Abstract
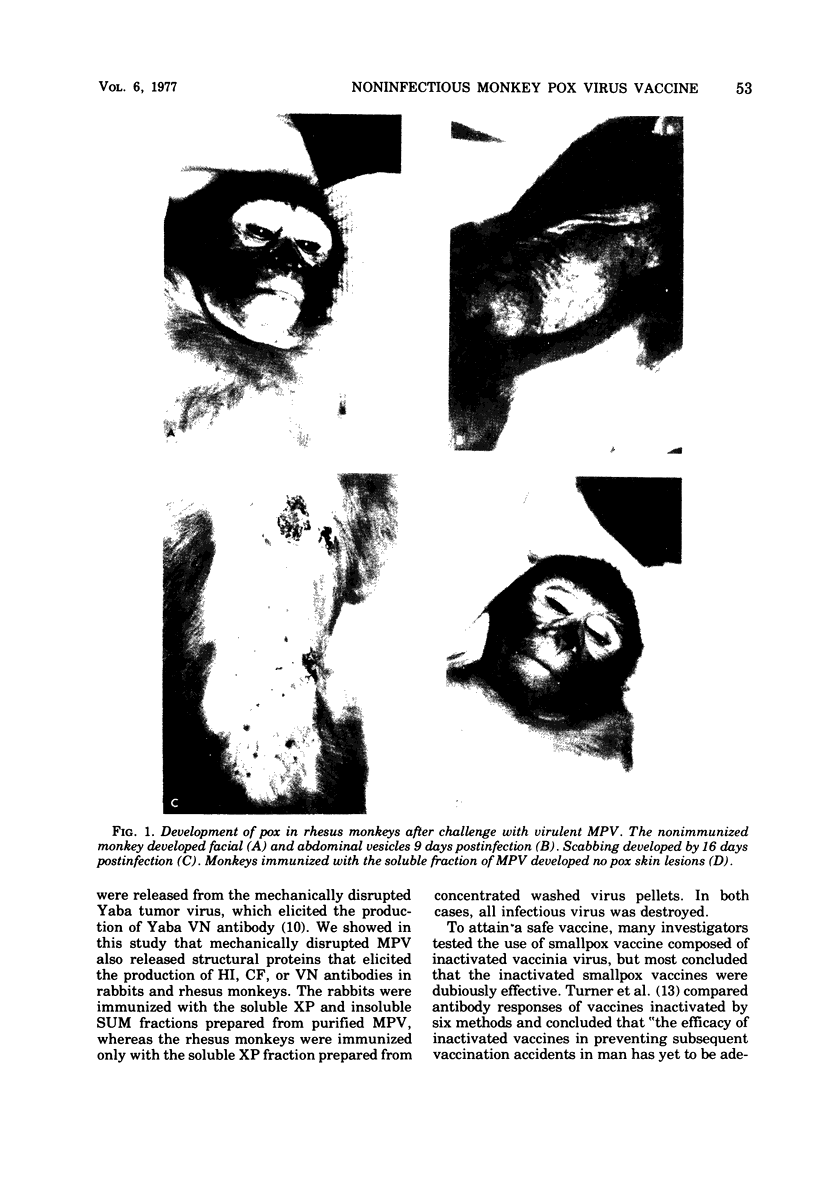
Monkey pox virus was mechanically disrupted by low temperature and high pressure into soluble and insoluble fractions. Soluble fractions elicited virus-neutralizing antibodies (1:20 to 1:160) in rabbits, whereas the insoluble (in saline) fractions did not (less than 1:5). No infectious virus was detected after the disruption procedure. Rhesus monkeys immunized with the soluble fraction elicited virus-neutralizing (1:1,200), complement-fixing (1:16), and hemagglutinating-inhibiting (1:80 to 1:160) antibody titers and were completely protected against monkey pox virus-induced disease. This model of monkey pox virus subunit vaccine preparation may prove to be useful in developing an efficacious noninfectious vaccinia vaccine for use in high-risk individuals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD G., ZWARTOUW H. T., WESTWOOD J. C. A PROTECTIVE ANTIGEN FROM THE POX-VIRUSES. I. REACTION WITH NEUTRALIZING ANTIBODY. Br J Exp Pathol. 1964 Apr;45:150–161. [PMC free article] [PubMed] [Google Scholar]
- Hall R. D., Olsen R. G., Pakes S. P., Yohn D. S. Differences in clinical and convalescent-phase antibodies of Rhesus monkeys infected with monkey pox, tanapox, and Yaba poxviruses. Infect Immun. 1973 Apr;7(4):539–546. doi: 10.1128/iai.7.4.539-546.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T. Standardized viral hemagglutination and hemagglutination-inhibition tests. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969 Nov;18(5):816–823. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marquardt J., Holm S. E., Lycke E. Immunoprecipitating factors of vaccinia virus. Virology. 1965 Oct;27(2):170–178. doi: 10.1016/0042-6822(65)90156-x. [DOI] [PubMed] [Google Scholar]
- Munyon W., Mann J., Grace J. T., Jr Protection of vaccinia from heat inactivation by nucleotide triphosphates. J Virol. 1970 Jan;5(1):32–38. doi: 10.1128/jvi.5.1.32-38.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. G., Yohn D. S. Immunodiffusion analysis of Yaba poxvirus structural and associated antigens. J Virol. 1970 Feb;5(2):212–220. doi: 10.1128/jvi.5.2.212-220.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. S., Squires E. J., Murray H. G. Inactivated smallpox vaccine. A comparison of inactivation methods. J Hyg (Lond) 1970 Jun;68(2):197–210. doi: 10.1017/s0022172400028679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnaar S. O., Knegt E., Charles R., Schilperoort R. A., Grose W. J. The preparation by alkaline treatment of a serumblocking antigen of vaccinia virus with immunizing potency. Arch Gesamte Virusforsch. 1965;17(5):577–584. doi: 10.1007/BF01262234. [DOI] [PubMed] [Google Scholar]