Abstract
Various subtypes of nicotinic cholinergic receptors are expressed in autonomic ganglia. The distinct functional roles of these receptors in autonomic ganglionic transmission to different target organs remain to be elucidated. In this study, we tested the sympathetic and parasympathetic cardiovascular responses to nicotinic agonist and antagonists in urethane-anesthetized mice. Intravenous injection with a nicotinic agonist, 1,1-Dimethyl-4-phenylpiperazinium iodide, induced a brief but pronounced decrease in heart rate, followed by significant increases in heart rate and arterial blood pressure. The bradycardic response was blocked by atropine whereas the pressor response was blocked by prazosine, confirming those responses were parasympathetic and sympathetic activities, respectively. The sympathetic response was blocked by Methyllycaconitine citrate, a selective α7 nAchR antagonist. The parasympathetic response was blocked by a selective α4β2 nAchR antagonist, dihydro-β-erythroidine hydrobromide. Moreover, injection with a selective α4β2 nAchR agonist, RJR2403 oxalate, induced a pronounced parasympathetic response with a smaller sympathetic response. Collectively, these data show that activations of α4β2 nAchRs elicits a parasympathetic cardiovascular response and activation of α7 nAchRs elicits a sympathetic cardiovascular response. These data suggest that specific subtypes of nicotinic receptors at the level of the ganglia may play distinct roles in mediating sympathetic or parasympathetic activation.
Keywords: nicotinic receptor, autonomic nerves, ganglia, heart rate, blood pressure
INTRODUCTION
Sympathetic (SNS) and parasympathetic (PSNS) nervous systems consist of pre-ganglionic and post-ganglionic fibers. Forming synapses with pre-ganglionic fibers, neurons at autonomic ganglia project post-ganglionic fibers to target organs. Increasing evidence suggests that the autonomic ganglia do not just simply relay the sympathetic and parasympathetic pre-ganglionic signals but play an integrative role in regulation of the autonomic function[4, 9, 23]. Moreover, there is evidence that anatomical and functional properties of autonomic ganglia may vary dependent on specific target organs[7, 15]. The different functional features of autonomic ganglia are associated with the diversity of nicotinic cholinergic receptors (nAChRs) that mediate autonomic ganglionic transmission[5, 23].
There are at least 7 α subunits and 3 β subunits cloned[1] in mammalian neuronal tissues. Various combinations of the subunits form the pentameric structure of the nAchRs. Many nAchR subunits, including α3, α4, α7, β2 and β4, have been found expressed in both sympathetic and parasympathetic ganglia, including parasympathetic intracardiac ganglia[18, 22]. However, the precise roles of different nAchR subtypes at the ganglionic level in sympathetic and parasympathetic regulation of particular target tissues/organs remain to be defined. In this study, we attempted to test the possible selective roles of α7 and α4β2 nAchRs in autonomic regulation of cardiovascular responses using specific nicotinic agonists and antagonists in anesthetized mice.
METHODS
Animals
Male Swiss Webster mice (Harlan Inc., Indianapolis, IN) aged 12–14 weeks old were used in this study. Mice were maintained on commercially available normal mouse chow (Harlan) and tap water in an environment with a 12:12-h light-dark cycle and ambient temperature (22°C). All experimental procedures in the present study were approved by Institutional Animal Care and Use Committee of the University of South Dakota, and all of the procedures were in accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH)].
Surgery and hemodynamic measurements
The mice were anesthetized with urethane (2g/kg, ip). This anesthesia ensures a consistent stable hemodynamic baseline without cardiac inhibition. The trachea was intubated to facilitate spontaneous breathing. The right common carotid artery was catheterized with Millar pressure catheter (Model SPV-1049, Millar Inc., Houston, TX). The arterial blood pressure and heart rate were measured via the catheter which was connected to the PowerLab data-acquisition system (ADIstruments Inc., Springfield, CO). In addition, the left common jugular vein was isolated and cannulated for intravenous injections with test substances.
Treatments and responses
nAchRs agonists and antagonists used in this study include: 1,1-Dimethyl-4-phenylpiperazinium iodide (DMPP, D5891, Sigma-Aldrich), a non-selective nAchR agonist; Hexamethonium (H0879, Sigma-Aldrich), a non-selective ganglionic nAchR blocker; Methyllycaconitine citrate (MLA, 1029, Tocris Bioscience), a selective α7 nAchR antagonist; Dihydro-β-erythroidine hydrobromide (DHβE, 2349, Tocris Bioscience), a selective α4β2 nAchRs antagonist; RJR2403 oxalate (1053, Tocris Bioscience), a selective α4β2 nAchRs agonist. Each drug was dissolved in normal saline to appropriate concentrations.
Prior to each treatment, baselines of heart rate and arterial blood pressure were recorded. Then a nAchRs agonist or antagonist was quickly injected via the jugular vein catheter and a recording was taken of the entire course of the response. The peak changes from the baseline were calculated and compared.
To test the role of α7 nAchRs in DMPP induced responses, the responses to DMPP alone and the responses to DMPP following the pretreatment with MLA were measured in one animal, with an interval of 15 minutes between the two DMPP administrations. The same protocol was used to test the responses to DMPP alone versus DMPP after the pretreatment with DHβE, and the responses to RJR alone versus RJR following pretreatment with DHβE. Five mice were used for each set of the above experiments.
Statistical analysis
The compiled data are expressed as means ± s.d. Comparison of data were made using one-way analysis of variance (ANOVA) followed by Student– Newman–Keuls test. Significance was accepted when P value was less than 0.05.
RESULTS
Intravenous injection with non-selective nAchR agonist DMPP (0.1mg/kg) induced a brief but pronounced decrease in heart rate, which was immediately followed by a dramatic increase in heart rate and blood pressure. The decreased heart rate was abolished by the muscarinic receptor antagonist, atropine (0.5mg/kg), whereas the pressor response was blocked by the adrenergic α1 receptor antagonist, prazosin (0.3 mg/kg) (Figure 1). These data confirm that the bradycardic response and the pressor responses were parasympathetic and sympathetic activities, respectively. Moreover, intravenous pre-administration of ganglionic blocker, hexamethonium (0.5 mg/kg), largely blocked both parasympathetic and sympathetic responses induced by DMPP, indicating that these responses were caused by the stimulation of nAchRs in ganglia (Figure 1).
Figure 1.
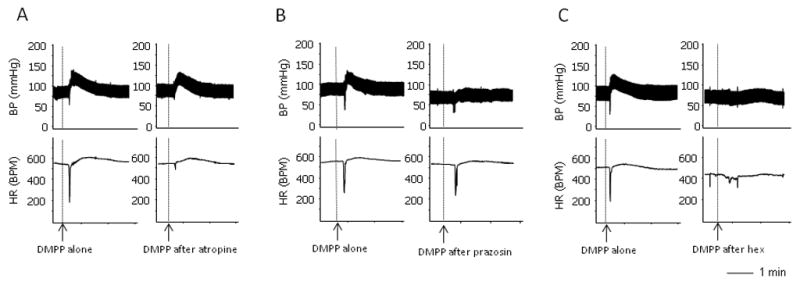
Raw recordings showing the DMPP-induced sympathetic and parasympathetic responses. A. DMPP induced a quick decrease in heart rate, followed by an increase in heart rate and blood pressure. Pre-administration of atropine blocked the bradycardic response with no effect on pressor response. B. Pre-administration of prazosine, eliminated the DMPP-induced pressor response. C. Pre-administration of ganglionic blocker hexamethonium largely blocked all components of the responses induced by DMPP.
It was found that α7 nAchRs was involved in autonomic activity[10]. We wanted to test whether the activation of α7 nAchRs are involved in these DMPP-induced responses. Pretreatment with MLA (1mg/kg), a selective α7 antagonist, significantly attenuated the pressor response induced by DMPP but elicited little effect on the parasympathetic bradycardic response (Figure 2A). This result suggests that the activation of α7 nAchRs is involved in sympathetic but not parasympathetic activities.
Figure 2.

A. Effects of α7 nAchRs antagonist MLA on the DMPP-induced responses. The raw recordings (top) show that pre-administration of MLA largely blocked the DMPP-induced sympathetic responses with no effect on the parasympathetic response. Mean data (bottom) show that compared with the treatment with DMPP alone (open bars), pre-administration of MLA (solid bars) significantly abolished the DMPP-induced pressor response and the increase in HR with no effect on the decrease in HR. B. Effects of α4β2 nAchRs antagonist DHβE on the DMPP-induced responses. Raw recordings (top) show that pre-administration of DHβE largely blocked the DMPP-induced parasympathetic response with no effect on the sympathetic response. Mean data (bottom) show that, compared with the DMPP alone (open bars), pre-administration of DHβE (solid bars) significantly attenuated the DMPP-induced decrease in heart rate with no effect on the pressor response and the increase in HR. “*” represents p<0.05 compared to the DMPP alone. n= 5
In contrast to MLA, the pretreatment with DHβE (1mg/kg), a selective α4β2 nAchR antagonist, blocked the bradycardic responses induced by DMPP. However, DHβE did not affect the pressor response induced by DMPP (Figure 2B).
Moreover, a selective α4β2 nAchRs agonist, RJR2403 oxalate, primarily induced a bradycardic response with a slight pressor response. The bradycardic response induced by RJR 2403 was blocked by DHβE (Figure 3). Collectively, these data suggest that the activation of α4β2 nAchRs are involved in the parasympathetic bradycardic response induced by the nicotinic stimulations.
Figure 3.
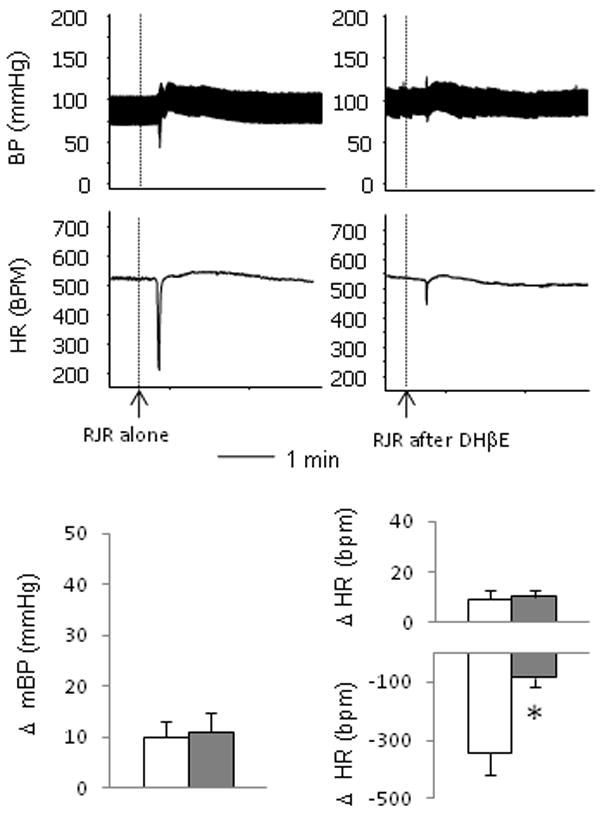
The α4β2 nAchRs agonist RJR 2403 oxalate (RJR) induced responses. Raw recordings (top) show that intravenous injection of RJR induced a major parasympathetic response with slight sympathetic responses. Pre-administration of DHβE largely blocked the parasympathetic bradycardic response. Mean data (bottom) show that, compared with the RJR alone (open bars), pre-administration of DHβE (solid bars) significantly attenuated the parasympathetic bradycardic response induced by RJR. “*” represents p<0.05 compared to the DMPP alone. n= 5
DISCUSSION
Emerging studies suggest that the multiple subtypes of nAChRs expressed in ganglia may have distinct functional roles. However, the detail of the mechanisms remains to be elucidated. In this study, we found that in the DMPP-induced dual responses, the α7 nAchRs antagonist MLA and α4β2 nAchRs antagonist DHβE selectively attenuated the sympathetic and parasympathetic cardiovascular responses, respectively, suggesting that these two types of nAchRs may play distinct roles at ganglia in mediating autonomic regulation of cardiovascular function.
Previous studies have shown that α7 nAchRs in ganglia are involved in the autonomic regulation of cardiovascular activities[10, 13]. In Langendorff preparation of rat hearts, nicotine-induced decrease in heart rate was blocked by a α7 nAchRs antagonist, α-bungarotoxin, suggesting that α7 nAchRs may play a role in intracardiac ganglia to mediate the negative chronotropic (parasympathetic) effects[13]. However, in α7 nAchRs deficient mice, it was found that lack of α7 nAchRs did not change the baroreflex-induced[10] and vagal stimulation-induced[6] bradycardic response in vivo, suggesting that α7 nAchRs do not play a major role in the parasympathetic control of heart rate. Instead, it was found that sympathetic activity was impaired in α7 deficient mice[10]. Our data showed that specific α7 antagonist MLA significantly attenuated the pressor response but not the bradycardic response induced by DMPP. These data are consistent with the findings in α7 nAchRs knockout mice, suggesting that α7 nAchRs are involved in sympathetic but not parasympathetic regulation of cardiovascular function.
α4 and β2 subunits are also expressed in both sympathetic and parasympathetic ganglia[5]. However their functional roles in autonomic regulation of cardiovascular function are largely unknown. Our study showed for the first time that the specific α4β2 antagonist DHβE selectively blocked the parasympathetic bradycardic response with no effect on the sympathetic pressor response induced by DMPP. Consistently, the selectiveα4β2 nAchR agonist RJR2403 mainly induced the parasympathetic bradycardic response with a little effect on the sympathetic activity. These data suggest that activation of α4β2 nAchRs are selectively involved in the parasympathetic response in the heart.
Collectively, data from this study suggest that α7 and α4β2 may have different functional roles at ganglia in autonomic regulation of cardiovascular activities. Notably, neither administration of MLA nor DHβE significantly changes the baseline of blood pressure and heart rate, suggesting that both α7 andα4β2 nAchRs may not play a major role for the resting autonomic tone. Instead, our data suggest that the activation of α7 nAchRs contributes to increased sympathetic activity and the activation of α4β2 nAchRs contributes to increased parasympathetic activity.
It should be emphasized that the results from this study only suggest a function of α7 and α4β2 in the autonomic cardiovascular regulation. Their roles in autonomic regulations of other visceral organs remain to be elucidated. There is evidence that the expression and functional properties of subtypes of nAchRs in autonomic ganglia are dependent on the specific target tissues and organs[7, 15]. It is important to define the specific roles of different nAchRs in autonomic regulations of different organs. Interestingly, studies showed that α4 subunits of nAchRs are present in the intracardiac ganglia[2, 8] but absent in the gut enteric ganglia[19]. These data suggest that α4β2 nAchRs may be specifically involved in parasympathetic control of heart rate.
The findings in this study are clinically relevant. Impaired autonomic activity is a hallmark for a variety of cardiovascular diseases, including heart failure[21], hypertension[14], and diabetes[12, 17]. During these disease states, autonomic dysfunctions are commonly characterized as elevated sympathetic activity and suppressed parasympathetic activity. Blockade of sympatho-excitation using beta blockers provides beneficial effects to a variety of cardiovascular diseases. On the other hand, studies[3, 16, 20], including ours[11], suggest that enhancement of suppressed parasympathetic activity is beneficial to cardiovascular diseases. It is conceivable to believe that the special nAchRs at ganglia could be appropriate targets to regulate the altered autonomic balance. For example, as suggested by this study, blockade ofα7 nAchRs could be a new approach to attenuate the increased sympathetic activity, whereas stimulation of α4β2 nAchRs could specifically increase parasympathetic action on the heart. Collectively, a better understanding of the functional roles of different nAchRs in regulation of autonomic activities could lead to a new avenue to treat abnormal autonomic function in a variety of cardiovascular diseases.
It is also important to point out the limitation of this work. Although MLA, DHβE, and RJR2403 are well described and widely used specific nAchRs antagonists, these pharmacological approaches cannot sufficiently rule out the involvements of other nAchRs. Rather, our data invite further investigations using different approaches to confirm these findings. Nevertheless, this study provides interesting novel information to support the idea that the sympathetic and parasympathetic pathways at the ganglionic level are mediated by different nAchRs[6]. Specifically, our data showed that the activation of α7 nAchRs are involved in the sympathetic pressor response whereas α4β2 nAchRs are important for parasympathetic negative chronotropic effect. More studies need to be done to better understand the properties and interactions of the different nAchRs at ganglia for regulation of autonomic activities.
Acknowledgments
This study was supported partially by American Heart Association SDG grant No.0835256N, NIH COBRE grants No. P20 RR01766 and No. P20 RR015567, NIH INBRE grant No. P20 RR016479 and the USD Division of BBS research bridge grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson KB, Florholmen G, Winer LH, Tonnessen T, Christensen G. Regulation of neuronal type genes in congestive heart failure rats. Acta Physiol (Oxf) 2006;186:17–27. doi: 10.1111/j.1748-1716.2005.01503.x. [DOI] [PubMed] [Google Scholar]
- 3.Behling A, Moraes RS, Rohde LE, Ferlin EL, Nobrega AC, Ribeiro JP. Cholinergic stimulation with pyridostigmine reduces ventricular arrhythmia and enhances heart rate variability in heart failure. Am Heart J. 2003;146:494–500. doi: 10.1016/S0002-8703(03)00319-3. [DOI] [PubMed] [Google Scholar]
- 4.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation. 1999;99:2958–63. doi: 10.1161/01.cir.99.22.2958. [DOI] [PubMed] [Google Scholar]
- 5.De Biasi M. Nicotinic mechanisms in the autonomic control of organ systems. J Neurobiol. 2002;53:568–79. doi: 10.1002/neu.10145. [DOI] [PubMed] [Google Scholar]
- 6.Deck J, Bibevski S, Gnecchi-Ruscone T, Bellina V, Montano N, Dunlap ME. Alpha7-nicotinic acetylcholine receptor subunit is not required for parasympathetic control of the heart in the mouse. Physiol Genomics. 2005;22:86–92. doi: 10.1152/physiolgenomics.00085.2004. [DOI] [PubMed] [Google Scholar]
- 7.Devay P, McGehee DS, Yu CR, Role LW. Target-specific control of nicotinic receptor expression at developing interneuronal synapses in chick. Nat Neurosci. 1999;2:528–34. doi: 10.1038/9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorakova M, Lips KS, Bruggmann D, Slavikova J, Kuncova J, Kummer W. Developmental changes in the expression of nicotinic acetylcholine receptor alpha-subunits in the rat heart. Cell Tissue Res. 2005;319:201–9. doi: 10.1007/s00441-004-1008-1. [DOI] [PubMed] [Google Scholar]
- 9.Felix B, Catalin D, Miolan JP, Niel JP. Integrative properties of the major pelvic ganglion in the rat. J Auton Nerv Syst. 1998;69:6–11. doi: 10.1016/s0165-1838(97)00133-1. [DOI] [PubMed] [Google Scholar]
- 10.Franceschini D, Orr-Urtreger A, Yu W, Mackey LY, Bond RA, Armstrong D, Patrick JW, Beaudet AL, De Biasi M. Altered baroreflex responses in alpha7 deficient mice. Behav Brain Res. 2000;113:3–10. doi: 10.1016/s0166-4328(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 11.Freeling J, Wattier K, Lacroix C, Li YF. Neostigmine and pilocarpine attenuated TNF{alpha} expression and cardiac hypertrophy in the heart with pressure overload. Exp Physiol. 2007 doi: 10.1113/expphysiol.2007.039784. [DOI] [PubMed] [Google Scholar]
- 12.Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr Metab Cardiovasc Dis. 2005;15:441–9. doi: 10.1016/j.numecd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Ji S, Tosaka T, Whitfield BH, Katchman AN, Kandil A, Knollmann BC, Ebert SN. Differential rate responses to nicotine in rat heart: evidence for two classes of nicotinic receptors. J Pharmacol Exp Ther. 2002;301:893–9. doi: 10.1124/jpet.301.3.893. [DOI] [PubMed] [Google Scholar]
- 14.Julius S. Autonomic nervous system dysregulation in human hypertension. Am J Cardiol. 1991;67:3B–7B. doi: 10.1016/0002-9149(91)90813-z. [DOI] [PubMed] [Google Scholar]
- 15.Levey MS, Brumwell CL, Dryer SE, Jacob MH. Innervation and target tissue interactions differentially regulate acetylcholine receptor subunit mRNA levels in developing neurons in situ. Neuron. 1995;14:153–62. doi: 10.1016/0896-6273(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 17.Lindmark S, Wiklund U, Bjerle P, Eriksson JW. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of Type 2 diabetes patients and control subjects. Diabet Med. 2003;20:399–405. doi: 10.1046/j.1464-5491.2003.00920.x. [DOI] [PubMed] [Google Scholar]
- 18.Mandelzys A, Pie B, Deneris ES, Cooper E. The developmental increase in ACh current densities on rat sympathetic neurons correlates with changes in nicotinic ACh receptor alpha-subunit gene expression and occurs independent of innervation. J Neurosci. 1994;14:2357–64. doi: 10.1523/JNEUROSCI.14-04-02357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obaid AL, Nelson ME, Lindstrom J, Salzberg BM. Optical studies of nicotinic acetylcholine receptor subtypes in the guinea-pig enteric nervous system. J Exp Biol. 2005;208:2981–3001. doi: 10.1242/jeb.01732. [DOI] [PubMed] [Google Scholar]
- 20.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–71. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 21.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF, Jr, Mohanty PK. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest. 1990;85:1362–71. doi: 10.1172/JCI114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rust G, Burgunder JM, Lauterburg TE, Cachelin AB. Expression of neuronal nicotinic acetylcholine receptor subunit genes in the rat autonomic nervous system. Eur J Neurosci. 1994;6:478–85. doi: 10.1111/j.1460-9568.1994.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 23.Skok VI. Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci. 2002;97:1–11. doi: 10.1016/s1566-0702(01)00386-1. [DOI] [PubMed] [Google Scholar]


