Abstract
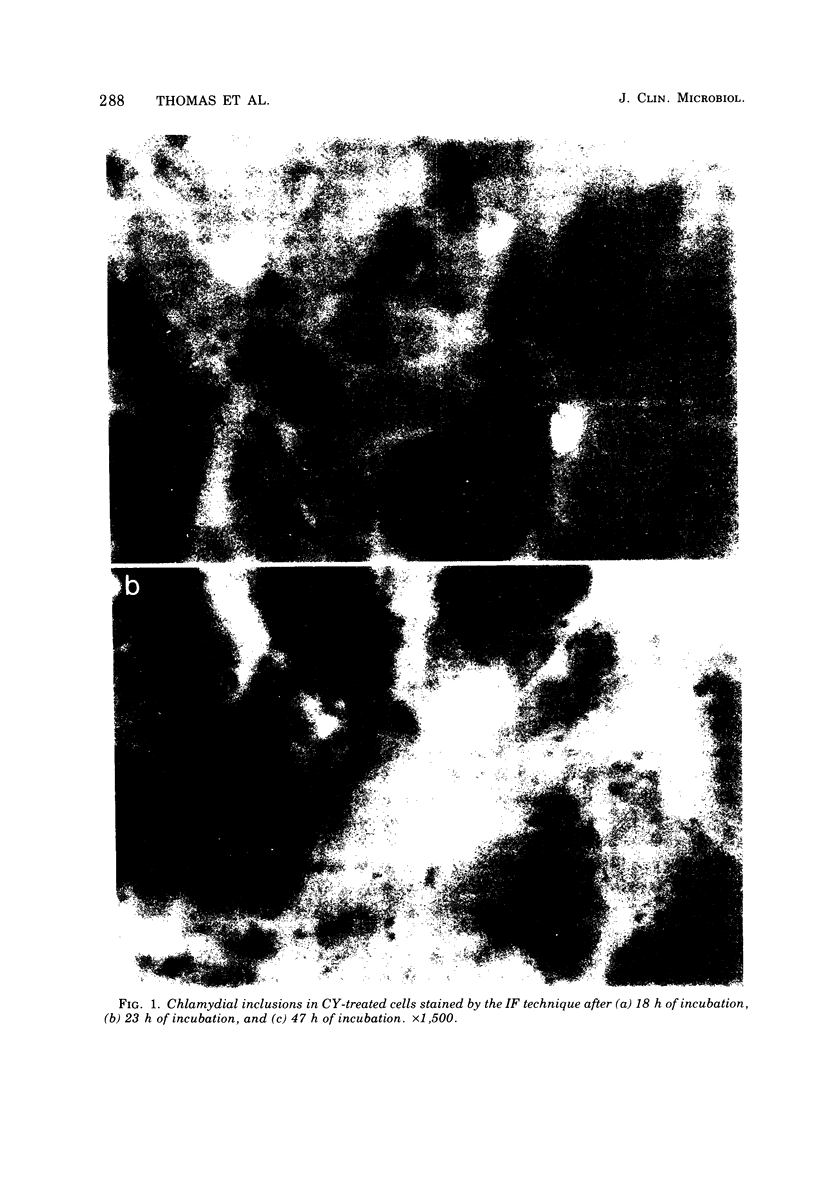
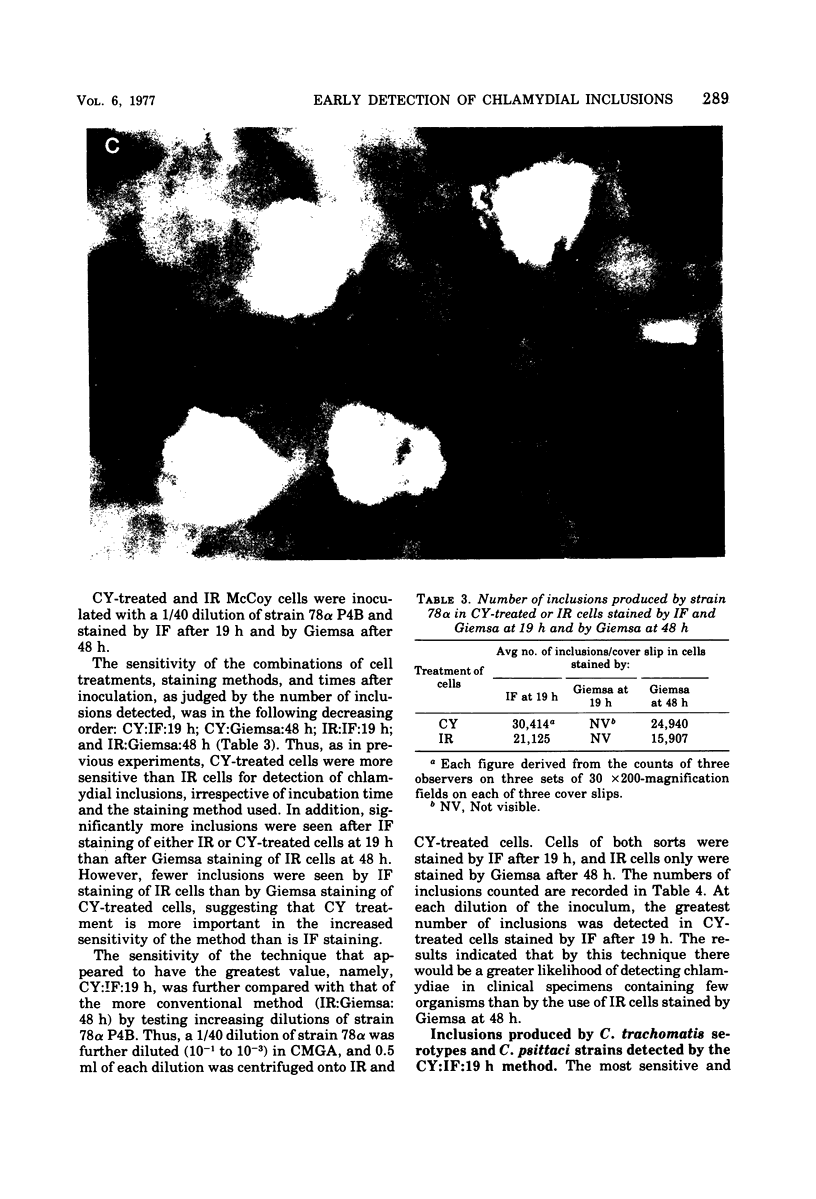
Detection of Chlamydia trachomatis inclusions only 21 h after a specimen reaches the laboratory has been achieved by the combined use of cycloheximide-treated McCoy cells and immunofluorescence staining. Moreover, cells exposed to cycloheximide were more sensitive for detecting chlamydial inclusions than those pretreated by irradiation, since larger numbers of inclusions were found in the former cells. The application of this rapid and sensitive method allows a diagnosis of chlamydial infection to be made before antibiotic therapy is started. In this way, it should enable the treatment of nonspecific genital infections to be placed on a more rational basis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blyth W. A., Taverne J. Cultivation of TRIC agents: a comparison between the use of BHK-21 and irradiated McCoy cells. J Hyg (Lond) 1974 Feb;72(1):121–128. doi: 10.1017/s0022172400023287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy T. R., Kuo C. C., Wang S. P. Comparative susceptibility of eleven mammalian cell lines to infection with trachoma organisms. J Clin Microbiol. 1975 May;1(5):434–439. doi: 10.1128/jcm.1.5.434-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. CYCLOHEXIMIDE: ASPECTS OF INHIBITION OF PROTEIN SYNTHESIS IN MAMMALIAN CELLS. Science. 1964 Dec 11;146(3650):1474–1476. doi: 10.1126/science.146.3650.1474. [DOI] [PubMed] [Google Scholar]
- Gordon F. B., Dressler H. R., Quan A. L., McQuilkin W. T., Thomas J. I. Effect of ionizing irradiation on susceptibility of McCoy cell cultures to Chlamydia trachomatis. Appl Microbiol. 1972 Jan;23(1):123–129. doi: 10.1128/am.23.1.123-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson D., Johnson F. W., Byng R. E. Isolation of Chlamydia trachomatis in tissue-culture of human thyroid. Lancet. 1976 Sep 18;2(7986):643–643. doi: 10.1016/s0140-6736(76)90723-6. [DOI] [PubMed] [Google Scholar]
- Hobson D., Johnson F. W., Byng R. E. The growth of the ewe abortion chlamydial agent in McCoy cell cultures. J Comp Pathol. 1977 Jan;87(1):155–159. doi: 10.1016/0021-9975(77)90091-3. [DOI] [PubMed] [Google Scholar]
- Hobson D., Johnson F. W., Rees E., Tait I. A. Simplified method for diagnosis of genital and ocular infections with Chlamydia. Lancet. 1974 Sep 7;2(7880):555–556. doi: 10.1016/s0140-6736(74)91879-0. [DOI] [PubMed] [Google Scholar]
- Johnson F. W., Hobson D. Factors affecting the sensitivity of replicating McCoy cells in the isolation and growth of chlamydia A (TRIC agents). J Hyg (Lond) 1976 Jun;76(3):441–451. doi: 10.1017/s0022172400055376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Harper I. A. Isolation of chlamydia in irradiated and non-irradiated McCoy cells. J Clin Pathol. 1975 Dec;28(12):1003–1004. doi: 10.1136/jcp.28.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Wentworth B. B., Grayston J. T. Primary isolation of TRIC organisms in HeLa 229 cells treated with DEAE-dextran. J Infect Dis. 1972 Jun;125(6):665–668. doi: 10.1093/infdis/125.6.665. [DOI] [PubMed] [Google Scholar]
- Nayyar K. C., O'Neill J. J., Hambling M. H., Waugh M. A. Isolation of Chlamydia trachomatis from women attending a clinic for sexually transmitted diseases. Br J Vener Dis. 1976 Dec;52(6):396–398. doi: 10.1136/sti.52.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice M. J., Taylor-Robinson D., Csonka G. W. Non-specific urethritis. A placebo-controlled trial of minocycline in conjunction with laboratory investigations. Br J Vener Dis. 1976 Aug;52(4):269–275. doi: 10.1136/sti.52.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve P., Owen J., Oriel J. D. Laboratory procedures for the isolation of chlamydia trachomatis from the human genital tract. J Clin Pathol. 1975 Nov;28(11):910–914. doi: 10.1136/jcp.28.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S. J. Letter: Growth of Chlamydia trachomatis in cell culture. Lancet. 1976 Apr 17;1(7964):865–865. doi: 10.1016/s0140-6736(76)90524-9. [DOI] [PubMed] [Google Scholar]
- Rota T. R., Nichols R. L. Chlamydin trachomatis in cell culture. I. Comparison of efficiencies of infection in several chemically defined media, at various pH and temperature values, and after exposure to diethylaminoethyl-dextran. Appl Microbiol. 1973 Oct;26(4):560–565. doi: 10.1128/am.26.4.560-565.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Richmond S. Growth of Chlamydia trachomatis in McCoy cells treated with cytochalasin B. Appl Microbiol. 1974 Dec;28(6):912–914. doi: 10.1128/am.28.6.912-914.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol. 1974 May;27(5):912–916. doi: 10.1128/am.27.5.912-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]