Abstract
Cognitive-attentional factors may moderate emotion deficits associated with psychopathy (Newman & Lorenz, 2003). This study examined the role of these factors in moderating fear-potentiated startle (FPS) as a function of Fearless Dominance and Impulsive Antisociality-personality dimensions with links to psychopathy. University students performed a task that required them to focus on a (1) threat dimension under low working memory load, (2) threat-irrelevant dimension under low load, or (3) threat-irrelevant dimension under high load. Attentional focus, but not working memory load, moderated the relationship between Fearless Dominance and FPS. Fearless Dominance was negatively correlated with FPS only when attention was directed away from the threat. There were no significant findings for Impulsive Antisociality. Results provide evidence that reduced fear response associated with psychopathy may result from an attentional mechanism.
Keywords: Psychopathy, Antisocial Behavior, Fear-Potentiated Startle, Attentional Processing, Working Memory
Psychopathy is a psychopathological syndrome that reflects a combination of self-serving interpersonal traits, shallow affect, impulsive irresponsible behavior, and an antisocial lifestyle. Historically, investigators have conceptualized psychopathy as a unitary syndrome comprised of diverse diagnostic indicators (e.g., Cleckley, 1976; Hare, 1970). Increasingly, however, investigators propose that the psychobiological correlates of psychopathy will be more readily identified by studying the specific components (i.e., traits or factors) that comprise the syndrome (e.g., Lilienfeld & Andrews, 1996; Patrick, 1994).
According to proponents of the factor approach, investigations that focus on specific psychopathic traits or components can reveal meaningful relationships that would otherwise be obscured when examining the total syndrome. For instance, according to the two-factor model of psychopathy that emerges from factor analysis of Hare's Psychopathy Checklist (PCL-R; 2003), psychopathy may be conceptualized as a combination of callous unemotional traits (Factor 1) and impulsive antisocial traits (Factor 2) with separate psychobiological correlates (Patrick, Bradley, & Lang, 1993; Patrick Cuthbert, & Lang, 1994). When the two-factor model is used to examine the association between the psychopathy factors and trait anxiety, for example, analyses commonly reveal a negative correlation between Factor 1 and anxiety and a positive correlation between Factor 2 and anxiety even though anxiety scores are uncorrelated with PCL-R total scores (Hare, 2003; Patrick, 1994). Another potential advantage of the component approach to psychopathy relates to the possibility of studying particular aspects of the syndrome using individuals from the community who may not satisfy diagnostic criteria for the disorder but may embody particular components of the syndrome.
For these and other reasons, we adopted the component approach in order to examine alternative hypotheses regarding the nature of threat processing differences that have been linked to psychopathy. Specifically, we calculated the Fearless Dominance and Impulsive Antisociality scales (see Benning, Patrick, & Salekin., 2005a) from scores on the brief form of the Multidimensional Personality Questionnaire (MPQ-BF; Patrick, Curtin, & Tellegen, 2002) and examined their association with fear response while manipulating attentional focus and working memory load.
We used these MPQ-BF derived scales instead of a standard measure of psychopathy for several reasons. First, previous research suggests that fear response deficits may be selectively associated with variance from Factor 1 as opposed to Factor 2 of the PCL-R (Patrick, et al, 1993; Levenston et al., 2000, Vanman, Mejia, Dawson, Schell, & Raine, 2003). Consequently, the orthogonal nature of the Fearless Dominance and Impulsive Antisociality scales, along with their unique relationships to Factor 1 and 2 of the PCL-R respectively, was advantageous. Second, to our knowledge, the Fearless Dominance scale is the only self-report measure that has been found to predict decreases in affect modulated startle that have been associated with Factor 1 of the PCL-R (Benning, Patrick, & Iacono, 2005b). Third, these MPQ-BF derived scales are suitable for use with university samples such as the one employed in this study. To the extent that subsequent research with psychopathic individuals yields similar results, it will support this use of the component approach for studying specific psychopathic deficits and provide additional evidence regarding the potential importance of the Fearless Dominance scale for characterizing psychopathy.
The alternative hypotheses addressed in this study pertain to the nature and magnitude of the fear response associated with Fearless Dominance. With regard to the deficient fear response of psychopathic individuals, Lykken (1995) proposed that they are biologically predisposed to experience low fear and that their diminished capacity for fear is sufficient to explain all of the primary symptoms of psychopathy outlined by Cleckley (1976). This proposal has been refined by Patrick and colleagues (Patrick et al., 1993; Patrick et al., 1994; Patrick, 1999) who have distinguished two types of emotion processing deficits—an amygdala-based fear processing deficit that correlates with PCL-R Factor 1 scores and an executive function deficit that undermines fear conditioning in high Factor 2 individuals (see also Patrick, 2007, Patrick & Lang, 1999; Raine, 1997; Raine, Lencz, Bihrle, LaCasse, & Coletti, 2000).
Until recently, there was general consensus that the fear response deficits associated with PCL-R Factor 1 scores of psychopathy were the result of a fundamental emotion deficit and that psychobiological reactions to threat stimuli were essentially independent of attentional and cognitive factors (Levenston, Patrick, Bradley, & Lang, 2000). More recently, however, both of these assumptions have been challenged. With regard to the nature of the deficit, Newman and Lorenz (2003)'s response modulation hypothesis (see also Gorenstein & Newman, 1980; Patterson & Newman, 1993) proposes that the emotion deficits associated with psychopathy are moderated by attentional set. More specifically, they propose that psychopathic individuals are deficient in processing threat and other emotion cues when these are peripheral to their primary focus of attention but that their sensitivity to such cues is equal to that of nonpsychopathic individuals when the cues are the focus of top-down attention. Moreover, there is increasing evidence that the subcortical regions involved in generating emotional responses interact extensively and are moderated by higher order cognitive processing (Simpson, Snyder, Gusnard, & Raichle, 2001a; 2001b; Curtin, Patrick, Lang, Cacioppo & Birbaumer, 2001). In fact, research demonstrates that subcortically-mediated emotion processes may be regulated by higher-order processes such as effortful evaluation and appraisal (Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003), and eliminated when attention is directed to another task (Pessoa et al., 2002). Such findings raise the possibility that disturbances in cognitive functions, such as attention and working memory, may underlie impairments in affective response (Curtin et al., 2001).
Furthermore, contemporary neuroscience has acknowledged the role of individual differences in moderating the interaction between cognitive and affective processing (Bishop, Duncan, & Lawrence, 2004; Simpson et al., 2001a; 2001b). In particular, investigators (e.g. Bishop et al., 2004; Dvorak-Bertsch, Curtin, Rubinstein, & Newman, 2007) have demonstrated that such individual differences may operate at the level of attentional engagement or working memory as opposed to general emotional reactivity, thus, suggesting that individual differences in affective response may relate to direction of attention and/or availability of working memory resources as well as emotional reactivity per se.
In light of these developments, there is a need to examine whether psychopathy-related variables, such as PCL-R Factor 1 and Fearless Dominance that have been linked to reduced fear response, reflect the effects of attentional focus and working memory load on threat processing or more fundamental differences in emotional reactivity. To this end, we used fear-potentiated startle to investigate the effects of individual differences and attentional / working memory manipulations on threat processing. Substantial evidence indicates that fear-potentiated startle is a sensitive and specific index of fear responding (Lang, 1995). For example, the startle response in humans is potentiated when viewing negatively valent photographic images (Vrana, Spence, & Lang, 1988), during negative emotional imagery (Miller, Patrick, & Levenston, 2002), and during the anticipation of electric shock (Grillon, Ameli, Foot, & Davis, 1993, Curtin et al., 2001). Startle potentiation during unpleasant stimuli has been observed to be greater in high vs. low trait fearful individuals (Cook, Davis, Hawk, Spence, & Gautier, 1992). Research also indicates that fear-potentiated startle during processing of threat cues is mediated by the amygdala (Davis et al., 1999), which provides an attractive connection to proposals that link psychopathy and amygdala dysfunction (Blair, 2005).
Furthermore, related research using startle response methodology has demonstrated that the magnitude of fear-potentiated startle to threat cues is moderated independently by focus of attention and working memory load (Dvorak-Bertsch, Curtin, Rubinstein, & Newman, 2007). Specifically, this research has demonstrated that fear response, as indexed by fear-potentiated startle, is significantly reduced when participants’ attentional focus is directed away from the stimulus dimension that connotes threat. Moreover, fear response is still further reduced when working memory demands of the task are increased. Thus, fear-potentiated startle appears well-suited to investigate the contribution of attention and working memory to psychopathy-related individual differences in affective response.
Thus, following Dvorak-Bertsch et al. (2007), we assessed participants’ fear response under three experimental conditions designed to selectively manipulate either attentional focus or working memory load: 1) threat focus/low-load (TF/LL), where participants are required to focus attention directly on threat cues and no substantial load is placed on working memory, 2) alternative focus/low-load (AF/LL), where participants are required to focus attention on threat-irrelevant information but working memory load remains low, and 3) alternative focus/high-load (AF/HL), where participants again are required to focus attention on threat-irrelevant information while a moderate to high load is simultaneously placed on working memory resources (Jonides, Schumacher, Smith & Lauber, 1997). Given this task design, the contrast between the threat focus/low-load and alternative focus/low-load conditions provides a specific manipulation of the effects of attentional focus, while holding working memory load relatively constant (i.e., an Attentional focus contrast). Similarly, the contrast between the alternative focus/low-load and alternative focus/high-load conditions provides a specific manipulation of the effects of working memory load, while holding the focus of attention constant (i.e., a Working memory load contrast).
The primary question evaluated in this study is the extent to which reduced fear response associated with Fearless Dominance are observed consistently across all task conditions or moderated by Attentional focus and/or Working memory load. Support for the thesis that the Fearless Dominance component of psychopathy is associated with a fundamental fear response reduction of the type that has been linked to amygdala dysfunction (Blair 2005) will be offered by a main effect of Fearless Dominance on fear-potentiated startle across all task conditions, including threat focus/low-load. In contrast, if the response modulation hypothesis has merit (Arnett, Howland, Smith, & Newman, 1993; Newman & Kosson, 1986; Newman and Lorenz, 2003), then the association between Fearless Dominance and fear response should be moderated by attentional focus. That is, Fearless Dominance should be negatively associated with fear response only when participants are focused on threat-irrelevant stimuli rather than a more global reduction in fear-potentiated startle.
A secondary question pertains to the putative role of executive function in the link between impulsive-antisocial traits and altered affective response (Patrick, 2007; Patrick & Lang, 1999; Raine, 1997; Raine, Lencz, Bihrle, LaCasse, & Coletti, 2000). A meta-analytic review by Morgan & Lilienfeld (2000) concluded that there is a robust relationship between antisocial behavior and deficits on neuropsychological tests of executive function (see also Ross, Benning, and Adams, 2007). Raine et al (1997, 2000) has suggested that reduced stress reactivity among antisocial individuals may result from prefrontal cortex dysfunction. Patrick et al (1999, 2007) have proposed that altered emotional response among individuals with antisocial traits may result from inadequate processing of emotion relevant cues in complex environments that place large demands on executive or other higher level cognitive functions to guide attention. Based on these proposals, we investigated the extent to which working memory load moderates the association between Impulsive Antisociality and fear-potentiated startle. We reasoned that individuals who are high on Impulsive Antisociality may display a greater reduction in fear responding as demands on working memory resources, an important component of executive function, are increased from the alternative focus/low-load to the alternative focus/high-load conditions (i.e., the Working memory contrast).
Finally, Fearless Dominance and Impulsive Antisociality, like psychopathy itself, may reflect contributions from more specific facets (e.g., stress reaction, social potency, and harm avoidance for Fearless Dominance; aggression, alienation, control, traditionalism, and social closeness for Impulsive Antisociality). Given the potential multidimensional nature of these two personality dimensions, a tertiary goal of this study was to parse any observed effects of these two dimensions into their constituent facets to elaborate their meaning.
Method
Participants
Participants were 55 right-handed undergraduates (20 female) between the ages of 17 and 25. All participants were unselected volunteers and they provided written informed consent according to the procedures set forth by the University of Wisconsin-Madison Human Subjects Committee. Data from thirty-five of the fifty-five participants in the current study were included in a previously published report (Dvorak-Bertsch et al., 2007). However, this previous report did not examine the effects of Fearless Dominance or Impulsive Antisociality on fear response. Four additional participants with outlying potentiated startle response magnitude (i.e., 2.5 standard deviations above the mean in one more conditions) were collected but not included in the final analyses for this experiment. Three other participants were excluded because of equipment or other measurement failure (e.g., EMG sensor detached during session).
Shock sensitivity evaluation
To control for individual differences in shock sensitivity, the intensity of shocks received during the experimental session was calibrated to participants’ individual subjective shock sensitivity. This procedure was conducted immediately prior to the start of the instructed fear conditioning task. Participants were administered a series of electric shocks of increasing intensity to the fingers of their left hand. Participants reported two intensity anchors: the first intensity that they considered uncomfortable and the maximum intensity level that they could tolerate. The series was terminated when they reached their maximum intensity level. The shock intensity administered during the experimental session was calibrated to the mid-point intensity between their discomfort level and their maximum intensity level.
Instructed fear conditioning task
During the instructed fear conditioning task, participants viewed a series of letter cues, each presented for 500ms with a variable inter-trial interval of 3−4s. Letter cues were either upper or lower case and colored red or green. Participants were instructed that in all three conditions, electric shocks would be administered on some trials following letter cues colored in red (CUE+), but that no shocks would follow green letters (CUE-). In fact, 200ms duration electric shocks were administered to adjacent fingers on the participant's left hand at 1750s post-cue onset on 20% of CUE+ trials in each condition, for a total of 30 shocks (10 shocks per condition). Serial position of the cues was balanced across cue types.
Letter cues were grouped into six task blocks of 50 trials. Task instructions for these blocks varied across three conditions: Threat focus/Low-load (TF/LL), Alternative focus/Low-load (AF/LL), and Alternative focus/High-load (AF/HL). For TF/LL, participants were instructed to attend to the color of the letter cue and press one of two buttons using their right hand to indicate letter color. This condition was designed to focus participants on the feature of the letter cue (i.e., color) that connoted threat of shock.
For AF/LL, participants were instructed to attend to the case of the letter cue and press one of the two buttons to indicate if the letter cue was in upper or lower case. Thus, letter color (threat information) was no longer part of the task-relevant feature set needed to perform this simple letter case identification condition. Neither TF/LL nor AF/LL conditions required maintenance or manipulation of information in working memory across trials and therefore both were considered to place an insubstantial load on working memory resources.
For AF/HL, participants were instructed to attend to the letter identity (i.e., f vs. c vs. r, etc) of each letter cue in the series and press one of the two buttons to indicate if the identity of the current letter matched the identity of the letter presented 2 trials back in the series. As in the AF/LL condition, letter color (threat information) was not part of the task-relevant feature set necessary to perform this “2-back” task (Jonides et al., 1997). Moreover, other research with this 2-back task has confirmed that it places substantial demand on working memory resources, requiring both maintenance and manipulation of information across trials (i.e., deletion, insertion, re-ordering and then maintenance of letter set on each trial). Moreover, increased activation of the neurobiological substrates of working memory in this 2-back task relative to simpler identification tasks such as used in the TF/LL and AF/LL conditions has been confirmed (Jonides et al., 1997).
As noted earlier, participants were instructed that electric shocks would be administered in all three conditions on some trials following letter cues colored in red, but that no shocks would follow green letters. Participants performed two consecutive blocks of each of these three tasks and task order was fully counterbalanced across participants. To further enhance the task-relevant feature set manipulation and to increase task motivation, participants were informed that speed and accuracy would influence the amount of shocks they received in the TF/LL condition and the likelihood of receiving a reward (i.e. one of three prizes) based on task performance in the AF/LL and AF/HL conditions1. However, the number of shocks participants actually received was not influenced by their behavioral performance.
As described earlier, these three task conditions were designed to provide independent manipulations of attentional focus and working memory load. Specifically, the contrast of the TF/LL vs. the AF/LL conditions was developed to manipulate the focus of attention across threat-relevant vs. threat-irrelevant features of the letter stimuli, while holding the working memory demands constant (and low). Similarly, the contrast of the AF/LL vs. the AF/HL conditions was developed to manipulate the load placed on working memory resources (low vs. high, respectively), while holding the focus of attention constant (on threat irrelevant features of the letter stimuli). These two contrasts were the focus of our analytic strategy when examining conditions effects, as described in more detail below.
Startle response elicitation and measurement
Forty-eight startle-eliciting noise probes (50ms, 102dB white noise burst with near instantaneous rise time) were presented 1750ms post cue onset. The noise probes were equally distributed across CUE+/CUE− trials in all three task conditions so that each participant experienced 16 noise probes (8 CUE+ and 8 CUE−) per condition. Serial position of the noise probes was balanced across cue type. Moreover, time between probes was roughly balanced across cue type (CUE−: range of 15.1 − 73.6s; CUE+: 16.6 − 68.7s). Probes never occurred on the same trial as shock administration. Startle eyeblink electromyographic activity was sampled at 2000Hz with a bandpass filter (30−500Hz; 24dB/octave roll-off) from electrodes placed on the orbicularis oculi muscle under the right eye. Offline processing included epoching (−50ms to 250ms surrounding noise probe), smoothing (signal rectification followed by 30Hz lowpass filter, 24dB/octave), and baseline correction. Startle blink magnitude was scored as the peak response between 20−120ms post-probe onset. Fear response to threat cues was indexed by fear-potentiated startle, calculated as the difference in blink-response magnitude to probes following CUE+ versus CUE− letters in each of the three task conditions.
Self-Report Measures
Following the completion of the experimental task, participants completed a 155-item version of the Multidimensional Personality Questionnaire (MPQ-BF; Patrick, Curtin & Tellegen, 2002). The MPQ comprises 11 primary trait scales that tap three independent higher order dimensions of personality: Positive Emotionality, Negative Emotionality, and Constraint. Following methods developed and described in detail by Benning et al. (2003), the Fearless Dominance and Impulsive Antisociality dimensions of psychopathy were calculated as linear combinations of specific standardized (i.e, z-scored) MPQ-BF primary trait scales. Specifically, Fearless Dominance was calculated as (0.34 * zSocial Potency) + (−0.42 * zStress Reaction) + (−0.21 * zHarm Avoidance). Impulsive Antisociality is calculated as (0.16 * zAggression) + (0.31 * zAlienation) + (−0.13 * zTraditionalism) + (−0.29 * zControl) + (−0.15 * zSocial Closeness). Both Fearless Dominance and Impulsive Antisociality were mean centered and standardized (z-scored) to aid interpretation. Prior research has demonstrated that in prisoners, Fearless Dominance was related selectively to the interpersonal-affective Factor 1 of the PCL-R (particularly its interpersonal features), whereas Impulsive Antisociality was related preferentially to the antisocial lifestyle Factor 2 of the PCL-R (Benning et al., 2005a). Furthermore, research suggests that these constructs provide a valid basis for evaluating the psychophysiological correlates of psychopathy in a nonincarcerated, community participant sample (Benning et al., 2005b).
Results
Data analytic strategy
Task performance (response time and error rate) and fear-potentiated startle measures were analyzed separately in General Linear Models (GLMs) with Condition (TF/LL vs. AF/LL vs. AF/HL) as a categorical repeated measures factor and the personality dimensions Fearless Dominance and Impulsive Antisociality as quantitative individual difference factors. In addition, interactions between Condition and each personality dimension were also included. Condition effects were parsed into two planned contrasts, an Attentional focus contrast (TF/LL – AF/LL) and a Working memory load contrast (AF/LL – AF/HL), as described earlier. Task order was included as a categorical covariate to increase power for analyses of fear potentiated startle because it was a significant factor for this dependent measure. Task order was unrelated to task performance measures and therefore was not included in those analysis models. Both raw score GLM coefficients (B) and partial eta-squared (pη2) indices are reported where appropriate to quantify the magnitude and direction of significant effects. All contrasts were coded such that raw-score GLM coefficients (B's) could be interpreted as the different in dependent measure means between for relevant conditions.
Task performance analyses
Response time and error rates were analyzed within a GLM with Fearless Dominance, Impulsive Antisociality and Condition as factors as described above. Means and standard deviations for response time and error rate are reported in Table 1. Both planned Condition contrasts were significant for task response time. Specifically, the Attentional focus contrast was significant, B= 72.1(SE=7.3), pη2= 0.65, F(1,52)= 97.68, p< .001, indicating that participants were approximately 72 ms slower to respond in the AF/LL condition relative to the TF/LL condition. Similarly, the Working memory load contrast was significant, B= 255.6(SE=22.6), pη2= 0.71, F(1,52)= 127.57, p< .001, indicating that participants were approximately 256 ms slower to respond in the AF/HL condition relative to the AF/LL condition. The overall effects of Fearless Dominance (p=.533) and Impulsive Antisociality (p=.696) were not significant. Moreover, neither of the Condition contrasts (attentional focus and working memory load) significantly moderated (i.e., interacted with) either Fearless Dominance (p's= .595 & .887 for interactions with attentional focus and working memory load contrasts, respectively) or Impulsive Antisociality effects (p's= .310 & .391, respectively).
Table 1.
Mean (and Standard Deviation) for Task Performance Measures by Condition
| Condition | Response Time (ms) | Error Rate (%) |
|---|---|---|
| Threat focus/Low-load | 529.8 (79.9) | 2.9% (2.1) |
| Alternative focus/Low-load | 601.9 (84.6) | 5.4% (3.3) |
| Alternative focus/High-load | 857.5 (195.5) | 17.8% (12.4) |
Note: Mean (SD). N=55
Both planned Condition contrasts were also significant for task error rate. Specifically, the Attentional focus contrast was significant, B= .025 (SE= .004), pη2= 0.43, F(1,52)= 39.27, p< .001, indicating that participants committed 2.5% more errors in the AF/LL condition relative to the TF/LL condition. Similarly, the Working memory load contrast was significant, B= .124(SE= .017), pη2= 0.51, F(1,52)= 55.03, p< .001, indicating that participants committed 12.4% more errors in the AF/HL condition relative to the AF/LL condition. The overall effects of Fearless Dominance (p= .365) and Impulsive Antisociality (p= .599) were not significant. Moreover, neither of the Condition contrasts moderated either Fearless Dominance (p's= .830 & .460, respectively) or Impulsive Antisociality effects (p's= .966 & .962, respectively).
These analyses provide two important manipulation checks. First, they demonstrate that both the attentional focus and working memory load manipulations were associated with greater cognitive demand as expected. More importantly, participants across the range of Fearless Dominance and Impulsive Antisociality scores performed comparably on the tasks across the three conditions.
Fearless Dominance, Impulsive Antisociality, and Fear-potentiated Startle
Fear potentiated startle was analyzed within a GLM with Fearless Dominance, Impulsive Antisociality, Condition & Task order as factors as described above2. Neither the main effect of Fearless Dominance, B= −2.7(SE= 2.1), pη2= 0.03, F(1,47) = 1.63, p= .208, nor Impulsive Antisociality, B= 0.0(SE=2.3), pη2= 0.00, F(1,47) = 0.00, p= .993, was significant, indicating that the overall magnitude of fear potentiated startle, collapsed across conditions, did not reliably vary by either of these two personality dimensions. Furthermore, a supplemental analysis of Fearless Dominance and Impulsive Antisociality effects limited to the condition that placed the fewest demands on attention and working memory resources (i.e., TF/LL) also failed to reveal any evidence of overall effects of either personality dimension on fear response (p= .955 & .945, respectively).
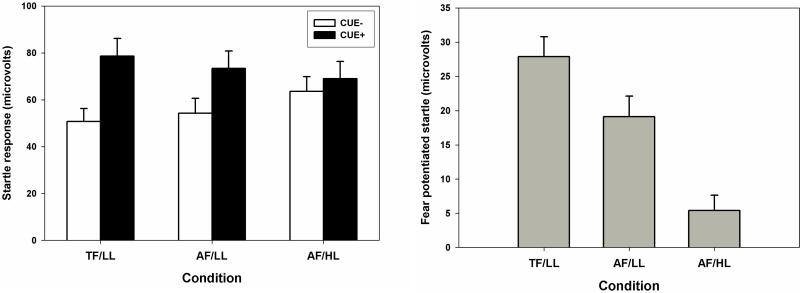
A main effect of Condition was observed for fear potentiated startle (see Figure 1, right panel), pη2= 0.37, F(2,94) = 28.07, p < .01. Within the Condition effect, the Attentional focus contrast was significant, B= 9.0(SE=3.0), pη2= 0.16, F(1,47)= 9.21, p = .004, indicating that fear-potentiated startle was reduced 9 microvolts by the task manipulation that shifted attention away from threat cues (i.e., in the AF/LL relative to the TF/LL condition). In addition, the Working memory load contrast was also significant B= 13.5(SE=3.2), pη2= 0.27, F(1,47) = 17.74, p < .001, indicating that fear potentiated startle was reduced another 13.5 microvolts by the task manipulation that increased working memory load in the AF/HL relative to AF/LL condition. Despite these reductions, fear-potentiated startle remained significantly elevated above 0 in all three conditions (TF/LL, p < .001; AF/LL p < .001; AF/HL p = .024).
Figure 1.
The left panel of the figure displays startle response magnitude separately for CUE− and CUE+ stimuli in the threat focus/low-load (TF/LL), alternative focus/low-load (AF/LL), and alternative focus/high-load (AF/HL) conditions.
The right part of the figure displays fear-potentiated startle in these same three conditions. Fear-poteniated startle is calculated as the difference between startle responses for CUE+ vs. CUE−.
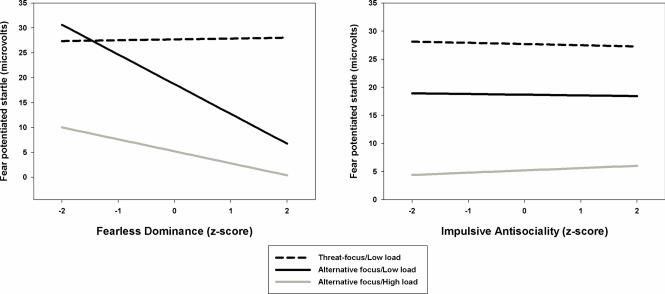
To evaluate predictions regarding the moderating effects of our attentional focus and working memory manipulations, we tested for interactions between the individual difference measures and the Attentional focus and Working memory contrasts. Attentional focus significantly moderated the effect of Fearless Dominance on fear potentiated startle, B= −6.1(SE=3.0), pη2= 0.08, F(1,47)= 4.13, p= .048. This interaction can be understood in two ways. First, it indicates that for every one standard deviation in increase in Fearless Dominance, the magnitude of the reduction in fear potentiated startle from the TF/LL to the AF/LL condition increased by 6.1 microvolts. Alternatively, it indicates that the strength of the relationship between Fearless Dominance and fear potentiated startle differs significantly (i.e., the negative relationship between Fearless Dominance and fear potentiated startle increased) across the TF/LL and AF/LL conditions (see Figure 2, left panel, slopes of dotted black vs. solid black lines). Supplemental simple effect analyses in which fear potentiated startle was regressed on Fearless Dominance scores separately in TF/LL and AF/LL conditions further explicate this interaction. No significant relationship was observed between Fearless Dominance and fear-potentiated startle in the TF/LL condition (p= .907). However, Fearless Dominance scores were negatively and significantly related to fear-potentiated startle in the AF/LL condition (B= −6.6[SE=3.0]; p= .034), indicating that as Fearless Dominance scores increased, participants displayed less fear-potentiated startle when focused on task-relevant but threat-irrelevant features (i.e. upper/lower case) of the letter cues. Although not contributing to this interaction contrast, no significant relationship was observed between Fearless Dominance and fear potentiated startle in the AF/HL condition (p= .272)
Figure 2.
The left panel of the figure displays fear potentiated startle across the range of scores for Fearless Dominance in the threat focus/low-load (TF/LL), alternative focus/low-load (AF/LL), and alternative focus/high-load (AF/HL) conditions. Analyses indicated that the magnitude of the relationship between Fearless Dominance and fear potentiated startle was significantly stronger in the AF/LL vs. TF/LL conditions.
The right panel of the figure displays fear potentiated startle across the range of scores for Impulsive Antisociality in the threat focus/low-load (TF/LL), alternative focus/low-load (AF/LL), and alternative focus/high-load (AF/HL) conditions. Analyses indicated no main effect or interactions involving Impulsive Antisociality.
In contrast to this significant interaction between attentional focus and Fearless Dominance, attentional focus did not moderate the effect of Impulsive Antisociality on fear potentiated startle, B= 0.1(SE=3.2), pη2= 0.00, F(1,47) = 0.00, p = .977 (see Figure 2, right panel). Moreover, the manipulation of working memory load did not moderate either the Fearless Dominance or Impulsive Antisociality (B's= 3.6[SE=3.3] & 0.5[SE=3.5], p's = .279 and .879, respectively).
As described in the method, fear potentiated startle is calculated as a difference score for startle response between threat (CUE+) vs. neutral (CUE−) stimuli (see also Figure 1). Supplemental analyses were conducted on startle response during CUE− only trials to confirm that the significant Fearless Dominance X Attentional focus interaction was not primarily the result of changes during the CUE− stimuli. Specifically, startle response during CUE− trials was analyzed in a GLM with Fearless Dominance, Impulsive Antisociality, Task order, and Condition. Critically, there was no evidence of a Fearless Dominance X Attentional focus interaction for startle response during CUE− trials, B= 0.0(SE=2.7), pη2= 0.00, F(1,47) = 0.00, p= .992. In fact, no main effects or interactions involving Fearless Dominance or Impulsive Antisociality were observed for startle response during CUE− trials (all p's > .616). A significant Working memory load contrast was observed, B= 10.4(SE=3.3), pη2= 0.18, F(1,47)= 10.13, p= .003, with startle response 10.4 microvolts greater during the AF/HL than AF/LL condition across all participants (see Figure 1, left panel). The Attentional focus contrast was not significant, indicating that startle response during CUE− trials was comparable across TF/LL and AF/LL conditions.
Examining Facets of Fearless Dominance
As observed above, the association between individual differences in Fearless Dominance and fear potentiated startle was moderated by attentional focus. Fearless Dominance is a composite of three primary trait scales from the MPQ-BF, Social potency, Stress reaction, and Harm avoidance. As such, each of these facets of Fearless Dominance are highly correlated with the Fearless Dominance total score (r's = 0.63, −0.71, & −0.40, respectively, p's < .01). In addition, all three facets were relatively independent of each other (p's > .41). To parse Fearless Dominance into the unique effects of each of its three facets, a GLM analysis was conducted with Condition (as described earlier) and the three facets of Fearless Dominance (Social potency, Stress reaction, and Harm avoidance) as quantitative individual difference factors. As before, interactions between Condition contrasts and each of these facets were also included and task order was included as a categorical covariate.
Paralleling the previously reported interaction effect involving Fearless Dominance, Social Potency and attentional focus significantly interacted to predict fear potentiated startle, B= −7.6(SE=3.0), pη2= 0.12, F(1,46)= 6.40, p= .015. As with Fearless Dominance, this indicates that the magnitude of the reduction in fear potentiated startle from TF/LL to AF/LL increased by 7.6 microvolts as for every one standard deviation in Social Potency and/or that the relationship between Social Potency and fear potentiated startled was significantly stronger in the AF/LL vs. the TF/LL condition. Neither Stress Reaction nor Harm Avoidance interacted with the Attentional focus contrast (p's = .268 & .234, respectively). As with overall Fearless Dominance in earlier analyses, none of the effects of its three facets were moderated by the Working memory contrast either (p's = .215, .312, .229, respectively).
In contrast to the absence of an overall effect for Fearless Dominance, a significant overall effect (across conditions) of Stress reaction was observed, B= 4.5(SE=2.1), pη2= 0.09, F(1,46)= 4.51, p= .039, indicating that for every one standard deviation increase in Stress Reaction, participants displayed a 4.5 micrvolt increase in fear potentiated startle on average across conditions. The overall effects of Social Potency and Harm Avoidance were not significant (p's = .858 & .725, respectively).
Discussion
The primary goal of this study was to investigate alternative hypotheses regarding the association between Fearless Dominance and threat processing. Specifically, the purpose of this study was to determine if diminished fear response associated with Fearless Dominance was selectively observed only in certain conditions associated with our attentional and working memory load manipulations on threat processing or a more generally across all conditions (e.g., a cross-situational reduction in emotional reactivity). To this end, we found that Fearless Dominance was not associated with reduced fear-potentiated startle in Condition TF/LL or with an overall reduction in fear-potentiated startle across all task conditions. Consequently, our findings are not consistent with conceptualizations that relate this component of psychopathy to a pan-situational fear deficit. Rather, we found that attentional engagement moderated the effect of Fearless Dominance on threat processing. Specifically, the magnitude of the relationship between Fearless Dominance and fear potentiated startle was significantly different across TF/LL vs. AF/LL conditions. Further analysis confirmed that this interaction was due to a selective negative relationship between Fearless Dominance and fear potentiated startle selectively in the AF/LL condition. Thus, it appears that Fearless Dominance is associated with reduced allocation of attentional resources to threat-relevant stimuli specifically when circumstances require processing of threat-irrelevant information.
Despite previous research suggesting that psychopathy, or more specifically Factor 1 of PCL-R is associated with a general deficit in threat processing, our findings for Fearless Dominance, a probable component of psychopathy, raise the possibility that the reduced fear response displayed by psychopathic individuals relates to allocation of attention rather than a more fundamental fear response deficit. Based on previous evidence that psychopathy predicts deficient passive avoidance and electrodermal reactivity when attention is directed away from a punishment contingency but not when attention is directed toward a punishment contingency (e.g., Arnett et al., 1993; Newman & Kosson, 1986), Newman and Lorenz (2003; see also Patterson & Newman, 1993) proposed that such affective changes reflect abnormal attention (i.e., deficient response modulation) rather than an intrinsic fear deficit. Our findings are consistent with this proposal.
The TF/LL and AF/LL conditions clearly differed on the attentional focus that was required of participants, threat (i.e., stimulus color) focused vs. threat-irrelevant (e.g., letter case), respectively. However, it is possible that working memory load may have inadvertently varied to some small degree between these conditions given the nature of the two tasks. Despite this, the full pattern of Fearless Dominance results across all three task conditions suggests that attentional focus and not working memory load is the critical moderator of the Fearless Dominance-fear potentiated startle response relationship. Specifically, working memory load was robustly manipulated across AF/LL and AF/HL conditions and this manipulation contrast did not significantly moderate the Fearless Dominance- fear potentiated statle relationship. Moreover, the direction of the working memory load moderation was not even in the correct direction. Directing the focus of attention away from the threat cues produced a significant negative relationship between Fearless Dominance and fear potentiated startle. Increasing working memory load reduced rather than strengthened this inverse relationship. Thus, it is unlikely that an inadvertent weaker change in load across TF/LL and AF/LL conditions could account for the attentional focus moderation effect.
A second goal of this study was to examine relations between impulsive-antisocial traits and affective response (Patrick et al 1999, 2007; Raine, 1997; Raine, Lencz, Bihrle, LaCasse, & Coletti, 2000). Based on a previous proposal that this association results from limitations of cognitive processing resources (Patrick 2007; Patrick & Lang, 1999), we investigated the extent to which working memory load moderates the association between Impulsive Antisociality and fear-potentiated startle. Contrary to expectation, working memory load did not moderate the relation of Impulsive Antisociality and fear potentiated startle. Of course, definitive conclusions cannot be reached from this data set alone. Our manipulation of working memory load was coarse (only low load vs. high load) and the high load condition substantially reduced fear response, raising some concern about floor effects in this condition (although it should be noted that significant fear potentiated startle was observed in all conditions including alternative set/high load). Future research should employ finer gradations in the manipulation of working memory load. Moreover, executive function is a complex construct involving numerous component processes (Cowan, 1999). Thus, it is possible that components of executive function that were not manipulated in our study may explain the association between Impulsive Antisociality and affective response. Finally, it is likely that the variance of scores on Impulsive Antisociality was somewhat restricted in our unselected undergraduate sample. This may have reduced power to detect a relationship between Impulsive Antisociality and affective response. Future research should examine this relationship in samples that include increased representation of more serious antisocial tendencies. Similar concerns about restriction of range apply to all other individual different dimensions (e.g., harm avoidance) in this unselected sample.
The third and final goal of this study was to parse any observed effects of Fearless Dominance and/or Impulsive Antisociality into its constituent facets. Fearless Dominance is composed of three constituent facets, Social potency, Harm avoidance, and Stress reaction. Analysis of these facets of Fearless Dominance revealed that the observed interaction between Fearless Dominance and attentional focus was carried primarily by Social Potency, such that increases in Social Potency were associated with reduced fear potentiated startle when attention was directed towards threat-irrelevant aspects of task stimuli without meaningfully taxing working memory. At first glance, this finding may seem surprising because the threat processing deficits associated with psychopathy are typically attributed to fearlessness (e.g., harm avoidance) or low anxiety (e.g., stress reactivity). Social Potency, by contrast, loads on the positive emotionality factor of the MPQ and is associated with being forceful and decisive, liking to influence others and leadership roles, and wanting to be the center of attention (Patrick et al., 2002; Tellegen, 1982). Although these attributes could reflect fearlessness or a lack of social anxiety, they may also reflect a lack of self-consciousness or uncertainty stemming from an ability to focus attention on one's immediate goals without much concern for how others perceive or respond to one's assumption of a leadership role. Although we can not say whether the association between Social Potency and threat processing during alternative focus is more accurately understood as reflecting attentional as opposed to core affective processes, the present data provide a justification for more specific investigation of the various possibilities.
Benning et al. (2005a) derived the Fearless Dominance and Impulsive Antisociality scales from the MPQ-BF to predict the factors of the Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996). In addition, they demonstrated that these MPQ-BF derived scales predict actual PCL-R factor scores, even when the two instruments are administered several years apart. Furthermore, preliminary research indicates that these scales provide a valid basis for evaluating the psychophysiological correlates of psychopathy in a nonincarcerated, community participant sample (Benning et al., 2005a; 2005b). While this line of research has provided valuable information regarding the assessment of psychopathic traits within a community setting, as well as the opportunity to explore potential physiological correlates of psychopathic traits, our reliance on these self-report measures requires cautious interpretation. Diminished fear response under attentional load in our study was uniquely associated with one putative dimension of psychopathy, Fearless Dominance. Although many psychopathy researchers believe that characterizing specific dimensions of psychopathy will provide insight into the construct of psychopathy as a whole, this strategy is potentially problematic because mechanisms that are uniquely associated with one factor may change when the factors are combined and may not relate to the construct of psychopathy as a whole. Thus, additional research is needed to determine whether the findings observed in this study generalize to the psychopathy construct as measured by the PCL-R (Hare, 2003) or a similarly well validated measure of the psychopathy construct.
In summary, this experiment has advanced understanding of cognitive-emotional interactions affecting fear responses in individuals varying on putative dimensions of psychopathy in several important ways. Research from our and other laboratories confirm that affective responding is not completely automatic and requires both attentional and working memory resources (e.g., Bertsch et al., 2007; Pessoa et al., 2002). The current findings demonstrate that individual differences in a putative affective-interpersonal component of psychopathy (Fearless Dominance) are not associated primarily with a global deficit in threat processing, as Fearless Dominance was associated with reduced fear response only when attention was directed away from, rather than towards, threat-relevant stimuli. In addition, when Fearless Dominance is further parsed, our data suggest that individual differences in Social Potency are primarily responsible for the reduced fear response associated with attentional shift to task stimuli that are threat irrelevant. Overall, these results provide further support for the hypothesis that psychopathy-related reductions in affective response may reflect the attentional demands of a situation rather than a global deficiency in threat processing (e.g., Newman & Lorenz, 2003).
Acknowledgments
Research described in this manuscript was supported by a grant from the National Institute of Mental Health (MH53041). We greatly appreciate Alex Shackman's insightful contributions to the preparation of this manuscript. We also thank Josh Zeier and Samantha Glass for their feedback and suggestions regarding the manuscript.
Footnotes
Performance contingencies were included in the experiment to motivate participants to comply with task instructions to manipulate attention focus and load robustly. However, the nature of attentional focus manipulation precluded the use of the same contingency across threat focus and alternative focus conditions. If monetary rewards were provided in the threat focused condition, it would have undermined participants focus on threat-related processing. Alternatively, if task performance was motivated by reducing the probability of electric shock in the alternative focus conditions, it would have placed threat into the focus of attention. Thus, the different contingencies were a critical part of the attentional focus manipulation itself. With respect to theory on BIS/BAS, Gray (e.g., 1987) and others (e.g., Fowles, 1980) are very clear that both active avoidance (i.e., actively responding to avoid aversive stimulation as in our TF/LL condition) and reward related responding (e.g., actively responding to earn monetary reward) activate the Behavioral Activation System. Thus, strictly speaking, our threat focused and alternative focused conditions are well-matched (i.e., theoretically equivalent) with respect to BAS activation. Nevertheless, we acknowledge that these conditions differ with regard to opportunities to earn reward and it is possible that individual differences in sensitivity to reward may have contributed to our findings. Despite this, we believe that the procedures employed in this study were the best available for addressing the hypotheses.
Gender was included as an additional factor in preliminary analyses. No significant main effect or interactions involving gender were observed. In other words, men and women did not differ significantly in the magnitude of their fear potentiated startle response. Moreover, the effects of Fearless Dominance, Impulsive Antisociality, and Condition on fear potentiated startle were comparable across genders (i.e., gender did not significantly moderate these effects). Finally, we confirmed that the magnitude of our primary significant effect, the Fearless Dominance X Attentional contrast interaction, was not significantly reduced when gender was included in the analysis (using a test of differences in regression coefficients; Cohen, Cohen, West, and Aiken, 2003). Based on these results and the interpretive ambiguity that results from attempts to statistically control for correlated factors (see Miller & Chapman, 2001), gender was not included in the final analyses.
References
- Arnett PA, Howland EW, Smith SS, Newman JP. Autonomic responsivity during passive avoidance in incarcerated psychopaths. Personality and Individual Differences. 1993;14(1):173–184. [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the Psychopathic Personality Inventory: Validity and implications for clinical assessment. Psychological Assessment. 2003;15(3):340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Salekin RT. Convergent and discriminant validity of psychopathy factors assessed via self-report: A comparison of three instruments. Assessment. 2005a;12(3):270–289. doi: 10.1177/1073191105277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005b;42(6):753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Blonigen DM, Hicks BM, Iacono WG. Estimating facets of Psychopathy from normal personality traits: A step toward community epidemiological investigations. Assessment. 2005c;12:3–18. doi: 10.1177/1073191104271223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient Fear Conditioning in Psychopathy: A Functional Magnetic Resonance Imaging Study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the Amygdala response to unattended threat-related stimuli. Jounral of Neuroscience. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology. 2005;17:3. doi: 10.1017/S0954579405050418. Special Issue: Integrating Cognitive and Affective Neuroscience and Developmental Psychopathology, 865−891. [DOI] [PubMed] [Google Scholar]
- Cook EW, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29(6):633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge University Press; Cambridge, UK: 1999. pp. 62–101. [Google Scholar]
- Curtin JJ, Patrick JP, Lang AL, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychological Science. 2001;12(6):527–531. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR, Patrick CJ, Stritzke WGK. Alcohol and fear-potentiated startle: The role of competing cognitive demands in the stress-reducing effects of intoxication. Journal of Abnormal Psychology. 1998;107:547–565. doi: 10.1037//0021-843x.107.4.547. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Neurophysiology and neuropharmacology of startle and its affective modification. In: Dawson M, Schell A, Bohmelt M, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. Cambridge University Press; Cambridge: 1999. pp. 95–113. [Google Scholar]
- Dvorak-Bertsch JD, Curtin JJ, Rubinstein TJ, Newman JP. Anxiety moderates the interplay between cognitive and affective processing. Psychological Science. 2007;18:699–705. doi: 10.1111/j.1467-9280.2007.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Spitznagel E, Raichle ME. Functional anatomical differences between major depressive subtypes. Journal of Cerebral Blood Flow and Metabolism. 1995b;15:S93. [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: Peripheral and central correlates. Psychophysiology. 2002;39:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: Implications of Gray's two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Gray JA. The Psychology of Fear and Stress. 2 ed. Cambridge; Cambridge, NY: 1987. A conceptual nervous system for avoidance behaviour. pp. 241–409. [Google Scholar]
- Grillon C, Ameli R, Foot M, Davis M. Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry. 1993;33(8−9):566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Hare RD. Acquisition and generalization of a conditioned fear response in psychopathic and nonpsychopathic criminals. Journal of Psychology. 1965a;59:367–370. doi: 10.1080/00223980.1965.10544625. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy, fear arousal and anticipated pain. Psychological Reports. 1965b;16:499–502. doi: 10.2466/pr0.1965.16.2.499. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy, autonomic functioning and the orienting response. Journal of Abnormal Psychology. 1968;73:1–24. doi: 10.1037/h0025873. Monograph Supplement, No. 3, part 2. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy: Theory and research. Wiley; New York: 1970. [Google Scholar]
- Hare RD, Quinn MJ. Psychopathy and autonomic conditioning. Journal of Abnormal Psychology. 1971;71(3):223–235. doi: 10.1037/h0031012. [DOI] [PubMed] [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist-Revised. 2nd ed. Multi-Health Systems; Toronto, Ontario, Canada: 2003. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutional population. Journal of Personality and Social Psychology. 1995;68:151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: Emotion and attention in picture processing. Journal of Abnormal Psychology. 2000;109(3):373–385. [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. Journal of Abnormal and Social Psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller MW, Patrick CJ, Levenston GK. Affective imagery and the startle response: Probing mechanisms of modulation during pleasant scenes, personal experiences and discrete negative emotions. Psychophysiology. 2002;39:519–529. doi: 10.1017/s0048577202394095. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986;95:252–256. [PubMed] [Google Scholar]
- Newman JP, Lorenz AR. Response modulation and emotion processing: Implications for psychopathy and other dysregulatory psychopathology. In: Davidson RJ, Scherer K, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford University Press; New York: 2003. pp. 1043–1067. [Google Scholar]
- Ogloff JR, Wong S. Electrodermal and cardiovascular evidence of a coping response in psychopaths. Criminal Justice and Behavior. 1990;17:231–245. [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102(1):82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Cuthbert BN, Lang PJ. Emotion in the criminal psychopath: Fear image processing. Journal of Abnormal Psychology. 1994;103(3):523–534. doi: 10.1037//0021-843x.103.3.523. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31(4):319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Getting to the heart of psychopathy. In: Herve H, Yuille JC, editors. The Psychopath: Theory, Research and Social Implications. Lawrence Erlbaum Associates; Hillsdale, NJ: 2007. pp. 207–252. [Google Scholar]
- Patrick CJ, Lang PJ. Psychopathic traits and intoxicated states: Affective concomitants and conceptual links. In: Dawson M, Schell A, Boehmelt AH, editors. Startle Modification: Implications for Clinical Science,Cognitive Science, and Neuroscience. Cambridge University Press; New York: NY: 1999. pp. 209–230. [Google Scholar]
- Patrick CJ, Berthot B, Moore JD. Diazepam blocks fear-potentiated startle in humans. Journal of Abnomral Psychology. 1996;105:89–96. doi: 10.1037//0021-843x.105.1.89. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Lang AR. Psychopathic Traits and intoxicated states: Affective concomitants and conceptual links. In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. Cambridge University Press; New York, N.Y., US: 1999. pp. 208–230. [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: Toward a psychological mechanism for the syndromes of disinhibition. Psychological Review. 1993;100:716–736. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morlan T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimaging. doi: 10.1016/j.neuroimage.2005.05.048. In press. (not cited) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Antisocial behavior and psychophysiology: A biosocial perspective and a prefrontal dysfunction hypothesis. In: Stoff DM, Breiling J, Maser JD, editors. Handbook of antisocial behavior. John Wiley & Sons Inc.; Hoboken, New Jersey: 1997. pp. 289–304. [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Ross SR, Benning SD, Adams Z. Symptoms of executive dysfunction are endemic to secondary psychopathy: An examination in criminal offenders and non-institutionalized young adults. Journal of Personality Disorders. 2007;21:384–399. doi: 10.1521/pedi.2007.21.4.384. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. PNAS. 2001;98(2):683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. PNAS. 2001;98(2):688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanman EJ, Mejia VY, Dawson ME, Schell AM, Raine A. Modification of the startle reflex in a community sample: Do one or two dimensions of psychopathy underlie emotional processing? Personality and Individual Differences. 2003;35:2007–2021. [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]