Abstract
The effects of BMP2 on bone marrow stromal cell differentiation and bone formation after bone marrow ablation were determined using C57 BL/6J (B6) mice. Inhibition of BMP2 expression with lentiviral BMP2 shRNA prevented both mineralized nodule formation in vitro and bone formation in vivo, and blocked the expression of Runx2 and osterix, transcriptional determinants of terminal osteogenic differentiation. No effect was observed on the expression of Sox9, a transcription factor, which is the one of the first transcriptional determinant to be expressed in committed chondroprogenitor and osteoprogenitor cells. In vitro studies showed that exogenously added BMP7 rescued the expression of osterix and enhanced the expression of Sox9, but had no effect on the expression of Runx2, while it only partially recovered the development of mineral deposition in the cultures. On the other hand, the exogenous addition of BMP2 rescued both Runx2 and osterix expression, did not enhance the expression of Sox9, but fully recovered the inhibition of mineral deposition in the cultures. Using antibodies against CD146 and Sox9, immunohistological examination of the cell populations found in the medullary space three days after bone marrow ablation, showed qualitatively equal numbers of cells expressing these skeletal progenitor and stem cell markers in control and BMP2 shRNA-treated animals. Fluorescence Activated Cell Sorting (FACS) analysis of the cells found with the marrow cavities at three days after marrow ablation using CD146 antibody showed near equal numbers of immunopositive cells in both control and shRNA treated animals. In summary, the differences observed in vitro for BMP2 and BMP7 on osteogenic gene expression and mineralization suggest that they have differing effects on bone cell differentiation. These results further demonstrate that in vivo BMP2 is a central morphogenetic regulator of post natal osteoprogenitor differentiation, but does not affect recruitment of progenitors to the osteoblastic lineage.
Keywords: BMP, Stem Cells, Bone Repair, Transcription Factors, Osterix, Runx2
Introduction
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGF-β) superfamily of proteins that have diverse effects on cells of the mesenchymal lineage. Several BMPs have been shown to promote chondrogenic and osteogenic differentiation of mesenchymal stem cells in vitro, and bone formation and repair in vivo [1-3]. Studies using non-targeted gene ablation of specific BMPs have shown that several of these molecules play diverse biological roles during organogenesis [4]. Global ablation of BMPs 2 and 4 lead to early embryonic lethality, while the loss of other BMPs produce less severe phenotypes and affect later stages of embryogenesis or postnatal development of various tissues [5-7]. Specific studies on the molecular functions of BMPs have shown that these factors regulate proliferation, differentiation, and apoptosis in diverse cell types [8-11]. The role of BMPs in skeletal tissues has been more specifically examined by the removal or expression of functional mutations of various BMP receptors in only skeletal cell lineages. Other approaches have used the selective overexpression of the BMP antagonist noggin in cartilage or bone. Using murine transgenic models, the targeted disruption in osteoblasts of either BMP receptor type IA or the expression of a dominant-negative BMP receptor type IB were found to both impair osteoblast function and reduce bone volume [12,13]. Other studies in which noggin was overexpressed in actively growing osteoblasts by using the bone specific segment of the Col1a1 promoter to drive its expression led to increased bone volume that was associated with a decreased rate of bone formation. This apparent paradox was related to reduced periosteal bone formation accompanied by reduced resorption of bone in the marrow spaces, and led to an increase in the volume of intramedullary bone. Overexpression of noggin also decreased osteoclast number and resorption suggesting that BMPs play an important role in calibrating coupled remodeling [14]. In contrast, when noggin overexpression was restricted to mature osteoblasts by placing it under the control of the osteocalcin promoter, a generalized severe osteopenia developed [15]. These different sets of results suggest that there is a complexity to BMP function that may be related to how these molecules affect different stages of osteogenic lineage development and lead to different effects on the remodeling of cortical and intramedullary bone.
A previous study from our laboratory has shown that osteogenic differentiation of bone marrow stromal cells is associated with the expression of multiple BMPs and that inhibition of BMP expression by either noggin or BMP2 antibody blockade prevents osteogenic differentiation [16]. In a recent study by Tsuji et al. [17], inactivation of BMP2 in limb bud mesenchymal tissues, before the onset of skeletal development, lead to the inability to repair post natal fractures. Interestingly, the loss of BMP2 in mouse limb bud mesenchymal tissues did not affect normal pattern formation of skeletal tissues or embryonic limb development. These results suggest that BMP2 plays a central role in post natal bone growth and repair.
Studies from our laboratory have shown that there is a coordinated expression of multiple BMPs and their receptors during fracture healing and during the in vitro osteogenic differentiation of bone marrow stromal cells [16,18]. Furthermore, BMP2 appears to regulate the autogenous expression of a number of other BMPs, and the osteogenic differentiation of it is associated with the increased expression of certain BMPs and the down regulation of others. Indeed, the in vivo efficacy of a given BMP’s activity might relate to the target population of cells that is most responsive to it. In this regard, it is generally thought that BMPs mediate stem cell recruitment and, as such, would be beneficial in attracting cells to the osteogenic lineage in the setting of skeletal repair. However, there has been little research to identify those stages in the progression of the skeletogenic lineage in which a given BMP will show the greatest efficacy of action in promoting osteogenic differentiation. There have also been few studies done to discriminate one BMP’s activity from another at a molecular level or to understand how multiple BMPs might work together to promote optimal healing.
In the studies presented here, a lentivirus that expressed BMP2 shRNA was employed to selectively inhibit only BMP2 mRNA expression during bone marrow stromal cell differentiation in vitro. To study the function of BMP2 in vivo, a bone marrow ablation model was used. In this in vivo model, robust intramembranous bone formation is induced by surgically reaming out the medullary space [20] and BMP2 expression was blocked after reaming by injecting into the marrow cavity the same BMP2 shRNA lentiviruses as used in vitro. Within the context of these studies, a comparison of the selective effects of the exogenous delivery of BMP2 or BMP7 on phenotype rescue in relation to the selective loss of BMP2 expression was also performed. This approach allowed us to determine if different BMP isotypes have comparable effects on osteogenic differentiation. Finally, these studies were designed to specifically identify those transcription factors that mediate the actions of BMPs and the specific stage in the osteogenic lineage at which these BMPs act to promote cell differentiation.
Materials and Methods
Cell Culture and Osteoinduction
Research was conducted in conformity with all Federal and USDA guidelines under an IACUC approved protocol. All cell culture studies were conducted with C57 BL/6J (B6) male mice of 8-10 weeks of age (Jackson Laboratories, Bar Harbor, ME), while the marrow ablation studies used 15 week old male mice. Primary bone marrow stromal cell cultures were prepared and osteogenesis was induced as previously reported [16]. Phenotype rescue experiments were performed by supplementing the osteoinductive media with 200ng/ml BMP7 or BMP2 protein. All experiments were performed with 3 separate cell preparations and all measurements from a given set of cells preparations were carried out with three replicates.
In Vitro Assay of Osteogenesis
Alkaline phoshatase (APase) activity was assayed using CSPD chemiluminescence substrate with sapphire 2 enhancer from the cell culture media. The change in alkaline phoshatase activity per minute was recorded with a luminometer. Alizarin red staining and osteogenesis quantification were performed by using a standard osteogenesis quantification kit (Millipore Billerica, MA). Nodule counting was as described by Edgar et al., 2007 [16].
Surgical Marrow Ablation
Tibia marrow ablation was performed as described by Suva [19] and modified by Gerstenfeld [20] for mice. Briefly, fifteen-week-old C57 BL/6J male mice with weights between 30-35 grams were used for this study using the same approach. Animals were anesthetized with 4% isoflurane, and their right hind legs were shaved and prepped with betadine solution. A longitudinal incision through the skin was made over the knee, and the medial half of the patellar tendon was incised to expose the joint. With the knee in maximal flexion, a 0.5 inch 25 gauge needle was used to create a starting portal centromedially in the tibial plateau. A 30 gauge needle was next inserted to find the path of the medullary space extending to the level of the middiaphyseal bow. Sequential reaming of the tibial canal was then performed using 27, 25, and 23 gauge needles respectively. The medullary cavity was flushed with 200 μl of sterile PBS, and the bone marrow contents were aspirated with a 27 gauge needle and syringe. To prevent backflow, a unicortical hole was created just distal to the bow of the tibia using a 0.5 inch 25 gauge needle. An intramedullary injection of 50 μl of saline (control), or various solutions of lentiviral particles was performed via a 30 gauge needle and syringe. The skin was closed in a single layer fashion using 4-0 vicryl suture. Animals were permitted immediate weight bearing as tolerated.
Micro Computer Assisted Tomography (μCT)
Specimens were scanned at a resolution of 16 μm using a Scanco μCT 40 system (Scanco Medical, Basserdorf, Switzerland). For these measurements, a region of interest (ROI) was defined for each of the experimental sample groups in the longitudinal axis by assessing a fixed distance from the proximal growth plate. This was established by taking 100 slices distal from this point of reference. Only intratrabecular bone was determined based on manual substraction of the surrounding cortical bone. The measurements were done as reported earlier [21]. Total Bone volume and average mineral density were each compared across groups using a Kruskal-Wallis test (analysis of variance by ranks) [22].
Demineralized Histology and Immunohistochemistry
For histological assessment, the tibiae were fixed, decalcified, sectioned, and stained as previously described [23]. Serial sections were generated and slides were taken every 100 μM. Slides were stained with hematoxylin and eosin. Similar immunohistological procedures to those that we used previously were carried in this study [21]. Polyclonal antibodies to CD146 polyclonal and Sox9 antibody were from Abcam. Mice that were injected with lentiviruses expressing β -Galactosidase were reacted with X-gal using a modification of the procedure reported by Mortlock et al [24]. Briefly, at the time of euthanasia the tibiae were directly collected into 4% paraformaldehyde and fixed overnight at 4°C. After fixation the tibiae were cut in half in the longitudinal plane with a scalpel blade and then rinsed 3-4 times for 30 min in wash buffer and stained in wash/staining buffer with 0.8 mg/mL X-gal for 24 h. Stained tibiae were rinsed several times in PBS and whole bones were digitally photographed.
Lentivirus Preparation
All work with lentiviruses was performed under BL2 conditions. All lentivirus based shRNA DNA clones and packaging cell lines that were used for making the viral transduction particles were from Sigma-Aldrich Inc., St. Louis, MO, USA. The lentiviral constructs expressing β-Galactosidase was generated in the laboratory of Dr. Inder Verma and was available from Addgene, Cambridge, MA, USA (plasmid 12108), while viral DNA plasmid containing the luciferase (pHR’EF1a-luciferase/WPRE-SIN) was kindly provided by Dr. Jeffrey Medin, University Health Network, Toronto, Ontario, Canada. Lentivirus particles were prepared using three-plasmid system from Addgene (http://www.addgene.org/pgvec1). In short, 293T cells were plated at density 2 × 106 293T cells in 10cm2 dishes containing 10 mL of media. After 24 hrs, the cells were overlaid with complex containing 3 plasmid system (PLKO shRNA, pCMV-VSV-G, and pCMV-dR8.2 dvpr) at the ratio 8:1:8 using Fugene 6. The supernatant was collected starting from 48 to 96 hrs post-transfection. The virus particles were concentrated by ultracentrifugation at 18,500 rpm for 1 hr 30 min with Beckman ultracentrifuge using SW28 rotor, resuspended in PBS and stored until use at -70°C. The physical titer of virus particles were determined by using p24 ELISA (Cell Biolabs, Inc San Diego, CA).
Lentivirus Particle Transduction
Experiments performed in bone marrow stromal cells were carried out in adherent cultures at 10 days after they had been isolated. At this time, the cells had been grown in osteoinductive media for four days. Viral transduction was performed in media containing 8 μg/ml hexadimethrine bromide for 12 hrs, at which time this media was replaced with osteoinductive media alone. The media was changed every 48 hrs thereafter with fresh osteoinductive media. Intra-tibial injection of lentivirus particles was carried out after surgical marrow ablation. After ablation, the cavity was washed three times with PBS containing 0.0032% polybrene in order to increase the transduction efficiency of the lentivirus. After the final wash, either 50 μl of PBS or 50 μl lentivirus 2 × 106 viral particles that contained coding sequences for either lacZ (LV-lacZ), luciferease (LV-Luc), a non target shRNA (NT), or shBMP2 were injected along with polybrene at a final concentration of 8μg/ml in PBS and the incision was closed. The animals were monitored for a week and tibias were removed at day 7 and either fixed and used for histological and micro-CT analysis or flash frozen in liquid nitrogen and used for RNA preparation.
Bioluminescent imaging of tibia in vivo
A subset of mice were imaged using an IVIS 200 system (Xenogen, Alameda, CA, USA). Anesthesia was administered in an induction chamber with 2.5% isoflurane in 100% oxygen at a flow rate of 1 L/min and maintained while the animal was in the IVIS imaging device with a 1.5% mixture at 0.5 L/min. Approximately, 150 μl of D-luciferin (30mg/ml dissolved in PBS was injected intraperitoneally. Bioluminescent images were taken from 1 min to 45 min and peak signals were observed around 20 min. The data are reported as the photon flux (p/s) from a defined region of interest in tibia of lentivirus expressing luciferase compared to noninjected control mice.
Isolation of mRNA and Molecular Biology Procedures
Tissues were collected and RNA was processed as previously described [25]. Messenger RNA levels were assessed by qRT-PCR as previously described [21,26].
Flow cytometry
Cell populations were prepared from the medullary space as described above from N = 3 animals per experimental group and repeated at least three times. Cells were stained as reported for CD146 [27]. Briefly, CD146 flow cytometry was performed by using CD146-PE monoclonal antibody or PE Mouse IgG1, k Isotype control (BD Bioscience, Bedford, MA). MSCs prepared as mentioned earlier, plated for overnight, trypsinsed next day, and stained with CD146-PE antibody for 30 min on ice. The cells were sorted by using a MoFlo instrument (Dako, Carpinteria, CA 93013). The data were analyzed by using summit software (Cytomation, DakoCytomation, Inc., Fort Collins, CO). All the experiments were repeated 2-3 times for each group. The sorted CD146 cells were plated in 6-well plate for reconfirmation of the osteoprogenitor population.
Results
Establishment of Bone Marrow Stromal Cell Transduction of VSV-G Pseudotyped Lentivirus Particles
Initial studies were focused on defining the optimal conditions of lentiviral transduction of bone marrow stromal cells and evaluated both the effect of lentivirus on cell viability and on the normal osteogenic development of bone marrow stromal cells. A lentivirus that expressed green fluorescent protein was first used to determine whether the marrow stromal cells could be efficiently transduced. Increasing numbers of cells were shown to take up the virus from a multiplicity of infection (MOI) of 10-100 with the optimal numbers of viable cells taking up the virus seen at an MOI of 30 (Fig. 1A). These data were validated by FACs analysis of numbers of viable cells in the transduced cultures which showed that a transfection efficiency of ∼75-95% was achieved (data not shown). Using a virus containing a nonsense sequence that was not targeted (NT) to any specific mRNA as a control, the long term viability of the MSCs cultures were tested after transduction, using lactate dehydrogenase activity assay. These data showed that the virally transduced marrow stromal cell cultures had identical long term viability as the control cultures at an MOI of 30 (Fig. 1B). The effect of viral transduction on terminal osteogenic differentiation was next assessed by examining the number of osteogenic nodules that formed in the MSCs in the presence and absence of non targeted (NT) virus. As can be seen in these studies, the number of nodules remained the same demonstrating that the lentivirus by itself had no adverse effect on osteogenic development (Fig. 1C).
Fig. 1. Characterization of Marrow Stromal Culture Transduction with Lentiviral BMP2 shRNA Expression Constructs.
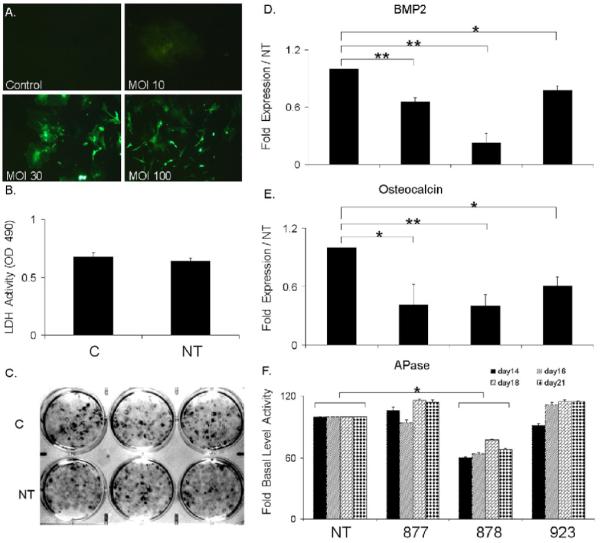
C= control no virus transduction and NT= transduced with non target virus. A) Assessment of the optimal multiplicity of infection (MOI) for lentiviral transduction by green fluorescent protein expression. Viral titers are as indicated in the figure. Representative phase contrast microscopy images taken under UV light excitation are depicted (100X magnification). Percentage optimal cell transduction was validated by FACs analysis. B) Effect of lentiviral transduction on cellar viability after 21days of culture growth. Viability was measured by LDH enzyme release as quantified by OD405nm showed no significant difference. . C) Effect of lentiviral transduction on formation of alizarin red stained osteogenic nodules. Representative images are of mineralized nodule formation in triplicate 33mm culture wells after 21 days of growth in mineralization media. D) Identification of the optimal BMP2 shRNA for BMP2 RNA silencing in MSC cultures. Cultures were separately transduced three different lentiviral constructs each containing a unique shRNA to BMP2. The expression of BMP2 mRNAs are presented as the relative value to NT transduced cultures. Values were determined at 21 days in culture. Numeric notation of the separate lentivirus constructs are as provided by Sigma Aldrich Corporation from The MISSION shRNA library of The RNAi Consortium. E) Functional effect of the various BMP2 shRNAs on osteocalcin mRNA expression relative to NT transduced cultures was determined at 21 days. F) Functional effect of effect of various BMP-2 shRNA on alkaline phospatase (Apase) activity. Activities were assessed at multiple time points as indicated in the figure. Lentivirus BMP2 shRNA clone 878 showed significant down regulation of BMP2 mRNA, osteocalcin mRNA and APase activity. Error bars represent standard deviation from replicates measurements from three experiments. P-value reflects the comparison of different treatment groups calculated by t-test and denoted as * p<0.05, ** p<0.01.
In the next series of studies, a group of five different shRNAs for BMP2 were screened to identify the one that was most efficient at inhibiting the expression of BMP2 mRNA. Groups of cell cultures were individually transduced with the separate lentiviruses for three shRNAs that were directed towards BMP2 at day 10, which was 8 days prior to peak BMP2 expression during the osteogenic differentiation of these cultures [16]. Three separate means of assessing the inhibitory activities of each of these shRNAs were examined. First, each of the shRNAs was tested for their ability to block the expression of BMP2 mRNA expression. Each of the shRNAs showed differing effects with the 878 sequence demonstrating ∼80-90% knock down in BMP2 mRNA expression. Since our previous antibody blockade studies had suggested that the osteogenic differentiation of the marrow stromal cell cultures were dependent on the autogenous expression of BMP2 expression, the functional consequences on osteogenic differentiation of each of BMP2 shRNAs was next examined. Two separate phenotypic markers of osteogenic differentiation osteocalcin expression and alkaline phosphatase (APase) activity were examined (Fig. 1E and 1F). Each clone showed consistent inhibition on these two functions that were relatable to the levels of knock down of the expression of BMP2 mRNA expression. Thus, clone 878 showed the greatest down regulation of BMP2, osteocalcin mRNA expression, and APase activity, which was statistically significant in comparison to the non targeted virus treated cultures. All subsequent experiments that were performed were then carried out using this clone.
BMP2 Knock Down Can Be Rescued by Administration of Exogenous BMP-7 Protein
Cultures that had been transduced with the sh-BMP2 virus were treated with BMP7 in order to assess if the osteogenic differentiation of the marrow stromal cell cultures were selectively dependent on BMP2 expression or if BMP7 was capable of serving a redundant function and would rescue cellular differentiation. It is important to note that marrow stromal cells do not express any observable mRNA for BMP7 [16] therefore allowing for the definitive assessment of its sole function in this culture model system. The levels of expression of BMP2 were first determined across the time course of marrow stromal cell osteogenic differentiation (Fig. 2A). Under normal differentiation conditions BMP2 mRNAs showed increasing expression until 16 days in culture, after which they began to fall. BMP7 treatment alone in the absence of BMP2 shRNA increased expression of BMP2, however the exogenous treatment of BMP7 did not have any effect on BMP2 expression in the BMP2 shRNA treated cultures, thereby showing the overall efficacy and persistence of the shRNA inhibition of the BMP2 expression in these cultures. Examination of the levels of APase activity showed that BMP7 alone would increase the overall levels of APase activity in the NT lentivirus transfected cultures indicating that the lentiviral transfection did not interfere with BMP7 signaling. The transfection of the BMP2 shRNA downregulated APase activity while treatment with BMP7 recovered the APase activity to that in NT controls (Fig. 2C).
Fig. 2. Rescue of Osteogenic Differentiation with Exogenous Addition of BMP7 in BMP2 shRNA Treated MSCs.
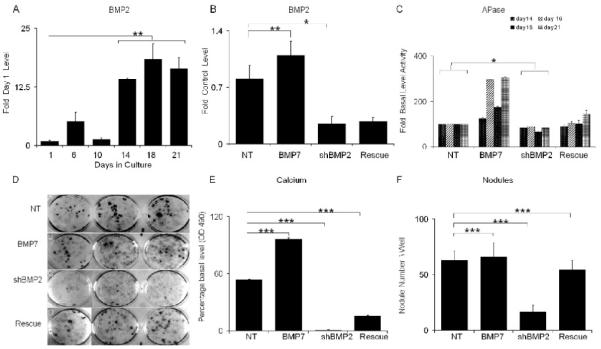
NT= cultures transduced with non targeted; BMP7= transduced with NT lentivirus and treated with 200ng/ml BMP7; shBMP2= cultures transduced with lentivirus containing BMP2 shRNA; and Rescued= cultures transduced with lentivirus containing BMP2 shRNA and treated with 200ng/ml BMP7. All RNA measurements made from three separate preparations of cells and error bars are the SD of the triplicate measurements from 3 separate cell preparations. A) Steady state BMP2 mRNA expression levels over the time course of in vitro osteogenic differentiation. RNA measurements are presented as a relative fold of expression to day 1. B) Steady state BMP2 mRNA expression levels after 21 days growth in cultures under various experimental conditions. RNA measurements are presented as a relative value to the NT control. C) APase activities were assessed at 21 days time points. Error bars are the SD from three separate cell culture preparations. D) Representative images of mineralized nodule formation in triplicate 33mm culture wells after 21 days of growth in mineralization media. E) Quantification of effect of lentiviral transduction on Ca++ accumulation. Calcium bound alizarin dye was released as described in the materials and absorbance was analyzed. F) Effect of lentiviral transduction on formation of Alizarin red stained nodules. Error bars represent standard deviation from replicates measurements from three experiments. P-value reflects the comparison of different treatment groups calculated by t-test and denoted as * p<0.05, ** p<0.01, *** p<0.001.
An examination of the accumulation of nodules and mineral contents in BMP7 and shRNA treated cultures (Fig. 2D-2F) showed that the exogenous addition of BMP7 by itself increased the total mineral accumulation in the culture but did not have an over effect on the number of nodules. The exogenous treatment of the cultures with BMP7 while fully recovering the number of nodules in the BMP2 shRNA treated cultures, did not fully rescue the accumulation of the total Ca that was observed in the BMP2 shRNA treated cultures, which only reached a third that of the NT treated cultures (Fig. 2D-2F).
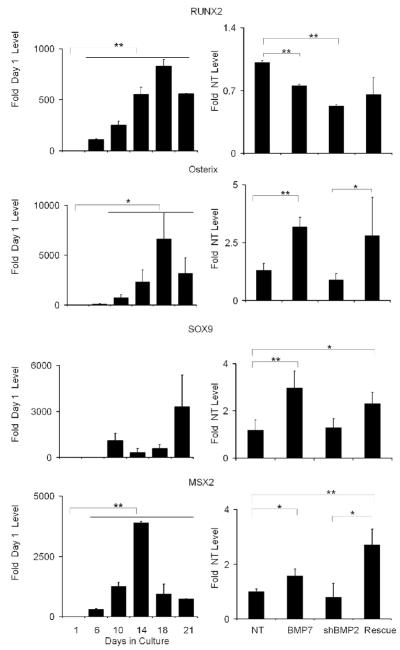
The molecular mechanisms by which BMP2 regulated osteogenic differentiation within the marrow stromal cell cultures were examined by assessing the expression of mRNAs that encode four transcription factors known to control skeletal progenitor differentiation into osteogenic cells (Fig. 3). These transcription factors are: SOX 9, which commits stem cells to the skeletal lineage and is common to both chondrogenic and osteogenic cells; Osterix, which directs the lineage commitment to osteogenesis; RunX2, which is involved in both terminal differentiation and promotes the development of phenotypic characteristics that promote mineralization of osteoid matrix [28]; and Msx2 which is found in proliferating but not fully differentiated osteogenic cells [29]. The temporal expression of each of these transcription factors was first assessed over the time course of the differentiation of the marrow stromal cultures (Fig. 3, left panels). These results may be compared to the end point measurements of each of these mRNAs in cultures treated with both exogenous BMP7, sh-BMP2 inhibition of BMP2 expression, and rescue of BMP2 inhibition with BMP7 treatment (Fig. 3, right panels).
Fig. 3. Effect of Exogenous Addition of BMP7 in BMP2 shRNA Treated MSCs on the Expression of Various Transcription Factors that Control Skeletal Stem Cell Lineage Progression.
The nature of each mRNA that was assayed is indicated in the figure. Left panels in the figure present the steady state mRNA expression levels of the various transcription factors over the time course of in vitro osteogenic differentiation. RNA measurements are presented as a relative fold of expression to day 1. Right panels show the transcription factor mRNA expression levels after 21 days growth in cultures under various experimental conditions. RNA measurements are presented as a relative value to the NT control. Mean values are measurements made from three separate preparations of cells. Error bars represent standard deviation from replicates measurements from three experiments. P-value reflects the comparison of different treatment groups calculated by t-test and denoted as * p<0.05, ** p<0.01, *** p<0.001.
Under normal growth conditions that promoted the osteogenic differentiation, the expression of Runx2 and osterix both showed increasing expression until day 16 after which their peak levels decreased. In contrast, Sox9 showed a transient increase at day 10 after which the levels decreased. Interestingly, the expression of Sox9 showed a very sharp increase at the end of culture period at day 21. Msx2 expression levels had a distinct peak at day 14, after which they fell throughout the remainder of the culture period. With the exception of Runx2, Sox9, osterix, and Msx2 all showed increased expression when the cultures were treated with BMP7. Inhibition of BMP2 expression decreased the expression of Runx2 and osterix, while having no effect the expression of Sox9 and Msx2. It is interesting to note that BMP7 when added to the BMP2 shRNA treated cultures, the expression of all of the transcription factors but Runx2 were returned to their pre-inhibition levels or as in the case of Sox9 and Msx2 even greater levels than those seen in the NT control cultures.
Rescue of BMP2 Knockdown Cultures by Administration of Exogenous BMP2 Shows Unique Differences from Rescue with BMP7
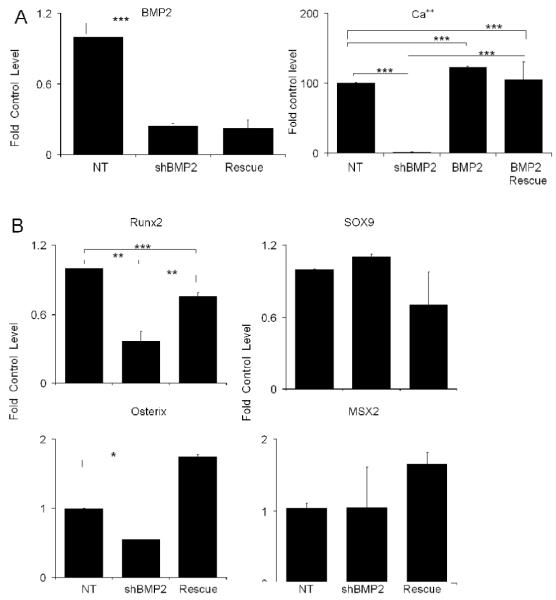
The rescue of the osteogenic phenotype with the exogenous addition of BMP7 did not completely recover the expression of either Runx2 or mineralization of the cultures, suggesting that the signaling processes that are mediated by BMP7 are in some manner different from those of BMP2. Possible explanations for these observations might be due to differences in this BMPs interaction with the available receptors on the marrow stromal cells and/or signal transduction processes that are mediated by BMP7 in comparison to BMP2. Alternatively, lentiviral transduction of the cells might interfere with the biological activities that are mediated by exogenously administered BMPs. In order to further examine these possibilities, cultures that had been transduced with lentiviral containing BMP2 shRNAs were treated with exogenously added BMP2. These results are shown in Fig. 4. Exogenous addition of BMP2 protein was not able to increase the expression of BMP2 mRNA expression that has been inhibited by the shRNA to BMP2. Comparisons of the effects of exogenous BMP2 treatment on the development of nodules and the overall mineralization of the cultures between the BMP2 and BMP7 show that BMP2 increased the number of nodules by about 50% over the NT treated cultures and fully restored mineral deposition. Like the exogenous addition of BMP7, the exogenous addition of BMP2 increased above control levels the expression of osterix, but more importantly the Runx2 expression was almost completely restored to its control levels. It is noteworthy that Sox9 levels were not increased to the same extent that was seen with BMP7 treatment while Msx2 were only slightly elevated.
Fig. 4. Affect of Exogenous Addition of BMP2 on BMP2 shRNA Treated Culture.
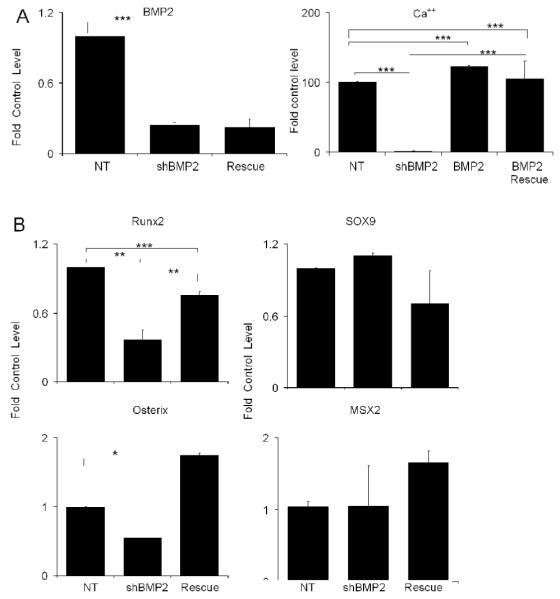
All data is presented as the fold of the control untreated MSC cultures at 21 days. A) The exogenous addition of BMP2 protein rescue of nodule and mineral deposition in BMP2 shRNA inhibited MSCs cultures. Left panel shows the fold change in BMP2 mRNA expression in response to both BMP2 shRNA treated and BMP2 shRNA in the presence of exogenous addition of BMP2. Right panel shows the total mineral accumulation as measured by alizarin dye uptake by BMP2 rescue in comparison of NT shRNA and NT shRNA with BMP2 protein. B) Affect of Exogenous Addition of BMP2 in BMP2 shRNA Treated MSCs on the Expression of Various Transcription Factors that Control Skeletal Stem Cell Lineage Progression. Error bars represent standard deviation from replicate measurements from three separate cell preparations. P-value reflects the comparison of different treatment groups calculated by t-test and denoted as * p<0.05, ** p<0.01, *** p<0.001.
Central Role of BMP-2 in Injury Induced Post Natal Bone Formation
In order to asses the role of BMP2 during osteogenic differentiation in vivo, a model of surgical marrow ablation was used to induce intramedullary bone formation. Two separate control experiments were carried out to assess both the uniformity of lentiviral infection within the developing bone tissues after their introduction into the medullary space at the time of surgery, and the persistence of lentiviral expression within these tissues. In order to assess the uniformity of the viral transduction gross staining of the bone tissue was carried out in tissues infected with a lentivirus expressing the β-galactosidase gene. Varying degrees of LacZ staining was seen at both time points within all six of the bones that were examined. Five out of the six specimens showed very intense reactions either in, throughout the medullary space, or on the endosteal lining surfaces of the cortical regions of the bone. Equal intensity was also seen for the LacZ staining at both day three and seven after the intramedullary reaming and introduction of the virus into the tissues (Fig. 5A). A quantitative assessment of the persistence of lentiviral gene expression was made by measuring the luciferase expression in vivo over the same 7 day period after transduction, which showed that the intensity of luciferase activity increased until day 7 (Fig. 5B). The effect of knockdown of BMP2 expression in vivo, during bone formation after marrow ablation is presented in Fig. 5C and 5D. MicroCT analysis (Fig. 5C) of the newly formed bone tissues showed robust osteogenesis in the saline injected groups after seven days. Injection of the non targeted lentivirus into the marrow space after ablation caused some reduction in new formation however this was significantly less than that seen in the BMP2 shRNA samples. Injection of BMP2 shRNA lentivirus caused a statistically significant decrease in the formation of mineralized bone tissue compared to either the NT lentivirus or the control PBS injections. The effect of BMP2 shRNA lentivirus on the unmineralized tissues and cells that were recruited into the medullary space after seven days, are seen in Fig. 5D. The histological analysis confirmed the microCT analysis, with the control and NT virus treated animals showing the development of an extensive network of trabeculated bone with islands of hematopoietic and myeloid tissues forming between the trabeculea. In contrast, the BMP2 shRNA lentivirus treated animals showed a large number of fibroblastic cells filling the medullary space with little evidence of condensation of these cells into trabecular structures. Interestingly, in the absence of bone formation there also appeared to be no evidence that large amounts of hematopoietic and myeloid tissues had formed either.
Fig. 5. In vivo Effects of BMP2 shRNA Inhibition on Bone Formation after Marrow Ablation.
A) Gross staining of tibiae to detect β-galactosidase activity. Individual tibia from mice injected with either PBS control or lentiviral particles that expression β-galactosidase. Representative images of three bones that had been cut in half and reacted with X-gal staining are shown. Staining is detected as dark green to green brown areas and is denoted with arrows. B) Representative in vivo measurements of bioluminescent intensities from mice injected with lentivirus overexpressing luciferase (lenti-Luc) compared to PBS injected controls. The data are reported as the photon flux (p/s) from a defined region of interest in tibia of lentivirus expressing luciferase compared to noninjected control mice. C) MicroCT assessment of endosteal bone formation in response to surgical marrow ablation. All images are orientated with the proximal end of the bone at the top and are from 7 days after the time of surgery. Representative μCT 3D renderings of bone formation from control mice and mice injected with NT and BMP2 shRNA lentiviral particles are shown in the top panels. Graphical measurements of percent bone tissue (BV/TV) as determined from the μCT analysis are presented in the below the microCT images. The Comparisons for all pairs using one way ANOVA showed statistically significant difference in mean in shBMP2 group (mean=0.66) compared to PBS (Mean =0.14) and NT (mean= 0.10) and p value<.0001 denoted as ***showing significant differences in each group. Analysis by t-test showed also showed significance for the comparison of shBMP2 with p <.05 whereas with PBS p<.0001. D) Histological Analysis of bone formation in response to surgical marrow ablation. All images are orientated with the proximal end of the bone at the right side of the micrographs. Sections were cut in longitudinal orientations and sections were selected from the central regions such that the surrounding cortices are seen. Sections were stained with H&E. In shRNA injected groups, there is observable loss of trabecular structure and bone loss indicated by arrow.
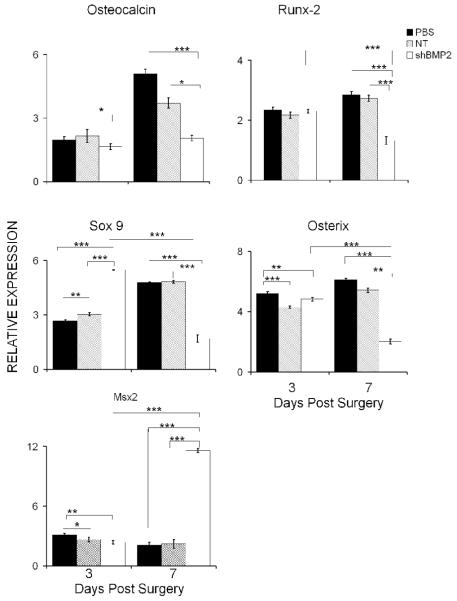
In order to assess the underlying effect of the inhibition of BMP2 expression on the in vivo differentiation of osteogenic cells after the surgically induced bone formation, the same panel of transcription factors were assayed as were examined in the marrow stromal cell cultures. These results are seen in Fig. 6. The inhibitory effect was first verified by examining the expression of osteocalcin, which is used as the marker of terminal osteogenic differentiation. At three days after ablation, there were no differences observed between the PBS and NT virus injected tissues, but even at this early time point there was a small decrease in osteocalcin expression in the BMP2 shRNA lentiviral injected tissues. By seven days post injection, levels of osteocalcin expression had risen significantly (2 to 2.5 fold) in both PBS and the NT virus injected tissues, but showed statistically less expression than either of these controls in BMP2 shRNA lentiviral injected tissues. Examination of the various transcription factors produced a more complex but similar set of expression data as was seen for the marrow stromal cells. Both osterix and Runx2 showed almost no differences in their expression at day three after surgery but both these genes showed significantly 50-75% lower levels of expression at seven days. In contrast, Sox9 expression showed almost 2 fold greater expression in the BMP2 shRNA treated samples at day three than either control but then showed a dramatic decrease in expression relative to either the PBS or NT virus controls, which showed a continued increase in Sox9 expression throughout the seven day post surgical treatment period. Interestingly, Msx2 showed a significant increase in the day seven treated samples suggesting that the cells had not progressed past their initial stages of differentiation.
Fig. 6. Effect of BMP2 shRNA Lentiviral Treatment on the Expression of Various Transcription Factors that Control Skeletal Stem Cell Lineage Progression within Bone Tissues Formed After Surgical Ablation.
Individual samples are as denoted in the figure and the legend. Levels of expression are relative to unoperated control tibia bones. Error bars represent standard deviation from replicates measurements from three experiments. P-value reflects the comparison of different treatment groups calculated by t-test and denoted as * p<0.05, ** p<0.01, *** p<0.001.
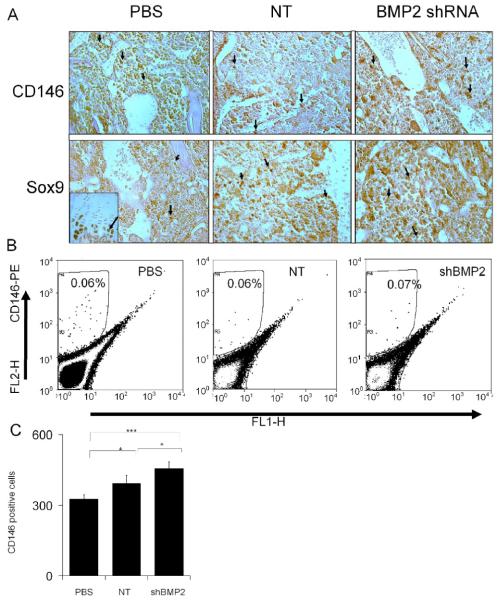
While the mRNA expression data present an average of the activity of all of the cells these data do not directly examine the activities in individual cells. The last set of studies that are presented directly examined the nature of the cell populations that were recruited into the marrow space three days after ablation. Immunohistological localization using either an antibody to Sox9 that will identify the progenitor cells that are committed to the skeletogenic lineage [21, 26] or CD146 [27], which interacts with a cell surface marker found of the population stem cells that gives rise to the osteogenic progenitors. However, the direct correlation between CD146 and Sox9 cells has not been established yet. These results are seen in Fig. 7. Unlike the distinct histological differences seen at day 7 between the three experimental groups, the day three specimens all had similar histological features showing a dense mass of cells filling the marrow space. Comparable numbers of cells showing similar morphologies were interspersed uniformly throughout the marrow space (Fig. 7A). Both antibodies qualitatively appeared to recognize a small percentage of the cells in the reamed marrow space with the Sox 9 antibody appearing to react with more cells. These immunopositive cells appeared to be uniformly interspersed throughout the cell population filling the space, and both antibodies recognized smaller rounded cells with the same general morphology. The specificity of Sox9 staining was corroborated by the intense levels of reaction seen in the growth plates of the same specimens (seen as an inset) and its strong immunostaining of the nucleus and control non immune antibodies showed no specificity to the intramedullary cell population (data not shown). It is interesting to note that the shBMP2 lentiviral treated specimens all appeared to have the greatest number and intensity of Sox9 reactive cells, consistent with the mRNA expression data seen in Fig. 6 that also showed that this mRNA was expressed at its highest levels of expression in the BMP2 animal group (Fig. 7A).
Fig. 7. Effect of BMP2 shRNA Lentiviral Treatment on the Development of Immunoreactive Cells Showing Stem Cell and Osteogenic Progenitor Marker Expression.
A) Immunohistology of CD146 and Sox9 reactive cells. Tibiae were collected three days post bone marrow ablation. Tissues were cut in longitudinal orientations and sections were selected from the central regions. Sections were reacted with antibodies directed against mouse specific CD146 or Sox9. All photomicrographs were taken at 200X magnification. Immunopositive cells stain reddish brown. The experimental group and antibody used for the immunostaining are indicated in the figure. The inset in left lower (PBS/Sox9) image depicts positive immunostaining for Sox 9 in the growth plate from the same specimen. B) Quantification of CD146 positive osteoprogenitor stems cells in different treatment groups. Representative FACs analysis of CD146 positive cells observed in the total population of cells flushed from the marrow space three days post surgery and plated overnight before FACs. The gated population was optimized for nonspecific stating by isotype control antibody. Left Panel Shows 0.06% CD146 positive cells in gate R4 from tibia of mice injected with PBS as a control. Middle panel shows this cell population in nontarget shRNA treated group (006%). Right panel shows that the BMP2 shRNA treatment produced a slight increase in CD146 cells (0.07%). The total number of CD146 cells sorted cells from three separate experiments plotted, showed statistical significant difference in shRNA treated groups compared to PBS (p= 0.00154) and NT (0.028139). Error bars are standard deviation from replicates measurements from three experiments. P-value reflects the comparison of different treatment groups calculated by t-test and denoted as * p<0.05, ** p<0.01, *** p<0.001.
In order to quantify the number of osteogenic stem cells found in the marrow space three days after ablation, FACs analysis was carried out using CD146 (Fig. 7B and 7C). These analysis showed no effect due to loss of endogenous BMP2 on the CD146 cells population after 3 days in the cells flushed from the medullary space with a range of 0.02 to 0.1% of the cells for all three experimental groups being positive. In the mice injected with PBS or non target shRNA an average of 0.06 percent positive cells were recovered whereas in BMP2 shRNA treated groups showed an average of 0.07%. The average number of cells sorted from three independent experiments showed a very small but statistically significant increase in CD146 population in BMP2 shRNA virus injected group compared to PBS and non-target shRNA. In summary, these data provide corroborating data to the mRNA analysis from both the cultured marrow stromal and the marrow ablation tissues that supports the conclusion that loss of BMP2 expression does not effect the recruitment of skeletogenic stem cells, but rather promotes the differentiation and expansion of these cells.
Discussion
Previous studies from our laboratory have shown that either the BMP antagonist noggin or BMP2 antibody blockade inhibited osteogenic differentiation of marrow stromal cells, while exogenously added BMP2 or BMP7 induced the endogenous expression of multiple BMPs and enhanced their osteogenic differentiation [16]. Because the exogenous addition of BMPs can not be used to rescue either noggin treatment or BMP2 antibody blockade, these methods of BMP2 inhibition can not isolate the effects of one BMP from another. Nor do these methods of BMP inhibition allow for the isolation of separate molecular effects that these different BMPs may have since both stimulate the expression endogenously produced BMPs by the marrow stromal cells. Recently it has been shown that both BMP7 and BMP2 regulate marrow stromal cell osteoblastic differentiation through different receptors such that BMP2/4 used predominantly BMPR1A whereas ACVR1A was the preferred type I for BMP-6/7 [30]. Thus, it remains unresolved whether different BMP isotypes have differential effects on the osteogenic differentiation of marrow stromal cells.
Use of shRNAs to block only BMP2 expression corroborated our previous findings regarding the central regulation of osteogenic differentiation by BMP2 while at the same time allowing us to demonstrate that BMP2 and BMP7 do not have identical effects on osteogenic differentiation. Thus, while both BMP2 and BMP7 equally promoted the expression of osterix, and the development of equal numbers of osteogenic nodules, the exogenous addition of BMP7 to the BMP2 shRNA-treated cultures could not fully rescue APase activity, mineral accumulation or the expression of Runx2. In contrast, exogenous BMP2 treatment of the BMP2 shRNA treated cultures did restore both Runx2 expression and total mineral accumulation. This finding is consistent with several studies that have shown that BMP2 signaling both requires and synergizes Runx2 activity to achieve maximal osteogenic differentiation in either C2C12 or C3H10T1/2 pluripotent mesenchymal precursor cell lines [31-33]. Furthermore, studies by Lee et al., 2001 [34], showed that BMP2 upregulated the expression of Runx2. Therefore, the additional effects of BMP2 compared to BMP7 on MSC osteogenic differentiation appear to be due to the direct effects that BMP2 has on Runx2 expression, while BMP7 does not appear to regulate Runx2 expression. Interestingly, in the control cultures in which BMP2 was not inhibited, BMP7 equally promoted osteogenic differentiation compared to BMP2 and our data suggest that this effect is due to upregulation of BMP2. Thus these subtle differences can only be seen when the endogenous stimulation of BMP2 expression was removed from the system by the BMP2 shRNA treatment.
The observation that BMP7, in the absence of BMP2 signaling, could partially promote osteogenic differentiation through the induction of osterix suggests a number of important implications. First, osterix can independently regulate specific aspects of osteogenesis independent of Runx2, even though osterix is induced by Runx2 and has been localized by genetic studies to be downstream to Runx2 in the cascade of transcriptional events that control embryonic skeletogenic development [28]. Second, BMP7 and BMP2 must have divergent or preferential signal transduction mechanisms that separately mediate some of their nuclear effects. In this regard, it is noteworthy that BMP2 and BMP7 appear to be part of two structurally different subgroups of the BMP superfamily with BMP2 and BMP4 forming one group and BMP5, BMP6, BMP7, and BMP8a/b forming the other [35]. Thus, while BMP2, BMP4, BMP5, BMP6, and BMP7 can utilize the same type I (Alk2, Alk3, and Alk6) and type II (ActRII, and ActRIIb) receptors [36] and can direct phosphorylation of the same set of BMP receptor-specific second signals (Smads 1, 5, or 8) [37-40], there are differences in the specificity of receptor binding which appear to discriminate between these two subgroups of ligands [30]. In studies by ten Dijke et al., 1994 [41], BMP4 was shown to bind to BMPR-1A but not Alk2, while BMP7 showed exactly the opposite specificity. Subsequent studies of Ebisawa et al., 1999 [35], confirmed these findings and showed that there were differences in both the combinations of BMP receptors that the various osteogenic and osteogenic progenitor cell lines expressed as well as differences in specificity of binding of BMP2 or BMP6 to the complement of receptors found in these different cell lines. Neither of these studies, however, investigated the down stream consequences of this differential receptor binding.
Finally, it is important to consider that the individual BMPs may synergize in a differential manner with other growth factors. In this regard, a number of studies have shown that IGF will enhance the osteogenic activities of both BMP7 and BMP2 [42-44]. In the studies of Celil et al., 2005 [42], IGF was shown to independently upregulate osterix separate from BMP2 and that the combination of BMP2 and IGF synergistically enhanced osteogenesis and MSC extracellular matrix mineralization. These studies further showed that while MAP kinase inhibition blocked both IGF and BMP2 induction of osterix and decreased matrix mineralization, that MAP kinase inhibition did not block Runx2 expression. These studies then clearly showed a dissociation of osterix regulation from Runx2 and suggested that in the context of our observations that BMP7’s activities are driven in part through the induction of BMP2 through its upregulation Runx2 expression.
As noted above, one aspect of BMP function that has not been extensively studied relates to the influence of these molecules at specific stages during cellular lineage progression. In the current study, two separate approaches were used to determine the lineage stage at which BMP2 acts. In one approach, we used the expression levels of four transcription factors (Sox9, Msx2, Runx2, and osterix) that have been shown to be lineage determinants for the progression of skeletogenic cells towards their terminal osteogenic state. Sox9 was examined because all osteo-chondroprogenitor cells, as well as lineage progenitors in a variety of tissues during mouse embryogenesis, express Sox9 [45]. Msx2, Runx2, and osterix, respectively, have been shown to be determinants that affect osteoblastic differentiation from the common Sox 9 expressing progenitor [28; 45-48]. For the in vivo studies, an immunohistological approach was also used. Measurement of Sox9 protein expression by immunohistological means corroborated the molecular findings while staining for CD146 positive cells in the intramedullary space was used as an independent means of identifying the osteoprogenitor population. This marker was shown in recent studies in human marrow stromal cell populations to identify the self renewing osteogenic stem cell population in the bone marrow space and these studies showed that human CD146 positive cells isolated from the bone marrow were both self renewing formed bone upon transplantation into an ectopic site, as well as support the development of a hematopoietic microenvironment [27]. In the context of the studies reported here, cells expressing this surface maker remained unaffected by BMP2 shRNA treatment and even showed a small increase in their numbers, which was consistent with our interpretation of the data related to the transcriptional factors as lineage stage determinants. These vivo results suggest that BMP2 does not act to expand stem cell numbers based on the expression of Sox9 and CD146, but rather that the target stage of its actions is at the level later than the committed chondro/osteo bipotential cell. Indeed BMP2, when added back to the shRNA-treated cultures, showed that the levels of Sox9 decreased slightly. It is interesting to note the differences in the comparison to BMP7 in that BMP7 rescue in BMP2 shRNA-treated cultures appeared to increase the expression of Sox9 suggesting that BMP7 might play a role in expanding the progenitor cell population. In this context, the differences in the effects of BMP2 and BMP7 on Runx2 expression may also play a role since increased Runx2 expression is reciprocally and mechanistically related to decreased proliferation and terminal commitment of the osteoprogenitors to their terminal state of differentiation [48].
These in vitro results were confirmed by the in vivo comparisons at three days after bone marrow ablation when the expression levels of Sox9 mRNA and the observed number of both Sox9 and CD146 immunopositive progenitors was actually greater or equal in both control and BMP2 shRNA-treated cell populations. However, differences between in vitro and in vivo results were observed at the later time points in that Sox9 levels along with those for Runx2 and osterix were dramatically inhibited in contrast to the levels of Msx2 which were greatly increased. While these results are inconsistent with our interpretation that BMP2 does not affect the recruitment of osteoblast progenitors, we interpret the diminished levels of mRNA expression at seven days to reflect either the failure of the progenitors to survive or an alternate stage of differentiation that expresses a phenotype that no longer expresses Sox9. This later interpretation would be consistent with the very large increase in levels of Msx2, the fact that expression Msx2 expression was associated with the prevention of osteoblastic differentiation and mineralization of the extracellular matrix whereas, antisense Msx2 RNA decreased proliferation and accelerated differentiation [29].
In summary, these results suggest that BMP2 acts after the stage of osteoprogenitor recruitment and primarily drives bone marrow stem cells towards terminal osteoblastic differentiation. BMP7, in the absence of BMP2, appears to increase the numbers of progenitors but is less efficient at promoting terminal differentiation. This primary difference in the effects of these two BMPs appears to be related to their differential actions on Runx2 and osterix expression. Three recent animal studies reported clinically-relevant findings that are potentially consistent with the findings and mechanisms that we suggest here. In one study, the co-administration of adenovirus-mediated gene transfer for both BMP2 and BMP7 showed comparatively more stable spinal fusion and higher levels of mineralization compared to the single, individual transfer of either BMP [50]. In the second study, a delay in the timing of injection of the adenoviral BMP2 expression vector until 5 or 10 days after surgery increased the percentage of critical-sized segmental defects that achieved radiological union and showed enhanced mineralization and greater mechanical strength [51]. In the third study, using a non human primate critical size defect model showed that delaying BMP2 treatment for up to one week after surgery gave the optimal therapeutic effects. These authors concluded that the accelerated healing seen in the delayed treatment groups was because of an increase in the number of responding cells that came into the surgical site which were then responsive to BMP2, which increased direct bone formation [52]. These observations, as well as recent clinical experiences in which recombinant human BMPs 2 and 7 (Osteogenic Protein 1; OP-1) showed less than consistent results in achieving fracture union suggest that a deeper understanding of their how BMPs act on specific stages of the osteogenic lineage may be required for more successful therapeutic application.[53]
Acknowledgments
Supported with grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (PO1AR049920) (TAE and LCG). Institutional support was provided by the Department of Orthopaedic Surgery Boston University School of Medicine and by Boston University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hollinger JO, Schmitt JM, Buck DC, Shannon R, Joh SP, Zegzula HD, Wozney J. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res. 1998;43:356–64. doi: 10.1002/(sici)1097-4636(199824)43:4<356::aid-jbm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [2].Tsiridis E, Morgan EF, Bancroft JM, Song M, Kain M, Gerstenfeld L, Einhorn TA, Bouxsein ML, Tornetta P. Effects of OP-1 and PTH in a new experimental model for the study of metaphyseal bone healing. J Orthop Res. 2007;25:1193–2033. doi: 10.1002/jor.20420. [DOI] [PubMed] [Google Scholar]
- [3].Welch RD, Jones AL, Bucholz RW, Reinert CM, Tjia JS, Pierce WA, Wozney JM, Li XJ. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J Bone Miner Res. 1998;13:1483–90. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- [4].Hogan BL. Bmps: multifunctional regulators of mammalian embryonic development. Harvey Lect. 1996;92:83–98. [PubMed] [Google Scholar]
- [5].Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–39. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- [6].Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- [7].Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- [8].Gambaro K, Aberdam E, Virolle T, Aberdam D, Rouleau M. BMP-4 induces a Smad-dependent apoptotic cell death of mouse embryonic stem cell-derived neural precursors. Cell Death Differ. 2006;13:1075–87. doi: 10.1038/sj.cdd.4401799. [DOI] [PubMed] [Google Scholar]
- [9].Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–52. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- [10].Nakashima K, Yanagisawa M, Arakawa H, Taga T. Astrocyte differentiation mediated by LIF in cooperation with BMP2. FEBS Lett. 1999;457:43–6. doi: 10.1016/s0014-5793(99)00997-7. [DOI] [PubMed] [Google Scholar]
- [11].Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–42. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- [12].Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, Hanks MC, Amling M, Pinero GJ, Harada S, Behringer RR. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004;279:27560–6. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- [13].Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol. 2002;157:1049–60. doi: 10.1083/jcb.200109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21:1022–33. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- [15].Devlin RD, Du Z, Pereira RC, Kimble RB, Economides AN, Jorgetti V, Canalis E. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972–8. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- [16].Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40:1389–98. doi: 10.1016/j.bone.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld LC, Einhorn TA, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- [18].Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–20. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- [19].Suva LJ, Seedor JG, Endo N, Quartuccio HA, Thompson DD, Bab I, Rodan GA. Pattern of gene expression following rat tibial marrow ablation. J Bone Miner Res. 1993;8:379–88. doi: 10.1002/jbmr.5650080315. [DOI] [PubMed] [Google Scholar]
- [20].Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–94. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- [21].Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/JBMR.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Theodorsson-Norheim E. Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput Methods Programs Biomed. 1986;23:57–62. doi: 10.1016/0169-2607(86)90081-7. [DOI] [PubMed] [Google Scholar]
- [23].Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–92. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- [24].Mortlock DP, Guenther C, Kingsley DM. A general approach for identifying distant regulatory elements applied to the Gdf6 gene. Genome Res. 2003;13(9):2069–81. doi: 10.1101/gr.1306003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang K, Vishwanath P, Eichler GS, Al-Sebaei MO, Edgar CM, Einhorn TA, Smith TF, Gerstenfeld LC. Analysis of fracture healing by large-scale transcriptional profile identified temporal relationships between metalloproteinase and ADAMTS mRNA expression. Matrix Biol. 2006;25:271–81. doi: 10.1016/j.matbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [26].Jepsen K, Price C, Silkman L, Nicholls F, Nasser P, Hu B, Hadi N, Alapatt M, Stapleton S, Kakar S, Einhorn TA, Gerstenfeld LC. Genetic Variation in the Patterns of Skeletal Progenitor Cell Differentiation and Progression During Endochondral Bone Formation Affects the Rate of Fracture Healing. J Bone Miner Res. 2008;23(8):1204–16. doi: 10.1359/JBMR.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- [28].Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–66. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- [29].Dodig M, Tadic T, Kronenberg MS, Dacic S, Liu YH, Maxson R, Rowe DW, Lichtler AC. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev Biol. 1999;209:298–307. doi: 10.1006/dbio.1999.9258. [DOI] [PubMed] [Google Scholar]
- [30].Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–58. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bae JS, Gutierrez S, Narla R, Pratap J, Devados R, van Wijnen AJ, Stein JL, Stein GS, Lian JB, Javed A. Reconstitution of Runx2/Cbfa1-null cells identifies a requirement for BMP2 signaling through a Runx2 functional domain during osteoblast differentiation. J Cell Biochem. 2007;100:434–49. doi: 10.1002/jcb.21039. [DOI] [PubMed] [Google Scholar]
- [32].Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–46. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang S, Wei D, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003;18:705–15. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–92. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112(Pt 20):3519–27. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- [36].Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002;2002:PE40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- [37].Korchynskyi O, Dechering KJ, Sijbers AM, Olijve W, ten Dijke P. Gene array analysis of bone morphogenetic protein type I receptor-induced osteoblast differentiation. J Bone Miner Res. 2003;18:1177–85. doi: 10.1359/jbmr.2003.18.7.1177. [DOI] [PubMed] [Google Scholar]
- [38].Noth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, Hickok NJ, Tuan RS. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291:201–11. doi: 10.1016/s0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]
- [39].Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, Luu HH, Park JY, Haydon RC, He TC. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90:1149–65. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- [40].ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–13. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- [41].ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–8. [PubMed] [Google Scholar]
- [42].Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J Biol Chem. 2005;280:31353–9. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- [43].Shoba LN, Lee JC. Inhibition of phosphatidylinositol 3-kinase and p70S6 kinase blocks osteogenic protein-1 induction of alkaline phosphatase activity in fetal rat calvaria cells. J Cell Biochem. 2003;88:1247–55. doi: 10.1002/jcb.10474. [DOI] [PubMed] [Google Scholar]
- [44].Yeh LC, Adamo ML, Olson MS, Lee JC. Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology. 1997;138:4181–90. doi: 10.1210/endo.138.10.5465. [DOI] [PubMed] [Google Scholar]
- [45].Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, de Crombrugghe B. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005;102:14665–70. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fu H, Doll B, McNelis T, Hollinger JO. Osteoblast differentiation in vitro and in vivo promoted by Osterix. J Biomed Mater Res A. 2007;83:770–8. doi: 10.1002/jbm.a.31356. [DOI] [PubMed] [Google Scholar]
- [47].Kaback LA, Soung do Y, Naik A, Smith N, Schwarz EM, O’Keefe RJ, Drissi H. Osterix/Sp7 regulates mesenchymal stem cell mediated endochondral ossification. J Cell Physiol. 2008;214:173–82. doi: 10.1002/jcp.21176. [DOI] [PubMed] [Google Scholar]
- [48].Zelzer E, Olsen BR. The genetic basis for skeletal diseases. Nature. 2003;423:343–8. doi: 10.1038/nature01659. [DOI] [PubMed] [Google Scholar]
- [49].Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi JY, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen AJ. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003;63:5357–62. [PubMed] [Google Scholar]
- [50].Zhu W, Rawlins BA, Boachie-Adjei O, Myers ER, Arimizu J, Choi E, Lieberman JR, Crystal RG, Hidaka C. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res. 2004;19:2021–32. doi: 10.1359/JBMR.040821. [DOI] [PubMed] [Google Scholar]
- [51].Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, Vrahas MS, Bouxsein ML, Evans CH. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14(13):1039–44. doi: 10.1038/sj.gt.3302956. Epub 2007. [DOI] [PubMed] [Google Scholar]
- [52].Seeherman H, Li R, Bouxsein M, Kim H, Li XJ, Smith-Adaline EA, Aiolova M, Wozney JM. rhBMP-2/calcium phosphate matrix accelerates osteotomy-site healing in a nonhuman primate model at multiple treatment times and concentrations. J Bone Joint Surg Am. 2006;88:144–60. doi: 10.2106/JBJS.D.02453. [DOI] [PubMed] [Google Scholar]
- [53].Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman HJ. Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am. 2005;87(6):1367–78. doi: 10.2106/JBJS.D.02585. [DOI] [PubMed] [Google Scholar]