Abstract
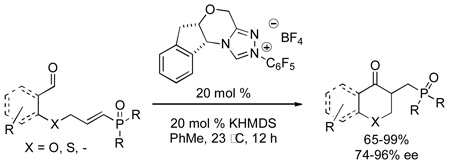
An intramolecular Stetter reaction of vinylphosphine oxides and vinylphosphonates has been developed. Treatment of an aldehyde with a nucleophilic N-heterocyclic carbene catalyst allows for addition of an acyl anion equivalent into a vinylphosphine oxide or vinylphosphonate Michael acceptor in yields up to 99% and ee’s up to 96%.
Organophosphorus compounds are abundant in many facets of chemistry including agrochemistry1 and medicinal chemistry.2 They have also found much utility as ligands in asymmetric catalysis.3 Owing to the multiple ways in which they can be employed, the synthesis of small molecules containing phosphorus has garnered significant attention.4 More importantly, the ability to generate phosphorus compounds with chiral centers at or near phosphorus in an asymmetric fashion is particularly attractive.5
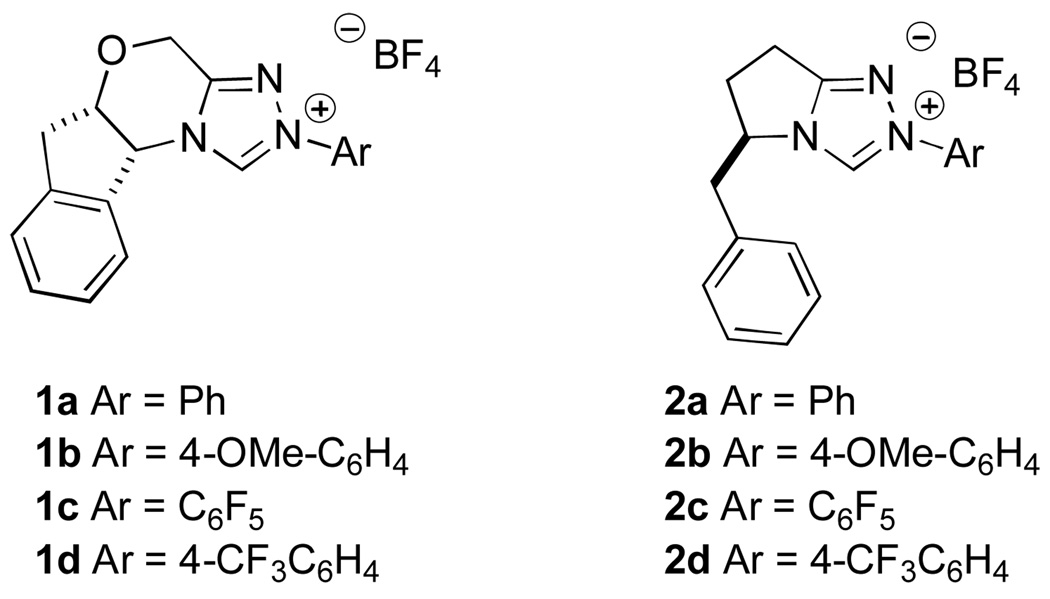
Since the first report of N-heterocyclic carbenes in 1991,6 carbene catalysis has become a burgeoning field. NHCs have been used to propagate Umpolung reactivity of aldehydes to form new carbon-carbon bonds catalytically and enantioselectively.7 Over the past several years, we have been engaged in developing a family of chiral nucleophilic carbenes (Figure 1)8 and applying them to a variety of transformations. These catalysts, in particular 1a–d and 2a–d have proven especially useful in the catalytic asymmetric Stetter reaction providing ketoester products in typically high yield and enantioselectivity.9
Figure 1.
Chiral triazolium salts
Although there are numerous examples that exploit the nucleophilic nature of the acyl anion equivalent,10 the α, β-unsaturated acceptors of the Stetter reaction11 have been largely limited to simple enones and enoates. We envisaged that vinylphosphine oxides and vinylphosphonates would make excellent acceptors for the intramolecular Stetter reaction due to their highly electrophilic nature at the β-position.12
While many nucleophiles add readily to the β-position of vinylphosphines and vinylphosphine oxides, few examples have been shown to do so asymmetrically.13 Recently, the first organocatalytic asymmetric transformation employing highly activated bis-phosphonates as electrophilic acceptors has been achieved by Alexakis and co-workers yielding chiral bis-phosphonates in good yields and enantioselectivities.14 During the preparation of this manuscript, Jørgensen also reported a method complimentary to Alexakis’ work utilizing cinchona alkaloid derived catalysts in an asymmetric Michael-type addition affording high yields and enantioselectivities.15
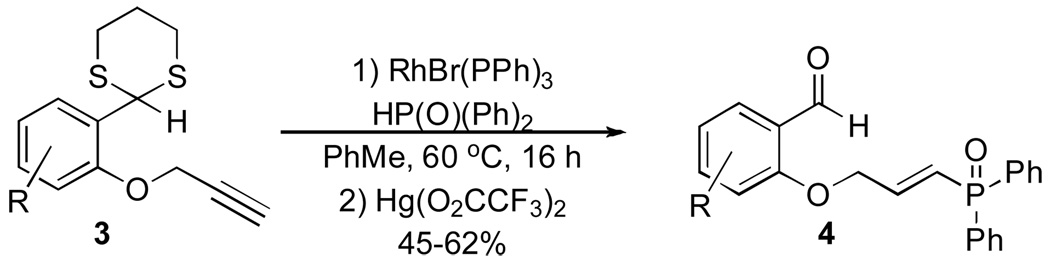
We decided to employ a series of vinylphosphine oxides and vinylphosphonates as electrophilic acceptors in the intramolecular Stetter reaction. The vinylphosphine oxides of type 4 were prepared using a modified procedure described by Tanaka and co-workers (Scheme 1).16 The use of the bromide salt of Wilkinson’s catalyst17 on alkynes 3 provides a smooth transformation to the desired vinylphosphine oxides in good yields. Deprotection of the 1,3-dithiane proved trivial and provided the desired aldehydes 4 in excellent yields.
Scheme 1.
Rhodium Catalyzed Hydrophosphinylation
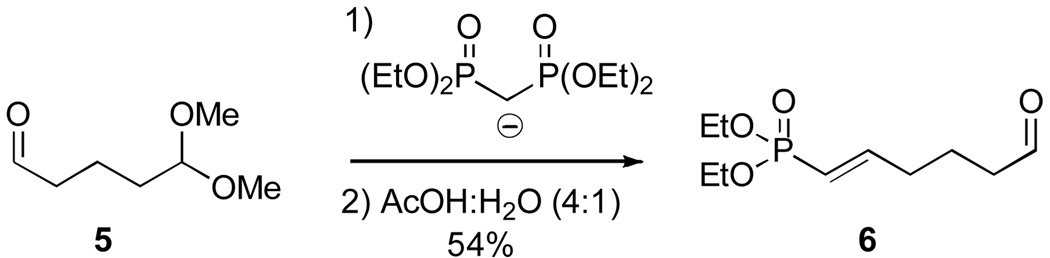
The requisite vinylphosphonates of type 6 were prepared by treatment of the corresponding aldehyde 5 with a preformed bis-phosphonate complex, as demonstrated by Ojea and Ruiz (Scheme 2).18
Scheme 2.
Aliphatic Vinylphosphonates
With a series of vinylphosphine oxides and vinylphosphonates prepared, we were able to test our hypothesis on the viability of these Michael acceptors for the intramolecular Stetter reaction.
Structures with an aromatic backbone employing a vinylphosphine oxide or vinylphosphonate as the electrophilic acceptor afford the Stetter product in typically high yields and enantioselectivities (Table 1). Vinylphosphine oxide 4a provides higher yields than its vinylphosphonate congener 8 while enantioselectivities are increased as well (entries 1 and 9). The presence of halogen substituents on the aromatic ring (entries 2–4) is well tolerated as long as reaction time is limited to 30 minutes. When the reaction time is extended to 12 hours, enantioselectivities drop significantly to 80% and 70% ee respectively, due to epimerization. Electron donating p-methoxy aldehyde 4e shows only a slight increase in selectivity over sigma withdrawing m-methoxy aldehyde 4d and only a slight decrease in chemical yield.19 The presence of sulfur in the tether (4f) leads to higher enantioselectivies, albeit in lower chemical yields than when an oxygen is used in the tether (e.g. 4a). Five membered carbocycle 7g was also obtained in excellent yield and high enantioselectivity.20,21
Table 1.
Scope of Aromatic Aldehyde Substrates
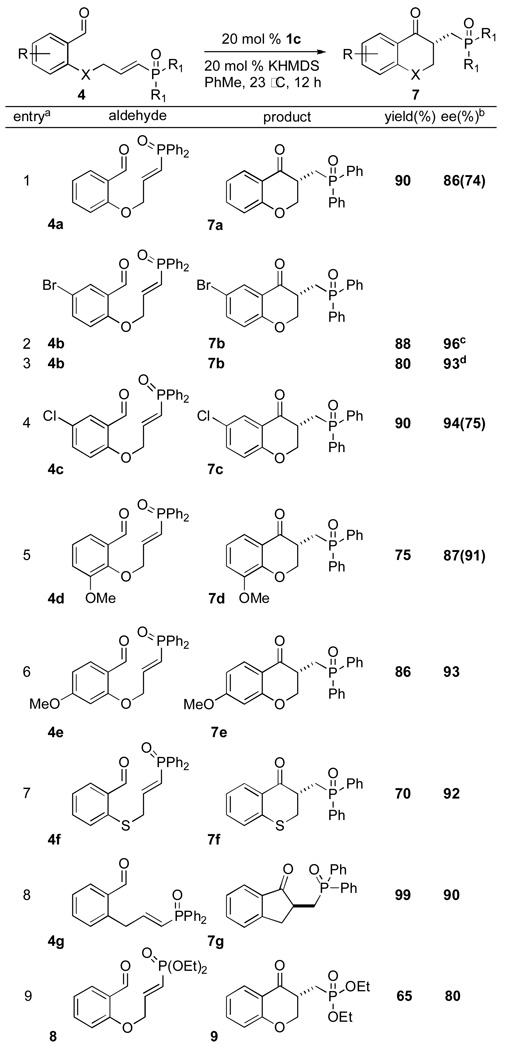 |
All reactions conducted in the presence of 20 mol % catalyst 1c, 20 mol % KHMDS (.25M in PhMe) in PhMe for 12 h at 23 °C.
Determined by HPLC using a chiral stationary phase - ee’s in parantheses obtained with catalyst 2c.
Using catalyst 1a, no reaction was observed.
10 mol % 1c and 10 mol % KHMDS.
Previously, we demonstrated that aliphatic aldehydes are competent substrates to undergo the intramolecular Stetter reaction.9a,f By exchanging the acceptor to a vinylphosphonate or vinylphosphine oxide, the intramolecular Stetter reaction proceeds smoothly to give the desired products in high yields and enantioselectivities (Table 2).
Table 2.
Scope of Aliphatic Aldehyde Substrates
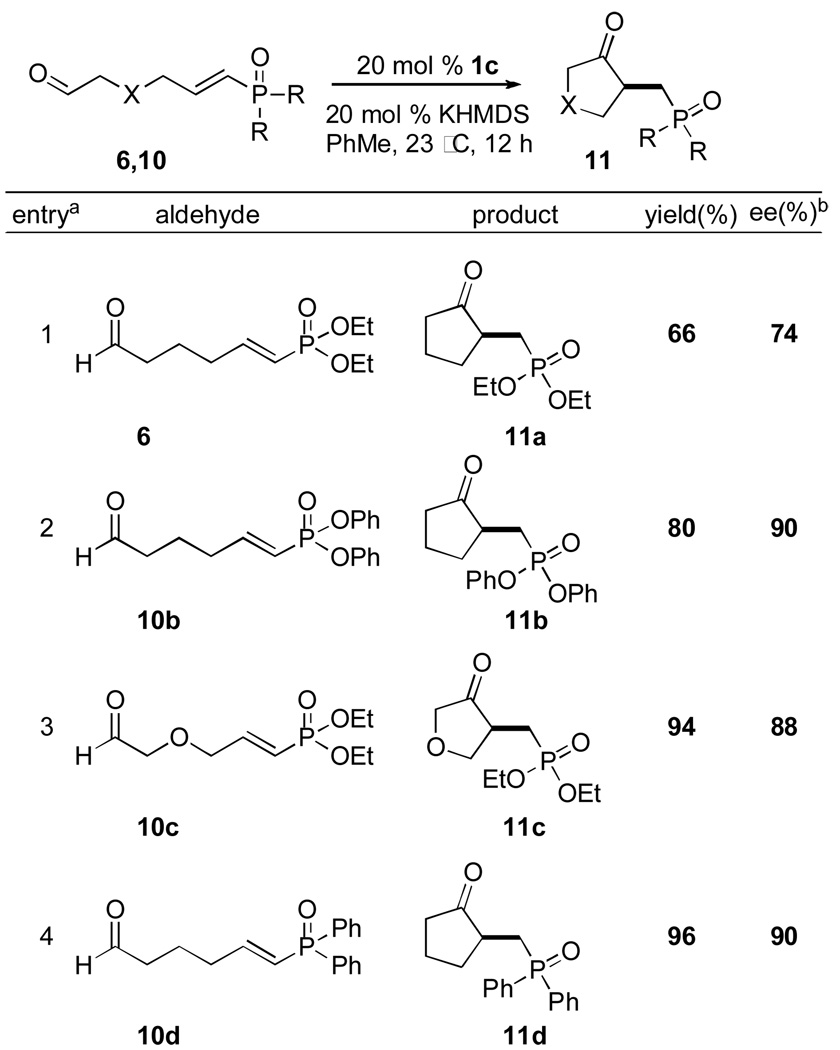 |
See footnotes Table 1
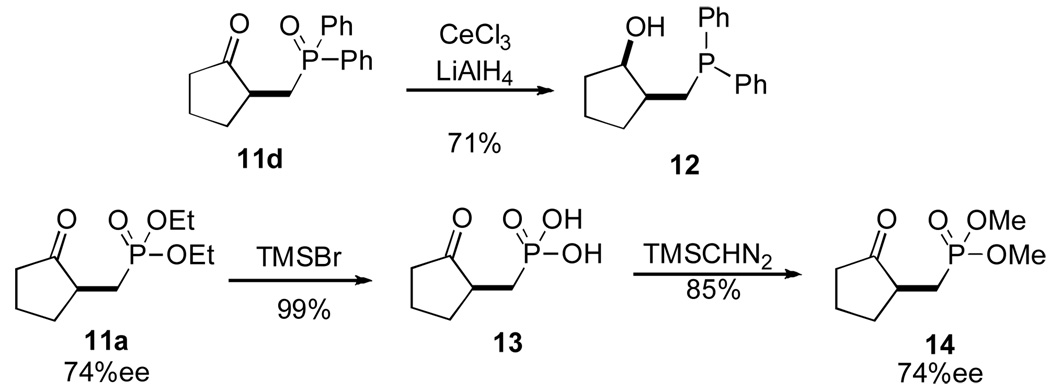
Phosphonate 11a was obtained in modest yield and enantioselectivity. Gratifyingly, when the diethyl vinylphosphonate was switched to the diphenyl phosphonate 10b, the yield and enantioselectivity of the desired product 11b were both improved substantially (entry 2). This increase in ee is most likely due to the increased steric demand of the diaryloxy group, but indicates that tuning of selectivities is possible using this approach.
Introduction of an oxygen in the aliphatic chain 10c gives higher yields and enantioselectivities in the Stetter product 11c compared to its alkyl congener 11a (entry 3 vs entry 1). It is also possible to form a carbocycle bearing a phosphine oxide moiety 11d which proceeds in good yield and enantioselectivity.22
Treatment of 11d under Luche-type conditions affords the global reduction product 12 (Scheme 3) bearing a syn relationship (>19:1), presumably due to the intervention of a Lewis acid chelation during the reduction step.23,24 Hydrolysis of 11a with bromotrimethylsilane in DCM overnight followed by a methanol quench affords phosphonic acid 13 in quantitative yield which was then subjected to esterification with (trimethylsilyl)diazomethane25 to generate methyl ester 14 with no loss of ee.
Scheme 3.
Elaboration of Substrates
In summary, we have developed an intramolecular Stetter reaction employing vinylphosphine oxides and vinylphosphonates as electrophilic acceptors. Both aromatic and aliphatic substrates are tolerated providing keto phosphonates and phosphine oxides in good to excellent yields and enantioselectivities. This extension of the Stetter reaction leads to interesting new enantioenriched scaffolds of phosphorus containing compounds not easily obtainable by other methods.
Supplementary Material
Acknowledgement
We thank NIGMS (GM72586) and the Herman Frasch Foundation for support of this research. We acknowledge Eli Lilly, Johnson & Johnson and Boehringer-Ingelheim for additional support. T. R. thanks the Monfort Family Foundation for a Monfort Professorship. We thank Donald Gauthier (Merck) for a generous gift of aminoindanol. We thank Brian Newell and Professor Matthew P. Shores (CSU) for X-ray analysis.
Footnotes
Supporting Information Available: Experimental procedures, 1H, 13C and 31P spectra and chiral separations. This material is available free of charge via the internet http://pubs.acs.org.
References
- 1.(a) Kukhar VP, Hudson HR. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity. Wiley; 2000. [Google Scholar]; (b) Eto M. Biosci. Biotech. Bioch. 1997;61:1. [Google Scholar]
- 2.(a) Krise JP, Stella VJ. Adv. Drug Deliver. Rev. 1996;19:287. [Google Scholar]; (b) Hwang JT, Choi JR. Drug Future. 2004;29:163–177. [Google Scholar]
- 3.(a) Tang WJ, Zhang XM. Chem. Rev. 2003;103:3029–3069. doi: 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]; (b) Zhou YG. Acc. Chem. Res. 2007;40:1357–1366. doi: 10.1021/ar700094b. [DOI] [PubMed] [Google Scholar]
- 4.(a) Mader MM, Bartlett PA. Chem. Rev. 1997;97:1281. doi: 10.1021/cr960435y. [DOI] [PubMed] [Google Scholar]; (b) Hanessian S, Rogel O. J. Org. Chem. 2000;65:2667. doi: 10.1021/jo991696l. [DOI] [PubMed] [Google Scholar]; (c) Bricklebank N. Organophosphorus Chem. 2003;33:289. [Google Scholar]; (d) McReynolds MD, Dougherty JM, Hanson PR. Chem. Rev. 2004;104:2239. doi: 10.1021/cr020109k. [DOI] [PubMed] [Google Scholar]; (e) Montchamp J-L. J. Organomet. Chem. 2005;690:2388. [Google Scholar]; (f) Baumgartner T, Réau R. Chem. Rev. 2006;106:4681. doi: 10.1021/cr040179m. [DOI] [PubMed] [Google Scholar]
- 5.(a) Denmark SE, Fu J. Chem. Rev. 2003;103:2763. doi: 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE, Fan Y. J. Am. Chem. Soc. 2003;125:7825. doi: 10.1021/ja035410c. [DOI] [PubMed] [Google Scholar]
- 6. Arduengo AJ, III, Harlow RL, Kline M. J. Am. Chem. Soc. 1991;113:361. Also see: Igau A, Grutzmacher H, Baceiredo A, Bertrand G. J. Am. Chem. Soc. 1988;110:6463.
- 7.For recent reviews see: Zeitler K. Angew. Chem. Int. Ed. 2005;44:7506. doi: 10.1002/anie.200502617. Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606. doi: 10.1021/cr068372z. Marion N, Díez-González S, Nolan SP. Angew. Chem. Int. Ed. 2007;46:2988. doi: 10.1002/anie.200603380. Rovis T. Chem. Lett. 2008;37:2.
- 8.Kerr MS, Read de Alaniz J, Rovis T. J. Org. Chem. 2005;70:5725. doi: 10.1021/jo050645n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Kerr MS, Read de Alaniz J, Rovis T. J. Am. Chem. Soc. 2002;124:10298. doi: 10.1021/ja027411v. [DOI] [PubMed] [Google Scholar]; (b) Kerr MS, Rovis T. Synlett. 2003:1934. [Google Scholar]; (c) Kerr MS, Rovis T. J. Am. Chem. Soc. 2004;126:8876. doi: 10.1021/ja047644h. [DOI] [PubMed] [Google Scholar]; (d) Liu Q, Rovis T. J. Am. Chem. Soc. 2006;128:2552. doi: 10.1021/ja058337u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Moore JL, Kerr MS, Rovis T. Tetrahedron. 2006;62:11477. [Google Scholar]; (f) Read de Alaniz J, Kerr MS, Moore JL, Rovis T. J. Org. Chem. 2008;73:2033. doi: 10.1021/jo702313f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Read de Alaniz J, Rovis T. J. Am. Chem. Soc. 2005;127:6284. doi: 10.1021/ja0425132. [DOI] [PubMed] [Google Scholar]
- 10. Takikawa H, Hachisu Y, Bode JW, Suzuki K. Angew. Chem. Int. Ed. 2006;45:3492. doi: 10.1002/anie.200600268. Mattson AE, Bharadwaj AR, Zuhl AM, Scheidt KA. J. Org. Chem. 2006;71:5715. doi: 10.1021/jo060699c. (c) See ref. 7f
- 11.Stetter H, Kuhlmann H. Org. React. 1991;40:407. [Google Scholar]
- 12.For 1,4 additions using: Amines, see: Collins DJ, Rowley LE, Swan JM. Aust. J. Chem. 1974;27:841. Pietrusiewicz KJ, Zablocka M. Tetrahedron Lett. 1988;29:1991. Cavalla D, Warren S. Tetrahedron Lett. 1987;52:2170. Oxygen, see: Johnson CR, Imamoto TJ. J. Org. Chem. 1987;52:2170. Kawashima T, Nakamura M, Inamoto N. Heterocycles. 1997;44:487. Sulfur, see: Pietrusiewicz KM, Wisniewski W, Zablocka M. Tetrahedron Lett. 1989;45:337. Carbanions, see Berlan J, Battioni JP, Koosha K. Tetrahedron Lett. 1976:3351. Pietrusiewicz KM, Zablocka M. Tetrahedron Lett. 1988;29:937.
- 13.(a) Minowa N, Hirayama M, Fukatsu S. Tetrahedron Lett. 1984;25:1147. [Google Scholar]; (b) Ruiz M, Vicente O, Shapiro G, Weber HP. Tetrahedron Lett. 1994;35:4551. [Google Scholar]; (c) Hayashi T, Senda T, Takaya Y, Ogasawara M. J. Am. Chem. Soc. 1999;121:11591. [Google Scholar]; (d) Fernández MC, Diaz A, Guillin JJ, Blanco O, Ruiz M, Vicente OJ. J. Org. Chem. 2006;71:6958. doi: 10.1021/jo061072x. [DOI] [PubMed] [Google Scholar]; (e) Kondoh A, Yorimitsu H, Oshima K. J. Am. Chem. Soc. 2007;129:6996. doi: 10.1021/ja071622o. [DOI] [PubMed] [Google Scholar]; (f) Nishida G, Noguchi K, Hirano M, Tanaka K. Angew. Chem. Int. Ed. 2008;47:3410. doi: 10.1002/anie.200800144. [DOI] [PubMed] [Google Scholar]
- 14.Sulzer-Mossé S, Tissot M, Alexakis A. Org. Lett. 2007;9:3749. doi: 10.1021/ol7015498. [DOI] [PubMed] [Google Scholar]
- 15.Capuzzi M, Perdicchia D, Jørgensen KA. Chem. Eur. J. 2008;14:128. doi: 10.1002/chem.200701317. [DOI] [PubMed] [Google Scholar]
- 16.Han L-B, Zhao C-Q, Tanaka M. J. Org. Chem. 2001;66:5929. doi: 10.1021/jo010337z. [DOI] [PubMed] [Google Scholar]
- 17. Osborn JA, Jardine FH, Young JF, Wilkinson G. J. Chem. Soc. (A) 1966;1711 Also see ref. 16.
- 18.Ruiz M, Fernandez MC, Diaz A, Quintela JM, Ojea V. J. Org. Chem. 2003;63:7634. doi: 10.1021/jo034707q. [DOI] [PubMed] [Google Scholar]
- 19.σ value for p-MeO = −.27, σ value for m-MeO = .12: Carey FA, Sundberg RJ. Advanced Organic Chemistry Part A: Structure and Mechanisms. 5th ed. New York: Springer; 2007. p. 339.
- 20.Substrate 4g was also cyclized on 0.72 mmol scale with 20 mol% 1c to provide 7g in 95% yield and 89% ee.
- 21.Absolute stereochemistry was unequivocally assigned for 7b and 7g by X-ray analysis; the rest were assigned by analogy to these products and those found in reference 9f. See Supporting Information for details.
- 22.Six membered aliphatic carbocycles bearing phosphonate Michael acceptors were not competent substrates in this reaction.
- 23.(a) Constantino MG, Matias LGD, da Silva GVJ, Barbieri E, Gambardella MTD. Quim. Nova. 1998;21:719. [Google Scholar]; (b) Dalpozzo R, Bartoli G, Bosco M, De Nino A, Procopio A, Sambri L, Tagarelli A. Eur. J. Org. Chem. 2001:2971. [Google Scholar]; (c) Kison C, Opatz T. Eur. J. Org. Chem. 2008:2740. [Google Scholar]
- 24.The use of NaBH4 provides the hydroxy phosphine oxide in lower diastereoselectivity (6:1).
- 25.Guy DJ, Jacobsen NE. J. Am. Chem. Soc. 2004;126:4102. doi: 10.1021/ja0494398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.