Abstract
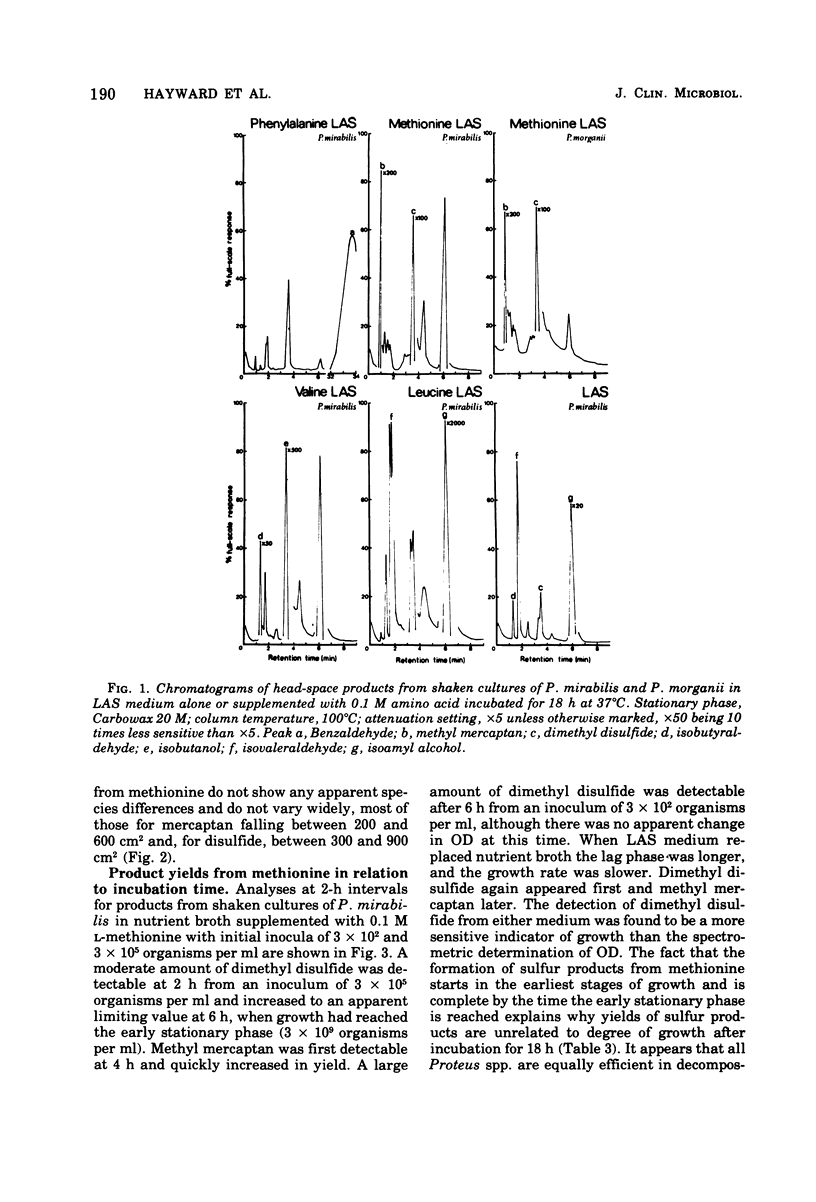
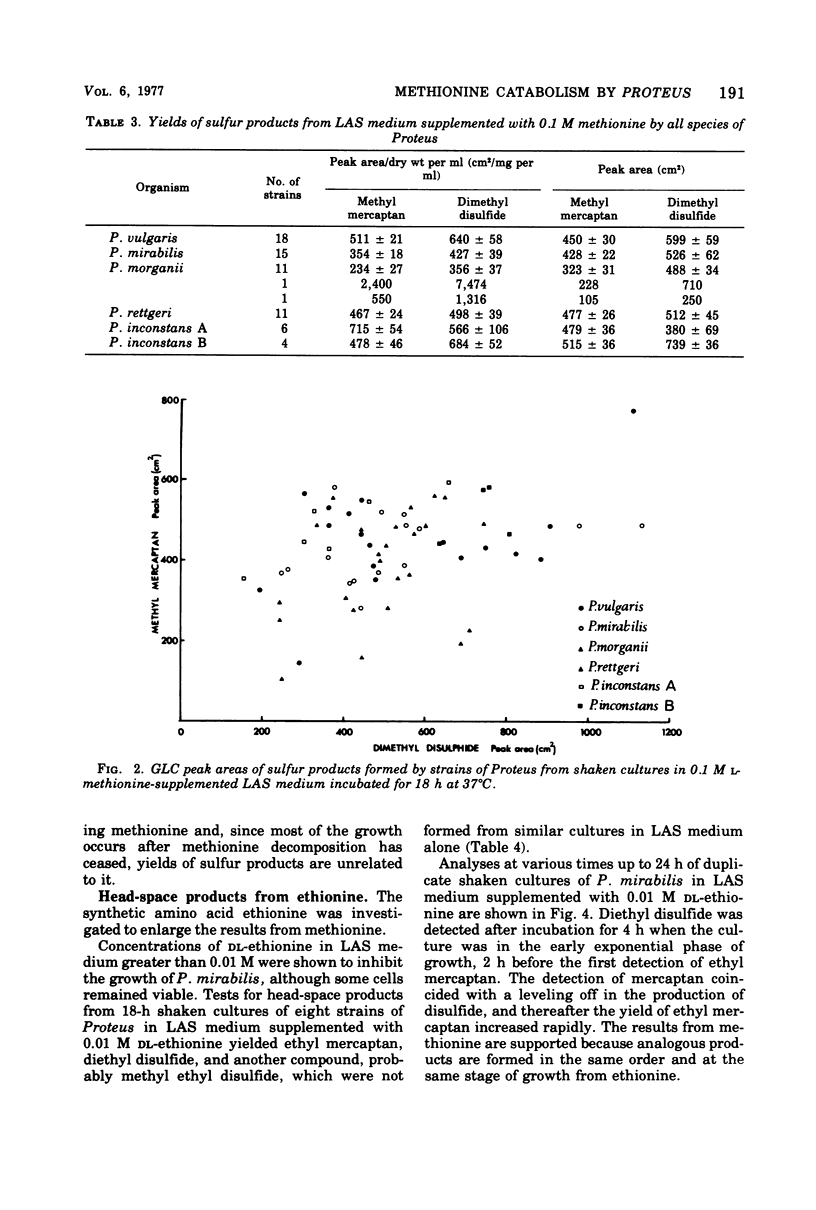
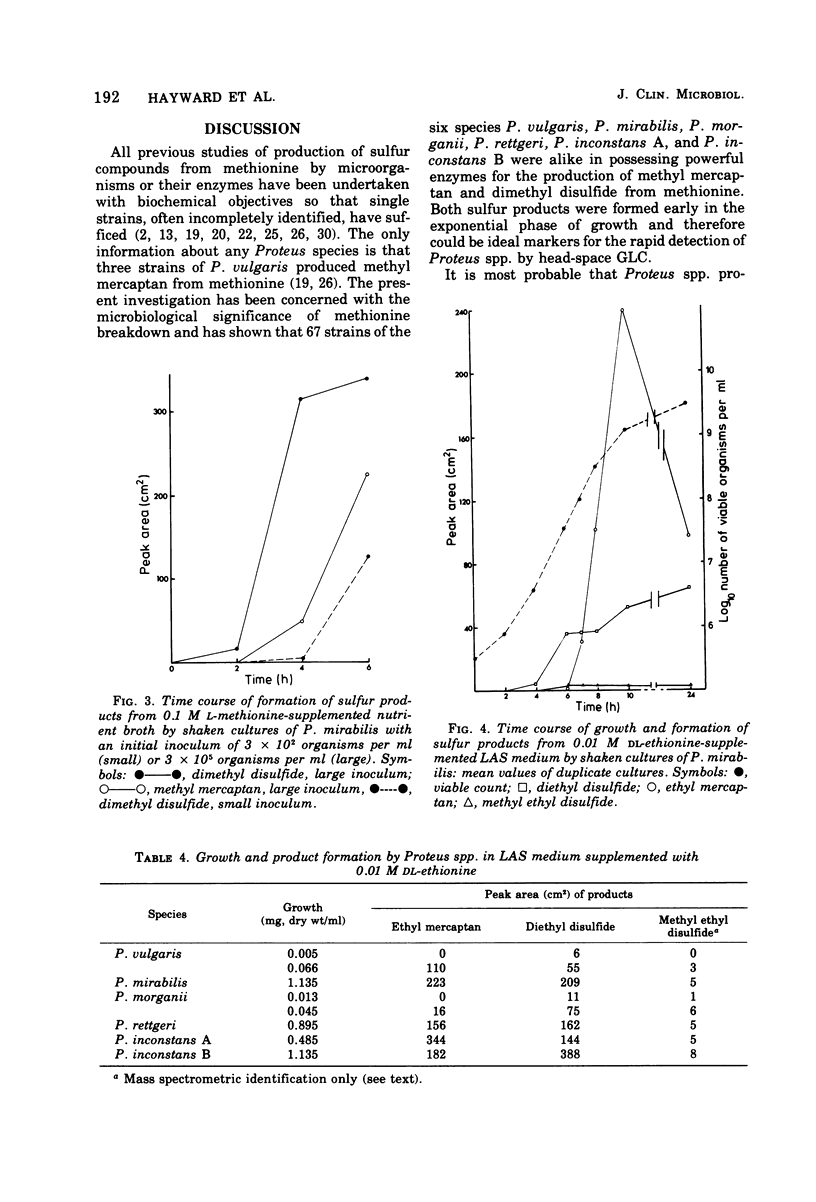
Head-space gas-liquid chromatography and mass spectrometry were used to detect and identify products formed by Proteus vulgaris, P. mirabilis, P. morganii, and P. rettgeri from a defined medium supplemented with either phenylalanine, methionine, valine, leucine, histidine, lysine, ornithine, threonine, asparagine, aspartic acid, or tryptophan. In a detailed study of the products formed by 68 strains of Proteus spp. from L-methionine, the production of large amounts of both dimethyl disulfide and methyl mercaptan was found to be a characteristic of the genus. Both sulfur products appeared within a few hours of inoculation. Dimethyl disulfide was a more sensitive indicator of growth than the spectrometric determination of optical density. This suggests that it could be useful for the rapid, automated detection of any species of Proteus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkinshaw J. H., Findlay W. P., Webb R. A. Biochemistry of the wood-rotting fungi: The production of methyl mercaptan by Schizophyllum commune Fr. Biochem J. 1942 Jun;36(5-6):526–529. doi: 10.1042/bj0360526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. B., Kellogg D. S., Alley C. C., Short H. B., Handsfield H. H., Huff B. Gas chromatography as a potential means of diagnosing arthritis. I. Differentiation between staphylococcal, streptococcal, gonococcal, and traumatic arthritis. J Infect Dis. 1974 Jun;129(6):660–668. doi: 10.1093/infdis/129.6.660. [DOI] [PubMed] [Google Scholar]
- CURRY A. S., HURST G., KENT N. R., POWELL H. Rapid screening of blood samples for volatile poisons by gas chromatography. Nature. 1962 Aug 11;195:603–604. doi: 10.1038/195603b0. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Simplified gas chromatographic procedure for identification of bacterial metabolic products. Appl Microbiol. 1973 Feb;25(2):287–289. doi: 10.1128/am.25.2.287-289.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini G. L., O'Brien R. T. Detection of Escherichia coli by gas chromatography. J Bacteriol. 1968 Mar;95(3):1205–1206. doi: 10.1128/jb.95.3.1205-1206.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. B. The identification of streptococci by gas-liquid chromatography. Microbios. 1972 Mar-Apr;5(18):109–112. [PubMed] [Google Scholar]
- MITSUHASHI S. Decomposition of thioether derivatives by bacteria; methylmercaptan formation and the properties of the responsible enzyme. Jpn J Exp Med. 1949 Sep;20(2):211–222. [PubMed] [Google Scholar]
- Mitruka B. M., Alexander M. Halogenated compounds for the sensitive detection of clostridia by gas chromatography. Can J Microbiol. 1972 Oct;18(10):1519–1523. doi: 10.1139/m72-232. [DOI] [PubMed] [Google Scholar]
- Mitruka B. M., Alexander M. Rapid and sensitive detection of bacteria by gas chromatography. Appl Microbiol. 1968 Apr;16(4):636–640. doi: 10.1128/am.16.4.636-640.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitruka B. M., Carmichael L. E., Alexander M. Gas chromatographic detection of in vitro and in vivo activities of certain canine viruses. J Infect Dis. 1969 Jun;119(6):625–634. doi: 10.1093/infdis/119.6.625. [DOI] [PubMed] [Google Scholar]
- Mitruka M., Kundargi R. S., Jonas A. M. Gas chromatography for rapid differentiation of bacterial infections in man. Med Res Eng. 1972;11(2):7–11. [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Cherry W. B. Use of gas chromatography for determining catabolic products of arginine by bacteria. Appl Microbiol. 1972 May;23(5):889–893. doi: 10.1128/am.23.5.889-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROOM H., KNIGHT B. C. The minimal nutritional requirements of some species in the genus Bacillus. J Gen Microbiol. 1955 Dec;13(3):474–480. doi: 10.1099/00221287-13-3-474. [DOI] [PubMed] [Google Scholar]
- Reiner E., Hicks J. J., Ball M. M., Martin W. J. Rapid characterization of salmonella organisms by means of pyrolysis-gas-liquid chromatography. Anal Chem. 1972 May;44(6):1058–1061. doi: 10.1021/ac60314a031. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Starkey R. L. Dissimilation of methionine by a demethiolase of Aspergillus species. J Bacteriol. 1969 Sep;99(3):764–770. doi: 10.1128/jb.99.3.764-770.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal W., Starkey R. L. Microbial decomposition of methionine and identity of the resulting sulfur products. J Bacteriol. 1969 Jun;98(3):908–913. doi: 10.1128/jb.98.3.908-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toan T. T., Bassette R., Claydon T. J. Methyl sulfide production by Aerobacter aerogenes in milk. J Dairy Sci. 1965 Sep;48(9):1174–1178. doi: 10.3168/jds.S0022-0302(65)88422-3. [DOI] [PubMed] [Google Scholar]
- WIESENDANGER S., NISMAN B. La Lméthionine démercapto-désaminase: un nouvel enzyme à pyridoxal-phosphate. C R Hebd Seances Acad Sci. 1953 Oct 5;237(14):764–765. [PubMed] [Google Scholar]
- Wade T. J., Mandle R. J. New gas chromatographic characterization procedure: preliminary studies on some Pseudomonas species. Appl Microbiol. 1974 Feb;27(2):303–311. doi: 10.1128/am.27.2.303-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka, Kitamura M., Tamura Z. Rapid gas chromatographic analysis of microbial volatile metabolites. Jpn J Microbiol. 1969 Mar;13(1):87–93. doi: 10.1111/j.1348-0421.1969.tb00438.x. [DOI] [PubMed] [Google Scholar]


