Abstract
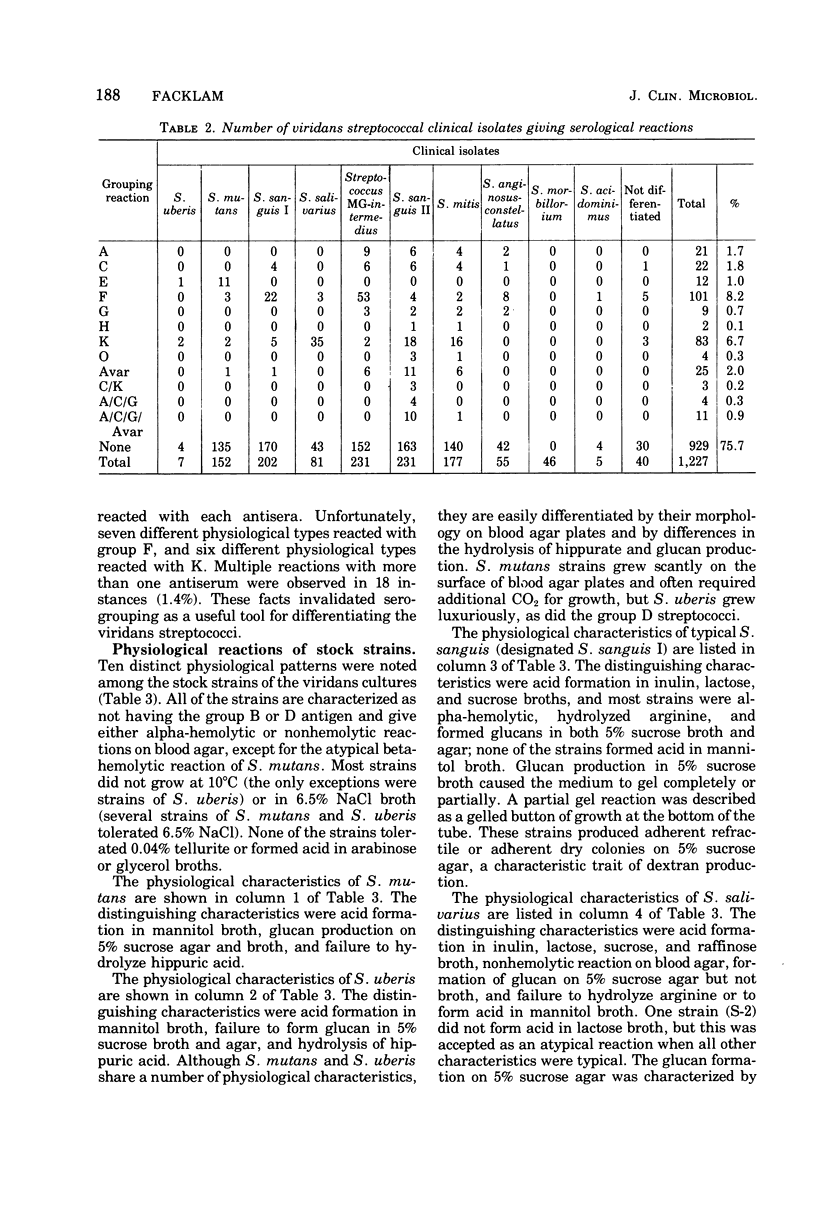
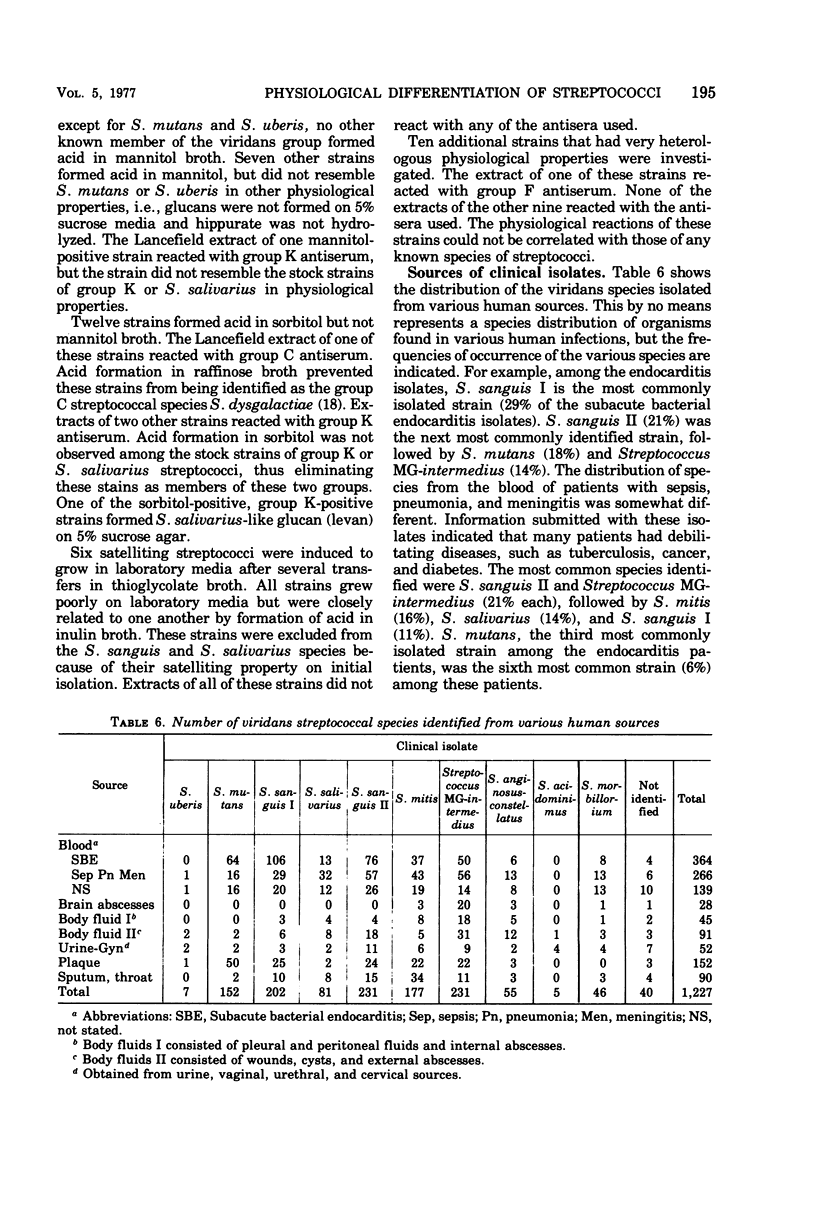
Twelve hundred and twenty-seven clinical isolates and eighty stock strains of viridans streptococci were tested for serological and physiological characteristics. Because the serological reactions of these strains varied, a differentiation scheme could not be based on these reactions. For the same reason, there could be no correlation of serological characteristics with physiological characteristics. Nearly 97% of the clinical isolates were speciated by differences in physiological characteristics. Ten different physiological species were recognized. The physiological speciation scheme was based on stable enzymatic reactions rather than on results of tolerance tests. The study included air-tolerant anaerobic streptococcal strains as well as viridans streptococcal strains not normally found in humans. The differentiation scheme and nomenclature of the author are related to those of other investigators. Differences in the distribution of species isolated from different clinical sources and human infections were also noted. A key for the differentiation of human isolates of viridans streptococci is proposed.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D., Pettersson B. M. Common and unique antigens of Streptococcus mutants. J Dent Res. 1976 Jan;55:A60–A64. doi: 10.1177/002203457605500123011. [DOI] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Cole R. M., Calandra G. B., Huff E., Nugent K. M. Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res. 1976 Jan;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- Colman G. The application of computers to the classification of streptococci. J Gen Microbiol. 1968 Jan;50(1):149–158. doi: 10.1099/00221287-50-1-149. [DOI] [PubMed] [Google Scholar]
- Colman G. Transformation of viridans-like streptococci. J Gen Microbiol. 1969 Aug;57(2):247–255. doi: 10.1099/00221287-57-2-247. [DOI] [PubMed] [Google Scholar]
- Colman G., Williams R. E. The cell walls of streptococci. J Gen Microbiol. 1965 Dec;41(3):375–387. doi: 10.1099/00221287-41-3-375. [DOI] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L., Specht P. A. DNA base sequence homologies among strains of Streptococcus sanguis. J Gen Microbiol. 1975 Nov;91(1):92–98. doi: 10.1099/00221287-91-1-92. [DOI] [PubMed] [Google Scholar]
- Cullen G. A. Classification of Streptococcus uberis with biochemical tests. Res Vet Sci. 1967 Jan;8(1):83–88. [PubMed] [Google Scholar]
- FARMER E. D. Serological subdivisions among the Lancefield group H streptococci. J Gen Microbiol. 1954 Oct;11(2):131–138. doi: 10.1099/00221287-11-2-131. [DOI] [PubMed] [Google Scholar]
- FARMER E. D. Streptococci of the mouth and their relationship to subacute bacterial endocarditis. Proc R Soc Med. 1953 Mar;46(3):201–208. [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Comparison of several laboratory media for presumptive identification of enterococci and group D streptococci. Appl Microbiol. 1973 Aug;26(2):138–145. doi: 10.1128/am.26.2.138-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Moody M. D. Presumptive identification of group D streptococci: the bile-esculin test. Appl Microbiol. 1970 Aug;20(2):245–250. doi: 10.1128/am.20.2.245-250.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Padula J. F., Thacker L. G., Wortham E. C., Sconyers B. J. Presumptive identification of group A, B, and D streptococci. Appl Microbiol. 1974 Jan;27(1):107–113. doi: 10.1128/am.27.1.107-113.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. Induction of dental caries in gnotobiotic rats with a levan-forming streptococcus and a streptococcus isolated from subacute bacterial endocarditis. Arch Oral Biol. 1968 Mar;13(3):297–308. doi: 10.1016/0003-9969(68)90128-3. [DOI] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. Physiological classification of oral viridans streptococci. J Dent Res. 1976 Jan;55:A166–A176. doi: 10.1177/002203457605500108011. [DOI] [PubMed] [Google Scholar]
- Harrell W. K., George J. R. Quantitative measurement of precipitating antibodies in streptococcal grouping antisera by the single radial immunodiffusion technique. Appl Microbiol. 1972 Jun;23(6):1047–1052. doi: 10.1128/am.23.6.1047-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmeier C., Washington J. A., 2nd Microbiological study of streptococcal bacteremia. Appl Microbiol. 1973 Oct;26(4):589–591. doi: 10.1128/am.26.4.589-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablon J. M., Zinner D. D. Differentiation of cariogenic streptococci by fluorescent antibody. J Bacteriol. 1966 Dec;92(6):1590–1596. doi: 10.1128/jb.92.6.1590-1596.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. 3. Immunochemical studies of the cross-reactivity between the cell wall antigens of a filamentous streptococcus and of pneumococcus. Infect Immun. 1973 Dec;8(6):969–976. doi: 10.1128/iai.8.6.969-976.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. II. Antigenic structure of provisional capsular types 89 and 83-89. Infect Immun. 1973 Dec;8(6):962–968. doi: 10.1128/iai.8.6.962-968.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Buettger C., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. I. Antigenic attributes of provisional capsular type 83 and its relationship to streptococci of so-called group M. Infect Immun. 1973 Dec;8(6):952–961. doi: 10.1128/iai.8.6.952-961.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast A. Comparative statistical investigations regarding incidence, etiology and topography of subacute bacterial endocarditis. Jpn Circ J. 1971 Oct;35(10):1203–1212. doi: 10.1253/jcj.35.1203. [DOI] [PubMed] [Google Scholar]
- Koshi G., John L. Lancefield group F streptococci causing liver abscess and empyema. Indian J Med Res. 1971 Jan;59(1):45–49. [PubMed] [Google Scholar]
- Kothari G. C., Willers J. M., Michel M. F. Immunochemistry of the carbohydrate antigens of some Streptococcus salivarius strains. J Gen Microbiol. 1971 Sep;68(1):77–86. doi: 10.1099/00221287-68-1-77. [DOI] [PubMed] [Google Scholar]
- Lai C., Listgarten M., Rosan B. Serology of Streptococcus sanguis: localization of antigens with unlabeled antisera. Infect Immun. 1973 Sep;8(3):475–481. doi: 10.1128/iai.8.3.475-481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejàre B., Edwardsson S. Streptococcus milleri (Guthof); an indigenous organism of the human oral cavity. Arch Oral Biol. 1975 Nov;20(11):757–762. doi: 10.1016/0003-9969(75)90048-5. [DOI] [PubMed] [Google Scholar]
- Montague E. A., Knox K. W. Antigenic components of the cell wall of Streptococcus salivarius. J Gen Microbiol. 1968 Dec;54(2):237–246. doi: 10.1099/00221287-54-2-237. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Chemical composition and immunological specificity of the streptococcal group O cell wall polysaccharide antigen. Infect Immun. 1972 May;5(5):707–714. doi: 10.1128/iai.5.5.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Hydrolysis of Arginine by Streptococci. J Bacteriol. 1942 Jun;43(6):651–660. doi: 10.1128/jb.43.6.651-660.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTENS H., WINKLER K. C. Indifferent and haemolytic streptococci possessing group-antigen F. J Gen Microbiol. 1962 Apr;28:181–191. doi: 10.1099/00221287-28-1-181. [DOI] [PubMed] [Google Scholar]
- PORTERFIELD J. S. Classification of the streptococci of subacute bacterial endocarditis. J Gen Microbiol. 1950 Jan;4(1):92–101. doi: 10.1099/00221287-4-1-92. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELBIE F. R., SIMON R. D., ROBINSON R. H. M. Serological classification of viridans streptococci from subacute bacterial endocarditis, teeth, and throats. Br Med J. 1949 Sep 24;2(4629):667–672. doi: 10.1136/bmj.2.4629.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman J. M., Niven C. F., Smiley K. L. Streptococcus salivarius and Other Non-hemolytic Streptococci of the Human Throat. J Bacteriol. 1943 Mar;45(3):249–263. doi: 10.1128/jb.45.3.249-263.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman R. D., Nord N., Brown R. W., Wessman G. E. Biochemical and serological characteristics of Lancefield groups E, P, and U Streptococci and Streptococcus uberis. Cornell Vet. 1972 Oct;62(4):540–568. [PubMed] [Google Scholar]
- WILLERS J. M., OTTENS H., MICHEL M. F. IMMUNOCHEMICAL RELATIONSHIP BETWEEN STREPTOCOCCUS MG, F 3 AND STREPTOCOCCUS SALIVARIUS. J Gen Microbiol. 1964 Dec;37:425–431. doi: 10.1099/00221287-37-3-425. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. E. Streptococcus salivarius (vel hominis) and its relation to Lancefield's group K. J Pathol Bacteriol. 1956 Jul;72(1):15–25. doi: 10.1002/path.1700720103. [DOI] [PubMed] [Google Scholar]
- Washburn M. R., White J. C., Niven C. F., Jr Streptococcus S.B.E.: Immunological Characteristics. J Bacteriol. 1946 Jun;51(6):723–729. doi: 10.1128/jb.51.6.723-729.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. C., Niven C. F., Jr Streptococcus S.B.E.: A Streptococcus Associated with Subacute Bacterial Endocarditis. J Bacteriol. 1946 Jun;51(6):717–722. doi: 10.1128/jb.51.6.717-722.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willers J. M., Alderkamp G. H. Loss of type antigen in a type 3 streptococcus and identification of the determinant disaccharide of the remaining antigen. J Gen Microbiol. 1967 Oct;49(1):41–51. doi: 10.1099/00221287-49-1-41. [DOI] [PubMed] [Google Scholar]


