Abstract
Objective
To compare the incidence of venous thromboembolism among women taking combined oral contraceptives before and after the October 1995 pill scare.
Design
Analysis of General Practice Research Database.
Setting
United Kingdom, January 1993 to December 1998.
Subjects
Women aged 15-49 taking combined oral contraceptives.
Main outcome measures
Incidence of venous thromboembolism.
Results
Use of so called “third generation” combined oral contraceptives fell from 53% during January 1993 to October 1995 to 14% during November 1995 to December 1998. There was no significant change in the incidence of venous thromboembolism between the two periods after age was adjusted for (incidence ratio 1.04, 95% confidence interval 0.78 to 1.39).
Conclusions
The findings are not compatible with the assertion that third generation oral contraceptives are associated with a twofold increase in risk of venous thromboembolism compared with older progestogens.
Introduction
In October 1995 the UK Committee on Safety of Medicines advised that combined oral contraceptives containing either gestodene or desogestrel were associated with twice the risk of venous thromboembolism compared with older products.1 The advice was based on their interpretation of three, then unpublished, studies.2–4 No confidence intervals were given for their estimate of the increase in risk. After the announcement, a large proportion of women taking these so called “third generation” combined oral contraceptives either discontinued use or changed to other formulations. In 1999 the Medicines Control Agency revised the estimate down to a 1.7-fold increase in risk.5 The rationale for the newer estimate was not included in its statement. Since 1995 several other studies and analyses have been published. Some of these support the hypothesis that there is a significant increase in risk associated with the newer progestogens,6–8 whereas others have found no difference.9–13 Two of the studies used the UK General Practice Research Database.3,13 Since the 1995 pill scare a further three years of data have been accumulated on this database. We used these data to quantify the change in use of combined oral contraceptives and the effect on the incidence of idiopathic venous thromboembolism among women taking oral contraceptives.
Methods
The General Practice Research Database comprises anonymous clinical data from general practices in the United Kingdom and has been described elsewhere.14 It is updated regularly. This investigation is restricted to the 304 practices that contributed data continuously throughout the study period (January 1993 to December 1998).
The study population consisted of women aged 15 to 49 who had taken combined oral contraceptives at any time within the study period. The population exposure to combined oral contraceptives was calculated from the number of 28 day cycles prescribed and ascribing use to each month within the study period. Cycles that were unused because of switching between products and cycles that would have been used outside the study period were discounted. Potential cases of idiopathic venous thromboembolism were identified by searching the database for women with a diagnosis of any deep venous thrombosis or pulmonary embolism. Women were included as cases only if they had evidence of treatment with oral anticoagulants (or had died from the event) and had a prescription for combined oral contraceptives current on the day that the thromboembolism was first detected. We excluded women who had evidence of previous venous thromboembolism or who, in the six weeks before the thromboemblolism, were pregnant, had lower limb fractures, or had surgery requiring immobilisation in the six weeks before the thromboembolism. Other exclusion criteria were malignancy, congenital heart disease, exposure to other sex hormones, less than six months of research standard data before the event, or drug overdose associated with the event. The methods and case identification are described fully elsewhere.14,15
The data were partitioned into exposures and events occurring between January 1993 and October 1995 (period 1) and those between November 1995 and December 1998 (period 2). We compared the overall use of combined oral contraceptives and the rates of idiopathic venous thromboembolism among women exposed to combined oral contraceptives in the two periods. We calculated the change in the numbers of cases of venous thromboembolism between the two periods that would have been expected had the risk of third generation formulations been twice that of the older formulations containing less than 50 μg oestrogen. The expected number of cases was standardised for year of age by using the data on overall use from the two periods.
Results
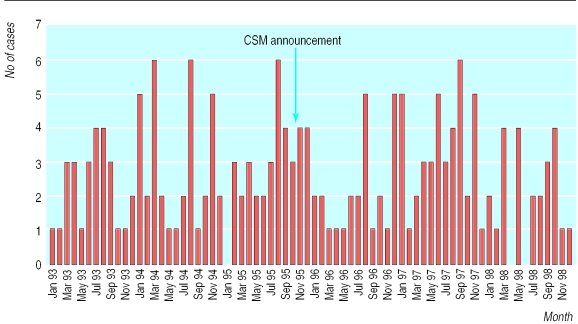
Between periods 1 and 2 the overall use of combined oral contraceptives fell by 14.1% among women aged 15-19 and by 11.7% among women aged 20-24. The smallest change was among women aged over 30. The percentage of prescribed combined oral contraceptives that contained either gestodene or desogestrel fell from 53.4% to 14.0% (table 1). The figure shows the number of cases of venous thromboembolism identified during each month from January 1993 to December 1998. There was no immediate increase in the numbers of cases after the announcement from the Committee on Safety of Medicines. The crude incidence of idiopathic venous thromboembolism remained stable between the two periods; the crude rate ratio was 1.09 (95% confidence interval 0.81 to 1.46), and the ratio adjusted for year of age by the Mantel-Haenszel method was 1.04 (0.78 to 1.39).16 Table 2 shows the rates of venous thromboembolism among women exposed to combined oral contraceptives and incidence ratios before and after October 1995 stratified by age.
Table 1.
Incidence of venous thromboembolism and use of combined oral contraceptives before and after October 1995
| Jan 1993-Oct 1995 (period 1) | Nov 1995-Dec 1998 (period 2) | |
|---|---|---|
| Observed population aged 15-49 (1000s woman years) | 1516 | 1677 |
| Exposed population aged 15-49 (1000s woman years) | 260.9 | 258.8 |
| No of cases of venous thromboembolism | 90 | 97 |
| Oral contraceptive years per 100 woman years | 17.2 | 15.4 |
| % of oral contraceptives containing gestodene or desogestrel | 53.4 | 14.0 |
| No of venous thromboembolisms/100 000 exposed woman years* | 34.5 | 37.5 (35.9†) |
Exposed to combined oral contraceptives.
Rate standardised to age distribution of period 1.
Table 2.
Rates of venous thrombolism per 100 000 woman years of exposure to combined oral contraceptives according to age, before and after October 1995
| Age (years) | Rate of venous thromboembolism
|
Rate ratio (95% CI) | Age adjusted ratio* (95% CI) | |
|---|---|---|---|---|
| Jan 1993-Oct 1995 | Nov 1995-Dec 1998 | |||
| 15-24 | 22.59 | 21.56 | 0.95 (0.51 to 1.79) | 0.96 (0.54 to 1.71) |
| 25-34 | 41.26 | 41.40 | 1.00 (0.67 to 1.51) | 0.99 (0.67 to 1.96) |
| 35-49 | 48.39 | 65.34 | 1.35 (0.68 to 2.75) | 1.31 (0.68 to 2.50) |
Adjusted by year of age.
The age standardised number of cases expected in period 2 based on the assertion that desogestrel and gestodene were associated with twice the risk of venous thromboembolism was calculated to be 69.3. The observed number of cases was 97, 1.4 times (95% confidence interval 1.14 to 1.71) that expected.
What is already known on this topic
Third generation combined oral contraceptives containing desogestrel or gestodene have been reported to carry increased risk of venous thromboembolism
Since this was reported in October 1995, the use of third generation oral contraceptives has fallen from 53% to 14% of total use
What this study adds
The change in patterns of use had no effect on the incidence of venous thromboembolism among women taking combined oral contraceptives
The findings are not consistent with third generation oral contraceptives doubling the risk of venous thromboembolism
Conclusions
The rate of venous thromboembolism among women taking oral contraceptives throughout the study period is consistent with that found in most other studies.17 If oral contraceptives containing gestodene or desogestrel had twice the risk of venous thromboembolism compared with older formulations, a reduction in their use would be expected to reduce the incidence of idiopathic venous thromboembolism. We found no such change. No evidence of a difference was seen in any of the age groups. Moreover, there was a substantial excess of cases compared with the number that would have been expected if third generation oral contraceptives doubled the risk of venous thromboembolism.
The detection rate could have increased because the 1995 “pill scare” alerted doctors to the probability of venous thromboembolism among women taking oral contraceptives. If this had happened, however, the number of cases would be expected to rise immediately after the 1995 announcement. No such increase was apparent.
Figure.
Number of cases of venous thromboembolism by month of occurrence, January 1993-December 1998
Acknowledgments
We thank Professor K D MacRae for his help with the statistical analyses.
Footnotes
Funding: The department is supported by grants from several pharmaceutical companies including NV Organon, Schering AG, and Wyeth. The companies have no control over the conduct of any research or over publications.
Competing interests: RDTF has been reimbursed expenses for attending conferences by pharmaceutical companies; he has also been paid fees for speaking and consultancy.
References
- 1.Committee on Safety of Medicines. Combined oral contraceptives and thromboembolism. London: CSM; 1995. [Google Scholar]
- 2.Poulter NR, Chang CL, Farley TMM, Meirek O, Marmot MG. World Health Organization collaborative study of cardiovascular disease and steroid hormone contraception. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case control study. Lancet. 1995;346:1575–1582. [PubMed] [Google Scholar]
- 3.Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and non-fatal venous thromboembolism in women using oral contraceptives with differing progestogen components. Lancet. 1995;346:1589–1593. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer WO, Lewis MA, Heinemann LA, Thorogood M, MacRae KD. Third generation oral contraceptives and risk of venous thromboembolic disorders: an international case-control study. Transnational Research Group on Oral Contraceptives and the Health of Young Women. BMJ. 1996;312:83–88. doi: 10.1136/bmj.312.7023.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayor S. Department of Health changes advice on third generation pills. BMJ. 1999;318:1026. doi: 10.1136/bmj.318.7190.1026a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin L, Skegg DCG, Wilson M, Herbison GP, Paul C. Oral contraceptives and fatal pulmonary embolism. Lancet. 2000;355:2133–2134. doi: 10.1016/S0140-6736(00)02382-5. [DOI] [PubMed] [Google Scholar]
- 7.Bloemenkamp KW, Rosendaal FR, Buller HR, Helmerhost FM, Colly LP, Vandenbroucke JP. Risk of venous thrombosis with use of current low-dose oral contraceptives is not explained by diagnostic suspicion and referral bias. Arch Int Med. 1999;159:65–70. doi: 10.1001/archinte.159.1.65. [DOI] [PubMed] [Google Scholar]
- 8.Mellemkjaer L, Sorenson HT, Dreyer L, Olsen J, Olsen JH. Admission for and mortality from primary venous thromboembolism in women of fertile age in Denmark, 1977-95. BMJ. 1999;319:820–821. doi: 10.1136/bmj.319.7213.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suissa S, Blais L, Spitzer WO, Cusson J, Lewis M, Heinemann L. First-time use of newer oral contraceptives and the risk of venous thromboembolism. Contraception. 1998;57:61–65. doi: 10.1016/s0010-7824(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 10.Lidegaard O, Edstrom B, Kreiner S. Oral contraceptives and venous thromboembolism: a case-control study. Contraception. 1998;57:291–301. doi: 10.1016/s0010-7824(98)00033-x. [DOI] [PubMed] [Google Scholar]
- 11.Lewis MA, MacRae KD, Kuhl-Habichl D, Bruppacher R, Heinemann LA, Spitzer WO. The differential risk of oral contraceptives: the impact of full exposure history. Hum Reprod. 1999;14:1493–1499. doi: 10.1093/humrep/14.6.1493. [DOI] [PubMed] [Google Scholar]
- 12.Farmer RDT, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83–88. doi: 10.1016/s0140-6736(96)07496-x. [DOI] [PubMed] [Google Scholar]
- 13.Farmer RDT, Lawrenson RA, Todd J-C, Williams TJ, MacRae KD, Tyrer F, et al. A comparison of the risks of venous thromboembolic disease in association with different combined oral contraceptives. Br J Clin Pharmacol. 2000;49:580–590. doi: 10.1046/j.1365-2125.2000.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jick H, Jick SS, Derby LE. Validation of information recorded on the general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrenson R, Todd J-C, Leydon GM, Williams TJ, Farmer RDT. Validation of the diagnosis of venous thromboembolism in general practice database studies. Br J Clin Pharmacol. 2000;49:591–596. doi: 10.1046/j.1365-2125.2000.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.StataCorp. Stata statistical software release 5.0. College Station, TX: Stata Corporation; 1997. [Google Scholar]
- 17.Lawrenson RA, Whalley A, Simpson E, Farmer RDT. DoH seems to have underestimated incidence of venous thromboembolism in users of combined oral contraceptives. BMJ. 1999;319:387. [PMC free article] [PubMed] [Google Scholar]