Abstract
Surrogate markers to detect vasculitic processes prior to organ compromise are lacking. To determine if specific populations among the fibronectin (FN) family of alternatively spliced proteins correlate with parameters of vasculitis in at-risk patients, we retrospectively evaluated the association of plasma levels of total FN (TFN) and FN bearing the alternatively spliced EIIIA segment (A+FN) with clinical vasculitis status and with levels of two putative vasculitis markers (C-reactive protein [CRP] and von Willebrand factor) in a previously-studied cohort of 27 patients with systemic inflammatory disease. We found that the percentage of TFN comprised by A+FN (%A+) and A+FN, but not TFN, correlated with plasma levels of CRP, the prototypic inflammation biomarker used to detect vasculitis. These findings suggest that different FNs may confer distinct clinical information, and that their simultaneous measurement merits further investigation in our efforts to identify soluble biomarker systems to detect vasculitis.
Keywords: vasculitis, fibronectin, alternative splicing, biomarkers
INTRODUCTION
Fibronectins (FNs) are a heterogeneous family of adhesion glycoprotein isoforms that differ as a consequence of alternative splicing. FN in plasma (pFN) has previously been considered to be a homogeneous molecular population derived from the liver (Tamkun et al. 1983, Hynes 1990). As such, pFN has been viewed as being devoid of molecules bearing the alternatively spliced 90 amino acid EIIIA (for “extra type III repeat A”) segment, since hepatocytes, which synthesize pFN, do not normally express FNs bearing this segment (Tamkun et al. 1983, Schwarzbauer 1991). However, small (less than 1% of total pFN pool) quantities of FN bearing the EIIIA segment (A+FN) are present in the circulation of healthy individuals. Additionally, A+FN has been reported to be increased above normal control levels in the circulation of patients and experimental animals with vascular tissue injury (Peters et al. 1986, Peters et al. 1988, Peters et al. 1989, Lockwood et al. 1990, Fijnheer et al. 1997, Peters et al. 2003). Although the source of such circulating A+FN remains uncertain, in situ hybridization and immunohistochemical analyses have shown increased A+FN expression and deposition at sites of blood vessel injury (Dubin et al. 1995, Nickeleit et al. 1995, Tan et al. 2004), and prominent A+FN expression has been observed at the surface of atheromatous lesions in Apo E null mice with increased plasma levels of A+FN (Tan et al. 2004). These findings raise the possibility that the increased circulating A+FN levels that occur in association with vascular tissue injury could result from synthesis and release from cells in blood vessel walls.
We hypothesize that simultaneous measurement of the total pool of pFN (TFN) and A+FN should yield two qualitatively different information sets, each reflecting distinct in situ “microenvironments”. Specifically, the majority of TFN is an acute phase reactant derived from the liver (Tamkun et al. 1983, Amrani et al. 1986, Pick-Kober et al. 1986, Hynes 1990), whereas A+FN is normally concentrated in blood vessel walls (Vartio et al. 1987, Peters et al. 1996). Therefore, potential sources of soluble circulating A+FN in health and disease include vascular endothelial cells, which have been observed to express EIIIA segment-enriched FN in vitro (Peters et al. 1990), and platelets, in which A+FN is similarly enriched relative to the total pool of alpha granule FN (Paul et al. 1986, Peters et al. 1995). Consistent with this distribution, circulating A+FN has gained a reputation as a marker for vascular injury or “endothelial activation”. In support of this, plasma A+FN levels have been observed to be elevated in several disorders involving vascular injury, including preeclampsia (Lockwood et al. 1990, Sikkema et al. 2002), subarachnoid hemorrhage (Frijns et al. 2002), sepsis (Peters et al. 1989, Fijnheer et al. 1997), acute major trauma (Peters et al. 1989), hemorrhagic transformation of acute ischemic stroke (Castellanos et al. 2004), thrombotic thrombocytopenic purpura (Fijnheer et al. 1997), and rheumatoid vasculitis (Voskuyl et al. 1998). Despite these findings, measurement of A+FN has seldom been performed simultaneously with TFN (Peters et al. 1988, Peters et al. 1989, Lockwood et al. 1990, Satoi et al. 1999), nor has the relationship between the two categories of FN previously been assessed for correlations with parameters of vascular injury or inflammation, including other putative biomarkers.
Sensitive and specific biomarker systems to detect and characterize vasculitis that occurs in the setting of systemic inflammatory disease (SID) or de novo are increasingly needed in this era of powerful therapeutic biologic agents with the capacity to target specific aspects of the immune system. Among current inflammatory biomarkers, the acute-phase protein CRP is recognized as the prototype. CRP and the erythrocyte sedimentation rate (ESR) are commonly measured together to gauge inflammatory and vasculitic activity in patients with SID and, with the advent of high sensitivity assays, CRP has also emerged as a strong predictor of atherosclerotic cardiovascular disease (Blake et al. 2003). Despite this, CRP is a non-specific marker for inflammation (Krishnamurthy et al. 2006) with a variable capacity to sense different SID-related vasculitides. For example, CRP has been observed to be more sensitive than erythrocyte sedimentation rate for detection of giant cell arteritis (GCA) (Hayreh et al. 1997), but may be only moderately raised in systemic lupus erythematosis (SLE) patients with very active disease (ter Borg et al. 1990). Clearly, additional sensitive and specific biomarkers to characterize and monitor the activity of vasculitides, SID-associated or not, would be helpful.
To assess whether A+FN and TFN could constitute soluble biomarkers for vascular tissue injury, we retrospectively examined a cohort of 27 patients with SID in whom the two FN pools had previously been simultaneously measured at a number of time points. We looked for evidence of correlations between these levels, or their relationship to one another (expressed as the percentage of the TFN pool comprised by A+FN [%A+]), with parameters indicating vasculitis. Specifically, levels of the two pools were examined for correlations with clinical evidence of vasculitis and with simultaneously measured levels of CRP and another putative biomarker for vascular injury, von Willebrand factor (vWF).
Materials and Methods
Study design
The original study was approved by human research oversight committee at the University of Bristol, England. Methods for plasma sample collection, preparation and quantitative immunoassays for TFN and A+FN were described in our previous descriptive report (Peters et al. 1989). 27 patients with SID, studied at the Bristol Royal Infirmary in 1986–1987, included individuals with rheumatoid arthritis (RA) (n=11), SLE (n=3), progressive systemic sclerosis (PSS) (n=3), polymyositis (n=2), GCA (n=2), polymyalgia rheumatica (n=2), Wegener’s granulomatosis (n=1), polyarteritis nodosa (n=1), eosinophilic fasciitis (n=1), and primary Sjogren’s Syndrome (n=1). 16 of the 27 patients exhibited clinical and/or histologic evidence of vasculitis, conforming with three general clinical categories: 1) “small vessel” in 6 (2 each with RA, SLE and PSS), 2) “systemic necrotizing” in 8 (5 with RA, 1 with polymyositis, 1 with polyarteritis nodosa, and 1 with Wegener’s), and “large vessel” in 2 (both with GCA) (Woolf et al. 1987). Of note, the original studies from which the current database is drawn were conducted prior to development of the Birmingham Vasculitis Activity Score (Luqmani et al. 1994) and Chapel Hill Consensus Conference classification (Jennette et al. 1994). Therefore, in addition to a specific SID diagnosis, the information available to us for each patient was limited to assessments regarding the presence and category (see above) of vasculitis, age, gender, presence or absence of glucocorticoid therapy, and plasma biomarker (CRP, vWF, A+FN, and TFN) levels. Each patient contributed between 1 and 10 plasma samples, at intervals ranging from 3 days to 1 month. A single sample of venous blood was also obtained from each of 10 healthy “control” subjects, who were drawn from the staff of the Bristol Royal Infirmary. The SID patients had originally been included in a study of the utility of vWF levels in the assessment of vasculitis, in which vWF and CRP levels were measured in most plasma samples, respectively by ELISA or radial immunodiffusion (Woolf et al. 1987). Levels of vWF and CRP, each expressed as a percentage of the average normal control level, were therefore respectively available for 62 (98%) and 42 (65%) of the 63 samples from 27 SID patients for which TFN and A+FN levels had previously been measured. In contrast, only FN levels were available for the 10 “control” samples (Peters et al. 1989).
Statistical analysis
T tests were used to compare age and number of blood draws between the vasculitic cases and the non-vasculitic control group. To assess the effect of vasculitis status upon average plasma concentrations of putative vascular injury markers, linear mixed effects models were fitted. The response variable was taken to be a given marker (e.g., A+FN concentration), whereas the predictor was vasculitis status. The intercept was taken to be random. Relationships between pairs of plasma protein components were analyzed by linear mixed effects models in order to estimate the degree to which one marker predicted the other at a particular blood draw, the degree to which a particular marker changed over time within and across patients, and to permit adjustment for diagnostic group via regression modeling. Linear mixed effects models were also used to compare SID patients with normal controls, and vasculitic with non-vasculitic groups.
Results
Subject characteristics
The control group of 10 healthy volunteers was composed of 5 females ranging in age from 23 to 37 (average age 29), and 5 males ranging in age from 29 to 35 (average age 32) with an overall average age of 30 years (Peters et al. 1989). In contrast, ages in the SID group ranged from 32 to 79 for the 26 subjects for whom age was recorded (age was not recorded for one individual). Within this group, the subgroup with vasculitis was younger (56.3 ± 13.6 years, n=15 [no age was available for patient 27, therefore n=15 instead of 16]) than the subgroup without vasculitis (60.8 ± 10.8 years, n=11) but the difference was not significant (p=0.35). Females accounted for 13 (86.7%) of the 15 vasculitis patients for whom gender was recorded (this information was not recorded for one of the vasculitis patients), and 10 (90.9%) of the 11 patients in the non-vasculitis subgroup (Table I). 13 (92.9%) of the 14 vasculitis patients for whom information was available were on steroid therapy, whereas such treatment information was not recorded for the remaining two patients in this group. In comparison, 5 (45.5%) of 11 patients in the non-vasculitis group were on steroid therapy. Patients with vasculitis underwent significantly more blood draws (3.2 ± 2.4) than the non-vasculitis group (1.2 ± 0.4) (p=0.007) (Table 1).
Table 1.
Characteristics of patients with systemic inflammatory disease without (controls) versus with vasculitis (cases)*
| Controls (without vasculitis) (n=11) | Cases (with vasculitis) (n=16) | |
|---|---|---|
| Age | 60.8 ± 10.8 | 56.3 ± 13.6 |
| Percent female | 90.9 | 86.7 |
| Percent on steroid treatment | 45.4 | 85.7 |
| Number of blood draws | 1.2 + 0.4 | 3.2 ± 2.4 |
In the non-vasculitis control group, no age was recorded for one patient, so age data was averaged for n=10. Similarly, in the vasculitis case group, gender information was unavailable for one patient, and steroid treatment information was missing for two patients. Therefore, gender and steroid data for this group were averaged for n=15 and n=14, respectively. Values are expressed as the mean ± standard deviation.
Relation of levels of A+FN and TFN to parameters of vasculitis in SID patients
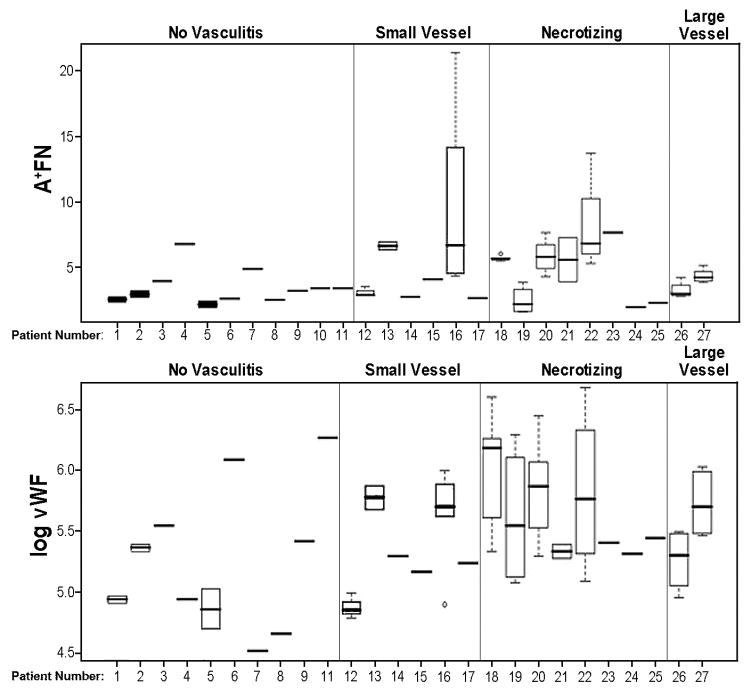
In comparison to healthy controls, patients exhibited significantly increased average circulating levels of A+FN (4.64 vs. 2.28, p=0.03), but not of %A+ (1.09% vs. 0.64%, p=0.06) or TFN (426.4 vs. 368.2, p=0.11) (not shown). Also, the average levels of A+FN, TFN, %A+, vWf and CRP were higher in SID patients with vasculitis than without vasculitis, but these differences did not attain statistical significance for any of the five markers (Table 2). Patients with vasculitis were further categorized as “small vessel”, “systemic necrotizing”, and “large vessel”. Consistent with previous observations (Woolf et al. 1987), patients with evidence of necrotizing vasculitis exhibited higher levels of vWF than non-vasculitis patients when a log transformation of vWF was used (57% greater; p=0.024), but such a difference was not evident for the “small vessel” or “large vessel” vasculitis subgroups. In comparisons between the three vasculitis subgroups, some variation in levels of plasma protein components was evident (see Figure 1 for A+FN and vWF), but small sample sizes and substantial variability within and between patients within diagnostic categories precluded having adequate statistical power to detect differences across vasculitis subtypes.
Table 2.
Effect of vasculitis status upon average plasma concentrations of putative vascular injury markers in systemic inflammatory disease patients*
| No Vasculitis |
Vasculitis |
||||
|---|---|---|---|---|---|
| Marker | estimated mean | estimated mean | estimated difference | standard error of difference | p-value |
| A+FN | 3.405 | 5.21 | 1.805 | 1.026 | 0.0908 |
| TFN | 404.783 | 438.344 | 33.561 | 36.448 | 0.366 |
| %A+ | 0.008 | 0.012 | 0.004 | 0.002 | 0.125 |
| VWF1 | 215.298 | 292.249 | 76.951 | 49.923 | 0.136 |
| CRP2 | 44.445 | 74.953 | 30.508 | 40.189 | 0.46 |
1In this model, one non-vasculitis patient was removed because no vWF was recorded. 2Total sample size for this model is 41 observations on 17 patients. A+FN refers to the plasma concentration (μg/ml) of FN isoforms bearing the EIIIA segment. TFN refers to the total concentration (μg/ml) of FN in plasma. %A+ refers to the percentage of TFN comprised of A+FN isoforms. vWF and CRP respectively refer to the plasma concentrations of vWF and CRP, expressed as the % of the average value in healthy individuals.
Figure 1.
Variation in plasma levels of A+FN and vWF between categories of vascular complications in SID, within category, and within person. Box plots show variation for repeated measures on same person; the ends of each box mark the 25th and 75th percentile of all observations for the person, and the whiskers show observations in the first and last quartile. Outliers are shown by open circles. Individuals with only one observation are shown by a single line segment. In A, the ordinate is A+FN concentration (micrograms/ml), whereas, in B, the ordinate is log of vWF concentration (expressed as % of average concentration in healthy controls). vWF was log transformed to stabilize variance and reduce skewness of data. Patient number 10 is missing from panel B, since no vWF concentration was available for this subject.
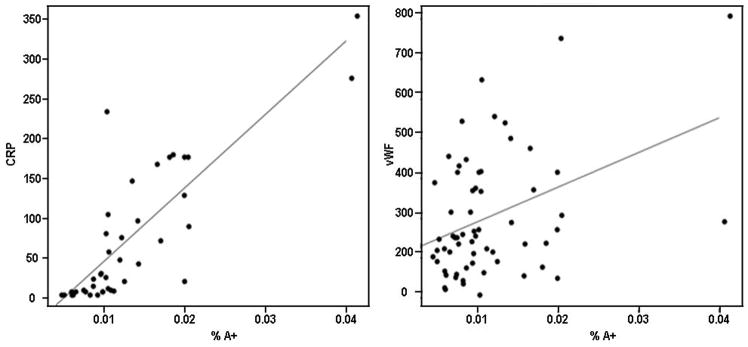
The relationship among putative vascular injury markers was assessed by fitting separate repeated measures regression models, first with one predictor marker alone (Table 3), and then with an indicator for vasculitis (Table 4), to determine whether significant correlations could be largely accounted for by changes in both markers limited to those with vasculitis. When the vasculitis status of subjects was not considered, both A+FN and %A+ correlated significantly with simultaneously-measured levels of CRP (p<0.001 for both comparisons) (Table 3). The correlations between A+FN and %A+ with CRP persisted even after removal of two large “outlier” values (Figure 2, left panel). When vasculitis status was considered, the association between elevated levels of %A+ and CRP remained strong (Table 4, p<0.001), suggesting that this association was not solely accounted for by differences associated with the presence of vasculitis. The relationship also persisted after removal of two outliers. The association between A+FN and CRP, however, was no longer significant when vasculitis status was considered (p=0.63, Table 4). No significant correlations were evident between TFN and CRP, either without considering vasculitis status (p=0.52, Table 3), or when vasculitis was added as a predictive factor (p=0.52, Table 4).
Table 3.
Correlations between levels of plasma biomarkers, irrespective of subject vasculitis classification*
| Outcome: CRP | Regression coefficient | std. error | p-value | Df |
|---|---|---|---|---|
| Predictor | ||||
| A+FN | 17.144 | 2.541 | <0.001 | 23 |
| TFN | −0.09 | 0.14 | 0.52 | 23 |
| %A+ | 9135.321 | 920.837 | <0.001 | 23 |
| VWF | 0.215 | 0.069 | 0.005 | 23 |
| Outcome: VWF | regression coefficient | std. error | p-value | Df |
| Predictor | ||||
| A+FN | 13.203 | 6.1 | 0.037 | 35 |
| TFN | 0.051 | 0.2 | 0.80 | 35 |
| %A+ | 8345.609 | 2695.398 | 0.004 | 35 |
The response variable is listed as the “outcome”. Vasculitis status is not included as a predictor. The random effect was included on the intercept. A+FN refers to the plasma concentration (μg/ml) of FN isoforms bearing the EIIIA segment. TFN refers to the total concentration (μg/ml) of FN in plasma. %A+ refers to the percentage of TFN comprised of A+FN isoforms. vWF and CRP respectively refer to the plasma concentrations of vWF and CRP, expressed as the % of the average value in healthy individuals.
Table 4.
Correlation between levels of plasma biomarkers, adjusting for subject vasculitis status (vasculitis included as a predictor)*
| Outcome: CRP | Regression coefficient | standard error | p-value |
|---|---|---|---|
| Predictor | |||
| A+FN | 17.404 | 32.569 | 0.63 |
| TFN | −0.093 | 0.14 | 0.515 |
| %A+ | 9382.463 | 920.837 | <0.001 |
| VWF | 0.212 | 0.071 | 0.007 |
| Outcome: VWF | Regression coefficient | standard error | p-value |
| Predictor | |||
| A+FN | 11.681 | 6.238 | 0.07 |
| TFN | 0.016 | 0.197 | 0.937 |
| %A+ | 7763.922 | 2750.941 | 0.008 |
The response variable is given as “outcome”. The two predictors are vasculitis status and the marker shown at the beginning of the row. A+FN refers to the plasma concentration (μg/ml) of FN isoforms bearing the EIIIA segment. TFN refers to the total concentration (μg/ml) of FN in plasma. %A+ refers to the percentage of TFN comprised of A+FN isoforms. vWF and CRP respectively refer to the plasma concentrations of vWF and CRP, expressed as the % of the average value in healthy individuals.
Figure 2.
Relationship between %A+, CRP and vWF in patients with SID. A. The relationship between %A+ and CRP is significant and persists after removal of outliers. B. The relationship between %A+ and vWF is significant but does not persist after removal of outliers. CRP and vWF are each expressed as the % of the average concentration in healthy controls, whereas %A+ represents the ratio of the concentration of A+FN to TFN (both in micrograms/ml).
vWF was significantly correlated with CRP, regardless of whether the vasculitis status was considered (p=0.007) (Table 4) or not (p=0.005) (Table 3). A+FN and %A+ were significant as predictors for vWF (p=0.037 and p=0.004, respectively) (Table 3), but the correlations for %A+ and A+FN did not persist after removal of the two outliers (Figure 2, right panel). A+FN also was not significantly correlated with vWF once the effects of vasculitis on vWF levels were considered (p=0.07, Table 4). In contrast, %A+ correlated significantly with vWF even after considering vasculitis status (p=0.008, Table 4), but the significance again was lost upon removal of two outliers. No significant correlation between TFN and vWF was evident in analyses unadjusted (p=0.80, Table 3) or adjusted for vasculitis status (p=0.94, Table 4). These findings were not materially changed in models adjusted for other covariates or restricted to patients with vasculitis (results not shown).
Plasma levels of A+FN and TFN have previously been observed to vary according age (Fyrand et al. 1978, Peters et al. 1989). However, when a linear mixed effects model was fitted with age as an additional predictor, the correlations between A+FN, TFN, CRP and vWF cited in Tables 3 and 4 were not affected. It was not possible to similarly assess whether gender or steroid usage played roles in the results, since the SID group included only three males, and most vasculitis patients were on steroid therapy.
Discussion
The observations of this study are consistent with our hypothesis that, based upon their spatially different tissue expression patterns, plasma levels of A+FN and TFN may provide distinct sets of clinical biomarker information. We have observed that A+FN, but not simultaneously-measured TFN, is significantly elevated in a cohort of SID patients in comparison to healthy controls, potentially reflecting incipient SID-related vascular injury or dysfunction. We have also found that both %A+ and A+FN correlate with levels of the prototypic inflammation marker CRP in patients with SID, whereas TFN does not. Specifically, %A+ predicted CRP not only overall but also independent of vasculitis status. In contrast, the correlation between A+FN and CRP was only significant if vasculitis status was not considered. These observations suggest that, while tissue expression analyses predict that plasma levels of A+FN should be a better marker for vascular inflammation and injury than TFN, the relationship between the “vascular injury marker” (A+FN) and the “acute phase reactant” (TFN) contributes importantly to the overall CRP-convergent %A+ signal.
Why should the portion of TFN comprised by A+FN (%A+) correlate with the development of vasculitis in patients with systemic inflammatory disorders? Production of FN in the setting of vascular inflammation is likely to occur both in the liver – where FN is synthesized as an acute phase reactant in response to inflammation at any site in the body, and in blood vessel walls in response to local injury (Clark et al. 1982, Hynes 1990). A variety of data, including histologic analyses (Vartio et al. 1987, Dubin et al. 1995, Peters et al. 1996, Tan et al. 2004), isolated perfused organ studies (Peters et al. 1986), and analyses of FN synthesis by endothelial cells (Peters et al. 1990, Peters et al. 1995), predict that vessel wall injury should lead to local production and release of FN that is enriched in A+ isoforms relative to the total pool of plasma FN (TFN). The vast bulk of the latter pool is derived from hepatocytes, which do not normally express the EIIIA segment (Hynes 1990). Therefore, in the absence of overt liver disease, in which hepatic production of A+FN could potentially occur (Jarnagin et al. 1994), %A+ should reflect the relationship between a numerator term (A+FN) denoting vascular inflammation, and a denominator term (the acute phase reactant TFN) reflecting generalized (vascular plus extravascular) inflammation. In patients with SID, %A+ should not only signify the presence of vasculitis (through release of A+FN from injured blood vessels), but should also reflect that prominence of vasculitic inflammation (embodied in the A+FN numerator) relative to the total inflammatory burden (embodied in the TFN denominator).
Plasma vWF, which is expressed only by endothelial cells and platelets/megakaryocytes, has also been observed to be increased in the plasma of patients with certain varieties of vasculitis (Woolf et al. 1987, Uddhammar 2000). Consistent with this, we found that levels of vWF correlated strongly with CRP in the SID cohort, both overall and after considering the effect of vasculitis on CRP levels. Although we observed trends for both %A+ and A+FN to similarly correlate with vWF, these did not attain significance, since much of the association was driven by a small subgroup of patients. In this respect, the subset of SID patients with vasculitis exhibited substantial heterogeneity both within and between subjects in almost every marker, suggesting that between-patient differences in the trajectory of vascular injury severity and inflammation may play important roles in differentiating subgroups clinically. The small number of patients and within-group variation limited ability for a priori comparisons, but descriptive analyses of subgroups suggested that necrotizing vasculitis patients may be at particular risk of high and persistent inflammatory marker levels (Figure 1).
Among the several limitations of the current study is that we have studied a small number of patients, rendering the analyses vulnerable to outliers and influential points, and precluding statistical comparisons between biomarkers according to small vessel, large vessel, and necrotizing vasculitis categories. Another potential concern is that A+FN levels could potentially have been increased, as least in part, as a consequence of injury resulting from vascular instrumentation. For example, the increased levels of A+FN observed in SID patients versus healthy controls could possibly reflect the fact that controls received only one blood draw, whereas the patients underwent multiple phlebotomies, potentially leading to elevations in plasma levels of endothelial injury markers. Finally, we have compared average values for groups of individuals in the current study, rather than for groups of samples, as we did in our previous descriptive study (Peters et al. 1989). For example, we previously reported that average A+FN and TFN plasma sample concentrations were significantly greater for the subgroup of SID patients with versus without vasculitis, we find in the current study that the average plasma concentrations of these two categories of FN did not differ significantly between individuals with and without vasculitis.
Specific plasma biomarkers or sets of biomarkers have proven to be of particular utility in the diagnosis of specific varieties of vasculitis, e.g. cytoplasmic antineutrophil antibody and antiproteinase-3 in Wegener’s granulomatosis (Krishnamurthy et al. 2006). It is therefore likely that we will need to rely on multiple circulating biomarkers to optimize diagnostic decisions regarding vascular inflammation. This study suggests that different categories of FN may confer unique sets of clinical information with regard to SID-associated vasculitis status, and that simultaneous measurement of A+FN and TFN merits further investigation in our efforts to identify soluble biomarker systems to detect and monitor response to therapy in these disorders. Specifically, the ratio of concentrations of A+FN to TFN (%A+), but neither level alone, correlated significantly with levels of the putative vascular injury marker CRP regardless of vasculitis status. The ratio between plasma levels of two FN pools is therefore recognized as a potential biomarker for SID-related vascular injury/inflammation. Further research with larger numbers of patients will be helpful to confirm the observations obtained in this study. Specifically, additional studies with larger sample size should help to characterize the trajectories of specific categories or combinations of circulating FNs, find how these trajectories differ across subgroups such as those with specific varieties of vasculitis, and determine whether these trajectories are correlated with clinical outcome.
Acknowledgments
We thank Charles G. Cochrane and Mark H. Ginsberg for leading us to study the role of extracellular matrix molecules in vascular injury. Statistical analysis was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. JHP was supported by a Merit Review award from the Department of Veterans Affairs, a gift from the Charles See Foundation, and a grant from the Nora Eccles Treadwell Foundation (JHP).
Supported by a Merit Review award from the Department of Veterans Affairs, a gift from the Charles See Foundation, and a grant from the Nora Eccles Treadwell Foundation (JHP).
References
- Amrani DL, Mauzy-Melitz D, Mosesson MW. Effect of hepatocyte-stimulating factor and glucocorticoids on plasma fibronectin levels. Biochemical Journal. 1986;238:365–371. doi: 10.1042/bj2380365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. C-reactive protein: a surrogate risk marker or mediator of atherothrombosis? Am J Physiol Regul Integr Comp Physiol. 2003;285:R1250–1252. doi: 10.1152/ajpregu.00227.2003. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, Davalos A. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–1676. doi: 10.1161/01.STR.0000131656.47979.39. [DOI] [PubMed] [Google Scholar]
- Clark RA, Quinn JH, Winn HJ, Lanigan JM, Dellepella P, Colvin RB. Fibronectin is produced by blood vessels in response to injury. Journal of Experimental Medicine. 1982;156:646–651. doi: 10.1084/jem.156.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin D, Peters JH, Brown LF, Logan B, Kent KC, Berse B, Berven S, Cercek B, Sharifi BG, Pratt RE, et al. Balloon catheterization induced arterial expression of embryonic fibronectins. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15:1958–1967. doi: 10.1161/01.atv.15.11.1958. [DOI] [PubMed] [Google Scholar]
- Fijnheer R, Frijns CJ, Korteweg J, Rommes H, Peters JH, Sixma JJ, Nieuwenhuis HK. The origin of P-selectin as a circulating plasma protein. Thrombosis and Haemostasis. 1997;77:1081–1085. [PubMed] [Google Scholar]
- Frijns CJ, Rinkel GJ, Castigliego D, Van Gijn J, Sixma JJ, Fijnheer R. Endothelial cell activation after subarachnoid hemorrhage. Neurosurgery. 2002;50:1223–1229. doi: 10.1097/00006123-200206000-00009. discussion 1229–1230. [DOI] [PubMed] [Google Scholar]
- Fyrand O, Munthe E, Solum NO. Studies on cold insoluble globulin. I Concentrations in citrated plasma in rheumatic disorders. Annals of the Rheumatic Diseases. 1978;37:347–350. doi: 10.1136/ard.37.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. American Journal of Ophthalmology. 1997;123:285–296. doi: 10.1016/s0002-9394(14)70123-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. Fibronectins. Springer-Verlag, Inc; New York: 1990. [Google Scholar]
- Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. Journal of Cell Biology. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis and Rheumatism. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy A, Naguwa SM, Cheema GS, Gershwin ME. Vasculitis: contemporary perspectives. Comprehensive Therapy. 2006;32:118–126. doi: 10.1385/comp:32:2:118. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Peters JH. Increased plasma levels of ED1+ cellular fibronectin precede the clinical signs of preeclampsia. American Journal of Obstetrics and Gynecology. 1990;162:358–362. doi: 10.1016/0002-9378(90)90385-k. [DOI] [PubMed] [Google Scholar]
- Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C, Adu D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–678. [PubMed] [Google Scholar]
- Nickeleit V, Zagachin L, Nishikawa K, Peters JH, Hynes RO, Colvin RB. Embryonic fibronectin isoforms are synthesized in crescents in experimental autoimmune glomerulonephritis. American Journal of Pathology. 1995;147:965–978. [PMC free article] [PubMed] [Google Scholar]
- Paul JI, Schwarzbauer JE, Tamkun JW, Hynes RO. Cell-type-specific fibronectin subunits generated by alternative splicing. Journal of Biological Chemistry. 1986;261:12258–12265. [PubMed] [Google Scholar]
- Peters JH, Chen GE, Hynes RO. Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhesion and Communication. 1996;4:127–148. doi: 10.3109/15419069609010767. [DOI] [PubMed] [Google Scholar]
- Peters JH, Ginsberg MH, Bohl BP, Sklar LA, Cochrane CG. Intravascular release of intact cellular fibronectin during oxidant-induced injury of the in vitro perfused rabbit lung. Journal of Clinical Investigation. 1986;78:1596–1603. doi: 10.1172/JCI112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Ginsberg MH, Case CM, Cochrane CG. Release of soluble fibronectin containing an extra type III domain (ED1) during acute pulmonary injury mediated by oxidants or leukocytes in vivo. American Review of Respiratory Disease. 1988;138:167–174. doi: 10.1164/ajrccm/138.1.167. [DOI] [PubMed] [Google Scholar]
- Peters JH, Loredo GA, Chen G, Maunder R, Hahn TJ, Willits NH, Hynes RO. Plasma levels of fibronectin bearing the alternatively spliced EIIIB segment are increased after major trauma. Journal of Laboratory and Clinical Medicine. 2003;141:401–410. doi: 10.1016/S0022-2143(03)00042-8. [DOI] [PubMed] [Google Scholar]
- Peters JH, Maunder RJ, Woolf AD, Cochrane CG, Ginsberg MH. Elevated plasma levels of ED1+ (“cellular”) fibronectin in patients with vascular injury. Journal of Laboratory and Clinical Medicine. 1989;113:586–597. [PubMed] [Google Scholar]
- Peters JH, Sporn LA, Ginsberg MH, Wagner DD. Human endothelial cells synthesize, process, and secrete fibronectin molecules bearing an alternatively spliced type III homology (ED1) Blood. 1990;75:1801–1808. [PubMed] [Google Scholar]
- Peters JH, Trevithick JE, Johnson P, Hynes RO. Expression of the alternatively spliced EIIIB segment of fibronectin. Cell Adhesion and Communication. 1995;3:67–89. doi: 10.3109/15419069509081278. [DOI] [PubMed] [Google Scholar]
- Pick-Kober KH, Munker D, Gressner AM. Fibronectin is synthesized as an acute phase reactant in rat hepatocytes. Journal of Clinical Chemistry and Clinical Biochemistry. 1986;24:521–528. doi: 10.1515/cclm.1986.24.8.521. [DOI] [PubMed] [Google Scholar]
- Satoi S, Hiramatsu Y, Kitade H, Kwon AH, Matsui K, Miyashita K, Sakashita E, Sekiguchi K, Takahashi H, Kamiyama Y. Different responses to surgical stress between extra domain A+ and plasma fibronectins. Clinical and Experimental Pharmacology and Physiology. 1999;26:225–229. doi: 10.1046/j.1440-1681.1999.03019.x. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991;13:527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- Sikkema JM, Franx A, Fijnheer R, Nikkels PG, Bruinse HW, Boomsma F. Semicarbazide-sensitive amine oxidase in pre-eclampsia: no relation with markers of endothelial cell activation. Clinica Chimica Acta. 2002;324:31–38. doi: 10.1016/s0009-8981(02)00215-2. [DOI] [PubMed] [Google Scholar]
- Tamkun JW, Hynes RO. Plasma fibronectin is synthesized and secreted by hepatocytes. Journal of Biological Chemistry. 1983;258:4641–4647. [PubMed] [Google Scholar]
- Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- ter Borg EJ, Horst G, Limburg PC, van Rijswijk MH, Kallenberg CG. C-reactive protein levels during disease exacerbations and infections in systemic lupus erythematosus: a prospective longitudinal study. Journal of Rheumatology. 1990;17:1642–1648. [PubMed] [Google Scholar]
- Uddhammar AC. Von Willebrand factor in polymyalgia rheumatica and giant cell arteritis. Clinical and Experimental Rheumatology. 2000;18:S32–33. [PubMed] [Google Scholar]
- Vartio T, Laitinen L, Narvanen O, Cutolo M, Thornell LE, Zardi L, Virtanen I. Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. Journal of Cell Science. 1987;88 ( Pt 4):419–430. doi: 10.1242/jcs.88.4.419. [DOI] [PubMed] [Google Scholar]
- Voskuyl AE, Emeis JJ, Hazes JM, van Hogezand RA, Biemond I, Breedveld FC. Levels of circulating cellular fibronectin are increased in patients with rheumatoid vasculitis. Clinical and Experimental Rheumatology. 1998;16:429–434. [PubMed] [Google Scholar]
- Woolf AD, Wakerley G, Wallington TB, Scott DG, Dieppe PA. Factor VIII related antigen in the assessment of vasculitis. Annals of the Rheumatic Diseases. 1987;46:441–447. doi: 10.1136/ard.46.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]