Abstract
Apolipoprotein E (apoE), a ligand for the low-density lipoprotein (LDL) receptor family, has been implicated in modulating glial inflammatory responses and the risk of neurodegeneration associated with Alzheimer’s disease. Glial cells activated by lipopolysaccharide (LPS) have decreased apoE levels and we recently showed that apoE itself can modulate the inflammatory response by reducing c-Jun N-terminal kinase (JNK) activation. Reduced JNK phosphorylation is vital to overcome the LPS-induced decrease in apoE expression, suggesting that JNK inhibition may be an effective way to increase apoE protein and protract its anti-inflammatory properties. This study investigates the impact of JNK inhibition on apoE production using two JNK inhibitors. Our work in primary glia and in vivo in mice injected with JNK inhibitor demonstrates that inhibition of JNK may be an effective way to increase apoE expression.
Keywords: apolipoprotein E, Alzheimer’s disease, glia, inflammation, lipopolysaccharide, signal transduction, JNK, ABCA1, cholesterol, astrocytes
INTRODUCTION
The leading genetic risk factor for Alzheimer’s disease (AD) is the cholesterol transport protein apolipoprotein E (apoE). Single nucleotide polymorphisms in the APOE gene result in three common isoforms termed apoE2, E3, and E4 [1], with E4 carriers having an increased risk of developing AD [2]. AD is characterized by the presence of extracellular amyloid plaques, intracellular neurofibrillary tangles, synaptic and neuronal loss [3], and an astrocyte and microglial inflammatory response [4]. Modulating the inflammatory response in AD has been a target of extensive research and apoE has been identified as an anti-inflammatory molecule in a number of studies [5; 6; 7; 8]. APOE knockout mice have increased brain inflammation after injury, compared to wild-type controls [6]. ApoE targeted replacement mice show isoform specific differences in inflammation, with apoE2 and apoE3 having greater anti-inflammatory properties than apoE4 [9]. Furthermore, exogenous apoE and apoE mimetic peptides formed from the receptor-binding region of apoE reduce inflammatory responses in vitro and in vivo [5; 6; 7; 8].
ApoE expression itself is regulated by brain injury and glial activation. ApoE in the CNS is expressed primarily in glia [10; 11; 12] and upregulated after injury [13]. ApoE is downregulated in some types of inflammatory responses; for example, activation of macrophages or glial cells by lipopolysaccharide (LPS) results in decreased apoE levels [8; 14; 15]. ApoE-induced activation of the low density lipoprotein receptor family in microglia counteracts LPS-induced microglial inflammation [8]. Activation of these receptors signals a decrease in c-Jun N-terminal kinase (JNK) activation in microglia, which was vital to overcome LPS-induced decrease in apoE expression [8]. These in vitro studies suggested that JNK inhibition alone may be an effective way to increase apoE protein, and this increase in apoE could have anti-inflammatory properties. Thus, our current study aims to further investigate the impact of JNK inhibition on apoE production in the brain.
MATERIALS AND METHODS
Primary Glial Culture
Primary mouse mixed glial cultures were prepared from postnatal day 1 Swiss-Webster mouse pups as previously described [8]. The composition of mixed glial cultures was determined by staining cultures with GFAP (astrocytes), OX42 or Iba1 (microglia), NeuN or MAP-2 (neurons), and APC (oligodendrocytes). Across cultures the composition of cells was about 85 % astrocytes, 15 % microglia, < 1 % oligodendrocytes, and < 1 % neurons.
Intrahippocampal Injections
Adult male Swiss-Webster mice (30-40g, Taconic, Hudson, NY) were anesthetized and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A single injection, 5 μl or 10 μl of control, 5 μl of 10mM SP600125 (11.01 μg), 10 μl of 10mM SP600125 (22.02 μg), or 5 μl of 10mM PD98059 (13.36 μg) was delivered to the right hippocampus (from bregma: -1.46 posterior, -1.0 mm lateral, and -2.0 mm ventral) at a constant flow of 0.5 μl/min. SP600125 and PD98059 were dissolved in 5 % DMSO and 50 % EtOH in PBS and, thus, the control injection was 5 % DMSO, 50 % EtOH in PBS. A total of 12 animals were treated with SP600125 and 10 with vehicle control.
Tissue Preparation
24 hrs after injection mice were sacrificed and perfused transcardially with PBS. Proteins from the hippocampi and cortices were extracted in RIPA buffer (50mM Tris-HCL, pH 8.0M NaCl, 0.1% Triton X-100) with phosphatase and protease inhibitors. Samples were sonicated for 10 sec, centrifuged at for 10 min at 14,000 rpm, and the supernatant was collected.
Western Blot Analyses
For all gels, 15-20 μg of protein from cell lysates or conditioned media were analyzed as described [8].
Antibodies
ApoE was detected by rabbit polyclonal antibody against rodent apoE (Abcam, Cambridge, MA). ABCA1 was detected by a monoclonal antibody (Biorad). Rabbit polyclonal antibodies against phospho-c-Jun (Ser 73) and phospho-JNK (Thr183/Tyr185) were from Cell Signaling Technology (Beverly, MA). All blots were probed with a monoclonal β-actin (Abcam) antibody to ensure equal protein levels in each lane.
Chemicals
SP600125 was purchased from Invitrogen. L-JNK1 and LPS were purchased from Calbiochem (San Diego, CA). Wortmannin and PD98059 were purchased from Sigma.
Quantitative RT-PCR
Total RNA was isolated from cultures using Stratagene Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA). cDNA was synthesized using Affinity Script QPCR cDNA Synthesis Kit. cDNA (1 μl) was amplified by real-time PCR using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Samples were standardized to β-actin message. Synthetic oligonucleotides ApoE (sense-TCGGAAGGAGCTGACTGG and antisense- CCAGGGTTGGTTGCTTTG) and β-actin (sense-TGACAGGATGCAGAAGGAGA and antisense- ACATCTGCTGGAAGGTGGAC) were used. Each individual sample was analyzed in triplicate and RNA levels are reported as fold change compared with control. Analysis of real-time amplification data was done on SDS 2.3 (Applied Biosystems) and relative quantities were calculated using RQ Manager software (Applied Biosystems).
Nitrite Quantification
The production of NO from glial cultures was assessed as previously described [8] using a colorimetric reaction with Griess reagent (Invitrogen).
Statistical Analysis
All experiments were repeated a minimum of three times. The data were analyzed using Graphpad Prism 4, performing either ANOVA with Newman-Keuls Multiple Comparison post-hoc test or Student’s t-test analysis with significance determined at a P value of <0.05.
RESULTS
JNK-Dependent Effects of Glial Stimulation on NO Production
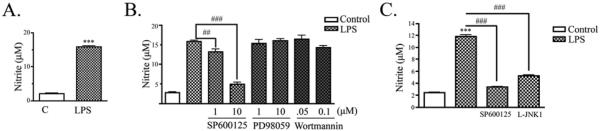
We have previously shown that LPS induces intracellular activation of JNK, downstream synthesis of inducible nitric oxide synthase (iNOS), and release of NO in microglia [8]. For the current experiments, mouse mixed glial cultures were treated with 100 ng/mL LPS in serum-free media. As expected, LPS treatment caused a significant increase in NO after 24 hrs of LPS stimulation (Fig. 1A). Primary mixed glia treated with LPS accumulated 16 ± 1 μM nitrite, compared with 2 ± 0.5 μM nitrite from untreated cells. We used inhibitors to the MAPK pathway family members, JNK (SP600125) and ERK (PD98059), and an inhibitor to the PI3K/Akt pathway (Wortmannin). Cells were treated with two doses of each inhibitor to test which signaling pathways mediated the NO response. LPS-activated mixed glia treated with SP600125 accumulated significantly less nitrite in a dose-dependent manner (13 ± 0.7 μM with 1 μM SP600126 and 5 ± 0.5 μM with 10 μM SP600125) when compared with control cultures (16 ± 0.3 μM nitrite) (Fig. 1B). Cultures treated with PD98059 or Wortmannin showed no attenuation of the LPS-induced nitrite response (Fig. 1B). We also tested whether a different class of JNK inhibitor, L-JNK1, would also overcome LPS-induced nitrite accumulation. As above, cells treated with LPS and SP600125 accumulated significantly less nitrite (3 ± 0.1 μM) than cells treated with LPS alone (12 ± 0.3 μM nitrite). Similarly, cells treated with LPS and L-JNK1 also accumulated significantly less nitrite (5 ± 0.1μM) than cells treated with LPS alone (Fig. 1C). Taken together, these findings show that JNK activation, but not ERK or PI3K/Akt activation, is important for LPS-induced NO accumulation in mixed glia.
Figure 1. Activation of glia by LPS increased NO production in a JNK-dependent manner.
A) In primary mixed glial cultures, LPS promoted nitrite accumulation in the conditioned media at 24 hr (mean ± SEM ; ***P < 0.001; n = 4). B) Primary glial cultures were treated with indicated doses of a JNK inhibitor (SP600125), ERK inhibitor (PD98059), or PI3K/Akt inhibitor (Wortmannin) and stimulated with LPS for 24 hrs. SP600125 treatment reduced nitrite production in a dose-dependent manner. PD98059 and Wortmannin did not effect LPS-induced nitrite production (mean ± SEM; ## P < 0.01, ### P < 0.001 compared to indicated cultures; n = 3). C) Primary glia were treated with 10 μM SP600125 or 1 μM L-JNK1 and stimulated with LPS for 24 hrs. Both JNK inhibitors reduced nitrite production (mean ± SEM; ***P < 0.001 compared to control culture; ###P < 0.001 compared to indicated cultures; n = 4).
Activation of glia decreases apoE production and secretion
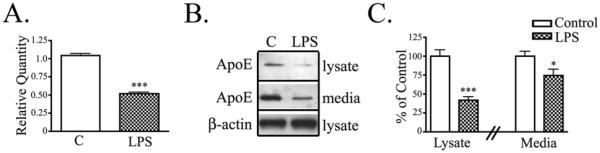
Previous reports show that apoE is downregulated in microglia activated with LPS [8; 15]. We tested whether there were similar effects in primary mixed glial cultures since most apoE in vivo is synthesized in astrocytes [13; 16; 17]. Mixed glial cultures treated with LPS for 24 hrs expressed 48% less APOE mRNA than control untreated cultures (Fig. 2A). LPS treatment also reduced endogenous apoE protein in cell lysates (by 58%) and conditioned media (by 26%) of primary glia (Fig. 2B, C). Thus, LPS decreased both APOE mRNA and protein levels in mixed astrocyte/microglial cultures.
Figure 2. Glial activation decreased apoE production and secretion.
Primary mixed glial cultures were treated with LPS for 24 hrs. A) APOE mRNA was measured by Real-Time PCR. Relative Quantity of APOE expression shows that LPS treatment reduced APOE mRNA (mean ± SEM; *** P < 0.001; n = 6). B) Cell lysates and conditioned media were analyzed by Western blotting with antibodies to apoE and β-actin. Representative blots show that LPS reduced apoE levels in the cell lysate and conditioned media. C) Western blot data were quantified as percent of control apoE in the cell lysate or media. Cells treated with LPS showed a decrease in lysate apoE and secreted apoE compared with control untreated cells (mean ± SEM; * P < 0.05, *** P < 0.001 compared to corresponding control cultures; n = 6).
Inhibition of JNK increases ApoE production and secretion
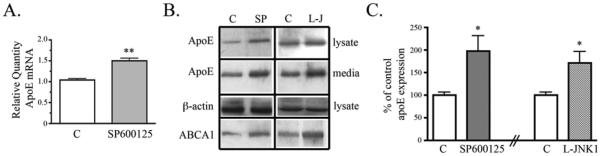
Data so far demonstrate that JNK inhibition decreased glial activation, and glial activation decreased apoE expression. We next tested whether JNK inhibition alone increased apoE expression. Glial cultures treated with SP600125 showed a significant 50% increase in APOE mRNA compared to control untreated glial cultures (Fig. 3A) and increased apoE protein in both cell lysates and conditioned media (Fig. 3B). SP600125 treatment increased apoE protein levels by 98% (Fig. 3C). Treatment of glial cultures with another JNK inhibitor, L-JNK1, also significantly increased apoE by 72% (Fig. 3B, C).
Figure 3. Inhibition of JNK increased apoE production and secretion in glia.
Primary glial cultures were treated with JNK inhibitors, SP600125 (10uM) or L-JNK1 (1uM), for 24 hrs. A) APOE mRNA was measured by Real-Time PCR. Relative Quantity of APOE expression shows SP600125 increased APOE mRNA (mean ± SEM; ** P < 0.01; n = 6). B) Cell lysates and conditioned media were analyzed by Western blotting with antibodies to apoE, ABCA1, and β-actin. Representative blots show that SP600126 (SP) and L-JNK1 (L-J) treatment increased apoE and ABCA1 levels in the cell lysate and increased apoE in the conditioned media. C) Western blot data were quantified as percent of control apoE in the cell lysate. Cells treated with SP600125 or L-JNK1 showed increased lysate apoE compared with control untreated cells (mean ± SEM; * P < 0.05 compared to corresponding control cultures; n = 6 for SP600125 treatment and n = 3 for L-JNK1 treatment).
We also studied expression of ABCA1, which transports cholesterol and phospholipids across the membrane to apoE [18]. We hypothesized that apoE and ABCA1 may be co-regulated by the JNK pathway. Indeed, we observed that inhibition of JNK with either SP600125 or L-JNK1 increased both apoE and ABCA1 (Fig. 3B).
Inhibition of JNK increases apoE expression in vivo
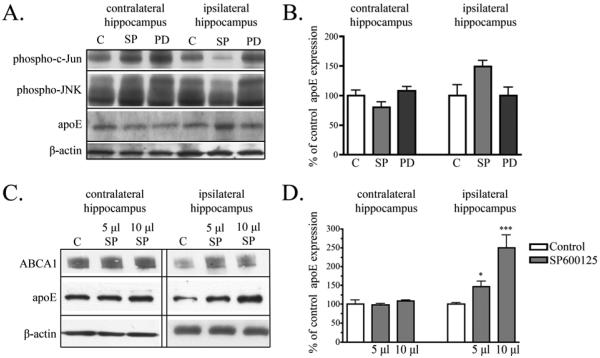
We have shown that inhibition of JNK increased APOE mRNA and protein expression in mixed glial cultures (Figs. 2 & 3). We next tested whether similar effects were observed in vivo. For our first experiments, we injected 5 μl SP600125 (10 mM), 5 μl PD98059 (10 mM) or 5 μl vehicle control into the right hippocampus of adult mice (n=3 for each condition). After 24 hrs, animals injected with SP600125 showed decreased phospho-c-Jun and phospho-JNK in the ipsilateral hippocampus compared to animals with either vehicle injection or compared to the contralateral hippocampus (Fig. 4A). These decreases signified that SP600125 was active in vivo.
Figure 4. Inhibition of JNK in vivo increased apoE.
A) 5μl vehicle control (C), 5μl SP600125 (SP) (10mM), or PD98059 (PD) (10mM) was injected into the hippocampus of adult mice. Western blot analysis revealed that injection of SP into the hippocampus decreased phospho-c-Jun and phospho-JNK expression. ApoE levels in the ipsilateral hippocampus increased with SP injection, but remained unchanged with PD injection. Across samples β-actin levels remained unchanged. B) Quantification of Western blot analysis revealed the apoE levels increased by 49 % with SP injection compared to control injection (mean ± SEM, n = 3). C) 5 μl or 10 μl SP (10mM) or vehicle control (5 or 10 μl) was injected into the hippocampus of adult mice. Representative blots show that apoE and ABCA1 levels were similar in the contralateral hippocampus from animals injected with either vehicle (C), 5μl or 10μl SP. ApoE and ABCA1 levels in the ipsilateral hippocampus increased in animals injected with SP. Levels of β-actin remained unchanged. D) Quantification of Western blot analysis revealed a significant increase in apoE with SP600125 (Mean ± SEM; * P < 0.05, *** P < 0.001 compared to vehicle control injected animals; n = 4 for vehicle control, n = 4 for 5μl SP600125, n = 2 for 10μl SP600125 injection).
We analyzed changes to apoE expression in these animals and found that animals injected with SP600125 showed a 49 % increase in apoE in the ipsilateral hippocampus when compared to control injected animals, although this increased did not reach statistical significance (p < 0.06). Levels of apoE in the contralateral hippocampus were similar to those in control ipsilateral hippocampus (Fig. 4A & B), and the ERK inhibitor PD98059 did not affect hippocampal apoE levels (Fig. 4A).
While these preliminary experiments did not demonstrate statistically significant differences with SP600125, we had observed a potentially large effect. We repeated the experiment, including a group with a larger amount of SP600125. For these experiments, we injected 5 μl or 10 μl SP600125 (10 mM) or vehicle control into the right hippocampus of mice. After 24 hrs, animals injected with either 5 μl or 10 μl SP600125 showed increased apoE expression in the ipsilateral hippocampus when compared to control injected animals (Fig. 4C), while apoE expression in the contralateral cortex remained unchanged (data not shown). Levels of hippocampal apoE were similar in the contralateral hippocampus from animals injected with vehicle or either 5 μl or 10 μl SP600125 (Fig. 4C). ApoE levels in the ipsilateral hippocampus significantly increased in both sets of animals injected with SP600125 when compared to vehicle injected animals (Fig. 4C). Mice injected with 5 μl SP600125 had more apoE than control injected mice (48 ± 14 %; P < 0.05), similar to the effect size seen in the previous set of animals (Fig. 4B). Mice injected with 10 μl SP600125 had a 150 ± 34 % increase in apoE (P < 0.001) (Fig. 4D). The contralateral hippocampus showed no differences in apoE between control, 5 μl or 10 μl SP600125 injection. We also observed that ABCA1 increased with apoE in mice injected with 5 μl or 10 μl SP600125 (Fig. 4C). ABCA1 expression was unchanged in the contralateral hippocampus.
In a third independent experiment, we injected 10 μl SP600125 into the hippocampus of 3 mice and compared them with 3 vehicle injected mice. This experiment also revealed that SP600125 injection into the hippocampus of mice increased apoE expression significantly when compared to control injected mice. The increase in apoE expression in the ipsilateral hippocampus of SP600125 mice was 120 ± 27 % compared to the ipsilateral hippocampus of control injected mice (data not shown). In this experiment, we also observed a small increase in the ipsilateral cortex after SP600125 compared to vehicle-injected controls (30 ± 10 %) (data not shown). Thus, we have consistently observed an increase in apoE levels with SP600125 in the mouse hippocampus, supporting the hypothesis that inhibition of JNK increases apoE expression in vivo.
DISCUSSION
We found that inhibition of JNK promotes expression of APOE mRNA and protein. When we inhibited JNK, APOE expression increased in mixed glial cultures in vitro and in mouse brains. We also demonstrated that an inflammatory response in glial cells stimulated with LPS reduced APOE mRNA and protein expression. The response to LPS was overcome by modulating JNK activation in glial cells. We hypothesize that LPS stimulation increases phospho-JNK, activating downstream c-Jun and potentially interfering with transcription of APOE mRNA.
The JNK family includes three isoforms (JNK1, JNK2, and JNK3) which are expressed in glial cells [19] and inhibited by the compounds used in this study. JNK phosphorylation leads to the activation of c-Jun and AP-1, a transcription factor composed of heterodimers of family members, which include Jun, Fos and ATF [20]. Overexpression of a dominant negative c-Jun by peripheral adenovirus-mediated gene transfer increased APOE mRNA levels in the liver and increased apoE plasma levels [21]. This work supports our findings that inhibition of c-Jun activation increases APOE expression.
We have previously shown that apoE protects against inflammatory signaling [8]. ApoE has been implicated in modulating the CNS inflammatory response in an isoform-dependent manner (apoE2 > apoE3 > apoE4) [9]. ApoE4 has major structural characteristics that distinguish if from apoE2 or apoE3 due to the presence of arginines at amino acid positions 112 and 158. The Arg112 affects the conformation of Arg61 side chain and a domain interaction between the carboxyl-terminal and amino-terminal domains of apoE4 [22]. The changes in apoE4 structure diminish the stability of apoE4 [23] leading to preferential degradation of apoE4 by glial cells [24]. In APOE ε2, APOE ε3, and APOE ε4 targeted replacement mice, levels of brain apoE protein vary, with decreased level of apoE in APOE ε4 targeted replacement mice [9; 24]. These findings suggest that the apoE isoform associated risks may be related to the expression of apoE protein. More recent work of APP transgenic mice crossed with APOE targeted replacement mice also demonstrated that apoE4 is associated with increased Aβ deposition [25]. These investigators speculated that increasing apoE levels could have beneficial effects in preventing Aβ accumulation [25]. Thus, the present work demonstrating that apoE can be increased by JNK inhibitors, which would have positive anti-inflammatory effects, may also affect Aβ accumulation in the brain.
A number of pharmacological JNK inhibitors have been discovered that target various portions of the JNK signaling pathway [26] and these compounds have entered clinical trials to treat leukemia and other cancers [26; 27]. In the CNS, various injuries trigger activation of JNKs, including pathological entities in AD [28], Parkinson’s disease [29], and ischemic injury [30]. Targeting JNK inhibition in the CNS may prove to protect against neurodegenerative insults, by either inhibiting JNK activation in neurons associated with increased apoptosis or preventing the inflammatory response in glial cells. JNK inhibitors may prove useful in part by increasing apoE and its protective anti-inflammatory properties.
ACKNOWLEDGEMENTS
The study was supported by NIH AG14473 to GWR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- [2].Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–81. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Selkoe DJ. Alzheimerʹs disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- [4].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimerșs disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- [5].Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, Bennett ER. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- [6].Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–13. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- [7].Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–33. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- [8].Pocivavsek A, Burns MP, Rebeck GW. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia. 2009;57:444–53. doi: 10.1002/glia.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grehan S, Tse E, Taylor JM. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci. 2001;21:812–22. doi: 10.1523/JNEUROSCI.21-03-00812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–61. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- [12].Uchihara T, Duyckaerts C, He Y, Kobayashi K, Seilhean D, Amouyel P, Hauw JJ. ApoE immunoreactivity and microglial cells in Alzheimerșs disease brain. Neurosci Lett. 1995;195:5–8. doi: 10.1016/0304-3940(95)11763-m. [DOI] [PubMed] [Google Scholar]
- [13].Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991;11:97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- [14].Gafencu AV, Robciuc MR, Fuior E, Zannis VI, Kardassis D, Simionescu M. Inflammatory signaling pathways regulating ApoE gene expression in macrophages. J Biol Chem. 2007;282:21776–85. doi: 10.1074/jbc.M611422200. [DOI] [PubMed] [Google Scholar]
- [15].Saura J, Petegnief V, Wu X, Liang Y, Paul SM. Microglial apolipoprotein E and astroglial apolipoprotein J expression in vitro: opposite effects of lipopolysaccharide. J Neurochem. 2003;85:1455–67. doi: 10.1046/j.1471-4159.2003.01788.x. [DOI] [PubMed] [Google Scholar]
- [16].Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–13. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murakami M, Ushio Y, Morino Y, Ohta T, Matsukado Y. Immunohistochemical localization of apolipoprotein E in human glial neoplasms. J Clin Invest. 1988;82:177–88. doi: 10.1172/JCI113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–72. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- [19].Hidding U, Mielke K, Waetzig V, Brecht S, Hanisch U, Behrens A, Wagner E, Herdegen T. The c-Jun N-terminal kinases in cerebral microglia: immunological functions in the brain. Biochem Pharmacol. 2002;64:781–8. doi: 10.1016/s0006-2952(02)01139-5. [DOI] [PubMed] [Google Scholar]
- [20].Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998;10:205–19. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- [21].Drosatos K, Sanoudou D, Kypreos KE, Kardassis D, Zannis VI. A dominant negative form of the transcription factor c-Jun affects genes that have opposing effects on lipid homeostasis in mice. J Biol Chem. 2007;282:19556–64. doi: 10.1074/jbc.M700986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dong LM, Weisgraber KH. Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J Biol Chem. 1996;271:19053–7. doi: 10.1074/jbc.271.32.19053. [DOI] [PubMed] [Google Scholar]
- [23].Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–54. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [24].Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–53. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–9. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–65. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- [27].Bogoyevitch MA, Arthur PG. Inhibitors of c-Jun N-terminal kinases: JuNK no more? Biochim Biophys Acta. 2008;1784:76–93. doi: 10.1016/j.bbapap.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–60. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Crocker SJ, Lamba WR, Smith PD, Callaghan SM, Slack RS, Anisman H, Park DS. c-Jun mediates axotomy-induced dopamine neuron death in vivo. Proc Natl Acad Sci U S A. 2001;98:13385–90. doi: 10.1073/pnas.231177098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–9. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]