Abstract
A major question in neuroscience concerns how widely separated brain regions coordinate their activity to produce unitary cognitive states or motor actions. To investigate this question, we employed multisite, multielectrode recording in rats to study how olfactory and motor circuits are coupled prior to the execution of an olfactory-driven, GO/NO-GO variant of a skilled, rapidly executed (~350–600 ms) reaching task. During task performance, we recorded multi-single units and local field potentials (LFPs) simultaneously from the rats’ olfactory cortex (specifically, the posterior piriform cortex) and from cortical and subcortical motor sites (the caudal forepaw M1, and the magnocellular red nucleus, respectively). Analyses on multi-single units across areas revealed an increase in beta-frequency spiking (12–30 Hz) during a ~100 ms window surrounding the Final Sniff of the GO cue before lifting the arm (the “Sniff-GO window”) that was seldom seen when animals sniffed the NO-GO cue. Also during the Sniff-GO window, LFPs displayed a striking increase in beta, low-gamma, and high-gamma energy (12–30, 30–50, and 50–100 Hz, respectively), and oscillations in the high gamma band appeared to be coherent across the recorded sites. These results indicate that transient, multispectral coherence across cortical and subcortical brain sites is part of the coordination process prior to sensory-guided movement initiation.
Keywords: Multielectrode, Multisite, Units, Local field potentials, Coherence, Wavelets
Introduction
Mammals are constantly assessing their sensory environment, motivational state, and possible actions, yet when the animal chooses a particular action, these ongoing, parallel processes momentarily converge to produce a coordinated movement. This is one example of the “large-scale integration problem” or the “coordination problem” in neuroscience (Bressler and Kelso 2001; Varela et al. 2001; Schnitzler and Gross 2005): the question of how widely scattered neuronal assemblies momentarily come into existence to produce one state or output, then rapidly transition so that a different neuronal assembly is active. Some progress has been made in understanding the mechanisms permitting transient, coordinated brain states. For example, over the last 10–15 years, Singer, Desimone and colleagues have demonstrated that increased gamma band (30–100 Hz) oscillations occur during the transient brain states that are associated with attention and stimulus recognition (Engel et al. 2001; Fries et al. 2001). More recently, hippocampal theta oscillations have been found to drive processing in the prefrontal cortex (Jensen 2005), and several studies have suggested that gamma oscillations nested within theta (4–12 Hz) oscillations play a role in working memory functions (Jensen and Lisman 2005). Also, substantial recent data suggest that corticothalamic (Llinas et al. 1998; Llinas and Ribary 2001; Neuenschwander et al. 2002; Ribary 2005) and hippocampal networks (Buzsaki 1997; Harris et al. 2003) make use of beta band (12–30 Hz) and gamma band (30–100 Hz) activity for long-distance transmission of information among task-related brain sites, although a number of those studies were carried out in brain slices or models (Kopell et al. 2000; Bibbig et al. 2002; Klausberger et al. 2003; Olufsen et al. 2003). These results suggest that task-related brain regions with differing network and cellular architectures may nonetheless display enhanced beta and gamma oscillations during attention to, and recognition of a rewarded stimulus, but to our knowledge this hypothesis had never been explicitly tested.
We therefore carried out experiments to test whether task-related cortical and subcortical brain sites would display increased beta and gamma energy despite their differing architectures as awake, behaving animals recognizing a GO cue in a GO/NO-GO task. The task was a GO/NO-GO variant of a skilled reaching task that was thought to be olfactory-driven (Whishaw and Tomie 1989). Rats were implanted with multielectrode arrays or bundles in their three-layered posterior piriform cortex, six-layered forelimb motor cortex, and midbrain motor red nucleus. They were then trained to perform a rapidly and regularly executed GO/NO-GO task in which banana- and chocolate-scented food pellets were the GO stimuli (S+), and visually identical, plastic-only or vanilla- or acetone-scented plastic beads were the NO-GO stimuli (S−). When rats detected an S+, they reached through a narrow slot to obtain the food pellet (Fig. 1a), whereas when they detected an S−, they inhibited reaching and froze. As rats performed this task, we simultaneously recorded multi-single units (Bressler et al. 1993; Nicolelis et al. 1995; Singer et al. 1996; Hoffman and McNaughton 2002) and local field potentials (LFPs; Bullock 1997; Laughlin and Sejnowski 2003) during the period of GO-cue or NO-GO cue evaluation (the “SniV-GO” and “SniV-NO-GO” periods) and ensuing forelimb reach or freezing periods, respectively. In previous work, we showed that the firing rates of large percentages of multi-single units in the rat motor cortex and red nucleus were significantly modulated on at least five videocoded task phases (Fig. 1b; Hermer-Vazquez et al. 2004).
Fig. 1.
a Depiction of the rat in the reaching chamber, shown making contact between its paw and the food pellet. b Phases of this task previously found to correlate with significant spike firing modulation in the forelimb primary motor cortex (M1), magno-cellular red nucleus (mRN), or both (Hermer-Vazquez et al. 2004), and approximate timeline for those task phases on real pellet (GO) trials
The results of the current experiments demonstrate that all three task-related brain sites display enhanced, transient, beta and gamma oscillations upon sniffing the GO cues relative to the NO-GO cues. Moreover, on GO trials the oscillations in the high gamma band are coherent across the recorded brain sites, suggesting that the increased rhythms play a role in coordinating these disparate brain regions prior to the onset of voluntary movement.
Methods
Behavioral training and testing
All experimental methods were approved by the SUNY Health Science Center Institutional Animal Care and Use Committee.
Twenty rats were screened for good olfactory acuity and low anxiety. Eleven rats who “passed” these tests were used in Experiment 1. Before surgery, they were taught a classic, skilled, reach-to-grasp-food task (Whishaw and Pellis 1990), in which all trials were GO trials, to determine handedness. For both experiments reported here, we employed a reaching apparatus of the same dimensions as that used in studies by Whishaw and Pellis (1990). In the skilled reaching task, rats learned to reach through a narrow plastic slot to grasp a 45 mg food pellet from one of two wells—the well contralateral to the reaching paw—located 13 mm beyond the slot on a plastic shelf at roughly the height of the rat’s snout (Fig. 1a). The slot’s width was such that the rat could place its nose directly behind the pellet in the well, thereby gaining information on the pellet’s odor quality and location, but still could not retrieve it with its tongue. Once rats had asymptoted at a stable, high performance level, which took 6–10 days for rats in this cohort, the animals sniffed the target for 1–3 respiratory cycles before withdrawing their snout, lifting their reaching paw off the ground and maneuvering it through the slot to a position just beyond where their snout had been, allowing them to grasp the pellet and bring it to their mouth. The pellets were either banana- or chocolate-flavored and served as the reward when rats successfully retrieved them. These trials constituted the experiments’ GO trials.
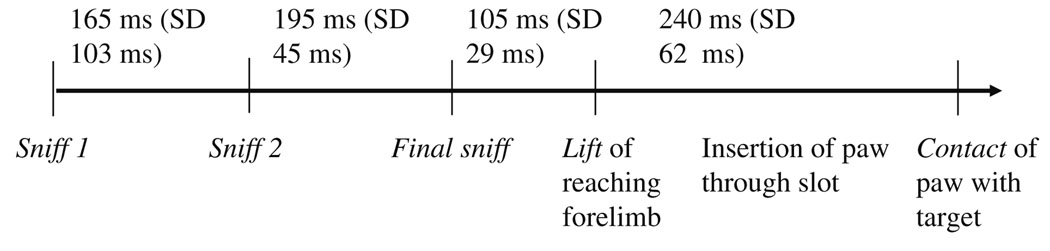
After recovery from surgery, the rats were taught the GO/NO-GO version of the task, on which, pseudorandomly, approximately 25% of trials were NO-GO trials. Odor presentation on GO and NO-GO trials was static. The task required rats to approach a narrow plastic slot in a rectangular chamber, beyond which was a shelf with two wells for food pellets. Randomly, on ~75% of trials, a real food pellet was present and rats had to evaluate the pellet’s odor and then reach for it through the slot. On the remaining ~25% of trials, a visually identical plastic pellet that was either plastic-only, or coated with a non-food odor (vanilla or ace-tone), was present, and rats had to inhibit reaching for, and eating, this non-food item. On GO trials, roughly equal proportions of chocolate or banana pellets were used. For NO-GO trials, we used vanilla-scented and plastic-only pellets on most trials, with a smaller proportion of acetone-scented trials. Although we did not test whether the rats reliably detected an odor associated with the plastic-only pellets, several human subjects whom we tested were able to do so. We used this GO/NO-GO variant of the Whishaw reaching task because of the relatively simplified and highly regular kinematics displayed by rats across trials (Hermer-Vazquez et al. 2004), which allowed us to perform both single-trial analyses and analyses on our neural data that averaged across trials. Once the task was well-learned, rats evaluated the pellet’s odor in one to three respiratory cycles (~100–300 ms, with ~100 ms between the Final Sniff of the pellet and reaching on GO trials), and executed the reaching maneuver on GO-trials in <300 ms (see Fig. 1b for timing from the Final Sniff onward).
All training and testing trials were videorecorded at 17 ms resolution. To ensure relatively regular kinematics across trials, we videocoded only those reaches that resulted, on the rat’s first try, in the forepaw contacting the pellet (regardless of whether the rat correctly retracted its paw and subsequently ate the food morsel). The rats had been shaped during the training phase not to attempt to grasp the pellet more than once if the first try led to failure. Thus, a large percentage of all GO trials (>80%) were retained and videocoded. Similarly, on a small percentage of NO-GO trials, rats started to lift their reaching paw and then placed it back on the ground. We also omitted those trials (<10% of the total) from further analysis. (For more information on coding the sniffs in particular, see below.)
To observe the nature of sensorimotor coordination prior to reaching, we needed a task in which the boundaries of the Sniff-GO and Sniff-NO-GO periods were as clear as possible. A potential confound in Experiment 1, in which neural activity was recorded with static presentation of the GO and NO-GO odors, was that the target’s odor plume would extend backwards into the reaching chamber, allowing rats to sample the odor while approaching the pellet for an extended and unknown length of time. To control for this possibility, in Experiment 2 we modified the task so that the rats’ decision point would have to occur during or after protrusion of the nose through the slot. To accomplish this, we used fans at the rear of the reaching box to provide positive pressure that would blow the odorant away from the rat and back out the slot at the front of the chamber, as well as a fan under the pellet well to provide negative pressure. This manipulation forced the rats to place their noses as close to the pellet as possible and sniff deeply to make an accurate discrimination. These deep sniffs were easily identifiable in the video records of Experiment 2, in which seven further rats were run, and made it possible to videocode up to three sniffs occurring after the rat had placed its nose far through the slot. Moreover, with the increased confidence this odor presentation method brought us, we performed additional analyses on the neural data in Experiment 2 (see Data Analysis section below).
Videocoding
All training and testing trials were videotaped at 30 frames/s, but the field-by-field analysis provided by our VCR system (Lafayette Instruments, Lafayette, IN, USA) allowed us to have 17 ms resolution between codable fields. The timestamping of our video fields was in turn provided by a time code generator that used the neural data acquisition’s master clock, so that all data acquisition was synchronized. Rats typically approached the front of the reaching chamber, sniffed 1–3 times, and then lifted their paw (nearly 100% of the time on GO trials) or froze (NO-GO trials). To code the sniffs, we recorded the timestamped constriction of the nostrils that followed an immediately prior field in which they were flared. The Final Sniff was designated as the last sniff prior to lifting the paw (GO trials) or freezing (NO-GO trials). To code the lifting of the paw on GO trials, we recorded the moment the wrist began to extend, beginning to straighten the paw as it moved upward.
Electrode implantation
Under isoflurane anesthesia, rats in Experiments 1 and 2 were stereotaxically implanted with eight-wire bundles in the posterior piriform cortex ipsilateral to the reaching hand (2.3 mm posterior to bregma, 5.0 mm lateral, and approximately 8.3 mm deep), 2 × 8 electrode arrays in the contralateral M1 (0.5 mm anterior to bregma, 4.0 mm lateral, and approximately 1.6 mm deep (Paxinos and Watson 2005) and eight-wire bundles in the contralateral magnocellular red nucleus (5.9 mm posterior to bregma, 2.9 mm lateral angled medially at 16°, and approximately 7.3 mm deep). We targeted the output layer 5 of M1 and the output layers 2/3 of the pPIR and confirmed satisfactory electrode implantation with post-mortem histology.
Our choice of recording sites reflected our study of a well-learned, reliably executed olfactomotor task. For instance, this task is known to be olfactory-driven (Whishaw and Tomie 1989). We therefore chose to record from the paleocortical pPIR because it is the main output region of the extended olfactory system (Litaudon et al. 1997; Haberly 2001) and it monosynaptically projects to the orbitofrontal cortex, entorhinal cortex, basolateral amygdala, and other sites involved in motivationally based decision-making (Johnson et al. 2000; Haberly 2001). Also, the pPIR has been strongly implicated in complex odor task learning (Chabaud et al. 1999; Haberly 2001; Mouly and Gervais 2002), that in rats is thought to be on a par with the executive-task abilities displayed by primates in the visual and auditory domains (Otto and Eichenbaum 1992; Dusek and Eichenbaum 1997; Dusek and Eichenbaum 1998; Schoenbaum and Setlow 2001). We recorded from the rat isocortical, caudal M1 and the subcortical, nuclear mRN because both areas (Kolb et al. 2000) are involved in the processing underlying successful performance of the skilled reaching maneuver required for GO trials on our task (Whishaw and Gorny 1996; Hyland 1998; Hermer-Vazquez et al. 2004). Also, the caudal M1 shows extensive plasticity as the skilled reaching task is learned (Kleim et al. 2002). We implanted the ipsilateral posterior piriform because piriform cortices have been shown to process bilateral information (Wilson 1997). We targeted the output layer 2 of the pPIR, the forepaw region of layer 5 of M1, and the center of the mRN. All electrodes were 50 µm at the tip, stainless steel, and Teflon-coated, fabricated by NB Labs (Denison, TX, USA). Most rats received implants in all three of the studied areas.
Recordings
Multi-single unit activity and local field potentials were recorded simultaneously using a Multi-neuron Acquisition Processor (MNAP) designed by Plexon, Inc. (Dallas, TX, USA). Units were high-pass filtered to remove 60 Hz artifact and sampled at 40 kHz. Waveform-based discrimination of single units was used, with the first two principal components analysis, K-means clustering and spike amplitude histograms employed in defining each cell. Local field potentials were low-pass filtered at 100 Hz and sampled at 1 kHz.
Confirmation of electrode placement
During electrode implantation surgery, we recorded units and LFPs as the implants descended and looked for several physiological markers of a correct trajectory toward each of the three target sites. For example, during implantation into the forelimb M1, tapping the contralateral forepaw should result in a punctuate, “ch–ch” response in layer 4, but a more complex train of action potentials in layer 5 (J. K. Chapin, unpublished data). Regarding the mRN, at the right dorso-ventral level one should begin seeing action potentials of extremely large amplitude, with a high yield of neurons on each wire (consistent with the large and densely packed cells in that area). Also, at light anesthesia levels, tapping all over the upper body should result in a (weak) somato-sensory neural response (R. Hermer-Vazquez and L. Hermer-Vazquez, unpublished data). Finally, descent into the pPIR is indicated by a very quiet zone just above layers 2/3, followed a few hundred microns deeper by action potentials of moderately large amplitude. If one accidentally passes through this area, odorant presentation under light anesthesia results in a visible LFP deflection in layer 1.
We present data from relatively newly implanted animals here (~within 2 months of implantation surgery), although in some cases animals’ implants yielded excellent unit and LFP data for considerably longer. Once the rats’ headcaps had become loose, we perfused all animals with 10% phosphate-buffered formalin, and either cut sections near the implant sites at 30 µm, and stained them with neutral red, or sent the brains out for professional histological processing, usually with thionin staining (Wax-It Histology Services, Vancouver, BC, Canada and Histology Tech Services, Gainesville, FL, USA). Data from implants found to have missed their targets were discarded. See Results for final numbers of rats, recording sites and units accepted for analysis.
Data analysis
All data analysis was performed with NeuroExplorer (Dallas, TX, USA), Statistica (Tulsa, OK, USA), Matlab (The Mathworks, Natick, MA, USA), and AutoSignal (SPSS Science, Chicago, IL, USA) software.
Before performing detailed data analyses, we screened all unit time series with autocorrelograms to ensure that a 1–3 ms refractory period was visible (approximately 90% of all unit time series were retained as a result), to determine that the data from a given “unit” did not contain spike times or waveforms from additional neurons. LFPs were screened against railing and 60 Hz artifact, and were linearly detrended to remove DC offset.
Testing of units for beta and gamma oscillations
For units from both experiments, we made autocorrelograms for spike firing during the Sniff-GO window versus the Sniff-NO-GO window. This window was defined the period from 50 ms before the videocoded Final Sniff time to 50 ms afterward, as determined by several factors: (1) prior results showing unit modulation during that task phase in that time window, (2) the correspondence between a sniff, in general, and the length of one theta cycle (Kepecs et al. 2006), and (3) Monte Carlo simulations to find a window in which oscillations for the two trial types (GO and NO-GO) were maximally distinguished. This period also correlated well with our prior finding that unit firing rate modulation tended to occur within a ~100 ms window (Hermer-Vazquez et al. 2004). We then systematically checked autocorrelograms at binwidths of 2, 5, 10, and 25 ms to determine the proportion of cells in each area for each rat that were oscillating above a 99% confidence level (above the background firing rate and assuming a Poisson distribution to calculate the window boundaries) in the beta, low gamma or high gamma band during the 100 ms Sniff-GO window versus the Sniff-NO-GO window. The tested binwidths were chosen to sample for oscillations with different periods (e.g. the smaller bindwiths would be sensitive to higher frequency oscillations, as per the Nyquist limit). After exploratory analyses with the different bin sizes, we settled on autocorrelograms for beta-frequency oscillations with a 10 ms binwidth, low gamma oscillations with a 5 ms binwidth, and high gamma oscillations with a 2 ms binwidth. To more easily view oscillations in each frequency range, exploratory Gaussian smoothing was also employed. In several cases during the analyzed sniff periods, cells displayed a higher frequency of oscillatory activity embedded in a lower frequency of oscillations. When such frequency nesting occurred, and the peaks were not characteristic of the spurious type of peaks discovered by Bar-Gad et al. (2001), both oscillatory frequencies were counted for the same unit.
We performed analyses of variance (ANOVAs) to test our hypothesis that more units would oscillate in the beta band during the Final Sniff-GO window. The dependent measure was the percentage of units oscillating in a given frequency range during the Final Sniff period (−50 to +50 ms). Independent factors for these analyses were rat (eight in Experiment 1 and seven in Experiment 2), brain area (3: pPIR, M1, and mRN), frequency band (3: beta, low gamma, and high gamma), and trial type (2: GO or NO-GO). We followed up on significant findings with Bonferroni-corrected t tests (i.e. where the post hoc P value is determined using (uncorrected P − α/N), where N is the total number of post hoc tests.
To rule out spurious oscillatory bursts caused by the interaction between a cell’s firing pattern and its refractory period, we only included units displaying statistically significant oscillations with a period greater than two times its apparent hyperpolarization phase (Bar-Gad et al. 2001).
The remainder of analyses in this report made use of local field potentials
LFP normalization prior to wavelet decomposition
The issue of normalizing for absolute power across local field potentials from different brain areas or rats is a technical challenge, and here we describe our approach. First, all LFPs were filtered with a 1-pole Butterworth filter between 0.3 and 93 Hz. All sampling of the LFPs was done at 1 kHz and all gains between sites and animals were kept constant. In 90% of LFPs, voltage values fell within consistent upper and lower bounds of ±100 µV, with few LFPs of significantly smaller amplitude. This gave us confidence that the dipoles generating the local field potentials were of sufficient strength to be directly compared across animals and brain areas. All LFPs were detrended for slow DC offset, centered with a mean of 0 µV, and screened against 60 Hz artifact as well as movement artifacts (to the extent that is possible, e.g. by checking for railing) during the reaching trials.
Confirmation of structure in the nonstationary LFP time series
To verify that the LFPs contained significant structure despite their “noisy” appearance, we employed a method—calculation of the Hurst coefficient (Bassingthwaighte and Raymond 1995) for each trial’s LFPs—designed to detect multi-scale structure in non-linear, nonstationary time series such as brain local field potentials. Virtually all our analyzed LFPs had Hurst coefficients ≥0.8, indicating a degree of long-term correlation well above the random value of 0.50. We therefore retained these LFPs for further analyses.
Wavelet decompositions
Once we carried out the above procedures, continuous wavelet transforms on the LFPs using the Morlet mother wavelet (Dear and Hart 1999) produced both real (Re) and imaginary (Im) coefficients reflecting the magnitude of the correlation between the mother wavelet used and the LFP. Coefficients were then converted to the decibel (dB) scale [10.0 × log10 (Re × Re + Im × Im)]. Zero dB was assigned to the time and frequency coefficients representing the highest energy zones (time/frequency), which for 98% of our LFPs occurred in a very specific frequency band, 3–12 Hz. This overlaps strongly with what is generally considered to be the theta band, and sniffing, heartbeat and movement tend to be synchronized when the animal is engaged in foraging or other exploratory behaviors. High energy theta oscillations were clearly evident in our LFPs, and since we normalized our data to the energy in that frequency band, we of course did not analyze relative energy in that band across GO and NO-GO trials.
We next determined how many dBs lower in energy we could get reliable signals from a given LFP before background noise was detected as a visible time-frequency event (i.e. in the color rendering, it differed from the no-energy color; see below). We used our ~100 Hz cutoff as a way to further normalize the LFPs: We adjusted the energy scale until we saw a lack of significant signal energy beyond approximately 120 Hz. When the dB range was too large, we would begin to see frequency-time artifacts—known as such because they were beyond our filter cutoff frequency.
Once the dB spread was calculated, a color scale having 24 equal partitions from high energy yellow to low energy light red, was used to render a bivariate spline interpolant used for smoothing the image. This procedure effectively allowed us to view the LFP’s signal energy (or frequency) evolve through time. A behaviorally defined frequency and time range corresponding to key task events, such as odor-induced sniffs, could then be isolated from the rest of the LFP time series and further analyzed.
In addition to constructing renderings of single-trial, wavelet-decomposed LFPs, we made wavelet figures for GO versus NO-GO trials that were averaged across multiple rats and trials. Because the rats’ total trial length as well as task phase length differed across trials and rats, we selected GO-trials with approximately the same overall length (±100 ms) in time (from the Final Sniff to Contact) and cross-correlated the sniff period across trials, thereby selecting a behaviorally determined, transient, high gamma event. Within this window the maximal cross-correlation coefficient was selected and used to “center” the time-series. We then averaged the waveforms over time and plotted the resultant continuous wavelet transform.
MANOVAs to test for differences across trial types, rats, and brain areas using the wavelet-decomposed, color-rendered LFP data
We performed multiple MANOVA-based analyses on the wavelet-decomposed LFPs across animals, brain areas, and single GO versus NO-GO trials for Experiment 1 and the more controlled Experiment 2. Because of the additional control in Experiment 2, a more extended set of analyses was conducted on these data. The first analyses were MANOVAs to assess the significance of wavelet-decomposed energy patterns between GO and NO-GO trials, brain regions, and rat. For Experiment 1 these MANOVAs were only conducted on the energy in the Final Sniff period, whereas for Experiment 2 they were conducted (1) separately on Sniff1, Sniff2, and Final Sniff data, and (2) on data from all the sniff periods together. When data from multiple sniff periods were used, “sniff number” was employed as a repeated-measures, within-subjects variable. Figure 2 graphically depicts the design of these MANOVAs, which are further described below.
Fig. 2.
Design of the multivariate analyses of variance (MANOVA) performed on the wavelet-decomposed, local field potential (LFP) measurements in Experiment 1 (only for Final Sniff data; last four ticks on the x-axis) and on data from all three sniff periods for Experiment 2. The x-axis depicts 25 ms bins of data surrounding Sniff1 (first four ticks with the videocoded Sniff1 time at the center), Sniff2 (the next four ticks with the videocoded Sniff2 time at the center), and the Final Sniff (last four ticks with the videocoded Final Sniff time at the center). The y-axis depicts frequency bands, ranging from 12–20 Hz (beta), 20–30 Hz (beta), and so on up through 90–100 Hz (high gamma). The wavelet correlation coefficients yielded by dividing the sniff period data into these squares were then averaged within each square, providing the dependent variables for the analysis. The colored rectangles dividing the grid into three regions show the frequencies (beta, low gamma and high gamma) used as an independent variable in the analyses. Other independent variables included rat, brain region and most importantly, trial type (GO or NO-GO)
To conduct these analyses, we first had to convert the continuous, color-rendered correlation coefficients for each time-frequency-decomposed LFP (i.e. the wavelet correlation coefficients across frequency and time) to a form suitable for MANOVAs. We prepared the data for these MANOVAs as follows, diagrammed in Fig. 2 for the more complex design used in Experiment 2. For both experiments, the x-axis was for time bins occurring just prior to, or just after, a videocoded sniff such as the Final Sniff and the y-axis was for frequencies from 12 to 100 Hz. For Experiment 1, four x-axis time bins for the Final Sniff period were designated: from 50 to 25 ms prior to the coded Final Sniff time, from 25 ms before the coded Final Sniff time to the actual coded time, from the coded time to 25 ms later, and from +25 ms to +50 ms after the coded time. For data from Experiment 2, a similar procedure was used except that data from the time periods centered around Sniff1 and Sniff2 were used in addition to the Final Sniff data, yielding 12 x-axis divisions rather than 4.
After this partitioning of the x-axis of the wavelet output rendering, the y-axis frequency data were divided into nine frequency ranges. The bottom row of the matrix was for 12–20 Hz coefficients (which ranged from 0 to 1.0) across the three sniff periods; the next row upward was for 20–30 Hz coefficients (with those two rows comprising the beta range); the next two rows were for 30–40 and 40–50 Hz, comprising the low-gamma range; and finally, rows from 50–60 up to 90–100 Hz comprised the high-gamma range. Therefore, each of the three coded sniffs had their own set of bins demarcated along the x-axis (sniff-time) and y-axis (frequency band).
The coefficient averages from each square were then used for the MANOVAs. The general design of these analyses was: rat (eight for Experiment 1 and six for Experiment 2) × brain region (3: pPIR, M1 and mRN) × frequency band (3: beta, low gamma and high gamma) × trial type (2: GO and NO-GO) (and for Experiment 2, 3:sniff number), with each square’s average energy correlation coefficient (from 0 to 1) across the four 25-ms time periods relating to each of the coded sniffs as the dependent variables. Given the large number of degrees of freedom used by these factors, and because when we normalized all the wavelet graphs so that the theta range had the maximum energy, we performed preliminary analyses to see whether we needed to retain rat and brain area as factors. As a result, for some analyses the design was simplified to brain region × frequency band × trial type. We performed the MANOVAs on the individual sniff periods first (e.g. on the Final Sniff data alone), and then combined all the data in Experiment 2 using a repeated-measures design with the additional factor of Sniff Number (3: Sniff1, Sniff2 and Final Sniff).
Transient phase-locking, entropy and coherence in single trials across pairs of recorded brain areas
Finally, with data from the more controlled Experiment 2, we examined whether LFP time series from pairs of brain regions exhibited transient phase-locking, and quantified the degree of phase-locking with both instantaneous correlation coefficients and entropy calculations. Recently, several methods for calculating transient phase-locking in nonstationary neural data have been published. We chose a method developed by Hurtado et al. (2004), which allowed us to calculate the instantaneous phase and amplitude for each oscillation. The difference in phase calculated between the oscillators is then presented as a time-evolving trajectory, emphasizing moment to moment changes in the phase relationship between the two oscillations. This further allowed us to use phase-locking indices such as coherence and entropy to quantify the phase relationship. We modified the above procedure by starting with a wavelet decomposition instead of a finite filter, so that we could both identify and filter the moment of gamma oscillations co-occurring with a given sniff period with one procedure.
Results
Behavioral results
The first phase of behavioral training took place before the surgery, when the rats were taught the GO-only version of the reaching task (i.e. every trial had a real chocolate or banana pellet in the well contralateral to the reaching paw). Since the behavioral results were nearly identical across the two experiments, Fig. 3 depicts the behavioral results averaged across both cohorts of rats, including the Sniff1 and Sniff2 times coded for rats in Experiment 2. The eight rats retained after postmortem histology in Experiment 1 as well as the seven rats retained in Experiment 2 (see below) reached their first day of asymptotic task accuracy on day 6 of training and were determined to have settled on that accuracy level, in which the food target was obtained on approximately 70% on trials, by day 10. In this phase of behavioral training, handedness was determined, and therefore also the brain hemisphere in which each electrode implant would be placed.
Fig. 3.
Timeline for real pellet (GO) trials averaged across animals in Experiment 1 (Final Sniff to Contact only), and averaged across animals in both experiments (for Sniff1 to Contact). Standard deviations are provided in parentheses, with the following ranges for the sniff and movement onset periods: Sniff 1 to Sniff 2, 101–370 ms; Sniff 2 to Final Sniff, 70–550 ms; and Final Sniff to Lift, 78–170 ms. The standard deviation for the Sniff1. The mean time between the Final Sniff and lifting the paw on GO trials was 105 ms, and the total trial length was 355 ms on average
The second phase of the behavioral portion of the experiment occurred after surgery, when rats were presented with the GO/NO-GO version of the task. Rats performed the GO/NO-GO aspect of this task with high accuracy, i.e. they reached on >95% of GO trials and abstained from reaching on NO-GO trials with a similar accuracy, after only one or two exposures to the vanilla, acetone, or plastic-only NO-GO targets. In videocoding these trials, it was found that all rats sniffed the target at least once before lifting their paw or freezing, and usually sniffed two times (although three and sometimes even more sniffs occurred). The inter-sniff times were generally consistent with a single theta (4–11 Hz) cycle. Also, the standard deviations of the time between task events (Sniff 1 and Sniff 2, for example, or Final Sniff and Lift) grew smaller as the time to lift approached, probably for two reasons: (1) because there were more Final Sniff-to-Lift periods than prior periods, and (2) because the behavioral variance, in concert with the neural variance analyzed in other studies with reaching tasks (Grammont and Riehle 1999), became smaller as the Lift approached (Fig. 3). Finally, it was found that the time period between the Final Sniff and the Lift was never shorter than twice the resolution of our videocoding session, indicating that all Final Sniffs and Lifts were physically separate events.
Experiment 1: Neurophysiological results
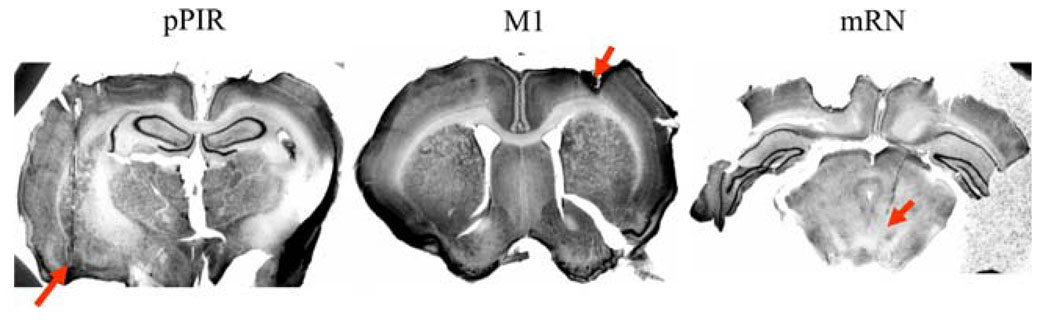
After analyzing the histological results from the 11 rats in Experiment 1, we retained eight rats’ M1 data, eight rats’ red nucleus data, and four rats’ posterior piriform unit and LFP data. Across these rats and brain areas, and after checking the units for sufficient isolation with autocorrelograms and interspike interval histograms, we recorded a total of 316 M1 neurons, 113 red nucleus neurons, and 88 posterior piriform neurons. Four rats had simultaneously recorded, well-isolated units plus high-quality LFPs in all three implanted sites. Figure 4 shows histology from the three implanted sites in a representative rat. Figure 5 shows representative units and LFPs simultaneously recorded in a single rat from the pPIR, M1 and mRN.
Fig. 4.
Thionin-stained sections of electrode placement in the posterior piriform cortex layers 2/3, the caudal forelimb motor cortex layers 5/6, and the dorsal portion of the magnocellular red nucleus. Correct anterior–posterior placement is indicated by the appearance of other structures in the images, such as the large caudate/putamen in the M1 image. The electrode tips extend slightly beyond the visible tracks. Differences in contrast across the images resulted from slightly different exposure times during image digitization. The rat presented here had pulled its cap out, resulting in some brain surface damage
Fig. 5.
Isolated units (which later displayed a ~2 ms notch at the center of their autocorrelograms) from wires located in the pPIR (left), M1 (center) and mRN (right), and single-trial LFPs from the same wires. Note that in two-dimensional principal components (PC) space, all units are not at the origin (indicating that they were not pure noise) and that for the M1 units, all three are well-separated in PC space. For the LFPs, note that they do not exceed ±100 µV and at the same time, they are all of high and similar magnitude across brain areas
Autocorrelograms of units during the Final Sniff period
We used autocorrelograms to determine (a) whether units oscillated in any frequency band during a 100-ms window centered on the Final Sniff of the pellet, (b) whether all recorded brain regions displayed neurons with similar or different oscillatory characteristics, and (c), whether spike oscillations in any frequency band in any area distinguished GO from NO-GO trials. Some neurons in each recorded area oscillated in the beta range (12–30 Hz), the low gamma range (30–50 Hz), and the high gamma range (50–100 Hz) during the time period from −50 to +50 ms surrounding the coded Final Sniffs. We collapsed the data across the different GO odors (a roughly equal number of banana and chocolate pellet trials), on the one hand, and the NO-GO odors (vanilla, acetone, and plastic-only beads, with substantially fewer acetone trials than vanilla or plastic-alone ones), on the other, for the remaining analyses. Also collapsing across trial type, we found that a mean of 35.0% of cells displayed beta oscillations (SD = 12.5%), and a mean of 16.2% cells displayed low-gamma oscillations (SD = 16.0%). Approximately 7% of units in each area displayed high gamma oscillations, consistent with the possibility that they were fast-spiking interneurons (Gibson et al. 2005), Fig. 6 shows representative Sniff-GO oscillations from each recorded brain area in each frequency band.
Fig. 6.
Examples from each recorded brain area (columns) of oscillatory firing during the 100 ms period centered around the Final Sniff on GO trials, in each frequency range (top row beta; middle row low gamma; bottom row high gamma). The dotted lines represent the upper 99% confidence limit as derived from the cell’s baseline firing rate. Autocorrelograms showing beta-frequency oscillations were determined using a 10 ms bin-width and were Gaussian-smoothed over five bins. Autocorrelograms for low- and high-gamma oscillations were determined using 5 and 2 ms binwidths, respectively, and were Gaussian-smoothed over three bins
Some units that oscillated at a lower frequency during the Sniff-GO window also demonstrated higher-frequency oscillatory behavior when viewed at shorter binwidths. The most common classes of this type were low gamma oscillations embedded in low beta oscillations, and high gamma oscillations embedded in low gamma oscillations (see Fig. 7 for an example of the latter class). When cells exhibited significant oscillatory behavior in more than one frequency band, all bands with statistically significant oscillations were counted (e.g., a single unit could count as having both beta and low gamma oscillations). However, such instances were relatively rare, occurring for at most 5–10% of neurons.
Fig. 7.
Example of a pPIR unit displaying high gamma oscillations nested within its low gamma oscillations. In both experiments, 5–10% of cells displayed oscillatory behavior in two frequency bands, as viewed with different binwidths
We performed an ANOVA to test whether any frequency occurred more commonly than the others, whether neurons from different brain regions exhibited different-frequency activity, and whether trial type was associated with different frequency modulations, during the 100 ms Final Sniff-GO interval. Importantly, the analysis found a significant main effect of trial type (F(3,136) = 11.78, P = 0.000079), with more cells oscillating above their background rates on GO trials than NO-GO trials. There was also a significant effect of frequency band (F(3,136) = 24.78, P < 0.000001), with cells in all three areas showing more beta oscillations than oscillations of other frequencies. Finally, there was a significant effect of brain area (F(2,136) = 9.28, P = 0.000017), with the pPIR displaying more oscillations overall. The analysis also revealed a single significant interaction among the independent measures: between frequency band and trial type (F(3,136) = 12.83, P < 0.00000001), with more units oscillating in the beta band on GO trials (41.4% of cells) than on NO-GO trials (29.0% of cells). Figure 8 displays these results. None of the other tests for interactions approached significance. A Bonferroni-corrected t test further supported the conclusion that there were more unit beta band oscillations during the Final Sniff on GO trials (t(159) = 3.50, corrected P = 0.0007). Bonferroni-corrected t tests also demonstrated that that in the pPIR, there were significantly more unit beta band oscillations during the Final Sniff on GO trials (t(7) = 4.01, corrected P = 0.0054), marginally more in M1 (t(13) = 2.16, corrected P = 0.08), and significantly more in the mRN (t(13) = 4.95, corrected P = 0.007; again, see Fig. 8).
Fig. 8.
Percentages for each recorded brain area of units oscillating in the beta frequency range (12–30 Hz) on real-pellet, GO trials (solid bars) versus NO-GO trials (horizontally striped bars) during the Final Sniff period. The single asterisk denotes a Bonferroni-corrected t test significant at P ≤ 0.05, and the double asterisk denotes significance at the P ≤ 0.01 level
Joint time-frequency analyses of LFPs on single trials
Having found a unit-level oscillatory difference between GO and NO-GO trials, we turned to analyses of the local field potentials. For the joint time-frequency analyses, we selected all of the NO-GO pellet trials and randomly chose an equal number of real pellet trials, yielding a total of 148 trials for joint time-frequency decomposition (with each of the eight rats supplying 2–8 trials for at least two of the three recorded brain regions). Figure 9a shows the continuous wavelet decomposition of LFPs from each brain region on the same single trial, normalized from highest to lowest energy using a dB. Figure 9b shows the same neural populations on a NO-GO trial. It can be seen that on both trial types, there is substantial energy in the theta and beta bands throughout most of the trial. In contrast, there is considerably less low gamma activity (30–50 Hz) throughout the NO-GO trial. But the key difference that we focus on here is in the high gamma range (50–100 Hz) during the Final Sniff period. As demarcated by the green dashed lines on the figure, there is abundant high gamma energy during this period on GO trials, and very little of this energy on NO-GO trials. This was true regardless of which odor (chocolate or banana) was the GO odor and which odor (vanilla, acetone, or plastic-only) was the NO-GO odor. Therefore, for analyses involving multiple wavelet outputs, we averaged across GO odor types, on the one hand, and NO-GO odor types on the other hand. Furthermore, this overall pattern of results was similar across the recorded brain regions, although for the further LFP analyses we did not average across brain regions.
Fig. 9.
Continuous wavelet decompositions of representative single- trial local field potentials from each recorded brain area on a real-pellet, GO trial (top row) and a plastic-only, NO-GO trial (bottom row). The x-axis shows time in milliseconds, and the y-axis shows a variable called “scale,” which corresponds to oscillatory frequency. The actual frequencies can be obtained by multiplying the y-axis numbers by 1,000, yielding a frequency range from 0 to 100 Hz (as indicated on the y-axis label). For more details on performing and rendering the wavelet decompositions, see Methods
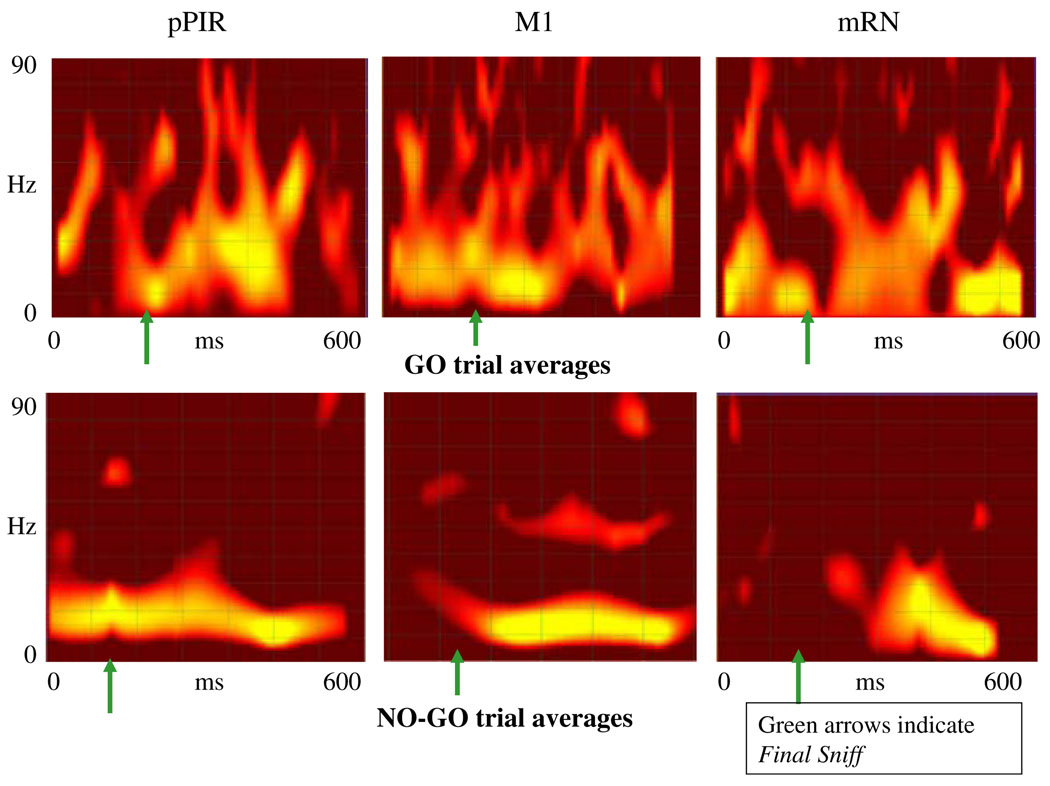
Figure 10 shows averaged wavelet renderings for each of the three areas on GO versus NO-GO trials from multiple rats and trials, aligned to the common reference point of the coded Final Sniff moment. The highly non-stationary nature of the data from these trials resulted in some jitter in the combined the images, but certain key differences between the GO and NO-GO averaged figures are clear nonetheless. In all three areas, above-background low gamma (30–50 Hz) and high gamma (50–100 Hz) activity can be seen during the Final Sniff window with the real pellet just before lift onset as well as throughout the rest of the trial. In contrast, NO-GO trials seldom appeared to contain detectable high-gamma energy during the Final Sniff period before target rejection. Although other differences in the LFP joint time-frequency graphs are visible between GO and NO-GO trials, we focus here on differences contained within the Sniff-GO and Sniff-NO-GO time periods.
Fig. 10.
Average wavelet figures for each brain region and trial type, summing across three trials from three animals for each image. The axes are the same as for the graphs in Fig. 9. For the averaging process, trials whose Final Sniff-to-Contact length was similar to within 100 ms were used. Their wavelet coefficients were then aligned to the Final Sniff moment
Amplitude in each energy band as a function of trial type
We quantitatively investigated these apparent frequency and energy differences with analyses of variance. Preliminary analyses found no effects of brain area (F(2,172) = 2.26, P = 0.116) or rat (F(7,167) = 1.62, P = 0.133), most likely a result of the normalization of all trials so that the theta band had the highest energy. We therefore dropped those factors from the analyses. MANOVAs performed on the energy intensity during the Final Sniff of the real food pellets versus the NO-GO pellets across the beta, low-gamma and high-gamma bands (12–30, 30–50, and 50–100 Hz, respectively) confirmed that while all three bands displayed an increase on real-pellet trials, high-gamma energy increased the most, by over 400% (Fig. 10, top graph: F(2,1578) = 10.06, P < 0.0001). Although the most energy overall was in the beta range, the greatest percentage increase in energy between real and artificial pellet trials was in the high-gamma range.
As a footnote analysis, we also checked for the significance of brain-wide high-gamma energy on GO trials and NO-GO trials, when either all three recorded sites displayed energy in this band or all three recorded sites did not. We found that out of 74 GO and 74 NO-GO trials, 100% of trials displaying high-gamma energy on the Final Sniff across all three brain sites were GO trials (F~∞). In contrast, on trials when no recorded brain area displayed any detectable high-gamma energy, 100% were NO-GO trials (F~∞).
Experiment 2: Neural correlates of the olfactory-driven GO/NO-GO task with controlled odorant delivery
This experiment was conducted to test whether Experiment 2’s neural results would be replicated in a task with more highly controlled odorant delivery. In this experiment, the rat could not detect the presence or absence of the GO odor prior to protrusion of its nose as far as possible through the slot, allowing a clearer determination of the boundaries of the Sniff-GO and Sniff-NO-GO windows. To carry out this experiment, we employed three fans at the rear of the reaching chamber to blow the odor plume away from the rat and out the front of the chamber through the front slot (at 2.8 m/s), plus an additional fan providing negative pressure below the pellet wells, sucking the pellet’s odor downward, as described in the Methods section. This resulted in a decrement in performance of the task: Unlike the rats in Experiment 1, rats in the fan condition reached on 86% of GO trials (rather than 96% of them as in Experiment 1) and inhibited reaching on 76% of NO-GO trials (rather than 100% of them as in Experiment 1). Rats performed the task while simultaneous recordings of pPIR, M1 and mRN activity were made, as in Experiment 1. Video analyses confirmed that rats in this experiment placed their noses as physically close as possible to the pellet wells and sniffed deeply on both GO and NO-GO trials, thus appearing to achieve our behavioral goal. Furthermore, the extended period of time during which the nose was projected through the slot made it easier to code sniffs prior to the Final Sniff. All trials in Experiment 2 had two sniffs while the nose protruded through the slot, and about 35% of trials had an earlier sniff. For the remainder of this report we refer to these sniffs as Sniff1, Sniff2 and Final Sniff. Figure 3 shows the timing of these additional sniffs.
Experiment 2: Neural results
After analyzing the histological results from the seven rats in this experiment, we retained six rats’ M1 data, all seven rats’ red nucleus data, and all seven rats’ posterior piriform data. Across these rats and brain areas, and after checking the units for sufficient isolation, our dataset included 48 M1 neurons, 68 red nucleus neurons, and 67 posterior piriform neurons. We also obtained local field potential recordings from each rat and area listed above, except for one rat whose LFPs in all implanted areas railed frequently. Six of the seven rats had acceptable units and LFPs in all three implanted areas. With Experiment 2’s better-designed behavioral task, we carried out an extended series of data analyses, as described in the Methods section. Overall, the neural results from Experiment 2 both replicated and extended Experiment 1’s findings.
Unit oscillations during the Sniff-GO and Sniff-NO-GO windows
Using our results from Experiment 1, we conducted planned t tests to test our hypotheses that (a) a larger subpopulation of units would fire in the beta range (12–30 Hz) on GO trials than on NO-GO trials, and (b) that there would be no other trial-type by frequency band interactions. These predictions were borne out in the data: A subpopulation of units across all recorded areas increased their beta-frequency (12–30 Hz) spiking during the Final Sniff on real pellet trials as compared to NO-GO pellet trials (43.8% of cells on banana or chocolate real pellet trials versus 28.4% of cells on plastic-only, vanilla or acetone pellet trials; t(38) = 7.606, P = 0.0135). In contrast, unit oscillations in the other frequency bands during the Sniff-GO versus the Sniff-NO-GO period did not differ significantly.
Local field potential analyses and results
Next, we prepared the wavelet-decomposed data for a series of MANOVAs as described in the Methods section and depicted in Fig. 2. First we performed the MANOVA on the Final Sniff data alone, as in Experiment 1. As before, we checked to see whether the factor rat needed to be retained, and it did not (F(12,4009) = 1.46, P = 0.132). The results of the MANOVA on the Final Sniff data are presented in Table 1. As the table and the Final Sniff portion of Fig. 11 show, there is a large effect of trial type, with more energy overall (i.e. higher wavelet coefficients) on GO than on NO-GO trials. There is also a large effect of frequency band, with much more energy in the lower bands, particularly in the beta range. There is a relatively small effect of brain region, which was not seen in Experiment 1, but which here came from the pPIR having overall higher wavelet coefficients across frequency bands. Finally, there was a small but significant interaction between trial type and frequency, with GO trials having more energy in the higher frequency bands.
Table 1.
Results of the MANOVA performed on the Final Sniff, wavelet-decomposed LFP data from Experiment 2
| rFactor/interaction | F ratio | Significance |
|---|---|---|
| Trial type | F(4,1581) = 100.00 | P < 0.00001* |
| Region | F(8,3162) = 3.20 | P = 0.001* |
| Frequency band | F(8,3162) = 156.50 | P < 0.00001* |
| Trial type × region | F(8,3162) = 1.20 | P = 0.288 |
| Trial type × freq | F(16,4831) = 1.94 | P = 0.050* |
| Region × freq | F(16,4831) = 1.20 | P = 0.263 |
| Trial type × region × freq | F(16,4831) = 1.19 | P = 0.30 |
Fig. 11.
Rendering of the wavelet decomposition correlation coefficients across sniff periods (x-axis) and frequencies (y-axis) for the MANOVAs including all three sniff periods. Dark blue depicts the lowest amounts of energy in a given frequency band and sniff period, and bright red depicts the highest amounts of energy. Top, average of all wavelet-decomposed GO trials in Experiment 2; bottom, average of all wavelet-decomposed NO-GO trials in the same experiment. For more details on the color rendering and analyses, see Methods
MANOVAs on Sniff1, Sniff2 and Final Sniff data
Although our assumption had been that the rat’s main “decision-making” period occurred between the Final Sniff and the Lift, we examined prior sniff data to gain insight about when the rats may have been recognizing the S+, “deciding” to move, and planning the movement (the design of this experiment does not allow us to view those processes separately). Rats had a codable Sniff1 on only about 35% of trials, whereas all rats in this experiment had a Sniff2 along with a Final Sniff. An initial MANOVA on the Sniff1 data found no effect of rat (F(12,1151) = 0.44, P = 0.948) so once again this factor was omitted from subsequent analyses. As can be seen in Table 2 and in the Sniff1 portion of Fig. 11, strikingly, during the Sniff1 period, GO and NO-GO trials did not differ in their overall amount of energy. In fact, the analysis found neither a main effect of trial type or interactions involving trial type with other significant factors. As before, however, there was an effect of brain region, with the pPIR exhibiting higher energy overall, and an effect of frequency band, with more energy in the lower bands. Also as before, there was a significant interaction between brain region and frequency band, with the mRN showing more energy in the beta band and the M1 and pPIR showing more energy in the low gamma band (data not shown). However, the main finding of this analysis, in contrast to analyses on the Final Sniff data, was a lack of effects or interactions involving GO versus NO-GO trials in the decomposed Sniff1 LFP data.
Table 2.
Results of the MANOVA performed on the Sniff1, wavelet-decomposed LFP data from Experiment 2
| Factor/interaction | F ratio | Significance |
|---|---|---|
| Trial type | F(4,510) = 0.40 | P = 0.814 |
| Region | F(8,1020) = 6.10 | P < 0.001* |
| Frequency band | F(8,1020) = 159.20 | P < 0.00001* |
| Trial type × region | F(8,1020) = 1.00 | P = 0.483 |
| Trial type × freq | F(16,1020) = .30 | P = 0.972 |
| Region × freq | F(16,1559) = 2.80 | P < 0.001 |
| Trial type × region × freq | F(16,4831) = 1.00 | P = 0.431 |
In contrast, it can be seen from Fig. 11 that the Sniff2 data differ markedly between GO and NO-GO trials. The factor rat was omitted from the final Sniff2 MANOVA even though it was marginally significant (F(12,4009) = 1.60, P = 0.084), to keep the Sniff2 analysis comparable to the Final Sniff and Sniff1 analyses. Table 3 presents the results of this analysis. Unlike Sniff1 GO and NO-GO trials, the trial types differed strongly during the Sniff2 phase of the task, with substantially more energy in all frequency bands on GO trials. There was also a large effect of frequency band, with more energy in the lower ranges, as seen before, and a relatively small main effect of brain region, with the pPIR having more energy overall (data not shown). Importantly, however, there was absolutely no interaction between trial type and brain region, indicating that all three areas displayed more LFP energy on GO trials There were significant interactions between trial type and frequency band (with the highest frequencies showing the greatest percentage increase (Fig. 11), and brain region and frequency band (with more high frequency energy in the pPIR, as in the Final Sniff data). A possible interaction among trial type, brain region and frequency was marginally significant, with the pPIR appearing to show more high frequency energy on GO trials. Overall, the results for the Sniff2 data resembled those for the Final Sniff.
Table 3.
Results of the MANOVA performed on the Sniff2, wavelet-decomposed LFP data from Experiment 2
| Factor/interaction | F ratio | Significance |
|---|---|---|
| Trial type | F(4,1581) = 64.60 | P < 0.00001* |
| Region | F(8,3162) = 3.80 | P < 0.001* |
| Frequency band | F(8,3162) = 166.40 | P < 0.00001* |
| Trial type × region | F(8,3162) = .50 | P = 0.879 |
| Trial type × freq | F(8,3162) = 2.63 | P = 0.018* |
| Region × freq | F(16,4831) = 1.90 | P = 0.015* |
| Trial type × region × freq | F(16,4831) = 1.50 | P = 0.084 |
Finally, we performed a repeated-measures MANOVA with the data from all three sniff periods, with the same design as before except for the addition of a within-subjects variable, Sniff Number (3: Sniff1, Sniff2 and Final Sniff). This analysis only used about 35% of the total data because of the paucity of codable Sniff1s. Table 4 presents the results, with many significant findings. Overall, there were large main effects of trial type (with much more energy on GO trials) and frequency band (with the greatest increase seen in the higher ranges), but not an effect of brain region. There were two interactions that appeared to result from higher energy in the pPIR, especially on GO trials: an interaction between brain region and frequency band, and an interaction among trial type, brain region and frequency band. Not surprisingly, given the obvious differences between the Sniff1 data, on the one hand, and the Sniff2 and Final Sniff data, on the other (as seen in Fig. 10), there was a significant main effect of sniff number and significant interactions among sniff number and trial type, sniff number and brain region, and sniff number and frequency band. Finally, there were significant interactions among sniff number, brain region and frequency band, and sniff number, trial type, brain region, frequency band.
Table 4.
Results of the MANOVA performed across all three sniff periods (with Sniff Number as a repeated measure) on the wavelet-decomposed LFP data from Experiment 2
| Factor/interaction | F ratio | Significance |
|---|---|---|
| Trial type | F(4,510) = 37.90 | P < 0.0001* |
| Region | F(8,1020) = 1.60 | P = 0.119 |
| Frequency band | F(8,1020) = 103.70 | P < 0.00001* |
| Trial type × region | F(8,1020) = 1.30 | P = 0.235 |
| Trial type × freq | F(8,1020) = .60 | P = 0.738 |
| Region × freq | F(16,1559) = 2.60 | P < 0.001* |
| Trial type × region × freq | F(16,1559) = 2.30 | P = 0.002* |
| Sniff # | F(8,506) = 40.60 | P < 0.0001* |
| Sniff # × trial type | F(8,506) = 20.40 | P < 0.0001* |
| Sniff # × trial type × freq | F(16,1012) = .70 | P = 0.792 |
| Sniff # × region × freq | F(32,1868) = 2.30 | P = 0.002* |
| Sn # × reg × trial type × freq | F(32,1868) = 1.50 | P = 0.029* |
Returning to the Final Sniff data alone, on trials when all three recorded areas in Experiment 2 displayed high-gamma energy on the Final Sniff, 100% were GO trials (F~∞), and when none of the three areas showed detectable high-gamma energy on the Final Sniff, 100% were NO-GO trials (F~∞).
Analyses of instantaneous phase-locking, entropy and coherence across recorded brain sites on single-trial, Final Sniff data
Using our modified version of the method developed by Hurtado et al. (2004), we found significant, transient phase-locking across all pairs of recorded brain sites (i.e. using LFPs from all combinations of the pPIR, M1 and mRN) in the Sniff-GO window in the high gamma band (r > 0.70). Figure 12 depicts these results across all pairs of brain sites for a single GO trial. For all pairs of brain areas, we analyzed ≥3 trials from each of three rats. We assessed the degree of phase-locking in two ways: with calculations of both coherence and entropy phase indices. These analyses found brief “phase slips” which are likely due to momentary aperiodic activity which has been observed in other neural studies. However, the high phase-locking demonstrated here overall appears to indicate a brain-wide, momentary coherence across all the recorded, task-related areas during the period immediately before and after the Final Sniff.
Fig. 12.
Dynamic phase-locking during sensory-motor coupling on GO trials. The forearm motor cortex, midbrain motor red nucleus and posterior piriform LFPs were compared on a pairwise basis for transient phase coupling in the detected high gamma range during the Final Sniff window. Normalized coherence and normalized entropy are plotted over the sniff-period time in milliseconds, with 1 on the y-axis indicating full phase-locking and 0 indicating no correlation [see the Methods section and Hurtado et al. (2004) for further details]. The gamma-band phase evolution in all three comparisons shows significant and marked phase-locking during the Final Sniff period at a specific frequency. The GO trial analyzed here was selected for display because it conveys the complexity consistently observed in nearly all analyzed GO trials in our experiments
Discussion
During our ~200 ms “coordination window” (Sniff2 and Final Sniff) prior to movement on GO trials of an olfactory GO/NO-GO task, we found an increase in beta-frequency spike oscillations, and an increase in high gamma-frequency local field potential oscillations, that were coherent across all recorded cortical and subcortical sites. Whereas theta and beta oscillations occurred with relatively high energy across both trial types, energy in the low gamma and especially the high gamma range increased markedly during the Sniff-GO window. Multiple psychological processes likely took place during this coordination window, including olfactory cue recognition, decision-making and movement planning. One or more of these processes therefore were associated with the striking, transient increase in coherent brain activity in multiple frequency bands. This finding is consistent with the results of other experiments showing increases in beta or gamma activity during cognition (Chapman et al. 1998; Kopell et al. 2000; Engel et al. 2001; Fries et al. 2001; Vanderwolf and Zibrowski 2001; Olufsen et al. 2003; Martin et al. 2005) and movement planning (Murthy and Fetz 1992; Donoghue et al. 1998).
Our results extend those findings in four important ways. First, our experiments revealed increased energy in multiple frequency bands simultaneously during the brief Sniff-GO coordination window, as detected by our joint time-frequency analyses (in this case, the wavelet decompositions). Although the high-gamma band contained the lowest energy overall, energy in this band showed the most striking increase on GO trials versus on NO-GO trials. Second, we found that similar amplitude increases in beta, low-gamma and high-gamma oscillations took place across all recorded sites—cortical and subcortical, sensory and motor—during a narrow time window revealed by our joint time-frequency analyses on the LFPs (the continuous wavelet decomposition). Third, we found that the Sniff-GO period’s oscillatory activity in our LFP recordings was dynamically coherent in the high gamma range across all recorded sites. These findings suggest that increased beta- and gamma-frequency activity are a hallmark of coordinated, task-related processing that occurs prior to the onset of voluntary movement. Third, the increases in beta unit oscillations and high gamma LFP oscillations on GO trials, independent of which odor was used within the GO or NO-GO odor classes, shows that an extensive network of brain circuits, from the early olfactory system, to memory encoding, to motor output is dynamically activated to solve sensory-guided, reward-seeking tasks (Schoenbaum and Eichenbaum 1995a, b; Kay and Laurent 1999). Fourth, the fact that these coherent modulations took place across sites with strikingly different micro-circuitry suggests that transient increases in multispectral oscillatory amplitude and coherence play a role in coordinating activity across many brain regions (Bressler and Kelso 2001; Varela et al. 2001; Schnitzler and Gross 2005), though these rhythms may be generated by different biophysical mechanisms in each area.
Prior to sensory-driven movement by an animal, task-related neurons must become coupled by mechanisms with a signal-to-noise ratio that permits accurate performance by the rat on a single-trial basis. Despite this fact, most experimenters average and analyze their data across trials. In contrast, our task design and data analyses permitted a view of potential coupling mechanisms that were robust enough to be detected in single trials, despite some inevitable trial-to-trial and rat-to-rat variation. We suggest that one reason for this is that although our experiment required decision-making at the beginning of each trial, the highly trained rats in our experiments performed the GO/NO-GO reaching task with little variance in the kinematics of odor sampling and the GO-trial reaching maneuver. This repeatability aided in detecting GO-trial-invariant modulations in beta, low-gamma and high-gamma energy in time-frequency decomposed LFPs. The detection of GO-trial-invariant coupling mechanisms was also aided by the use of analytical methods suited to nonstationary data such as LFP time series (e.g. the continuous wavelet decomposition).
We do not yet know whether the oscillatory modulations described here play a causal role in coordinating the three recorded areas prior to voluntary movement; alternatively, for example, they may be a covariant sign of coordination via other mechanisms. Numerous possibilities for large-scale coordination across different brain circuits are actively and fruitfully being explored, e.g. hypotheses concerning corticothalamic interactions (Crick 1984; Jones 2001). Even if the modulations reported here do play a causal, coordinating role, these integrative mechanisms could occur in tandem with mechanisms proposed by other laboratories.
Our experiments also do not allow a determination of the mechanisms that generate the beta and gamma oscillations in each recorded area, although this is an important and ongoing area of investigation. For instance, in the rodent olfactory system, it has traditionally been thought that gamma oscillations result from excitatory-inhibitory connections between mitral and granule cells. However, a recent report suggests that there are multiple generators of gamma rhythms in the olfactory system: The classic mitral-granule mechanism may underlie sniff cycle-generated oscillations, whereas during alert immobility, a different mechanism appears to produce gamma oscillations in a lower frequency range (Kay 2003). We hypothesize that the enhanced gamma energy observed during the Sniff-GO window resulted from the classic mechanism and was subthreshold, as has also been indicated in prior reports (Ketchum and Haberly 1993). This would be consistent with the observed, greater manifestation of fast oscillations in the local field potentials than in the units. However, it is also possible that our unit recordings simply failed to detect a representative proportion of local or projection neurons firing in that range.
Our finding of increased unit and LFP beta oscillations upon GO odor sniffing contributes to a relatively new group of reports of learning-related beta oscillations in the olfactory system (Martin et al. 2004, 2005). Recently, odorant-induced beta oscillations were found to be synchronous over several millimeters of the rat brain, and they have been observed to occur simultaneously across the olfactory bulb, piriform cortices, entorhinal cortex and dentate gyrus (Chapman et al. 1998). This is consistent with modeling, brain slice, and whole-animal studies suggesting that beta oscillations may play a particularly important role in long-distance transmission of information in the nervous system (Kopell et al. 2000; Brovelli et al. 2004). The large number of beta-oscillating cells that we found not only in the pPIR but M1 and the mRN, and the substantial increase in beta oscillatory activity upon GO odor sniffing in all three regions, strongly supports and extends this view.
In summary, we have demonstrated that in a narrow time window for cue recognition, decision-making and movement planning, increased beta band unit spiking and increased beta- and gamma band LFP oscillations take place across multiple task-related sites. These modulations occur transiently and coherently across sites with widely different architectures—a subcortical motor nucleus (the mRN), an ancient three-layered olfactory cortex (the pPIR) and six-layered sensorimotor neocortex (the rat’s caudal, forelimb M1). Our findings argue that beta and gamma coupling form part of a common “code” for coordinating task-related sensory, motivational and motor microcircuitry prior to voluntary action, possibly via different mechanisms in each region.
Acknowledgments
We thank Steve Bressler, Paul Carney, Juan Aggio, David Root, Raj Rajagovindan and Aaron Smith for their helpful comments on prior versions of this manuscript, and Larry Andrews of NB Labs for custom electrode fabrication. This work was supported by a University of Florida Opportunity Seed Fund Grant to L. H.-V., DARPA-ONR grant 02SCNSF1015 to J.K.C., DARPA-ONR grant N000149911097 to J.K.C., and NIH grant 2P50NS24707 to K.A.Moxon.
Contributor Information
Raymond Hermer-Vazquez, Behavioral Neuroscience Program, Department of Psychology, University of Florida, Gainesville, FL 32611, USA rayhv@ufl.edu.
Linda Hermer-Vazquez, Behavioral Neuroscience Program, Department of Psychology, University of Florida, Gainesville, FL 32611, USA lindahv@ufl.edu.
Sridhar Srinivasan, Department of Electrical Engineering, University of Florida, Gainesville, FL 32611, USA.
John K. Chapin, Department of Physiology and Pharmacology, SUNY Downstate Medical Center, Brooklyn, NY 11203, USA
References
- Bar-Gad I, Ritov Y, Bergman H. The neuronal refractory period causes a short-term peak in the autocorrelation function. J Neurosci Methods. 2001;104:155–163. doi: 10.1016/s0165-0270(00)00335-6. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte JB, Raymond GM. Evaluation of the dispersional analysis method for fractal time series. Ann Biomed Eng. 1995;23:491–505. doi: 10.1007/BF02584449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbig A, Traub RD, Whittington MA. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol. 2002;88:1634–1654. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Coppola R, Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature. 1993;366:153–156. doi: 10.1038/366153a0. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Kelso JA. Cortical coordination dynamics and cognition. Trends Cogn Sci. 2001;5:26–36. doi: 10.1016/s1364-6613(00)01564-3. [DOI] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci USA. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH. Signals and signs in the nervous system: the dynamic anatomy of electrical activity is probably information-rich. Proc Natl Acad Sci USA. 1997;94:1–6. doi: 10.1073/pnas.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Functions for interneuronal nets in the hippocampus. Can J Physiol Pharmacol. 1997;75:508–515. [PubMed] [Google Scholar]
- Chabaud P, Ravel N, Wilson DA, Gervais R. Functional coupling in rat central olfactory pathways: a coherence analysis. Neurosci Lett. 1999;276:17–20. doi: 10.1016/s0304-3940(99)00773-9. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Xu Y, Haykin S, Racine RJ. Beta-frequency (15–35 Hz) electroencephalogram activities elicited by toluene and electrical stimulation in the behaving rat. Neuroscience. 1998;86:1307–1319. doi: 10.1016/s0306-4522(98)00092-x. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear SP, Hart CB. Synchronized cortical potentials and wavelet packets: a potential mechanism for perceptual binding and conveying information. Brain Lang. 1999;66:201–231. doi: 10.1006/brln.1998.2031. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN, Hatsopoulos NG, Gaal G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J Neurophysiol. 1998;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and transverse patterning guided by olfactory cues. Behav Neurosci. 1998;112:762–771. doi: 10.1037//0735-7044.112.4.762. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2005;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- Grammont F, Riehle A. Precise spike synchronization in monkey motor cortex involved in preparation for movement. Exp Brain Res. 1999;128:118–122. doi: 10.1007/s002210050826. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hermer-Vazquez L, Hermer-Vazquez R, Moxon KA, Kuo KH, Viau V, Zhan Y, Chapin JK. Distinct temporal activity patterns in the rat M1 and red nucleus during skilled versus unskilled limb movement. Behav Brain Res. 2004;150:93–107. doi: 10.1016/S0166-4328(03)00226-2. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Hurtado JM, Rubchinsky LL, Sigvardt KA. Statistical method for detection of phase-locking episodes in neural oscillations. J Neurophysiol. 2004;91:1883–1898. doi: 10.1152/jn.00853.2003. [DOI] [PubMed] [Google Scholar]
- Hyland B. Neural activity related to reaching and grasping in rostral and caudal regions of rat motor cortex. Behav Brain Res. 1998;94:255–269. doi: 10.1016/s0166-4328(97)00157-5. [DOI] [PubMed] [Google Scholar]
- Jensen O. Reading the hippocampal code by theta phase-locking. Trends Cogn Sci. 2005;9:551–553. doi: 10.1016/j.tics.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Kay LM. Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci. 2003;2:31–44. doi: 10.1142/s0219635203000196. [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Ketchum KL, Haberly LB. Synaptic events that generate fast oscillations in piriform cortex. J Neurosci. 1993;13:3980–3985. doi: 10.1523/JNEUROSCI.13-09-03980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kolb B, Cioe J, Whishaw IQ. Is there an optimal age for recovery from motor cortex lesions? II. behavioural and anatomical consequences of unilateral motor cortex lesions in perinatal, infant, and adult rats. Restor Neurol Neurosci. 2000;17:61–70. [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301:1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaudon P, Datiche F, Cattarelli M. Optical recording of the rat piriform cortex activity. Prog Neurobiol. 1997;52:485–510. doi: 10.1016/s0301-0082(97)00027-0. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann NY Acad Sci. 2001;929:166–175. [PubMed] [Google Scholar]
- Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Gervais R, Hugues E, Messaoudi B, Ravel N. Learning modulation of odor-induced oscillatory responses in the rat olfactory bulb: a correlate of odor recognition? J Neurosci. 2004;24:389–397. doi: 10.1523/JNEUROSCI.3433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Gervais R, Chabaud P, Messaoudi B, Ravel N. Learning-induced modulation of oscillatory activities in the mammalian olfactory system: the role of the centrifugal fibres. J Physiol Paris. 2005;98:467–478. doi: 10.1016/j.jphysparis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Gervais R. Polysynaptic potentiation at different levels of rat olfactory pathways following learning. Learn Mem. 2002;9:66–75. doi: 10.1101/lm.45602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander S, Castelo-Branco M, Baron J, Singer W. Feed-forward synchronization: propagation of temporal patterns along the retinothalamocortical pathway. Philos Trans R Soc Lond B Biol Sci. 2002;357:1869–1876. doi: 10.1098/rstb.2002.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- Olufsen MS, Whittington MA, Camperi M, Kopell N. New roles for the gamma rhythm: population tuning and preprocessing for the Beta rhythm. J Comput Neurosci. 2003;14:33–54. doi: 10.1023/a:1021124317706. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–334. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res. 2005;150:127–142. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995a;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995b;74:751–762. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Integrating orbitofrontal cortex into prefrontal theory: common processing themes across species and subdivisions. Learn Mem. 2001;8:134–147. doi: 10.1101/lm.39901. [DOI] [PubMed] [Google Scholar]
- Singer W, Kreiter AK, Engel AK, Fries P, Roelfsema PR, Volgushev M. Precise timing of neuronal discharges within and across cortical areas: implications for synaptic transmission. J Physiol Paris. 1996;90:221–222. doi: 10.1016/s0928-4257(97)81427-1. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Zibrowski EM. Pyriform cortex beta-waves: odor-specific sensitization following repeated olfactory stimulation. Brain Res. 2001;892:301–308. doi: 10.1016/s0006-8993(00)03263-7. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gorny B. Does the red nucleus provide the tonic support against which fractionated movements occur? A study on forepaw movements used in skilled reaching by the rat. Behav Brain Res. 1996;74:79–90. doi: 10.1016/0166-4328(95)00161-1. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Olfaction directs skilled forelimb reaching in the rat. Behav Brain Res. 1989;32:11–21. doi: 10.1016/s0166-4328(89)80067-1. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Binaral interactions in the rat piriform cortex. J Neurophysiol. 1997;78:160–169. doi: 10.1152/jn.1997.78.1.160. [DOI] [PubMed] [Google Scholar]