, Wightman P, Sato S, Akira S, Puel A, Casanova J, Su B, Li X. TLR8-mediated NF-kB and JNK activation are TAK-1 independent and MEKK3-dependent. J Biol Chem. 2006;281:21013–21021. doi: 10.1074/jbc.M512908200. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal S, Kandimalla ER. Synthetic agonists of Toll-like receptors 7, 8, and 9. Biochem Soc Trans. 2007;35:1461–1467. doi: 10.1042/BST0351461. [DOI] [PubMed] [Google Scholar]
- 47.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Writh T, O'Neill LAJ. Bruton's tyrosine kinase is a Toll/Interleukin receptor domain-binding protein that participates in nuclear factor kB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 48.Sochorová K, Horváth R, Rozková D, Litzman J, Bartunková J, Sedivá A, Spísek R. Impaired Toll-like receptor 8–mediated IL-6 and TNF- production in antigen-presenting cells from patients with X-linked agammaglobulinemia. Blood. 2007;109:2553–2556. doi: 10.1182/blood-2006-07-037960. [DOI] [PubMed] [Google Scholar]
- 49.Le Poole IC, ElMasri WM, Denman CJ, Kroll TM, Bommiasamy H, Eiben GL, Kast WM. Langerhans cells and dendritic cells are cytotoxic towards HPV16 E6 and E7 expressing target cells. Cancer Immunol Immunother. 2008;57:789–797. doi: 10.1007/s00262-007-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Sting G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]