Summary
The Abl-family non-receptor tyrosine kinases are essential regulators of the cytoskeleton. They transduce diverse extracellular cues into cytoskeletal rearrangements that have dramatic effects on cell motility and morphogenesis. Recent biochemical and genetic studies have revealed several mechanisms that Abl-family kinases use to mediate these effects. Abl-family kinases stimulate actin polymerization through the activation of cortactin, hematopoietic lineage cell-specific protein (HS1), WASp- and WAVE-family proteins, and Rac1. They also attenuate cell contractility by inhibiting RhoA and altering adhesion dynamics. These pathways impinge on several physiological processes, including development and maintenance of the nervous and immune systems, and epithelial morphogenesis. Elucidating how Abl-family kinases are regulated, and where and when they coordinate cytoskeletal changes, is essential for garnering a better understanding of these complex processes.
Keywords: Abl-family non-receptor tyrosine kinases, Actin polymerization, Cell adhesion, Cell motility and morphogenesis, Cytoskeleton, Nervous- and immune-system maintenance
Introduction
The Abl non-receptor tyrosine kinase has been the focus of intense research ever since mutant forms of Abl were identified as the transforming determinant of Abelson murine leukemia virus and the cause of chronic myelogenous leukemia (CML) (Wong and Witte, 2004). Subsequent studies indicated that Abl-family kinases are essential transducers of signals from growth-factor, adhesion and axon-guidance receptors to the cytoskeleton. Of the 90 mammalian tyrosine kinases, Abl and Arg (Abl-related gene) are the only two that are known to interact directly with the cytoskeleton. Thus, in addition to phosphorylating substrates and interacting with effectors, Abl and Arg have the unique ability to both create and react to cytoskeletal changes that affect cell shape and movement.
Abl-family kinases include the vertebrate Abl (Abl1) and Arg (Abl2), Drosophila melanogaster dAbl, and Caenorhabditis elegans Abl (ABL-1). These proteins regulate several biological processes, including cell motility and morphogenesis, development and maintenance of the nervous and immune systems, response to genotoxic stress, and apoptosis. Their dysregulation leads to several pathological states. In addition to leukemia [CML and acute myeloid leukemia (AML)], recent evidence suggests possible roles in breast-cancer invasiveness (Srinivasan and Plattner, 2006; Srinivasan et al., 2008), neurological disorders (Derkinderen et al., 2005) and microbial pathogenesis (Backert et al., 2008; Newsome et al., 2006). Deciphering how these kinases are regulated and what pathways they modulate is crucial for understanding how to proceed in treating these diseases.
The most prominent and well-characterized processes that are coordinated by Abl-family kinases are changes in the cytoskeleton that lead to alterations in cell shape and movement. Cell migration and morphogenesis are complex processes that require the careful balance of numerous forces (Ridley et al., 2003; Vicente-Manzanares et al., 2005). For example, actin polymerization provides the protrusive force at the leading edge of a cell to propel the membrane forward (Mitchison and Cramer, 1996; Pollard and Borisy, 2003; Ponti et al., 2004). Following membrane protrusion, integrin receptors mediate cell adhesion to the extracellular matrix (ECM). These adhesions either turn over or mature into larger focal adhesions through the action of the RhoA GTPase. Actomyosin contractility then provides the force required to pull the cell body forward, and must be coordinated with the release of adhesions at the rear of the cell (de Rooij et al., 2005; Gupton and Waterman-Storer, 2006; Jay et al., 1995; Jockusch et al., 1995; Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996). Abl-family kinases play important regulatory roles in each of these steps.
In addition to regulating cell-matrix adhesion and motility, Abl-family kinases also play a prominent role in regulating cell-cell adhesion. They are essential modulators of epithelial cell-cell adhesion, as well as of both neuronal and immune synapses. The role of Abl-family kinases in each of these contexts will be discussed in this Commentary.
Several recent advances in our understanding of the molecular mechanisms by which Abl-family kinases modulate the cytoskeleton and cell shape and movement have only strengthened the interest in the field. These advances, coupled with emerging evidence that Abl-family kinases play roles in several disease states, underscore the timeliness of reviewing what we know about Abl-family kinases and, importantly, what we still need to understand. In this Commentary, we review the basic architecture of Abl-family kinases, the biochemical and genetic factors that regulate their activity, the recent identification of several mechanisms that underlie their ability to modulate the cytoskeleton, and lastly the physiological processes that require Abl-family kinase function. Although there is also a rich literature describing the functions of Abl in the cell nucleus, this Commentary will focus solely on the cytoplasmic functions of the kinases.
Domain structure
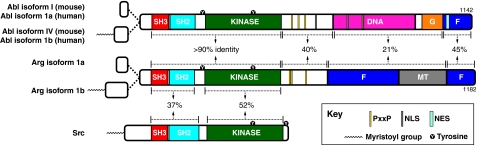
The N-terminal half of both Abl and Arg contains a `cap' region that is followed by sequentially arranged Src-homology 3 (SH3), SH2 and kinase domains (Fig. 1). There are two major isoforms of Abl in vertebrates, the non-myristoylated isoform 1a in humans (Abl isoform I in mice) and the myristoylated human isoform 1b (Abl isoform IV in mice); likewise, Arg has a non-myristoylated (1a) and a myristoylated (1b) isoform. The SH3, SH2 and kinase domains of Abl and Arg are greater than 90% identical (Kruh et al., 1990) and share over 75% identity with dAbl. Although Abl-family kinases are structurally very similar in this region to Src-family kinases (Nagar et al., 2003), they share only 52% identity in their kinase domain, and 37% identity in their SH3 and SH2 domains, when compared to Src.
Fig. 1.
Domain structure of Abl-family kinases. Abl and Arg each have two isoforms, the shorter non-myristoylated isoform 1a (Abl isoform I in mice) and the longer myristoylated isoform 1b (Abl isoform IV in mice). Both Abl and Arg have a `cap' region that extends from their N-terminus to their SH3 domain. C-terminal to the cap, they have sequential SH3 (red), SH2 (blue) and kinase (green) domains. Abl has four PxxP motifs (yellow stripes) and Arg has three. Abl also has nuclear localization sequences (NLS, gray stripes), a DNA-binding region (DNA, magenta) and a nuclear export sequence (NES, light-blue stripe). Additionally, Abl has G-actin (G, orange) and F-actin (F, purple)-binding domains. Arg has two F-actin-binding domains and an MT-binding domain (MT, gray). Abl and Arg isoforms 1b are 1142 and 1182 amino acids in mice, respectively. They share >90% sequence identity in their SH3, SH2 and kinase domains, 40% identity in their proline-rich regions, 45% identity in their C-terminal F-actin-binding domain, but only 21% identity in the remaining regions in the C-terminus. The related tyrosine kinase Src is myristoylated and contains SH3 and SH2 domains and a kinase domain, which share 37% and 52% identity, respectively, to the corresponding domains in Abl-family kinases. Phosphorylation of the linker and activation-loop tyrosine residues (Y in black circles) of Abl and Arg are required for full kinase activation. The linker tyrosine is between the SH2 and kinase domains (Abl Y245, Arg Y272), and the activation-loop tyrosine is in the kinase domain (Abl Y412, Arg Y439). Src also has an activation-loop tyrosine residue in its kinase domain (Src Y416). Additionally, Src has a tyrosine residue near its C-terminus that stabilizes its auto-inhibited conformation when phosphorylated (Src Y527).
Their unusual extended C-terminal halves are what make Abl-family kinases unique in the world of tyrosine kinases (McWhirter and Wang, 1993; Miller et al., 2004; Van Etten et al., 1994; Wang et al., 2001). There is only 29% sequence identity overall between the C-termini of Abl and Arg (Kruh et al., 1990), which highlights their unique functions in these regions. Abl has four proline-x-x-proline (PxxP) motifs following its kinase domain, and Arg has three, which allow interactions with SH3-domain-containing proteins (Antoku et al., 2008; Feller et al., 1994; Ren et al., 1994; Lapetina et al., 2009). These proline-rich regions are 40% identical between Abl and Arg.
Abl has a calponin homology (CH) domain at its extreme C-terminus that binds to filamentous (F)-actin (McWhirter and Wang, 1993; Van Etten et al., 1994), and a globular (G)-actin binding domain. Abl uses these domains to bundle F-actin in vitro (Van Etten et al., 1994; Wang et al., 2001). Arg has an analogous F-actin-binding domain at its C-terminus (which shares 45% identity with that in Abl), but also contains an [I/L]WEQ F-actin-binding domain internally (Wang et al., 2001) and can also bundle F-actin. Uniquely, Arg contains a microtubule (MT)-binding domain, which it uses in conjunction with its F-actin-binding domains to crosslink F-actin and MTs in vitro (see below) (Miller et al., 2004). Additionally, Abl contains a DNA-binding region (David-Cordonnier et al., 1998; Kipreos and Wang, 1992; Miao and Wang, 1996), and nuclear-localization (Wen et al., 1996) and -export (Taagepera et al., 1998) sequences, allowing it to shuttle between the nucleus and cytosol, whereas Arg and dAbl are primarily localized to the cytoplasm (Bennett and Hoffmann, 1992; Koleske et al., 1998).
Mechanism of regulation
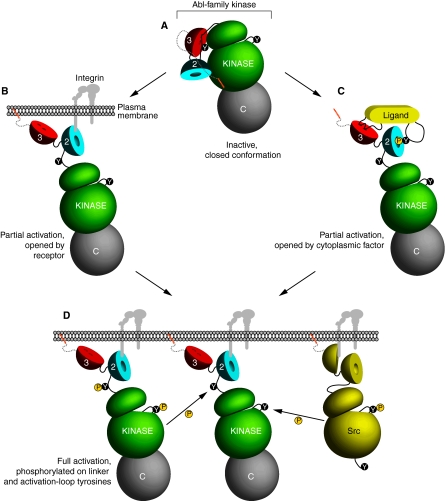
Similar to Src-family kinases, Abl-family kinases are regulated via a `latch-clamp-switch' mechanism (Harrison, 2003), in which their SH3 and SH2 domains act as a `clamp', folding back on their kinase domain to hold it in a rigid, locked and inactive conformation with the aid of a molecular `latch' (Nagar et al., 2003) (Fig. 2A). For Src-family kinases, the latch is an intramolecular interaction between its SH2 domain and phosphorylated Y527 in its C-terminal tail (Cooper et al., 1986; Liu et al., 1993; Thomas et al., 1991). Abl and Arg do not contain a similar tyrosine residue; however, X-ray crystallographic data of mouse Abl isoform IV show that a myristoyl group docks into a hydrophobic pocket in the kinase domain (Nagar et al., 2003). This latching mechanism might only apply to the myristoylated forms of Abl and Arg. However, the myristoyl group was added in trans in the solved Abl structure, implying that endogenous lipids in the cell might contribute to kinase inhibition by binding to this pocket on Abl or Arg. Abl isoform 1a also has additional hydrophobic residues at its N-terminus that are not present in isoform 1b; these residues might play an inhibitory role (Nagar et al., 2003). Indeed, there are reports that the cap inhibits kinase function (Pluk et al., 2002). In addition to the intramolecular mechanisms of kinase inhibition, Abl-family kinases are possibly inhibited in trans by co-inhibitors that stabilize the inactive conformation (Shi et al., 1995; Wang, 2004; Welch and Wang, 1993; Wen and Van Etten, 1997; Woodring et al., 2001; Xiong et al., 2008).
Fig. 2.
Regulation of kinase activity of Abl and Arg. (A) Abl-family kinases are held in an inactive closed conformation, in which their SH3 (3, red) and SH2 (2, blue) domains fold back on their kinase domain (green). The N-terminal cap (dotted gray line) makes additional inhibitory contacts with the SH3 and SH2 domains, and allows docking of the myristoyl group (orange) in a hydrophobic pocket in the kinase domain. The C-terminal half is shown in gray. (B,C) Partial activation is achieved through interaction with receptors, such as (B) an activated integrin heterodimer (gray) or (C) cytoplasmic PxxP and/or phosphotyrosine (P in yellow circle attached to Y in black circle)-containing ligands (yellow) that open the auto-inhibited conformation. (D) Full activation is achieved by phosphorylation of the linker tyrosine (between the SH2 and kinase domains) and the activation-loop tyrosine (between the two lobes of the kinase domain) in trans by other Abl-family kinases and/or by Src-family kinases (yellow). In this example, integrin clustering brings Abl- and Src-family kinases into close proximity.
Stimulation of Abl and Arg kinase activity is initiated by release of the latch and clamp. It is likely that a ligand for the SH3 and/or SH2 domain binds to Abl or Arg, releasing the hold of these domains on the kinase domain. The intracellular domain of an activated receptor or a bound adaptor protein might act as this ligand (Fig. 2B,C), because this mechanism is employed by several kinases. Phosphopeptides that interact with the Abl SH2 phosphotyrosine-binding pocket activate the kinase (Hantschel et al., 2003), and a similar mechanism is defined for both SH3- and SH2-binding ligands for Src-family kinases (Arias-Salgado et al., 2003; Moarefi et al., 1997; Porter et al., 2000; Sun et al., 2002).
For full kinase activation, Abl and Arg must also be phosphorylated on two key tyrosine residues, one in the linker region between the SH2 and kinase domains (Abl Y245, Arg Y272), and the other in the activation loop of the kinase domain (Abl Y412, Arg Y439) (Brasher and Van Etten, 2000; Tanis et al., 2003). These phosphotyrosines prevent reversion to the locked conformation and properly orient the catalytic site, respectively (Dorey et al., 2001; Johnson et al., 1996; Schindler et al., 2000). These `switch' tyrosines are phosphorylated by Src-family kinases or autophosphorylation in trans by a neighboring Abl or Arg molecule (Brasher and Van Etten, 2000; Tanis et al., 2003) (Fig. 2D).
Pathways that activate Abl-family kinases
Identifying the receptors and pathways that engage Abl-family kinases is essential to understand how, and in what cellular contexts, they are regulated. Abl-family kinases are stimulated by a variety of upstream stimuli, including growth factors (Furstoss et al., 2002; Plattner et al., 2003; Plattner et al., 1999; Plattner et al., 2004), cell-matrix and cell-cell adhesion (Hernandez et al., 2004; Huang et al., 2008; Lewis et al., 1996; Renshaw et al., 2000; Zandy et al., 2007), DNA damage (Kharbanda et al., 1995), oxidative stress (Cao et al., 2001; Sun et al., 2000), and bacterial invasion (Backert et al., 2008; Burton et al., 2005; Newsome et al., 2006; Poppe et al., 2007; Tammer et al., 2007). Exciting recent work has identified several mechanisms that involve both receptors and soluble proteins that enhance Abl and Arg kinase activity, and these are discussed below.
Activation by growth factors
Abl is phosphorylated and activated following stimulation of the epidermal growth factor receptor (EGFR) or platelet-derived growth factor receptor (PDGFR) with EGF or PDGF, respectively (Dorey et al., 2001; Plattner et al., 1999). This activation is dependent on Src-family kinases, because Src–/– Yes–/– Fyn–/– cells do not display increased Abl activity when treated with these growth factors (Plattner et al., 1999). PLCγ plays a role in this pathway, possibly by locally decreasing phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] levels, which might contribute to inhibition of Abl-family kinases (see above) (Plattner et al., 2003). Additionally, PDGF stimulates Abl- and Arg-mediated phosphorylation of the actin-regulating protein cortactin (see below) (Boyle et al., 2007) in a Src-dependent manner.
Recent reports show that dysregulation of either of the ErbB-family receptors (EGFR or Her-2), or of insulin-like growth factor receptor (IGFR) leads to constitutive activation of Abl-family kinases in invasive breast-cancer cell lines (Srinivasan and Plattner, 2006; Srinivasan et al., 2008). Treatment with the Abl-family-kinase-specific inhibitor STI-571 leads to reduced cell invasion. However, there are also reports that the EphB4 receptor signals to Abl-family kinases and reduces breast-cancer-cell invasion and viability through the Abl-Arg substrate CrkII (see below) (Noren et al., 2006). Abl and Arg are known to bind to both EphB4 and EphB2 receptors directly via their SH2 domain and sequences in their C-termini, leading to reciprocal activation (Yu et al., 2001). Although further study is required to understand these mechanisms, contributions from Src or increased expression of Abl in certain breast-cancer cell lines might explain the conflicting reports.
Activation through integrin-dependent adhesion
Fibroblasts plated on the ECM component fibronectin (an integrin ligand) or on an α5-integrin cross-linking antibody show increased Abl activation, as well as translocation of Abl from the nucleus to focal adhesions (sites of integrin-dependent cell adhesions to the ECM) (Lewis et al., 1996). Plating fibroblasts on fibronectin also induces Abl-family-kinase-dependent membrane protrusions (Lapetina et al., 2009; Miller et al., 2004; Woodring et al., 2002; Radha et al., 2007; Jin and Wang, 2007) that are abrogated by treatment with STI-571. These effects are independent of Src-family kinases (Woodring et al., 2002). Furthermore, Abl-family kinases promote neurite branching in primary cortical neurons when plated on laminin (an integrin ligand) (Moresco et al., 2005); this branching is significantly reduced by genetic or chemical inhibition of both Abl and Arg, or by treatment with the integrin inhibitor echistatin. Integrin adhesion also leads to the phosphorylation of several Abl-family-kinase substrates, including p190RhoGAP-A (Bradley et al., 2006; Hernandez et al., 2004) and cortactin (Lapetina et al., 2009).
Interestingly, the short cytoplasmic tails of β-integrins can act as non-canonical SH3 or SH2 ligands for Src- and Syk-family kinases, respectively (Arias-Salgado et al., 2003; Woodside et al., 2002). In both these cases, the integrin tails stimulate kinase activity. It is likely that Abl-family kinases employ a similar mechanism of activation by integrins (Fig. 2D). Once localized to a region of integrin adhesion, they might interact with the integrin tails, releasing their inhibitory clamp. Subsequent phosphorylation in trans or by co-clustered Src-family kinases could lead to full kinase activation.
Other receptors
Several other receptors are implicated in the activation of Abl-family kinases, but the biochemical mechanisms that mediate these interactions are less clear. Abl interacts functionally with cadherin receptors in both C. elegans (Sheffield et al., 2007) and Drosophila (Emerson and Van Vactor, 2002; Loureiro and Peifer, 1998; Rhee et al., 2007), and a recent report suggests that cadherin-based adhesion induces Abl activity through the GTPase Rac1 (Rac) (see below) (Zandy et al., 2007). Abl also binds to the nerve growth-factor receptor TrkA (Yano et al., 2000) and can be activated downstream of insulin-receptor signaling (Frasca et al., 2007), as well as upon T-cell receptor engagement (Gu et al., 2007; Huang et al., 2008; Zipfel et al., 2004). Several studies in Drosophila have genetically linked dAbl to several receptors that are required for nervous-system development, including the adhesion receptor dLAR and the chemorepellent receptor Robo (Moresco and Koleske, 2003). More recently, dAbl was found to interact genetically with the chemoattractant netrin receptor Frazzled (Dorsten et al., 2007; Forsthoefel et al., 2005). dAbl binds directly to Frazzled, possibly modulating dAbl kinase activity.
The role of adaptor proteins
Activated surface receptors often engage intracellular signaling pathways by means of adaptor proteins. Several adaptor proteins are known to activate Abl-family kinases, including Nck1 (Smith et al., 1999), CrkII (Reichman et al., 2005; Shishido et al., 2001) and Rin1 (Cao et al., 2008; Hu et al., 2005).
Nck1 can bind to the PxxP motifs in Abl (Ren et al., 1994), but these motifs are not required for Nck1-mediated Abl kinase activation (Smith et al., 1999), suggesting an indirect mode of regulation in which Nck1 recruits other proteins to stimulate Abl kinase activity. Similar to Nck1, CrkII binds to the PxxP motifs of Abl (Ren et al., 1994), and plays a role in actin polymerization and Abl-mediated membrane protrusions (Antoku et al., 2008). Overexpression of wild-type CrkII leads to a modest enhancement of Abl-mediated phosphorylation of cellular proteins. Mutation of CrkII at Y221, which is normally phosphorylated by Abl (Feller et al., 1994), leads to a much greater enhancement of Abl kinase activity (Shishido et al., 2001). This implies the existence of a negative-feedback loop in which CrkII can activate Abl, which then phosphorylates CrkII to prevent further Abl activation. It is clear that Nck1 and CrkII play a role in modulating Abl-family kinases, but physiological stimuli and mechanisms that initiate these interactions are unclear.
Rin1 is an effector of active Ras that acts as a guanine-nucleotide exchange factor (GEF) for Rab5 and a trans-activator of Abl and Arg (Bliss et al., 2006). Rin1 binds to the SH3 domain of Arg, is phosphorylated by it and subsequently binds to the Arg SH2 domain (Hu et al., 2005). Rin1 binding enhances Arg activity, which is further increased on expression of a constitutively active Ras, demonstrating regulatory crosstalk between Ras and Abl-family kinases.
Regulation of actin polymerization and cell shape by Abl-family kinases
Changes in cell shape, mediated by actin polymerization, are arguably the most pronounced effects of Abl-family kinase activation. Following PDGF treatment, cells in culture normally undergo actin-mediated dorsal ruffle formation and peripheral membrane ruffling. Cells lacking Abl and/or Arg exhibit dramatically reduced ruffling, which is restored by re-expression of the kinases (Boyle et al., 2007; Plattner et al., 1999). Similarly, fibroblasts deficient in Abl or Arg show reduced F-actin microspike extension (Woodring et al., 2004) and lamellipodial protrusions (Lapetina et al., 2009; Miller et al., 2004) when plated on fibronectin. Loss of Abl or Arg function also reduces actin-driven neurite branching of both neuroblastoma cells (Hernandez et al., 2004) and primary neurons (Moresco et al., 2005) following integrin adhesion. In each of these cases, reconstitution with Abl-family kinases restores the wild-type phenotype. Recently, work has begun to elucidate the biochemical mechanisms by which Abl-family kinases engage and activate the actin-polymerization machinery, and aspects of this work are discussed below.
Abl-family kinases activate WASp and WAVE proteins
There are five members of the WASp (Wiskott-Aldrich syndrome protein) and WAVE (WASp-family verprolin-homologous protein) family of proteins in mammals: WASp, neural (N)-WASp, WAVE1, WAVE2 and WAVE3 (Takenawa and Suetsugu, 2007; Pollitt and Insall, 2009). These proteins use a domain in their C-terminus (the VCA domain) to interact with actin monomers and the Arp2/3 complex, which nucleates the formation of new actin branches from the sides of existing filaments. Their actin-polymerization-inducing function is implicated in a number of processes, including filopodial and lamellipodial extension, dorsal ruffling, podosome and invadopodium formation, and neurite extension.
WASp and N-WASp are regulated through an intramolecular interaction that leads to the occlusion of the VCA domain, preventing its binding to monomeric actin or Arp2/3 (Kim et al., 2000). Binding of the small GTPase Cdc42 to N-WASp relieves this auto-inhibition, allowing N-WASp to stimulate Arp2/3-mediated actin polymerization (Rohatgi et al., 1999). The adaptor Nck1, which binds to Abl and Arg (see above) (Antoku et al., 2008), also stimulates N-WASp activity in a Cdc42-independent manner (Rivera et al., 2004; Rohatgi et al., 2001). Subsequent to effector binding, tyrosine phosphorylation of N-WASp prevents a return to its auto-inhibited state, thereby further stimulating actin polymerization (Suetsugu et al., 2002; Torres and Rosen, 2003). Several non-receptor tyrosine kinases, including Abl (Burton et al., 2005), phosphorylate N-WASp. In fact, Abl phosphorylation of N-WASp is essential for the ability of the bacterium Shigella flexneri to highjack cellular N-WASp in a Cdc42-independent manner to induce actin-comet formation (Burton et al., 2005). This raises the intriguing possibility that Nck1 and Abl-family kinases work in a pathway that is parallel to the Cdc42-dependent pathway to stimulate N-WASp-mediated actin polymerization.
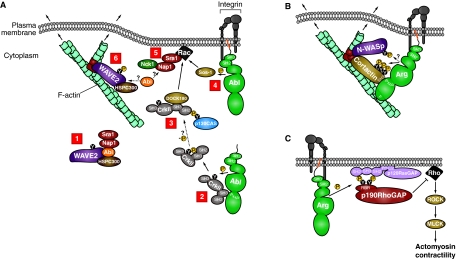
In contrast to WASp proteins, WAVE proteins are not held in an auto-inhibited conformation. Instead they are inhibited through the interaction of their N-terminal WAVE-homology domain (WHD) with an inhibitory complex comprising the proteins Abi1 or Abi2 (Abi1/2), Nap1, Sra1/CYFIP1 or PIR121 (CYFIP2), and HSPC300 (Eden et al., 2002) (Fig. 3A). Similar complexes exist for all three WAVE proteins (Gautreau et al., 2004; Stovold et al., 2005). These complexes are thought to act as trans-inhibitors, preventing WAVE from stimulating the Arp2/3 complex, although there are conflicting reports that indicate that the inhibitory complex is just as active as WAVE alone (Innocenti et al., 2004). Nck1 and active Rac interact with Nap1 and Sra1, respectively, to release the inhibitor complex (Eden et al., 2002). This frees WAVE, allowing it to interact with the Arp2/3 complex and stimulate actin polymerization.
Fig. 3.
Abl-family kinases modulate cytoskeletal signaling pathways. (A) Abl modulates WAVE2-mediated actin polymerization. [1] WAVE2 (purple) is held in a trans-inhibited complex with HSPC300 (brown), Abi (orange), and Nap1 and Sra1 (red). [2] Abl (green) uses a PxxP motif to bind to a CrkII SH3 domain (gray). Abl phosphorylates CrkII, leading to an intramolecular interaction between CrkII's SH2 domain and the phosphotyrosine residue (Y221). Phosphorylated CrkII then localizes to the cell periphery. [3] At the cell periphery, CrkII is possibly dephosphorylated, allowing interaction with p130CAS (blue) and the Rac GEF DOCK180 (light brown). [4] Abl also phosphorylates and activates the Rac GEF Sos-1 (light brown). [5] Active Rac (black) interacts with Sra1, and Nck1 (dark green) interacts with Nap1, which releases the inhibitory Sra1-Nap1 sub-complex from WAVE2. It is unclear whether Abi remains bound to active WAVE2 or is released with the inhibitory sub-complex. [6] Active WAVE2 remains bound to HSPC300, and possibly Abi, can be phosphorylated by Abl and interacts with the Arp2/3 complex (red) to stimulate actin polymerization at a nascent F-actin branch (turquoise). (B) Arg modulates cortactin- and N-WASp-mediated actin polymerization. Arg (green) uses a PxxP motif to bind to the SH3 domain of cortactin (light brown) and an F-actin-binding domain (`F') to bind to F-actin (turquoise). Cortactin interacts with the Arp2/3 complex (red) and modestly stimulates actin polymerization on a nascent F-actin branch. Following integrin (gray) adhesion, active Arg phosphorylates (P in yellow circle) cortactin and possibly N-WASp (purple), leading to a greater enhancement of actin polymerization and membrane protrusion. Arg can also use its SH2 domain to interact with phosphotyrosine residues on cortactin (not shown). Cortactin and N-WASp can also bind directly to each other, as well as to the Nck1 adaptor protein (not shown). (C) Arg modulates p190RhoGAP-mediated inhibition of RhoA. Following integrin (gray) adhesion, Arg phosphorylates the RasGAP-binding-region (RBR) of p190RhoGAP (p190; red), which then interacts with the SH2 domains of p120RasGAP (p120; light purple). p120RasGAP has phospholipid-binding domains (PH and C2), which allow the p190-p120 complex to localize to the membrane. Once in proximity to active Rho (black), p190 inhibits Rho by stimulating Rho's weak intrinsic GTPase activity. Active Rho normally activates ROCK (light brown), which, in turn, activates MLCK (light brown) to phosphorylate myosin, leading to increased actomyosin contractility. Therefore, p190 acts as a brake on actomyosin contractility.
Similar to the phosphorylation of N-WASp, tyrosine phosphorylation of WAVE proteins can enhance their activity. The inhibitory-complex component Abi1 plays a central role in WAVE2 phosphorylation (Innocenti et al., 2004), because it acts as a bridge between WAVE2 and Abl (Leng et al., 2005; Stuart et al., 2006), allowing Abl to phosphorylate WAVE2 in response to PDGF or to adhesion to fibronectin (Fig. 3A). Initial work reported that Abi2 is released from the WAVE inhibitory complex along with Nap1 and Sra1 to allow WAVE activation (Eden et al., 2002). However, more recent work suggests that phosphorylation of WAVE2 enhances its ability to stimulate actin polymerization, and also enables increased association between Abi1 and WAVE2 (Innocenti et al., 2004; Leng et al., 2005; Stuart et al., 2006). This implies that Abi1 is still bound to active WAVE2, and also suggests that a positive-feedback loop exists that allows sustained WAVE2 phosphorylation by Abl. Mutation of the Abl-phosphorylation site in WAVE2 prevents enhanced actin polymerization, suggesting that a WAVE2-phosphorylation event either prevents reassociation of the full inhibitory complex or attracts additional stimulatory factors. Abl can also phosphorylate WAVE3 in an Abi1-independent manner, which indicates additional modes of interaction (Sossey-Alaoui et al., 2007).
Abl-family kinases stimulate the GTPase Rac1
In addition to phosphorylating WAVE proteins directly, Abl-family kinases can also activate WAVE indirectly by stimulating Rac. Following treatment of fibroblasts with EGF, Abl is activated and phosphorylates the dual-specificity Ras-Rac GEF Sos-1 (Sini et al., 2004) (Fig. 3A). This phosphorylation is reduced, but not eliminated, in cells treated with STI-571 or in Abl–/– Arg–/– fibroblasts, probably because EGFR can also directly phosphorylate Sos-1. Tyrosine phosphorylation of Sos-1 is required for its Rac GEF activity, as is binding to Eps8 and Abi1 (Scita et al., 1999), although it is not known whether Abl-family kinases are also required for the assembly of this complex.
Abl-family kinases interface with another regulator of Rac activity, the CrkII adaptor protein (Ren et al., 1994). As described above, Abl uses its PxxP motifs to interact with the SH3 domains of CrkII (Antoku et al., 2008), and can phosphorylate CrkII on residue Y221 (Feller et al., 1994) (Fig. 3A). When Y221 is phosphorylated, CrkII forms an intramolecular interaction with its SH2 domain (Feller et al., 1994; Kain and Klemke, 2001; Rosen et al., 1995), preventing interaction with p130CAS and DOCK180, which, together with CrkII, form a Rac-activating complex (Kiyokawa et al., 1998a; Kiyokawa et al., 1998b). This suggests that Abl-mediated phosphorylation of CrkII inhibits Rac activation, yet CrkII phosphorylation is required for its localization to the cell membrane and for the activation of Rac (Abassi and Vuori, 2002). In support of this, Abl-mediated CrkII phosphorylation is known to activate Rac in response to cell-cell adhesion (Zandy et al., 2007) and bacterial invasion (Burton et al., 2003). It is likely that, following its proper localization, subsequent cycling of CrkII phosphorylation modulates several cellular responses. More work is necessary to decipher this pathway. It is important to remember that, regardless of how CrkII affects Rac activity, cells lacking Abl and/or Arg exhibit a reduction in membrane protrusions (Hernandez et al., 2004; Lapetina et al., 2009; Miller et al., 2004; Moresco et al., 2005; Plattner et al., 1999; Woodring et al., 2004), suggesting that these kinases have a net positive effect on actin polymerization.
Cortactin and HS1 are substrates of Abl-family kinases
Cortactin binds to the Arp3 subunit of the Arp2/3 complex, leading to modest stimulation of actin polymerization and stabilization of F-actin filaments (Bryce et al., 2005; Weaver et al., 2002; Weaver et al., 2001); however, cortactin can also synergize with N-WASp to greatly enhance actin polymerization (Kowalski et al., 2005; Martinez-Quiles et al., 2004; Mizutani et al., 2002; Weaver et al., 2002). It has long been known that cortactin is tyrosine phosphorylated (Thomas et al., 1995; Wu et al., 1991), but the mechanistic consequences of this phosphorylation have only recently been identified. Although it was originally identified as a Src substrate (Thomas et al., 1995; Wu et al., 1991), cortactin is phosphorylated in vitro with a lower Michaelis constant (KM) by Abl and Arg (23 and 80 nM, respectively) than by Src (320 nM) (Boyle et al., 2007). Cortactin phosphorylation downstream of Abl- and Src-family kinases is required for its ability to stimulate formation of dynamin- and F-actin-rich dorsal ruffles following PDGF treatment in fibroblasts (Krueger et al., 2003; Boyle et al., 2007). It is likely that phosphorylated cortactin acts as a scaffold to recruit additional SH2-domain-containing proteins to modulate actin polymerization. Indeed, Nck1 binds selectively to phospho-cortactin (Lapetina et al., 2009).
As well as being phosphorylated in response to PDGF, cortactin is phosphorylated by Arg in fibroblasts upon integrin-mediated adhesion to fibronectin, which leads to increased formation of lamellipodial protrusions (Lapetina et al., 2009). Arg acts as both a scaffold to bind to cortactin and a kinase to phosphorylate it, and both functions are required for the ability of cortactin to stimulate Arp2/3-mediated cell-edge protrusion (Fig. 3B). As mentioned above, it is known that cortactin and N-WASp can act synergistically to promote actin polymerization. Abl-family kinases can also phosphorylate and activate N-WASp (see above) (Burton et al., 2005). These observations raise the intriguing possibility that Arg employs both its scaffold and kinase activities to engage and activate cortactin, and that the Arg-cortactin complex subsequently recruits and activates N-WASp and Nck1 to further stimulate Arp2/3-dependent actin polymerization (Fig. 3B).
Abl and Arg also interact with and phosphorylate the hematopoietic-specific cortactin homolog HS1 to stimulate actin polymerization in lymphocytes (Huang et al., 2008). The role that this pathway plays in the reorganization of the actin cytoskeleton, both following T-cell adhesion to B cells and during chemokine-stimulated T-cell migration, is discussed below. Further studies are required to determine whether Abl-family kinases, cortactin or HS1, WASP and/or WAVE, and adaptors such as Nck1 act together or in parallel complementary pathways to regulate actin polymerization.
Abl-family kinases regulate Ena/VASP protein localization
Ena/VASP (Enabled/vasodilator-stimulated phosphoprotein) proteins regulate actin-filament elongation by preventing capping of the barbed end (Bear and Gertler, 2009). Genetic experiments in flies first identified Ena as a suppressor of lethality associated with zygotic Abl mutations, suggesting that Ena and Abl have antagonistic functions (Gertler et al., 1990). In support of this, several studies demonstrate that reduced dAbl function leads to increased or ectopic accumulation of Ena, and this is associated with an increased size or frequency of associated actin-based structures (Grevengoed et al., 2003; Gates et al., 2007). For example, RNA-interference-based depletion of dAbl from cultured Drosophila cells leads to increased localization of Ena to filopodial tips, accompanied by increases in filopodial number and length (Gates et al., 2007).
The exact mechanism by which Abl-family kinases regulate Ena localization is unclear. dAbl can phosphorylate Ena on at least six tyrosine residues, and this reduces the ability of Ena to bind to SH3-domain-containing proteins (Comer et al., 1998). These phosphorylation events are likely to disrupt interactions between Ena and a protein that tethers it to sites of action. Abl can target a single tyrosine residue in Mena (mammalian Enabled), but it is unclear whether this event alters Mena localization or its interactions with other proteins (Tani et al., 2003).
Regulation of adhesion and contractility
Tight control over the protrusive, adhesive and contractile forces in the cell is required for productive directed movement. As discussed above, Abl-family kinases stimulate actin polymerization, leading to cellular protrusion, the first step in cell motility. Surprisingly, however, fibroblasts that are deficient for Abl and/or Arg migrate faster than wild-type controls (Kain and Klemke, 2001; Peacock et al., 2007), and reconstitution with Abl or Arg slows these cells. Here, we describe evidence that Abl-family kinases act through the GTPase RhoA and other mechanisms to attenuate cell migration by modulating cell adhesion and contractility.
RhoA
The GTPase RhoA (Rho) is the master regulator of cell contractility (Chrzanowska-Wodnicka and Burridge, 1996; Ridley and Hall, 1992), mediates F-actin stress-fiber assembly and focal-adhesion maturation, and stimulates actomyosin contractility that powers the translocation of the cell body forwards. Active Rho signals to Rho kinase (ROCK), which phosphorylates and activates myosin-light-chain kinase (MLCK), which, in turn, phosphorylates and activates myosin II and localizes it to actin filaments (Chrzanowska-Wodnicka and Burridge, 1996). Rho activity was originally thought to be localized only at the rear of the cell, but it is now apparent that Rho activity also cycles at the front of the cell (Pertz et al., 2006). For a cell to sample its environment by means of membrane protrusions, local Rho activity and contractility at the front of the cell must be relieved.
The Rho-specific GTPase-activating protein p190RhoGAP-A (p190) is a substrate of Arg (Hernandez et al., 2004), and its Arg-dependent phosphorylation dictates its subcellular localization and therefore its activity (Bradley et al., 2006). The level of tyrosine phosphorylation of p190 in the adult Arg–/– mouse brain is significantly reduced compared with wild-type littermates. When Arg–/– fibroblasts are plated on fibronectin, they not only show a reduction in phospho-p190, but also a significant elevation of active Rho and increased F-actin stress fibers (Bradley et al., 2006). p190 is known to interact with p120RasGAP (p120) (Chang et al., 1995; Hu and Settleman, 1997; Roof et al., 1998), which contains both PH and C2 phospholipid-binding domains. Phosphorylation of p190 by Arg drives formation of the p190-p120 complex and is essential for p190 localization to the cell periphery (Bradley et al., 2006) (Fig. 3C). Disruption of the complex by genetic loss of Arg, expression of a dominant-negative p120 or expression of non-phosphorylatable p190 prevents complex formation and proper p190 localization. Because active Rho is membrane-bound, Arg dictates the ability of p190 to inhibit Rho by modulating p190 localization. With elevated Rho activity, Arg–/– fibroblasts are highly contractile (Peacock et al., 2007). Reconstitution of these cells with wild-type, but not kinase-inactive, Arg restores p190 phosphorylation and localization, and reduces Rho activity and contractility to normal levels.
Although Rho is one of the main stimulators of cell contractility, it ultimately signals to myosin to achieve this. A recent report identified myosin IIB as a substrate of Arg (Boyle and Koleske, 2007), which raises the possibility that tyrosine phosphorylation of myosin IIB is an additional way for Arg to regulate the contractile state of the cell directly. Interestingly, dAbl and myosin II were recently shown to interact functionally in the control of Drosophila growth-cone steering (Dorsten et al., 2007). Further work is warranted to understand precisely how tyrosine phosphorylation affects myosin-IIB function and overall cell contractility.
Regulation of focal adhesions
Abl has long been known to localize to focal adhesions (Lewis et al., 1996), yet only recently have we learned more about the role of Abl-family kinases in regulating adhesions. Interestingly, Arg–/– fibroblasts exhibit enlarged focal adhesions with altered dynamic properties (Peacock et al., 2007). Loss of Arg leads to less frequent adhesion turnover, and an uncoupling of adhesions from the ECM. So, despite being larger than wild-type adhesions, adhesions in Arg–/– cells are not well anchored and tend to slide in large groups within the cell. This, in conjunction with increased contractility, allows Arg–/– cells to move much faster than their properly balanced wild-type counterparts. It is important to note that the loss of Arg function disrupts the balance between protrusion, adhesion and contractility, and hence the cells migrate in an uncoordinated fashion in which they cannot properly sample their environment. Occasionally, Arg–/– cells initiate migration in two directions and, because they are hypercontractile, the cells tear themselves in half (Peacock et al., 2007). These findings underscore the importance of Arg in coordinating protrusive, adhesive and contractile forces to achieve proper directed cell migration. Future studies must focus on the molecular mechanisms by which Arg coordinates these processes in migrating cells.
Regulation of cell-cell adhesion
Cell-cell adhesion is essential for the organization and maintenance of tissue. Cells achieve this type of adhesion through specialized adherens junctions that link neighboring cells via interactions between cadherin receptors (Schock and Perrimon, 2002). Cadherins are also linked to the actin cytoskeleton through the catenin complex (α- and β-subunits), and are regulated by Rho GTPases (Arthur et al., 2002; Yamada and Nelson, 2007; Nelson, 2008).
Interestingly, genetic loss, knockdown or pharmacological inhibition of both Abl and Arg leads to disruption of N-cadherin- and E-cadherin-based cell-cell contacts (Zandy and Pendergast, 2008; Zandy et al., 2007). Conversely, cell-cell adhesion enhances Abl-family kinase activity through an as-yet-unknown mechanism, which leads to increased CrkII phosphorylation and subsequent Rac activation (Abassi and Vuori, 2002; Burton et al., 2005). Recent reports indicate that Rac-induced lamellipodia are necessary for initial cell-cell contact, and that cycling of Rac activity is required for expansion and maturation of this contact (Yamada and Nelson, 2007). One model for the role of Abl and Arg in this process includes a feedback loop in which activated Rac stimulates further Abl-family kinase activity, leading to enhanced phosphorylation of CrkII (Zandy and Pendergast, 2008). This feedback loop might lead to a further strengthening of the cell-cell junction.
As well as activating Rac following cell-cell adhesion, Abl-family kinases also modulate Rho activity (see above for a discussion of the mechanism of Rho regulation). Disruption of Abl and Arg signaling leads to increased Rho activity and actomyosin contractility, resulting in dissolution of cell-cell contacts. Rho activity is important in generating and maintaining cell-cell adhesions, as it plays a role in the clustering of receptors and expansion of the adhesion zone (Braga, 2000; Yamada and Nelson, 2007). Its ability to generate contractility is important for reshaping the cells, but too much active Rho is detrimental to adhesion stability, as seen in cells lacking Abl and Arg (Zandy et al., 2007). It remains to be seen whether this Rho-modulating activity is direct (via activation of p190RhoGAP, as described above) (Noren et al., 2003; Wildenberg et al., 2006) or indirect (via modulation of another Rac-Rho crosstalk pathway).
Direct interaction with the cytoskeleton
Abl-family kinases have the unique ability among tyrosine kinases to bind directly to the cytoskeleton. Both Abl and Arg can bind to and bundle F-actin in vitro. For Arg, both of its F-actin-binding domains (the CH domain at the C-terminus and the internal [I/L]WEQ domain) are essential for its ability to localize to and concentrate at the cell periphery (Miller et al., 2004).
A low-resolution electron-microscopy-derived structure of Arg bound to F-actin reveals that the [I/L]WEQ domain imposes a distortion on F-actin that mimics an ATP-bound closed nucleotide-binding cleft (Galkin et al., 2005). This distortion might be functionally important, as it might stabilize actin filaments by preventing severing by cofilin, which only binds to and severs ADP-bound actin. Arg binding to ADP-bound actin might mimic the ATP-bound state of actin, preventing cofilin binding and subsequent actin severing. Conversely, the CH F-actin-binding domain of Arg imposes a tilt to the F-actin filament, similar to cofilin. Changing the tilt and twist of F-actin filaments is thought to be one of the mechanisms by which cofilin severs actin (McGough et al., 1997; Galkin et al., 2001), but there are no reports that Arg severs actin filaments (Galkin et al., 2005). Although these structural data are interesting, the functional consequences of the distortions to actin filaments when Arg is bound remain unknown.
In addition to its two F-actin-binding domains, Arg, but not Abl, contains an MT-binding domain (Miller et al., 2004). Using these three domains, Arg cooperatively binds to F-actin, bundles it, and crosslinks F-actin and MTs in vitro. In fibroblasts, Arg is concentrated at sites at which F-actin and MTs colocalize and, given its crosslinking ability, this raises the possibility that Arg couples the two cytoskeletal structures in vivo. Arg also concentrates at sites of lamellipodial protrusion. Several studies have linked MT extension and capture at the cell periphery as a necessity for cell protrusion (Rodriguez et al., 2003). An Arg mutant with impaired MT-binding ability still localizes to the cell periphery, but appears more diffuse, and these cells show reduced lamellipodial dynamics when plated on fibronectin (Miller et al., 2004). These observations suggest that Arg acts as a peripheral target for MTs after their extension to the cell periphery, and that it might act as a capturing molecule. High-resolution live videomicroscopy of Arg trafficking in relation to MT and F-actin dynamics should show how Arg coordinates cytoskeletal interactions at the cell periphery that lead to protrusions.
Physiological processes that require Abl-family kinases
Abl-family kinases are essential regulators of cell morphogenesis and migration in developing metazoans. In the following sections, we review physiological processes that require Abl-family kinase function for their proper execution, and explore how the diverse Abl-regulated pathways of cytoskeletal control contribute to these morphogenetic and migratory events.
Lymphocyte function
The first descriptions of the Abl–/– mouse indicated that Abl-family kinases have crucial roles in lymphocyte development and function (Tybulewicz et al., 1991; Schwartzberg et al., 1991). Abl-deficient mice exhibit a depletion of both B and T cells, a condition known as lymphopenia. Specifically, the most severe and well-characterized lymphocyte reduction in Abl–/– mice occurs in the early pro-B and pre-B cell differentiating stages. B cells isolated from Abl–/– mice do not respond normally to cytokine or mitogenic stimuli (Hardin et al., 1995; Hardin et al., 1996) and have an increased susceptibility to pro-apoptotic stimuli (Dorsch and Goff, 1996). Abl-family kinases are also required for T-cell development, survival and function (Gu et al., 2007; Huang et al., 2008; Silberman et al., 2008; Zipfel et al., 2004).
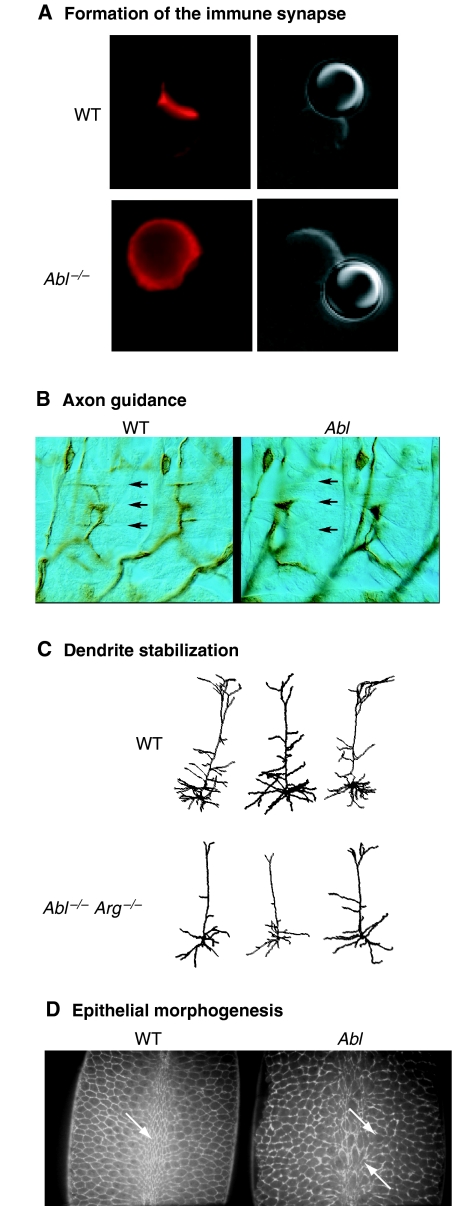
Abl plays an especially important role in the formation of the immune synapse, the stable adhesive and signaling complex that forms between T cells and antigen-presenting cells. An F-actin-rich scaffold forms at the immune synapse, providing structural stability and helping to organize signaling events. Genetic loss or chemical inhibition of Abl-family kinases significantly compromises F-actin assembly at the immune synapse (Huang et al., 2008). Lack of a functional Abl protein probably prevents HS1 from activating actin polymerization in this setting, although WAVE2 localization to the immune synapse is also compromised in these cells. It is possible that Abl-mediated phosphorylation of the adaptor protein Abi, or of WAVE2 itself, drives proper WAVE2 localization (Leng et al., 2005; Zipfel et al., 2006). Conflicting studies in T cells in which Abl is knocked down show that WAVE2 localization and actin organization are unaltered when Abl expression is reduced (Nolz et al., 2008). Different experimental conditions or residual expression of Abl in knockdown cells might account for this discrepancy.
Abl-family kinases also regulate lymphocyte trafficking, although this has been less extensively studied. Abl-deficient T cells exhibit reduced chemotactic responses, possibly due to deficits in the organization and persistence of lamellipodial protrusions (Huang et al., 2008) (Fig. 4A). Defects in T-cell trafficking probably contribute to the deficits in T-cell-mediated responses, including antibody production and tumor rejection, that are observed in mice with Abl-deficient T cells (Gu et al., 2007; Silberman et al., 2008).
Fig. 4.
Physiological roles for Abl-family kinases. (A) Abl is required for proper F-actin assembly during immune-synapse formation. Wild-type (WT, top) or Abl–/– (bottom) T cells were incubated with latex beads (right panels) coated with anti-T-cell-receptor antibody, and were then stained with phalloidin to reveal the F-actin cytoskeleton (left panels). Wild-type T cells form an immune-synapse-like concentration of F-actin at the bead contact site. The F-actin cytoskeleton remains diffusely localized in Abl–/– T cells. Figure courtesy of Ann Huang and Janis Burkhardt (University of Pennsylvania, Philadelphia, PA). (B) dAbl is required for proper axon guidance. In abdominal segments of wild-type (WT, left) Drosophila embryos, intersegmental neuron group b (ISNb) axons make normal neuromuscular junctions with longitudinal muscles, including muscle 12 (arrows). ISNb growth cones stop short and fail to contact muscle 12 in an Abl mutant (right). Adapted with permission from figure 3 in Wills et al. (Wills et al., 1999b). Figure courtesy of David Van Vactor (Harvard Medical School, Boston, MA). (C) Abl and Arg are required for dendrite stabilization. Camera lucida representations of wild-type (WT, top) or Abl–/– Arg–/– (bottom) cortical-layer-5 pyramidal neurons. Atrophy of dendrite arbors in Abl–/– Arg–/– neurons results in smaller dendrite arbors. (D) dAbl is essential for normal epithelial morphogenesis. Wild-type Drosophila embryos (WT, left) exhibit uniform apical constriction at the ventral furrow during gastrulation (arrow). Cell morphology is visualized using a moesin-GFP that binds to F-actin. An Ablmz mutant depleted for both maternal and zygotic Abl (right) exhibits non-uniform constriction. Figure courtesy of Don Fox and Mark Peifer (University of North Carolina, Chapel Hill, NC).
Axon guidance
At the tip of developing axons, a filopodium-rich growth cone navigates the extracellular milieu by reading axon-guidance cues (molecules that tell the growth cones to stop, come closer or move away). These cues are detected by cell-surface receptors, which coordinate downstream changes in cytoskeletal and adhesive structures to steer axonal pathfinding. A clear role for Abl-family kinases in tissue morphogenesis was first described in the regulation of axon guidance in the developing Drosophila nervous system. In flies, dAbl is enriched in developing axons (Bennett and Hoffmann, 1992), where it regulates the development of proper central nervous system (CNS) structure (Crowner et al., 2003; Dorsten et al., 2007; Elkins et al., 1990; Gertler et al., 1989; Giniger, 1998; Liebl et al., 2000; Liebl et al., 2003; Wills et al., 2002). Altering Abl function can lead to defects in axon attractant responses (Forsthoefel et al., 2005), repellent responses (Hsouna et al., 2003; Lee et al., 2004; Wills et al., 2002), and defasciculation and turning responses (Wills et al., 1999a; Wills et al., 1999b) (Fig. 4B), depending on the neuron type and genetic background. These observations clearly demonstrate that dAbl does not dictate a single type of growth-cone response, but coordinates diverse downstream responses to different cues.
dAbl `wires up' the fly nervous system using biochemical mechanisms similar to those employed by Abl and Arg in vertebrate cells. Growth-cone protrusion is powered by actin polymerization at the leading edge. In response to the proper cues, dAbl can promote growth-cone protrusion by locally directing actin polymerization. In this context, dAbl interacts genetically with several key actin-polymerization regulators including Ena (see above) (Gertler et al., 1995), profilin (Wills et al., 1999b), the adenylate cyclase associated protein (Wills et al., 2002) and the Rho/Rac GEF Trio (Liebl et al., 2000).
Advancement of the growth-cone central domain also requires actomyosin contractility exerted at points of adhesive contact. dAbl might modulate growth-cone advancement or contraction akin to Arg in fibroblasts (discussed above) by modulating actomyosin contractility or adhesion dynamics. Indeed, a recent study indicates that dAbl can modulate myosin-II activity to protect against improper targeting (Dorsten et al., 2007). However, pharmacological inhibition of both Abl and Arg blocks ephrin-A5-mediated growth-cone collapse, a process that is known to depend on Rho signaling through ROCK (Harbott and Nobes, 2005). Thus, in contrast to the loss of Arg function in fibroblasts, which elevates Rho activity, loss of both Abl and Arg function might compromise Rho signaling in growth cones.
The emerging picture is that dAbl regulates axon-guidance decisions by controlling the balance of protrusive, adhesive and contractile forces within the growth cone. The balance of these forces is most probably determined by which effector pathways dAbl engages, and these are likely to be regulated by the local physicochemical environment the growth cone encounters. Although Abl and Arg can promote neurite branching (Fig. 4C) and elongation when expressed in cultured mouse cortical neurons (Moresco et al., 2005; Woodring et al., 2002; Zukerberg et al., 2000), specific roles for Abl and Arg in mammalian axon guidance have not yet been described.
Synapses and dendrites
Abl overexpression promotes dendrite elaboration in cultured hippocampal neurons (Jones et al., 2004). On this basis, it was proposed that Abl-family kinases regulate dendritogenesis in vivo. However, cortical and hippocampal dendrites develop normally in mice whose neurons lack Abl, Arg or both kinases, reaching their full mature size and shape (Moresco et al., 2005; Sfakianos et al., 2007). Interestingly, however, loss of dAbl function does lead to a slight, but statistically significant, increase in dendrite branches in dendritic arborization (DA) sensory neurons in Drosophila (Li et al., 2005).
Abl-family kinases play important roles in synapse function and stability. Abl, Arg and p190RhoGAP-A are enriched in synapses, particularly in the postsynaptic side, although some Abl and Arg might be presynaptic (Moresco et al., 2003; Sfakianos et al., 2007). Abl–/– or Arg–/– hippocampal synapses are defective in a form of short-term synaptic plasticity that reflects deficiencies in vesicle release during closely spaced stimuli (Moresco et al., 2003). The cellular mechanisms that underlie this defect are unclear.
Abl-family kinases also seem to be important for the organization, maturation and stability of synapses. Excitatory synapses in the brain form on dendritic spines, which are specialized F-actin-rich protrusions that emerge from the dendrite shaft. Hippocampal synapses develop normally in Arg–/– mice, reaching wild-type numbers by postnatal day (P)21. However, 35% of these synapses are lost by P31, and this reduction is followed by a commensurate loss of dendrite arbor (Sfakianos et al., 2007). These degenerative events are accompanied by a progressive deficit in hippocampal-dependent memory tasks in Arg–/– mice. After initial normal development, severe cortical dendrite atrophy is also noted in both Arg–/– and Abl–/– Arg–/– mice, which probably results from a similar synaptic degeneration (Moresco et al., 2005).
p190RhoGAP activity is decreased in Arg-deficient hippocampi, leading to an elevation of Rho activity. Exactly how elevated Rho activity causes synapse degeneration is unclear. Dendritic spines in weanling mice are heterogeneous in size and shape, but undergo a transition to a smaller, more uniform size and shape as the mice reach adulthood. Of the dendritic spines that remain in Arg–/– mice, this maturation step does not occur. Instead, the spines remain larger overall and more heterogeneous than in wild-type animals (Sfakianos et al., 2007). These data indicate that the synaptic cytoskeleton and possibly synaptic adhesions might be distorted. The synaptic cytoskeleton organizes the postsynaptic adhesion and signaling machinery, and thus these distortions probably compromise synapse function and stability by disrupting their proper organization in Arg–/– mice. An analogous situation might occur at the neuromuscular junction, where Abl and Arg activation is required for agrin-induced clustering of acetylcholine receptors on the postsynaptic side (Finn et al., 2003). In addition, knockout or knockdown of the Abl-Arg substrates Abi2, cortactin or Rin1 disrupts normal dendritic-spine shape, number and/or function, suggesting that Arg also acts through these substrates to regulate synapse stability (Dhaka et al., 2003; Grove et al., 2004; Hering and Sheng, 2003).
Epithelial morphogenesis
Abl-family kinases are crucial for the proper formation and remodeling of epithelial tissues. The neuroepithelial cells that make up the vertebrate neural tube have an apical actin latticework, and constriction of this network is essential for neurulation (the process of neural-tube closure). Abl and Arg colocalize to the apical actin network in epithelial cells, where they regulate the formation of actin-rich structures (Koleske et al., 1998). Mouse embryos deficient in both Abl and Arg exhibit gross defects in the neuroepithelial cytoskeleton. In addition to patchy disruptions of the apical actin structures, disordered actin-rich structures were found at the basolateral/marginal surface of Abl–/– Arg–/– neuroepithelium (Koleske et al., 1998).
dAbl helps orchestrate several essential morphogenetic transformations of epithelial structures in the fly. Defects in cellularization, ventral-furrow formation and dorsal closure have all been described in flies lacking both maternal and zygotic sources of dAbl (Grevengoed et al., 2001; Grevengoed et al., 2003; Fox and Peifer, 2007; Gates et al., 2007). In particular, the coordinated cell-shape changes that are required for ventral-furrow formation and dorsal closure are disrupted in these mutants (Grevengoed et al., 2001; Gates et al., 2007; Fox and Peifer, 2007). In both cell types, the normal continuous uniform apical actin structures are non-uniform and in some cases patchy disruptions are evident. Defects in the dynamic leading-edge processes are also apparent in cells undergoing dorsal closure (Gates et al., 2007). As a result, both processes are disrupted or uncoordinated (Fig. 4D).
Epithelial morphogenesis requires a precise coordination between cell-cell interactions and the dynamic assembly and disassembly of actin structures. Abl-family kinases probably influence epithelial morphogenesis by regulating both processes. The loss of dAbl function leads to excessive accumulation and abnormal clustering of Ena at the leading edges of cells undergoing dorsal closure and at the apical surface of ventral-furrow cells (Grevengoed et al., 2001; Gates et al., 2007; Fox and Peifer, 2007). This altered Ena distribution is likely to be responsible for the observed distortions to cytoskeletal structures in the respective cell types (Fox and Peifer, 2007). Ena dysregulation also contributes to the distortions observed in actin networks during syncytial development and cellularization in Abl mutants (Grevengoed et al., 2003), as well as the defects in dorsal closure that result from ectopic expression of activated Bcr-Abl transgenes (Stevens et al., 2008).
Abl-family kinases also contribute to epithelial morphogenesis by regulating cell-cell adhesion. This idea stems from the observation that mutations in the fly catenin armadillo synergize with Abl mutations to enhance CNS defects (Loureiro and Peifer, 1998). Subsequent work has demonstrated that loss of Abl and Arg function prevents adherens-junction formation in fibroblasts (discussed above) (Zandy et al., 2007), in part because of hyperactive Rho signaling. p190RhoGAP-A is an obvious candidate for mediating Rho suppression by Abl and Arg (Hernandez et al., 2004). Indeed, p190RhoGAP-A knockdown disrupts adherens-junction formation (Wildenberg et al., 2006), and p190RhoGAP-A-knockout mouse embryos exhibit defects in neural-tube formation (Brouns et al., 2000). These defects are not as severe as those observed in Abl–/– Arg–/– embryos, but p190RhoGAP-B might play a compensatory role here. However, changes in p190RhoGAP phosphorylation during cell-cell adhesion have not been detected (Zandy et al., 2007) and this lack of resolution warrants further investigation of this issue.
Conclusions and perspectives
Abl-family kinases have received much attention since the first identification of Abl as an oncogene. Emerging evidence indicates that the kinases play an important role in several diseases, including leukemia, breast cancer, immune-system and neurological disorders, and microbial pathogenesis. Genetic studies indicate that Abl-family kinases are essential regulators of cell migration and tissue morphogenesis. Recent biochemical studies have begun to reveal the cellular and molecular mechanisms by which Abl-family kinases coordinate dynamic rearrangements in cytoskeletal structure. Together, these studies have expanded our knowledge of how Abl-family kinases are regulated and how they communicate with specific signaling pathways. These gains will undoubtedly aid us in understanding the cellular mechanisms that underlie the different relevant disease states and, more importantly, how to proceed in developing therapies to treat these diseases.
The studies reviewed here reveal that Abl-family kinases lie at the crossroads of several intersecting cytoskeleton and adhesion control pathways. These pathways must be carefully coordinated to achieve precise biological outcomes. A major unresolved issue moving forward is whether Abl-family kinases provide a direct physical link between these seemingly distinct control pathways. In one scenario, distinct pools of Abl-family kinases are dedicated to specific control pathways within a given cell. If this is the case, how are these distinct pools managed and maintained? Alternatively, Abl-family kinases might serve as a physical link between separate signaling pathways. For instance, can the same Arg molecule both promote actin polymerization via cortactin and inhibit contractility via p190RhoGAP? Answering these questions will require the combination of high-resolution live-cell microscopy, fluorescent probes of biological activity and some cleverness on the part of researchers. We have learned a great deal over the past several years regarding how Abl-family kinases function, and the ongoing challenge is to understand when, where and how these mechanisms are employed to ensure proper organismal development and homeostasis.
We thank David Van Vactor, Don Fox, Mark Peifer, Ann Huang and Janis Burkhardt for helpful discussion and figures. We thank two anonymous reviewers for thoughtful advice and criticism on improving this Commentary. We thank Brian Couch, Stacey MacGrath, Chris Mader, Matthew Miller, Sloan Warren and the rest of our colleagues for many helpful discussions and feedback. Work in the Koleske laboratory is supported by grants from the NIH (NS39475 and CA133346), from the Elsa U. Pardee Foundation, and from an anonymous donor. W.D.B. is supported by NIH Predoctoral NRSA (NS58086). A.J.K. is an Established Investigator of the American Heart Association. Deposited in PMC for release after 12 months.
References
- Abassi, Y. A. and Vuori, K. (2002). Tyrosine 221 in Crk regulates adhesion-dependent membrane localization of Crk and Rac and activation of Rac signaling. EMBO J. 21, 4571-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoku, S., Saksela, K., Rivera, G. M. and Mayer, B. J. (2008). A crucial role in cell spreading for the interaction of Abl PxxP motifs with Crk and Nck adaptors. J. Cell Sci. 121, 3071-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Salgado, E. G., Lizano, S., Sarkar, S., Brugge, J. S., Ginsberg, M. H. and Shattil, S. J. (2003). Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA 100, 13298-13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, W. T., Noren, N. K. and Burridge, K. (2002). Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol. Res. 35, 239-246. [DOI] [PubMed] [Google Scholar]
- Backert, S., Feller, S. M. and Wessler, S. (2008). Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem. Sci. 33, 80-90. [DOI] [PubMed] [Google Scholar]
- Bear, J. E. and Gertler, F. B. (2009). Ena/VASP: towards resolving a pointed controversy at the barbed end. J. Cell Sci. 122, 1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, R. L. and Hoffmann, F. M. (1992). Increased levels of the Drosophila Abelson tyrosine kinase in nerves and muscles: subcellular localization and mutant phenotypes imply a role in cell-cell interactions. Development 116, 953-966. [DOI] [PubMed] [Google Scholar]
- Bliss, J. M., Venkatesh, B. and Colicelli, J. (2006). The RIN family of Ras effectors. Methods Enzymol. 407, 335-344. [DOI] [PubMed] [Google Scholar]
- Boyle, S. N. and Koleske, A. J. (2007). Use of a chemical genetic technique to identify myosin IIb as a substrate of the Abl-related gene (Arg) tyrosine kinase. Biochemistry 46, 11614-11620. [DOI] [PubMed] [Google Scholar]
- Boyle, S. N., Michaud, G. A., Schweitzer, B., Predki, P. F. and Koleske, A. J. (2007). A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr. Biol. 17, 445-451. [DOI] [PubMed] [Google Scholar]
- Bradley, W. D., Hernandez, S. E., Settleman, J. and Koleske, A. J. (2006). Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol. Biol. Cell 17, 4827-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, V. (2000). Epithelial cell shape: cadherins and small GTPases. Exp. Cell Res. 261, 83-90. [DOI] [PubMed] [Google Scholar]
- Brasher, B. B. and Van Etten, R. A. (2000). c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 275, 35631-35637. [DOI] [PubMed] [Google Scholar]
- Brouns, M. R., Matheson, S. F., Hu, K. Q., Delalle, I., Caviness, V. S., Silver, J., Bronson, R. T. and Settleman, J. (2000). The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development 127, 4891-4903. [DOI] [PubMed] [Google Scholar]
- Bryce, N. S., Clark, E. S., Leysath, J. L., Currie, J. D., Webb, D. J. and Weaver, A. M. (2005). Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15, 1276-1285. [DOI] [PubMed] [Google Scholar]
- Burton, E. A., Plattner, R. and Pendergast, A. M. (2003). Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 22, 5471-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, E. A., Oliver, T. N. and Pendergast, A. M. (2005). Abl kinases regulate actin comet tail elongation via an N-WASP-dependent pathway. Mol. Cell. Biol. 25, 8834-8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, C., Ren, X., Kharbanda, S., Koleske, A., Prasad, K. V. and Kufe, D. (2001). The ARG tyrosine kinase interacts with Siva-1 in the apoptotic response to oxidative stress. J. Biol. Chem. 276, 11465-11468. [DOI] [PubMed] [Google Scholar]
- Cao, X., Tanis, K. Q., Koleske, A. J. and Colicelli, J. (2008). Enhancement of ABL kinase catalytic efficiency by a direct binding regulator is independent of other regulatory mechanisms. J. Biol. Chem. 283, 31401-31407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. H., Gill, S., Settleman, J. and Parsons, S. J. (1995). c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 130, 355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M. and Burridge, K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer, A. R., Ahern-Djamali, S. M., Juang, J. L., Jackson, P. D. and Hoffman, F. M. (1998). Phosphorylation of Enabled by the Drosophila Abelson tyrosine kinase regulates the in vivo function and protein-protein interactions of Enabled. Mol. Cell. Biol. 18, 152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J. A., Gould, K. L., Cartwright, C. A. and Hunter, T. (1986). Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science 231, 1431-1434. [DOI] [PubMed] [Google Scholar]
- Crowner, D., Le Gall, M., Gates, M. A. and Giniger, E. (2003). Notch steers Drosophila ISNb motor axons by regulating the Abl signaling pathway. Curr. Biol. 13, 967-972. [DOI] [PubMed] [Google Scholar]
- David-Cordonnier, M. H., Hamdane, M., Bailly, C. and D'Halluin, J. C. (1998). Determination of the human c-Abl consensus DNA binding site. FEBS Lett. 424, 177-182. [DOI] [PubMed] [Google Scholar]
- de Rooij, J., Kerstens, A., Danuser, G., Schwartz, M. A. and Waterman-Storer, C. M. (2005). Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J. Cell Biol. 171, 153-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkinderen, P., Scales, T. M., Hanger, D. P., Leung, K. Y., Byers, H. L., Ward, M. A., Lenz, C., Price, C., Bird, I. N., Perera, T. et al. (2005). Tyrosine 394 is phosphorylated in Alzheimer's paired helical filament tau and in fetal tau with c-Abl as the candidate tyrosine kinase. J. Neurosci. 25, 6584-6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka, A., Costa, R. M., Hu, H., Irvin, D. K., Patel, A., Kornblum, H. I., Silva, A. J., O'Dell, T. J. and Colicelli, J. (2003). The RAS effector RIN1 modulates the formation of aversive memories. J. Neurosci. 23, 748-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey, K., Engen, J. R., Kretzschmar, J., Wilm, M., Neubauer, G., Schindler, T. and Superti-Furga, G. (2001). Phosphorylation and structure-based functional studies reveal a positive and a negative role for the activation loop of the c-Abl tyrosine kinase. Oncogene 20, 8075-8084. [DOI] [PubMed] [Google Scholar]
- Dorsch, M. and Goff, S. P. (1996). Increased sensitivity to apoptotic stimuli in c-abl-deficient progenitor B-cell lines. Proc. Natl. Acad. Sci. USA 93, 13131-13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsten, J. N., Kolodziej, P. A. and VanBerkum, M. F. (2007). Frazzled regulation of myosin II activity in the Drosophila embryonic CNS. Dev. Biol. 308, 120-132. [DOI] [PubMed] [Google Scholar]
- Eden, S., Rohatgi, R., Podtelejnikov, A. V., Mann, M. and Kirschner, M. W. (2002). Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790-793. [DOI] [PubMed] [Google Scholar]
- Elkins, T., Zinn, K., McAllister, L., Hoffmann, F. M. and Goodman, C. S. (1990). Genetic analysis of a Drosophila neural cell adhesion molecule: interaction of fasciclin I and Abelson tyrosine kinase mutations. Cell 60, 565-575. [DOI] [PubMed] [Google Scholar]
- Emerson, M. M. and Van Vactor, D. (2002). Robo is Abl to block N-Cadherin function. Nat. Cell Biol. 4, E227-E230. [DOI] [PubMed] [Google Scholar]
- Feller, S. M., Knudsen, B. and Hanafusa, H. (1994). c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 13, 2341-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, A. J., Feng, G. and Pendergast, A. M. (2003). Postsynaptic requirement for Abl kinases in assembly of the neuromuscular junction. Nat. Neurosci. 6, 717-723. [DOI] [PubMed] [Google Scholar]
- Forsthoefel, D. J., Liebl, E. C., Kolodziej, P. A. and Seeger, M. A. (2005). The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development 132, 1983-1994. [DOI] [PubMed] [Google Scholar]
- Fox, D. T. and Peifer, M. (2007). Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development 134, 567-578. [DOI] [PubMed] [Google Scholar]
- Frasca, F., Pandini, G., Malaguarnera, R., Mandarino, A., Messina, R. L., Sciacca, L., Belfiore, A. and Vigneri, R. (2007). Role of c-Abl in directing metabolic versus mitogenic effects in insulin receptor signaling. J. Biol. Chem. 282, 26077-26088. [DOI] [PubMed] [Google Scholar]
- Furstoss, O., Dorey, K., Simon, V., Barila, D., Superti-Furga, G. and Roche, S. (2002). c-Abl is an effector of Src for growth factor-induced c-myc expression and DNA synthesis. EMBO J. 21, 514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin, V. E., Orlova, A., Lukoyanova, N., Wriggers, W. and Egelman, E. H. (2001). Actin depolymerizing factor stabilizes an existing state of F-actin and can change the tilt of F-actin subunits. J. Cell Biol. 153, 75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin, V. E., Orlova, A., Koleske, A. J. and Egelman, E. H. (2005). The Arg non-receptor tyrosine kinase modifies F-actin structure. J. Mol. Biol. 346, 565-575. [DOI] [PubMed] [Google Scholar]
- Gates, J., Mahaffey, J. P., Rogers, S. L., Emerson, M., Rogers, E. M., Sottile, S. L., Van Vactor, D., Gertler, F. B. and Peifer, M. (2007). Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development 134, 2027-2039. [DOI] [PubMed] [Google Scholar]
- Gautreau, A., Ho, H. Y., Li, J., Steen, H., Gygi, S. P. and Kirschner, M. W. (2004). Purification and architecture of the ubiquitous Wave complex. Proc. Natl. Acad. Sci. USA 101, 4379-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler, F. B., Bennett, R. L., Clark, M. J. and Hoffmann, F. M. (1989). Drosophila abl tyrosine kinase in embryonic CNS axons: a role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell 58, 103-113. [DOI] [PubMed] [Google Scholar]
- Gertler, F. B., Doctor, J. S. and Hoffman, F. M. (1990). Genetic suppression of mutations in the Drosophila abl proto-oncogene homolog. Science 248, 857-860. [DOI] [PubMed] [Google Scholar]
- Gertler, F. B., Comer, A. R., Juang, J. L., Ahern, S. M., Clark, M. J., Liebl, E. C. and Hoffmann, F. M. (1995). enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 9, 521-533. [DOI] [PubMed] [Google Scholar]
- Giniger, E. (1998). A role for Abl in Notch signaling. Neuron 20, 667-681. [DOI] [PubMed] [Google Scholar]
- Grevengoed, E. E., Loureiro, J. J., Jesse, T. L. and Peifer, M. (2001). Abelson kinase regulates epithelial morphogenesis in Drosophila. J. Cell Biol. 155, 1185-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed, E. E., Fox, D. T., Gates, J. and Peifer, M. (2003). Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J. Cell Biol. 163, 1267-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove, M., Demyanenko, G., Echarri, A., Zipfel, P. A., Quiroz, M. E., Rodriguiz, R. M., Playford, M., Martensen, S. A., Robinson, M. R., Wetsel, W. C. et al. (2004). ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol. Cell. Biol. 24, 10905-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J. J., Zhang, N., He, Y. W., Koleske, A. J. and Pendergast, A. M. (2007). Defective T cell development and function in the absence of Abelson kinases. J. Immunol. 179, 7334-7343. [DOI] [PubMed] [Google Scholar]
- Gupton, S. L. and Waterman-Storer, C. M. (2006). Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 125, 1361-1374. [DOI] [PubMed] [Google Scholar]
- Hantschel, O., Nagar, B., Guettler, S., Kretzschmar, J., Dorey, K., Kuriyan, J. and Superti-Furga, G. (2003). A myristoyl/phosphotyrosine switch regulates c-Abl. Cell 112, 845-857. [DOI] [PubMed] [Google Scholar]
- Harbott, L. K. and Nobes, C. D. (2005). A key role for Abl family kinases in EphA receptor-mediated growth cone collapse. Mol. Cell Neurosci. 30, 1-11. [DOI] [PubMed] [Google Scholar]
- Hardin, J. D., Boast, S., Schwartzberg, P. L., Lee, G., Alt, F. W., Stall, A. M. and Goff, S. P. (1995). Bone marrow B lymphocyte development in c-abl-deficient mice. Cell Immunol. 165, 44-54. [DOI] [PubMed] [Google Scholar]
- Hardin, J. D., Boast, S., Schwartzberg, P. L., Lee, G., Alt, F. W., Stall, A. M. and Goff, S. P. (1996). Abnormal peripheral lymphocyte function in c-abl mutant mice. Cell Immunol. 172, 100-107. [DOI] [PubMed] [Google Scholar]
- Harrison, S. C. (2003). Variation on an Src-like theme. Cell 112, 737-740. [DOI] [PubMed] [Google Scholar]
- Hering, H. and Sheng, M. (2003). Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J. Neurosci. 23, 11759-11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, S. E., Settleman, J. and Koleske, A. J. (2004). Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr. Biol. 14, 691-696. [DOI] [PubMed] [Google Scholar]
- Hsouna, A., Kim, Y. S. and VanBerkum, M. F. (2003). Abelson tyrosine kinase is required to transduce midline repulsive cues. J. Neurobiol. 57, 15-30. [DOI] [PubMed] [Google Scholar]
- Hu, H., Bliss, J. M., Wang, Y. and Colicelli, J. (2005). RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr. Biol. 15, 815-823. [DOI] [PubMed] [Google Scholar]
- Hu, K. Q. and Settleman, J. (1997). Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO J. 16, 473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Comiskey, E. O., Dupree, R. S., Li, S., Koleske, A. J. and Burkhardt, J. K. (2008). The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood 112, 111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti, M., Zucconi, A., Disanza, A., Frittoli, E., Areces, L. B., Steffen, A., Stradal, T. E., Di Fiore, P. P., Carlier, M. F. and Scita, G. (2004). Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat. Cell Biol. 6, 319-327. [DOI] [PubMed] [Google Scholar]
- Jay, P. Y., Pham, P. A., Wong, S. A. and Elson, E. L. (1995). A mechanical function of myosin II in cell motility. J. Cell Sci. 108, 387-393. [DOI] [PubMed] [Google Scholar]
- Jin, H. and Wang, J. Y. (2007). Abl tyrosine kinase promotes dorsal ruffles but restrains lamellipodia extension during cell spreading on fibronectin. Mol. Biol. Cell 18, 4143-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch, B. M., Bubeck, P., Giehl, K., Kroemker, M., Moschner, J., Rothkegel, M., Rudiger, M., Schluter, K., Stanke, G. and Winkler, J. (1995). The molecular architecture of focal adhesions. Annu. Rev. Cell Dev. Biol. 11, 379-416. [DOI] [PubMed] [Google Scholar]
- Johnson, L. N., Noble, M. E. and Owen, D. J. (1996). Active and inactive protein kinases: structural basis for regulation. Cell 85, 149-158. [DOI] [PubMed] [Google Scholar]
- Jones, S. B., Lu, H. Y. and Lu, Q. (2004). Abl tyrosine kinase promotes dendrogenesis by inducing actin cytoskeletal rearrangements in cooperation with Rho family small GTPases in hippocampal neurons. J. Neurosci. 24, 8510-8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain, K. H. and Klemke, R. L. (2001). Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J. Biol. Chem. 276, 16185-16192. [DOI] [PubMed] [Google Scholar]
- Kharbanda, S., Ren, R., Pandey, P., Shafman, T. D., Feller, S. M., Weichselbaum, R. R. and Kufe, D. W. (1995). Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376, 785-788. [DOI] [PubMed] [Google Scholar]
- Kim, A. S., Kakalis, L. T., Abdul-Manan, N., Liu, G. A. and Rosen, M. K. (2000). Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404, 151-158. [DOI] [PubMed] [Google Scholar]
- Kipreos, E. T. and Wang, J. Y. (1992). Cell cycle-regulated binding of c-Abl tyrosine kinase to DNA. Science 256, 382-385. [DOI] [PubMed] [Google Scholar]
- Kiyokawa, E., Hashimoto, Y., Kobayashi, S., Sugimura, H., Kurata, T. and Matsuda, M. (1998a). Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 12, 3331-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa, E., Hashimoto, Y., Kurata, T., Sugimura, H. and Matsuda, M. (1998b). Evidence that DOCK180 up-regulates signals from the CrkII-p130(Cas) complex. J. Biol. Chem. 273, 24479-24484. [DOI] [PubMed] [Google Scholar]
- Koleske, A. J., Gifford, A. M., Scott, M. L., Nee, M., Bronson, R. T., Miczek, K. A. and Baltimore, D. (1998). Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 21, 1259-1272. [DOI] [PubMed] [Google Scholar]
- Kowalski, J. R., Egile, C., Gil, S., Snapper, S. B., Li, R. and Thomas, S. M. (2005). Cortactin regulates cell migration through activation of N-WASP. J. Cell Sci. 118, 79-87. [DOI] [PubMed] [Google Scholar]
- Krueger, E. W., Orth, J. D., Cao, H. and McNiven, M. A. (2003). A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14, 1085-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh, G. D., Perego, R., Miki, T. and Aaronson, S. A. (1990). The complete coding sequence of arg defines the Abelson subfamily of cytoplasmic tyrosine kinases. Proc. Natl. Acad. Sci. USA 87, 5802-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina, S., Mader, C. C., Machida, K., Mayer, B. J. and Koleske, A. J. (2009). Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J. Cell Biol. 185, 503-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger, D. A. and Horwitz, A. F. (1996). Cell migration: a physically integrated molecular process. Cell 84, 359-369. [DOI] [PubMed] [Google Scholar]
- Lee, H., Engel, U., Rusch, J., Scherrer, S., Sheard, K. and Van Vactor, D. (2004). The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron 42, 913-926. [DOI] [PubMed] [Google Scholar]
- Leng, Y., Zhang, J., Badour, K., Arpaia, E., Freeman, S., Cheung, P., Siu, M. and Siminovitch, K. (2005). Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proc. Natl. Acad. Sci. USA 102, 1098-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J. M., Baskaran, R., Taagepera, S., Schwartz, M. A. and Wang, J. Y. (1996). Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc. Natl. Acad. Sci. USA 93, 15174-15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Li, Y. and Gao, F. B. (2005). Abelson, enabled, and p120 catenin exert distinct effects on dendritic morphogenesis in Drosophila. Dev. Dyn. 234, 512-522. [DOI] [PubMed] [Google Scholar]
- Liebl, E. C., Forsthoefel, D. J., Franco, L. S., Sample, S. H., Hess, J. E., Cowger, J. A., Chandler, M. P., Shupert, A. M. and Seeger, M. A. (2000). Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio's role in axon pathfinding. Neuron 26, 107-118. [DOI] [PubMed] [Google Scholar]
- Liebl, E. C., Rowe, R. G., Forsthoefel, D. J., Stammler, A. L., Schmidt, E. R., Turski, M. and Seeger, M. A. (2003). Interactions between the secreted protein Amalgam, its transmembrane receptor Neurotactin and the Abelson tyrosine kinase affect axon pathfinding. Development 130, 3217-3226. [DOI] [PubMed] [Google Scholar]
- Liu, X., Brodeur, S. R., Gish, G., Songyang, Z., Cantley, L. C., Laudano, A. P. and Pawson, T. (1993). Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene 8, 1119-1126. [PubMed] [Google Scholar]
- Loureiro, J. and Peifer, M. (1998). Roles of Armadillo, a Drosophila catenin, during central nervous system development. Curr. Biol. 8, 622-632. [DOI] [PubMed] [Google Scholar]
- Martinez-Quiles, N., Ho, H. Y., Kirschner, M. W., Ramesh, N. and Geha, R. S. (2004). Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell. Biol. 24, 5269-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough, A., Pope, B., Chiu, W. and Weeds, A. (1997). Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell Biol. 138, 771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter, J. R. and Wang, J. Y. (1993). An actin-binding function contributes to transformation by the Bcr-Abl oncoprotein of Philadelphia chromosome-positive human leukemias. EMBO J. 12, 1533-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, Y. J. and Wang, J. Y. (1996). Binding of A/T-rich DNA by three high mobility group-like domains in c-Abl tyrosine kinase. J. Biol. Chem. 271, 22823-22830. [DOI] [PubMed] [Google Scholar]
- Miller, A. L., Wang, Y., Mooseker, M. S. and Koleske, A. J. (2004). The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J. Cell Biol. 165, 407-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison, T. J. and Cramer, L. P. (1996). Actin-based cell motility and cell locomotion. Cell 84, 371-379. [DOI] [PubMed] [Google Scholar]
- Mizutani, K., Miki, H., He, H., Maruta, H. and Takenawa, T. (2002). Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 62, 669-674. [PubMed] [Google Scholar]
- Moarefi, I., LaFevre-Bernt, M., Sicheri, F., Huse, M., Lee, C. H., Kuriyan, J. and Miller, W. T. (1997). Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature 385, 650-653. [DOI] [PubMed] [Google Scholar]
- Moresco, E. M. and Koleske, A. J. (2003). Regulation of neuronal morphogenesis and synaptic function by Abl family kinases. Curr. Opin. Neurobiol. 13, 535-544. [DOI] [PubMed] [Google Scholar]
- Moresco, E. M., Scheetz, A. J., Bornmann, W. G., Koleske, A. J. and Fitzsimonds, R. M. (2003). Abl family nonreceptor tyrosine kinases modulate short-term synaptic plasticity. J. Neurophysiol. 89, 1678-1687. [DOI] [PubMed] [Google Scholar]
- Moresco, E. M., Donaldson, S., Williamson, A. and Koleske, A. J. (2005). Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J. Neurosci. 25, 6105-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar, B., Hantschel, O., Young, M. A., Scheffzek, K., Veach, D., Bornmann, W., Clarkson, B., Superti-Furga, G. and Kuriyan, J. (2003). Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 112, 859-871. [DOI] [PubMed] [Google Scholar]
- Nelson, W. J. (2008). Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 36, 149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome, T. P., Weisswange, I., Frischknecht, F. and Way, M. (2006). Abl collaborates with Src family kinases to stimulate actin-based motility of vaccinia virus. Cell Microbiol. 8, 233-241. [DOI] [PubMed] [Google Scholar]
- Nolz, J. C., Nacusi, L. P., Segovis, C. M., Medeiros, R. B., Mitchell, J. S., Shimizu, Y. and Billadeau, D. D. (2008). The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. J. Cell Biol. 182, 1231-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N. K., Arthur, W. T. and Burridge, K. (2003). Cadherin engagement inhibits RhoA via p190RhoGAP. J. Biol. Chem. 278, 13615-13618. [DOI] [PubMed] [Google Scholar]
- Noren, N. K., Foos, G., Hauser, C. A. and Pasquale, E. B. (2006). The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat. Cell Biol. 8, 815-825. [DOI] [PubMed] [Google Scholar]
- Peacock, J. G., Miller, A. L., Bradley, W. D., Rodriguez, O. C., Webb, D. J. and Koleske, A. J. (2007). The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin. Mol. Biol. Cell 18, 3860-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz, O., Hodgson, L., Klemke, R. L. and Hahn, K. M. (2006). Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069-1072. [DOI] [PubMed] [Google Scholar]
- Plattner, R., Kadlec, L., DeMali, K. A., Kazlauskas, A. and Pendergast, A. M. (1999). c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 13, 2400-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner, R., Irvin, B. J., Guo, S., Blackburn, K., Kazlauskas, A., Abraham, R. T., York, J. D. and Pendergast, A. M. (2003). A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-gamma1. Nat. Cell Biol. 5, 309-319. [DOI] [PubMed] [Google Scholar]
- Plattner, R., Koleske, A. J., Kazlauskas, A. and Pendergast, A. M. (2004). Bidirectional signaling links the Abelson kinases to the platelet-derived growth factor receptor. Mol. Cell. Biol. 24, 2573-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluk, H., Dorey, K. and Superti-Furga, G. (2002). Autoinhibition of c-Abl. Cell 108, 247-259. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D. and Borisy, G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465. [DOI] [PubMed] [Google Scholar]
- Pollitt, A. Y. and Insall, R. H. (2009). WASP and SCAR/WAVE proteins: the drivers of actin assembly. J. Cell Sci. 122, 2575-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti, A., Machacek, M., Gupton, S. L., Waterman-Storer, C. M. and Danuser, G. (2004). Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782-1786. [DOI] [PubMed] [Google Scholar]
- Poppe, M., Feller, S. M., Römer, G. and Wessler, S. (2007). Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene 26, 3462-3472. [DOI] [PubMed] [Google Scholar]
- Porter, M., Schindler, T., Kuriyan, J. and Miller, W. T. (2000). Reciprocal regulation of Hck activity by phosphorylation of Tyr(527) and Tyr(416). Effect of introducing a high affinity intramolecular SH2 ligand. J. Biol. Chem. 275, 2721-2726. [DOI] [PubMed] [Google Scholar]
- Radha, V., Rajanna, A., Mitra, A., Rangaraj, N. and Swarup, G. (2007). C3G is required for c-Abl-induced filopodia and its overexpression promotes filopodia formation. Exp. Cell Res. 313, 2476-2492. [DOI] [PubMed] [Google Scholar]
- Reichman, C., Singh, K., Liu, Y., Singh, S., Li, H., Fajardo, J. E., Fiser, A. and Birge, R. B. (2005). Transactivation of Abl by the Crk II adapter protein requires a PNAY sequence in the Crk C-terminal SH3 domain. Oncogene 24, 8187-8199. [DOI] [PubMed] [Google Scholar]
- Ren, R., Ye, Z. S. and Baltimore, D. (1994). Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 8, 783-795. [DOI] [PubMed] [Google Scholar]
- Renshaw, M. W., Lewis, J. M. and Schwartz, M. A. (2000). The c-Abl tyrosine kinase contributes to the transient activation of MAP kinase in cells plated on fibronectin. Oncogene 19, 3216-3219. [DOI] [PubMed] [Google Scholar]
- Rhee, J., Buchan, T., Zukerberg, L., Lilien, J. and Balsamo, J. (2007). Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat. Cell Biol. 9, 883-892. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J. and Hall, A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389-399. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T. and Horwitz, A. R. (2003). Cell Migration: Integrating Signals from Front to Back. Science 302, 1704-1709. [DOI] [PubMed] [Google Scholar]
- Rivera, G. M., Briceno, C. A., Takeshima, F., Snapper, S. B. and Mayer, B. J. (2004). Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr. Biol. 14, 11-22. [DOI] [PubMed] [Google Scholar]
- Rodriguez, O. C., Schaefer, A. W., Mandato, C. A., Forscher, P., Bement, W. M. and Waterman-Storer, C. M. (2003). Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5, 599-609. [DOI] [PubMed] [Google Scholar]
- Rohatgi, R., Ma, L., Miki, H., Lopez, M., Kirchhausen, T., Takenawa, T. and Kirschner, M. W. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221-231. [DOI] [PubMed] [Google Scholar]
- Rohatgi, R., Nollau, P., Ho, H. Y., Kirschner, M. W. and Mayer, B. J. (2001). Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J. Biol. Chem. 276, 26448-26452. [DOI] [PubMed] [Google Scholar]
- Roof, R. W., Haskell, M. D., Dukes, B. D., Sherman, N., Kinter, M. and Parsons, S. J. (1998). Phosphotyrosine (p-Tyr)-dependent and -independent mechanisms of p190 RhoGAP-p120 RasGAP interaction: Tyr 1105 of p190, a substrate for c-Src, is the sole p-Tyr mediator of complex formation. Mol. Cell. Biol. 18, 7052-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, M. K., Yamazaki, T., Gish, G. D., Kay, C. M., Pawson, T. and Kay, L. E. (1995). Direct demonstration of an intramolecular SH2-phosphotyrosine interaction in the Crk protein. Nature 374, 477-479. [DOI] [PubMed] [Google Scholar]
- Schindler, T., Bornmann, W., Pellicena, P., Miller, W. T., Clarkson, B. and Kuriyan, J. (2000). Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289, 1938-1942. [DOI] [PubMed] [Google Scholar]
- Schock, F. and Perrimon, N. (2002). Molecular mechanisms of epithelial morphogenesis. Annu. Rev. Cell Dev. Biol. 18, 463-493. [DOI] [PubMed] [Google Scholar]
- Schwartzberg, P. L., Stall, A. M., Hardin, J. D., Bowdish, K. S., Humaran, T., Boast, S., Harbison, M. L., Robertson, E. J. and Goff, S. P. (1991). Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell 65, 1165-1175. [DOI] [PubMed] [Google Scholar]
- Scita, G., Nordstrom, J., Carbone, R., Tenca, P., Giardina, G., Gutkind, S., Bjarnegard, M., Betsholtz, C. and Di Fiore, P. P. (1999). EPS8 and E3B1 transduce signals from Ras to Rac. Nature 401, 290-293. [DOI] [PubMed] [Google Scholar]
- Sfakianos, M. K., Eisman, A., Gourley, S. L., Bradley, W. D., Scheetz, A. J., Settleman, J., Taylor, J. R., Greer, C. A., Williamson, A. and Koleske, A. J. (2007). Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J. Neurosci. 27, 10982-10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield, M., Loveless, T., Hardin, J. and Pettitt, J. (2007). C. elegans Enabled exhibits novel interactions with N-WASP, Abl, and cell-cell junctions. Curr. Biol. 17, 1791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., Alin, K. and Goff, S. P. (1995). Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 9, 2583-2597. [DOI] [PubMed] [Google Scholar]
- Shishido, T., Akagi, T., Chalmers, A., Maeda, M., Terada, T., Georgescu, M. M. and Hanafusa, H. (2001). Crk family adaptor proteins trans-activate c-Abl kinase. Genes Cells 6, 431-440. [DOI] [PubMed] [Google Scholar]
- Silberman, I., Sionov, R. V., Zuckerman, V., Haupt, S., Goldberg, Z., Strasser, A., Ben-Sasson, Z. S., Baniyash, M., Koleske, A. J. and Haupt, Y. (2008). T cell survival and function requires the c-Abl tyrosine kinase. Cell Cycle 7, 3847-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sini, P., Cannas, A., Koleske, A. J., Di Fiore, P. P. and Scita, G. (2004). Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat. Cell Biol. 6, 268-274. [DOI] [PubMed] [Google Scholar]
- Smith, J. M., Katz, S. and Mayer, B. J. (1999). Activation of the Abl tyrosine kinase in vivo by Src homology 3 domains from the Src homology 2/Src homology 3 adaptor Nck. J. Biol. Chem. 274, 27956-27962. [DOI] [PubMed] [Google Scholar]
- Sossey-Alaoui, K., Li, X. and Cowell, J. K. (2007). c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J. Biol. Chem. 282, 26257-26265. [DOI] [PubMed] [Google Scholar]
- Srinivasan, D. and Plattner, R. (2006). Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 66, 5648-5655. [DOI] [PubMed] [Google Scholar]
- Srinivasan, D., Sims, J. T. and Plattner, R. (2008). Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene 27, 1095-1105. [DOI] [PubMed] [Google Scholar]
- Stevens, T. L., Rogers, E. M., Koontz, L. M., Fox, D. T., Homem, C. C., Nowotarski, S. H., Artabazon, N. B. and Peifer, M. (2008). Using Bcr-Abl to examine mechanisms by which abl kinase regulates morphogenesis in Drosophila. Mol. Biol. Cell 19, 378-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovold, C. F., Millard, T. H. and Machesky, L. M. (2005). Inclusion of Scar/WAVE3 in a similar complex to Scar/WAVE1 and 2. BMC Cell Biol. 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, J. R., Gonzalez, F. H., Kawai, H. and Yuan, Z. M. (2006). c-Abl interacts with the WAVE2 signaling complex to induce membrane ruffling and cell spreading. J. Biol. Chem. 281, 31290-31297. [DOI] [PubMed] [Google Scholar]
- Suetsugu, S., Hattori, M., Miki, H., Tezuka, T., Yamamoto, T., Mikoshiba, K. and Takenawa, T. (2002). Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev. Cell 3, 645-658. [DOI] [PubMed] [Google Scholar]
- Sun, G., Ramdas, L., Wang, W., Vinci, J., McMurray, J. and Budde, R. J. (2002). Effect of autophosphorylation on the catalytic and regulatory properties of protein tyrosine kinase Src. Arch. Biochem. Biophys. 397, 11-17. [DOI] [PubMed] [Google Scholar]
- Sun, X., Majumder, P., Shioya, H., Wu, F., Kumar, S., Weichselbaum, R., Kharbanda, S. and Kufe, D. (2000). Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J. Biol. Chem. 275, 17237-17240. [DOI] [PubMed] [Google Scholar]
- Taagepera, S., McDonald, D., Loeb, J. E., Whitaker, L. L., McElroy, A. K., Wang, J. Y. and Hope, T. J. (1998). Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc. Natl. Acad. Sci. USA 95, 7457-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa, T. and Suetsugu, S. (2007). The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37-48. [DOI] [PubMed] [Google Scholar]
- Tammer, I., Brandt, S., Hartig, R., Konig, W. and Backert, S. (2007). Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology 132, 1309-1319. [DOI] [PubMed] [Google Scholar]
- Tani, K., Sato. S., Sukezane, T., Kojima, H., Hirose, H., Hanafusa, H. and Shishido, T. (2003). Abl interactor protein 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase. J. Biol. Chem. 278, 21685-21692. [DOI] [PubMed] [Google Scholar]
- Tanis, K. Q., Veach, D., Duewel, H. S., Bornmann, W. G. and Koleske, A. J. (2003). Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol. Cell. Biol. 23, 3884-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. E., Soriano, P. and Brugge, J. S. (1991). Phosphorylation of c-Src on tyrosine 527 by another protein tyrosine kinase. Science 254, 568-571. [DOI] [PubMed] [Google Scholar]
- Thomas, S. M., Soriano, P. and Imamoto, A. (1995). Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature 376, 267-271. [DOI] [PubMed] [Google Scholar]
- Torres, E. and Rosen, M. K. (2003). Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell 11, 1215-1227. [DOI] [PubMed] [Google Scholar]
- Tybulewicz, V. L., Crawford, C. E., Jackson, P. K., Bronson, R. T. and Mulligan, R. C. (1991). Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153-1163. [DOI] [PubMed] [Google Scholar]
- Van Etten, R. A., Jackson, P. K., Baltimore, D., Sanders, M. C., Matsudaira, P. T. and Janmey, P. A. (1994). The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J. Cell Biol. 124, 325-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares, M., Webb, D. J. and Horwitz, A. R. (2005). Cell migration at a glance. J. Cell Sci. 118, 4917-4919. [DOI] [PubMed] [Google Scholar]
- Wang, J. Y. (2004). Controlling Abl: auto-inhibition and co-inhibition? Nat. Cell Biol. 6, 3-7. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Miller, A. L., Mooseker, M. S. and Koleske, A. J. (2001). The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin-binding domains to bundle F-actin. Proc. Natl. Acad. Sci. USA 98, 14865-14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, A. M., Karginov, A. V., Kinley, A. W., Weed, S. A., Li, Y., Parsons, J. T. and Cooper, J. A. (2001). Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11, 370-374. [DOI] [PubMed] [Google Scholar]
- Weaver, A. M., Heuser, J. E., Karginov, A. V., Lee, W. L., Parsons, J. T. and Cooper, J. A. (2002). Interaction of cortactin and N-WASp with Arp2/3 complex. Curr. Biol. 12, 1270-1278. [DOI] [PubMed] [Google Scholar]
- Welch, P. J. and Wang, J. Y. (1993). A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell 75, 779-790. [DOI] [PubMed] [Google Scholar]
- Wen, S. T. and Van Etten, R. A. (1997). The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity. Genes Dev. 11, 2456-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, S. T., Jackson, P. K. and Van Etten, R. A. (1996). The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 15, 1583-1595. [PMC free article] [PubMed] [Google Scholar]
- Wildenberg, G. A., Dohn, M. R., Carnahan, R. H., Davis, M. A., Lobdell, N. A., Settleman, J. and Reynolds, A. B. (2006). p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127, 1027-1039. [DOI] [PubMed] [Google Scholar]
- Wills, Z., Bateman, J., Korey, C. A., Comer, A. and Van Vactor, D. (1999a). The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron 22, 301-312. [DOI] [PubMed] [Google Scholar]
- Wills, Z., Marr, L., Zinn, K., Goodman, C. S. and Van Vactor, D. (1999b). Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron 22, 291-299. [DOI] [PubMed] [Google Scholar]
- Wills, Z., Emerson, M., Rusch, J., Bikoff, J., Baum, B., Perrimon, N. and Van Vactor, D. (2002). A Drosophila homolog of cyclase-associated proteins collaborates with the Abl tyrosine kinase to control midline axon pathfinding. Neuron 36, 611-622. [DOI] [PubMed] [Google Scholar]
- Wong, S. and Witte, O. N. (2004). The BCR-ABL story: bench to bedside and back. Annu. Rev. Immunol. 22, 247-306. [DOI] [PubMed] [Google Scholar]
- Woodring, P. J., Hunter, T. and Wang, J. Y. (2001). Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J. Biol. Chem. 276, 27104-27110. [DOI] [PubMed] [Google Scholar]
- Woodring, P. J., Litwack, E. D., O'Leary, D. D., Lucero, G. R., Wang, J. Y. and Hunter, T. (2002). Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J. Cell Biol. 156, 879-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodring, P. J., Meisenhelder, J., Johnson, S. A., Zhou, G. L., Field, J., Shah, K., Bladt, F., Pawson, T., Niki, M., Pandolfi, P. P. et al. (2004). c-Abl phosphorylates Dok1 to promote filopodia during cell spreading. J. Cell Biol. 165, 493-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside, D. G., Obergfell, A., Talapatra, A., Calderwood, D. A., Shattil, S. J. and Ginsberg, M. H. (2002). The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin beta cytoplasmic domains. J. Biol. Chem. 277, 39401-39408. [DOI] [PubMed] [Google Scholar]
- Wu, H., Reynolds, A. B., Kanner, S. B., Vines, R. R. and Parsons, J. T. (1991). Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 11, 5113-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X., Cui, P., Hossain, S., Xu, R., Warner, B., Guo, X., An, X., Debnath, A. K., Cowburn, D. and Kotula, L. (2008). Allosteric inhibition of the nonMyristoylated c-Abl tyrosine kinase by phosphopeptides derived from Abi1/Hssh3bp1. Biochim. Biophys. Acta 1783, 737-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, S. and Nelson, W. J. (2007). Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 178, 517-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, H., Cong, F., Birge, R. B., Goff, S. P. and Chao, M. V. (2000). Association of the Abl tyrosine kinase with the Trk nerve growth factor receptor. J. Neurosci. Res. 59, 356-364. [DOI] [PubMed] [Google Scholar]
- Yu, H. H., Zisch, A. H., Dodelet, V. C. and Pasquale, E. B. (2001). Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene 20, 3995-4006. [DOI] [PubMed] [Google Scholar]
- Zandy, N. L. and Pendergast, A. M. (2008). Abl tyrosine kinases modulate cadherin-dependent adhesion upstream and downstream of Rho family GTPases. Cell Cycle 7, 444-448. [DOI] [PubMed] [Google Scholar]
- Zandy, N. L., Playford, M. and Pendergast, A. M. (2007). Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc. Natl. Acad. Sci. USA 104, 17686-17691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel, P. A., Zhang, W., Quiroz, M. and Pendergast, A. M. (2004). Requirement for Abl kinases in T cell receptor signaling. Curr. Biol. 14, 1222-1231. [DOI] [PubMed] [Google Scholar]
- Zipfel, P. A., Bunnell, S. C., Witherow, D. S., Gu, J. J., Chislock, E. M., Ring, C. and Pendergast, A. M. (2006). Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr. Biol. 16, 35-46. [DOI] [PubMed] [Google Scholar]
- Zukerberg, L. R., Patrick, G. N., Nikolic, M., Humbert, S., Wu, C. L., Lanier, L. M., Gertler, F. B., Vidal, M., Van Etten, R. A. and Tsai, L. H. (2000). Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 26, 633-646. [DOI] [PubMed] [Google Scholar]