When facing a problem, we would ideally like to be able to eliminate its source. If that is not feasible, we focus our efforts on damage control, by attempting to minimize the consequences of the problem at hand. Likewise, host defense from infections can employ two distinct strategies of protection: one aims to reduce or eliminate the invading pathogen, whereas the other reduces the damage to the host inflicted by a given pathogen burden. The two strategies are referred to as resistance and tolerance, respectively (1). Resistance mechanisms are generally mediated by the immune system. Mechanisms of tolerance (not to be confused with immunological tolerance) are far less well understood, particularly in animal hosts (2). The report by Seixas et al. (3) in this issue of PNAS describes a mechanism of host tolerance used during infection with Plasmodium parasites. This study provides one of the first insights into molecular mechanisms of infection tolerance in mammals and is likely to be applicable, at least in principle, to a broad range of infectious diseases.
Resistance and tolerance have long been recognized as distinct host defense strategies used by plants to deal with their pests. The distinction between the two strategies is fundamentally important, because they rely on discrete molecular mechanisms and entail different consequences for the evolutionary dynamics of host–pathogen interactions (4). Surprisingly, the concepts of resistance and tolerance are seldom applied to studies of animal immunity, where research has traditionally focused almost exclusively on mechanisms of resistance. Consequently, very little is known about the underlying mechanisms of tolerance and their utility for host protection. Tolerance mechanisms do exist in animals, however, as documented by the few studies reported to date: Råberg et al. (5) described a variation in tolerance to Plasmodium infection in several inbred mouse strains, which was probably the first clear description of tolerance in animals, and Schneider and colleagues (6–9) in a series of elegant studies dissected the contributions of resistance and tolerance to Drosophila defense from infections. Together, these studies have indicated the existence of powerful but poorly understood defense mechanisms that contribute to host survival from infections. Clearly, this line of research will have to be continued and significantly expanded to reveal what is likely to be a plethora of mechanisms that contribute to host tolerance to infections.
Some basic conceptual aspects of host tolerance have been discussed in detail in recent excellent reviews (1, 2, 10). To provide a context for the discussion of the new report by Seixas et al. (3), a couple of points need to be made. First, the symptoms of infectious diseases can be either due to direct damage to the host inflicted by the pathogen, or due to immunopathology—the collateral damage to the host tissues caused by the immune response. Accordingly, tolerance mechanisms may be broadly divided into mechanisms that protect the host tissues from direct pathogen-induced damage, for example, caused by microbial toxins, and the mechanisms that limit immunopathology. The latter in turn can be subdivided into immunoregulatory mechanisms, which control the intensity and duration of the immune and inflammatory responses, and the mechanisms that render tissues more resistant to inflammatory damage. Second, the relative contribution of the two types of mechanisms most likely depends on the replication, transmission, and host adaptation strategies of microorganisms. An extreme example illustrating this point is the nature of host–commensal interactions. Here, the host tolerates microbial colonization of enormous proportions without any adverse effects (in fact, with a number of essential benefits). In large part, this is due to potent immunoregulatory mechanisms that maintain a host–commensal homeostasis at the sites of colonization, such as the colon. If the normal immunoregulatory state is disrupted, however, the ensuing problems are due almost entirely to the inappropriate immune and inflammatory responses triggered by the commensal microbes.
Most pathogenic infections will elicit both types of tissue damage, and therefore the host may commonly employ both types of tolerance mechanisms. The case in point is infection with Plasmodium, the causative agent of malaria. This protozoan parasite can cause direct damage to the host through hemolysis and anemia, and indirect damage attributable to immunopathology. The latter is responsible for some forms of severe malaria, including cerebral malaria, which is caused by the disruption of the blood–brain barrier followed by the often lethal inflammatory response in the brain (11). During the blood stage of infection, Plasmodium invades red blood cells (RBCs), where it degrades hemoglobin, resulting in the release of free heme. Because free heme is toxic to the host and to the parasite, both try to convert it to more innocuous chemical forms. Plasmodium converts heme into a polymer called hemozoin, which is nontoxic to the parasite. This conversion can be blocked by chloroquine, which presumably explains its therapeutic properties in malaria patients and the popularity of gin and tonic among European settlers in the parts of the world where malaria is common. Circulating heme has pro-inflammatory properties: it induces reactive oxygen species (ROS) (12, 13) and activates Toll-like receptor 4 on macrophages (14). Heme detoxification occurs through the induction of heme oxygenase-1 (HO-1), which converts it into biliverdin, carbon monoxide (CO), and iron. Biliverdin is further metabolized into bilirubin, which is excreted in bile. Importantly, HO-1 has previously been found to play an essential role in protection from the experimental cerebral malaria by preventing heme-induced destabilization of the blood–brain barrier (13).
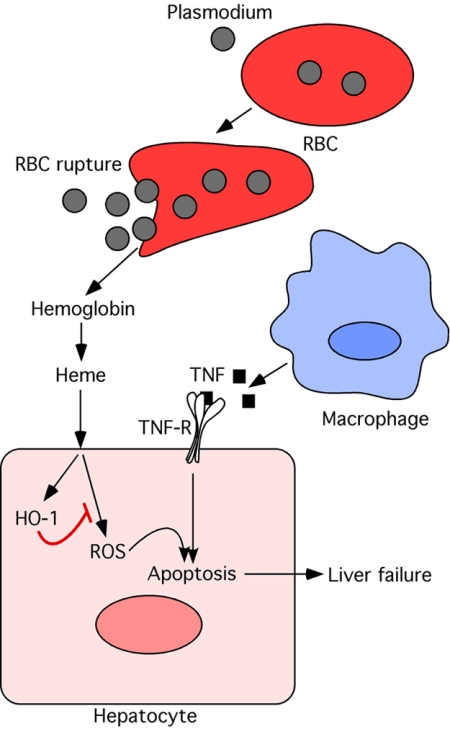
In the new study, Seixas et al. (3) demonstrate that the heme detoxification activity of HO-1 is a critical component of host tolerance to Plasmodium infection-associated liver failure. Infection with Plasmodium chabaudi chabaudi (Pcc) resulted in hepatic failure in susceptible mouse strains due to extensive hepatocyte apoptosis caused by tumor necrosis factor (TNF) produced during the infection. Expression of HO-1 determined susceptibility to this form of severe malaria, as demonstrated by the analyses of HO-1-deficient mice. Free heme was found to sensitize hepatocytes for TNF-induced apoptosis through a mechanism involving ROS generation. Degradation of heme by HO-1 was required to prevent Pcc induced hepatic failure and mortality (Fig. 1). Importantly, this protective activity of HO-1 was found to have no effect on parasite burden, thus clearly demonstrating its key contribution to host tolerance without affecting host resistance to Plasmodium infection. Remarkably, Seixas et al. found this tolerance mechanism to be
HO-1 is a critical component of host tolerance to Plasmodium infection-associated liver failure.
extremely potent, as it could afford a complete protection from Pcc infection-associated mortality. Furthermore, elimination of heme-induced ROS by administration of a pharmacological antioxidant, N-acetylcysteine (NAC), had a potent therapeutic effect, even when NAC was administered 4 days after infection. Importantly, although administration of NAC protected the otherwise susceptible mice from Pcc-induced mortality, it had no effect on parasite burden. This finding thus not only offers an attractive therapeutic strategy but also provides an interesting example of a pharmacological manipulation of host tolerance. A more familiar example of the same strategy is the use of cyclooxygenase inhibitors (such as aspirin or ibuprofen) during influenza virus infections. The fever-lowering effect of these drugs enhances host tolerance and presumably has no direct effect on the virus. It could be expected that blocking different aspects of the inflammatory response is likely to have a positive effect on host tolerance in a variety of infectious diseases, especially when boosting the antimicrobial activity is not an option, or when the presence of the pathogen is easily tolerated in the absence of immunopathology.
Fig. 1.
Role of HO-1 in cytoprotection during Plasmodium infection. Plasmodium infection results in lysis of red blood cells (RBC) followed by release of hemoglobin and free heme. Free heme triggers production of reactive oxygen species (ROS). ROS sensitizes hepatocytes to undergo apoptosis in response to tumor necrosis factor (TNF) produced during infection. Heme oxygenase-1 (HO-1) is induced in hepatocytes and degrades free heme, thus protecting hepatocytes from apoptosis. HO-1 thus plays a critical role in tissue protection during Plasmodium infection.
The study by Seixas et al. (3) has identified heme-induced ROS as a major component of host susceptibility to Pcc infection-associated liver damage. Excessive ROS levels are likely to play an important role in determining host tolerance in a variety of infectious diseases, particularly when an excessive inflammatory response is a limiting factor. Although immunoregulatory mechanisms that limit the excessive immune and inflammatory responses have been studied extensively, the mechanisms that render tissues resistant to immune and inflammatory damage are poorly understood. The importance of the study by Seixas et al. is that it describes just such a mechanism, which operates in the context of infection with a deadly parasite.
Footnotes
The author declares no conflict of interest.
See companion article on page 15837.
References
- 1.Råberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc London Ser B. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seixas E, et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci USA. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rausher MD. Co-evolution and plant resistance to natural enemies. Nature. 2001;411:857–864. doi: 10.1038/35081193. [DOI] [PubMed] [Google Scholar]
- 5.Råberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 6.Ayres JS, Freitag N, Schneider DS. Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics. 2008;178:1807–1815. doi: 10.1534/genetics.107.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 2008;6:2764–2773. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009;7:e1000150. doi: 10.1371/journal.pbio.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon MD, Ayres JS, Schneider DS, Nusse R. Pathogenesis of Listeria-infected Drosophila wntD mutants is associated with elevated levels of the novel immunity gene edin. PLoS Pathog. 2008;4:e1000111. doi: 10.1371/journal.ppat.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read AF, Graham AL, Råberg L. Animal defenses against infectious agents: Is damage control more important than pathogen control. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 12.Balla J, et al. Endothelial-cell heme uptake from heme proteins: Induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamplona A, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 14.Figueiredo RT, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]