Abstract
Methionine sulfoxide reductase A (MsrA) repairs oxidized methionine residues within proteins and may also function as a general antioxidant. Previous reports have suggested that modulation of MsrA in mice and mammalian cell culture can affect the accumulation of oxidized proteins and may regulate resistance to oxidative stress. Thus, under the oxidative stress theory of aging, these results would predict that MsrA regulates the aging process in mammals. We show here that MsrA−/− mice are more susceptible to oxidative stress induced by paraquat. Skin-derived fibroblasts do not express MsrA, but fibroblasts cultured from MsrA−/− mice were, nevertheless, also more susceptible to killing by various oxidative stresses. In contrast to previous reports, we find no evidence for neuromuscular dysfunction in MsrA−/− mice in either young adult or in older animals. Most important, we found no difference between MsrA−/− and control mice in either their median or maximum life span. Thus, our results show that MsrA regulates sensitivity to oxidative stress in mice but has no effect on aging, as determined by life span.—Salmon, A. B., Pérez, V. I., Bokov, A., Jernigan, A., Kim, G., Zhao, H., Levine, R. L., Richardson, A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span.
Keywords: longevity, free radical, stress resistance, aging, antioxidant
The oxidative stress theory of aging suggests that the accumulation of oxidation damage to cellular macromolecules is a key determinant of aging rate (1). This damage may arise from imbalances between the deleterious reactions that produce free radicals and the ability of the cell or tissue to protect against injury and repair damaged macromolecules (2). More than 50 yr of study has attempted to define the most important regulators of life span under the oxidative stress theory of aging. Proteins represent some of the most likely targets of oxidation in the cell, and among the pool of available amino acids, methionine residues may be the most sensitive to oxidation (3, 4). Oxidation of methionine leads to the formation of methionine sulfoxide in either the S- or R-epimer. Unlike most other forms of oxidative damage, specific enzymes have evolved to repair methionine sulfoxides in vivo; methionine sulfoxide reductase A (MsrA) is responsible for the reduction of the S-epimer of methionine sulfoxide, while methionine sulfoxide reductase B (MsrB) is known to reduce the R-epimer (3, 4).
MsrA appears to play an important role in the accumulation of protein oxidative damage. For example, it has been shown that the loss of MsrA in Escherichia coli, yeast, and mammals causes higher levels of accumulated oxidative damage to proteins in response to oxidative stress (5,6,7,8,9,10). Conversely, the overexpression of MsrA has been shown to protect proteins against the effects of oxidative stress in yeast (11), Drosophila (12), and mammalian cell lines (7, 13, 14). Levine et al. (15) proposed that the oxidation of methionine residues, which can be reduced by MsrA, may serve as a free radical sink, thereby protecting other macromolecules from oxidation. For example, MsrA levels have been correlated to protein carbonyls, as well as methionine sulfoxide (6), and a decrease in methionine residues in proteins increased the susceptibility of E. coli to oxidative stress (16).
Because of its role in repairing protein oxidative damage, MsrA would be predicted to be a determinant of life span according to the oxidative stress theory of aging. Indeed, overexpression of MsrA in yeast (17) and in Drosophila (12) has been shown to extend life span ∼25 and 70%, respectively. In mammals, MsrA activity has been suggested to contribute to the rate of progression of several potentially age-related phenotypes, including hippocampal neurodegeneration (18), the development of cataracts (9, 10), and cardiac dysfunction (14, 19). MsrA also has been suggested to regulate life span in mammals; mutant mice lacking MsrA (MsrA−/−) were reported to live ∼40% shorter than control mice, suggesting that the accumulation of oxidative damage, particularly to proteins, may be an important determinant of mammalian life span (8).
In this report, we show that mice lacking MsrA are sensitive to oxidative stress, assessed by paraquat challenge. Despite their oxidative stress sensitivity, we found that MsrA−/− mice did not differ from control mice in neuromuscular function when measured in either young or adult animals, and, notably, these mice showed no difference from control mice in life span.
MATERIALS AND METHODS
Animals
Generation of MsrA−/− mice on a 129/SvJ background was described by Moskovitz et al. (8), and the mice used for this study were obtained from the original colony at the Laboratory of Biochemistry, National Heart, Lung, and Blood Institute (Bethesda, MD, USA). The original study on the life span of MsrA−/− mice used mice that were the progeny of 129/SvJ and C57BL/6 chimeras and thus were of mixed background. To replicate the original study, we used the same mixed-background mice, although they had undergone multiple generations of interbreeding prior to our study. We also used these animals for the Rota-rod studies. However, for studies on resistance to oxidative stress, we used mice that had been backcrossed for 10 generations into the C57BL/6 background. By breeding male and female mice heterozygous for the MsrA gene (MsrA+/−), we obtained MsrA+/−, MsrA+/+, and MsrA−/− littermates that were used in this study. Mice were genotyped using primers for the neocassette in the targeted construct; in addition, we randomly checked mice for expression of MsrA by RT-PCR and for the presence of the MsrA protein by Western blot analysis. All procedures involving the mice were approved by the Subcommittee for Animal Studies at the Audie L. Murphy Veterans Administration Hospital at San Antonio and the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
Assessment of life span
The life span of 37 control mice (comprising both MsrA+/− and MsrA+/+) and 25 MsrA−/− mice was determined, and these animals were maintained as previously described (20, 21). In these survival studies, we used only the male littermates of all genotypes. Mice were housed under barrier conditions using microisolator cages, 4 animals/cage, and were fed ad libitum a standard NIH-31 chow diet (Harlan Teklad, Madison, WI, USA) on a 12-h dark-light cycle. The mice in the survival groups were maintained until they died spontaneously, and the life span was calculated from this information.
Western methods
Chemiluminescent detection was employed for detection of antioxidant proteins other than MsrA. Glutathione peroxide, catalase, and thioredoxin reductase antibodies were purchased from Cell Signaling (Beverly, MA, USA) and used at a dilution of 1:1000 in 5% powdered milk and 0.1% Tween 20 in 1× PBS. Actin primary antibody (Sigma, St. Louis, MO, USA) was used at a dilution of 1:5000 in 3% BSA and 0.1% Tween 20 in 1× PBS. Alkaline phosphatase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were then used at a dilution of 1:10,000 in 1% BSA and 0.1% Tween 20 in 1× PBS, and bands were detected using ECL+ reagent (GE Healthcare, Piscataway, NJ, USA). Total protein was isolated in liver and kidney samples from <6-mo-old mice and from fibroblast cell lines using a modified RIPA buffer and stored at −20°C until analysis. Total protein content was measured by the Pierce BCA assay (Bio-Rad, Hercules, CA, USA). For blots, 10 or 50 μg of total cellular protein extract was prepared in 6× SDS sample buffer, subjected to SDS-polyacrylamide electrophoresis, and transferred to PVDF membrane (Millipore, Billerica, MA, USA). The membrane was blocked in 5% powdered milk with 0.1% Tween 20 in 1× PBS. MsrA was not detected in cultured fibroblasts with the chemiluminescent method, so we employed detection by infrared fluorescence, which in our hands is typically 2 orders of magnitude more sensitive. A total of 10 μg of protein was loaded in each lane of a 10–20% Invitrogen Tris-glycine gradient gel. After transferring to 0.45-μm nitrocellose membrane, the blot was incubated with a 1:25,000 dilution of rabbit anti-mouse MsrA primary antibody raised against recombinant MsrA (GK). The membrane was held at 4°C overnight, washed 3 times, and then incubated for 1 h at room temperature with 1:10,000 Alexa Fluor 680 goat anti-rabbit IgG (Invitrogen A21109). Detection and quantitation of MsrA by fluorescence was performed with an Odyssey infrared scanner in the 700-nm channel (Li-Cor Biosciences, Lincoln, NE, USA).
Cell culture and assessment of cellular stress resistance
Primary fibroblast cell lines were derived from tail biopsies taken from newborn (<1 wk old) MsrA−/− and control (MsrA+/+) mice, as described previously (22, 23). All cell cultures were grown in complete medium (CM) made up of Dulbecco’s modified eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated FCS, antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Sigma), and 0.25 μg/ml of fungizone (Biowhittaker-Cambrex Life Sciences, Walkersville, MD, USA). All cell lines were maintained in CM at 37°C in a humidified incubator with 5% CO2 and ambient (∼21%) O2 air.
Cells were assayed for stress resistance, as described previously (22, 23); all assays were performed with cells that had undergone an equal number of cell passages. Cell lines were seeded in duplicate into 96-well tissue culture-treated plates at a concentration of 2.5 × 104 cells/100 μl CM. Following overnight incubation, cells were incubated in DMEM supplemented with 2% BSA, antibiotics, and fungizone (SD) for 24 h. Cells were then exposed to doses of each cellular stress agent diluted in SD for 6 h, followed by a DPBS wash, and 18-h incubation in fresh SD. Cell survival was measured using a test based on oxidative cleavage of the tetrazolium dye WST-1 (Roche Applied Science, Indianapolis, IN, USA) to a formazan product using the protocol suggested by the manufacturer. At each dose of the stressor, the mean survival relative to the untreated control was calculated for each cell line. The LD50, i.e., dose of stress agent that led to survival of 50% of the cells, was then calculated using probit analysis as implemented in NCSS software (NCSS, Kaysville, UT, USA).
Assessment of mouse resistance to paraquat
Young adult male and female mice between 3 and 6 mo of age were used to test whole-animal resistance to paraquat. Paraquat (methyl viologen; Sigma) was dissolved in sterile saline and administered by intraperitoneal injection at a dose of 50 mg paraquat/kg body weight. Animals were monitored for survival for 140 h following paraquat injection.
Rota-rod performance
Rota-rod performance was measured with a Rotamex 4/8 (Columbus Instruments, Columbus, OH, USA), using an accelerating rod protocol, as described by Shahbazian et al. (24). An infrared sensor was used to measure the fall from the rod. The initial speed of the rod was set to 2 rpm, with a linear acceleration to 40 rpm over 5 min. At each age tested, mice were given a learning period on the Rota-rod prior to measurement of performance; this learning period occurred on the day prior to performance evaluation and consisted of 4 trials on the rod with 20-min rest between trials. This learning protocol was repeated on the following day for evaluation of Rota-rod performance; the latency to fall was measured (no animals were able to stay on the rod for the entire 5 min) for each of 4 consecutive trials. Data were then analyzed as the average of these 4 trials for each genotype.
Statistical analyses
Genotype differences in cellular stress resistance to a specific agent were determined by 2-sample t tests comparing the LD50 value for MsrA−/− cell lines with those of control cell lines. Differences in survival of mice following paraquat injection were tested statistically using log-rank survival analysis (for the effects of paraquat in each sex independently) and by Cox proportional-hazards regression (for the effect of genotype). For Rota-rod studies, genotype differences were assayed for each trial by two-sample t tests comparing the latency to fall of each group. Lifespan curves were analyzed using the Cox-Mantel log-rank test (Kaplan-Meier test), and lifespan parameters were analyzed using Winmodest software (25) in the R language. The score test (26) was used to determine whether the survival of MsrA−/− or control mice differed significantly at various quantiles from the overall survival.
RESULTS
Lack of MsrA in mice increases sensitivity to oxidative stress
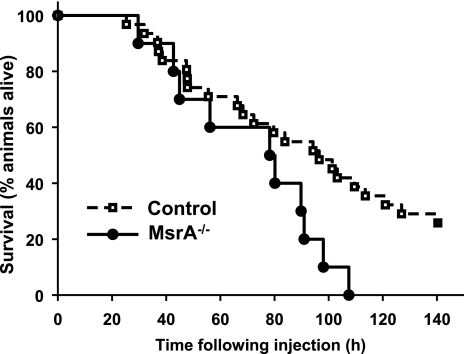
To test whether the loss of MsrA in mice is correlated with in vivo oxidative stress resistance, we assayed the survival of young adult MsrA−/− and control mice following intraperitoneal injection of paraquat, a compound known to generate superoxide radicals in vivo (27). In this experiment, we pooled MsrA+/+ and MsrA+/− mice as the control because we found no difference in paraquat toxicity between these two genotypes. In Fig. 1, we show the survival curves for control and MsrA−/− mice following injection with paraquat. The mean survival time of the 10 MsrA−/− mice that we tested was 71.8 h following injection, and all mice lacking MsrA had died by 108 h following paraquat treatment. In contrast, the mean survival time of 23 control mice following paraquat injection was 92.5 h, and 7 control mice were still alive at the conclusion of our experiment at 140 h. Because this data set consisted of both male and female mice, we analyzed the data by Cox proportional-hazards regression, which can analyze the effect of several risk factors (i.e., genotype and sex) on survival. Using this model, we found no difference in the hazards of paraquat-induced death for male or female mice (P=0.99) but did find a significant effect of genotype (MsrA−/− vs. control) on length of survival following paraquat (P=0.03). Thus, these data show that the lack of MsrA in mice increases in vivo susceptibility to paraquat.
Figure 1.
Mice lacking MsrA are more sensitive to paraquat toxicity. Mice (both male and female mice for each genotype) were given paraquat (50 mg/kg body weight). Control animals consist of both MsrA+/+ and MsrA+/− genotypes; no difference in survival was observed between these genotypes following paraquat (data not shown). Each circle represents time of death of a single animal; control (n=22) animals are represented by dashed line with open squares; MsrA−/− mice (n=10) are represented by solid line with closed circles. Survival of MsrA−/− animals was found to be significantly different than for control animals by Cox regression analysis (P=0.03 for genotype, P=0.99 for sex).
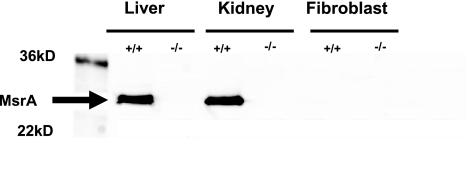
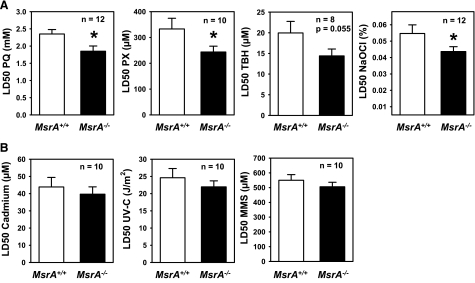
Previous reports have shown that oxidative stress resistance of mammalian cell lines can be modulated by altering the levels of MsrA expression (7, 9, 11, 14). We therefore tested whether primary cells derived from mice lacking MsrA differed from those derived from control mice in their sensitivity to oxidative stress. However, we observed that primary fibroblasts isolated from the skin of wild-type MsrA+/+ mice do not express MsrA, although it was readily detected in kidney and liver as expected (Fig. 2). We therefore expected to find no differences in resistance to oxidative stress between the cells from MsrA+/+ and MsrA−/− mice when assayed using methods previously described (22, 28). Surprisingly, we found that fibroblasts from MsrA−/− mice were significantly more sensitive to cell death caused by several different agents thought to induce oxidative stress through different mechanisms than were cell lines from MsrA+/+ mice (Fig. 3A). For example, MsrA−/− cells were significantly more sensitive to paraquat, thought to be toxic due to generation of intracellular superoxide (27, 29). These cells also were sensitive to hydrogen peroxide, the toxicity of which is thought to be derived from lipid peroxidation, lipid membrane perturbation, and the dissociation of peroxide into hydroxyl and superoxide radicals (30). Similarly, fibroblasts from MsrA−/− mice tended to be more sensitive to t-butyl hydrogen peroxide (P=0.055, 2-tailed t test), which is thought to act in a similar manner to hydrogen peroxide. Lastly, MsrA−/− cells were significantly sensitive to sodium hypochlorite, which is thought to oxidize primarily methionine and cysteine residues within proteins (31). The increased sensitivity of fibroblasts from MsrA−/− mice was relatively small in all cases; e.g., the difference in average LD50 between cells from MsrA−/− and MsrA+/+ mice ranged from ∼20% for sodium hypochlorite to 33% for hydrogen peroxide. The raw LD50 values for oxidative stress sensitivity measured in this study were similar to previous reports utilizing primary cultures of skin-derived fibroblasts from mice (22, 23, 28, 32,33,34). The diminished stress resistance of MsrA−/− cells seems to be specific to oxidative stress resistance; we found no statistically significant difference between MsrA−/− cells and those from MsrA+/+ mice in their sensitivity to cell death caused by the heavy metal cadmium or to DNA-damaging agents like UV-C light and methyl methanesulfonate (Fig. 3B).
Figure 2.
MsrA in liver, kidney, and fibroblasts. Molecular mass markers are in left-most lane. In other lanes, 10 μg of total protein was loaded. MsrA is readily detected in wild-type liver and kidney, but not in fibroblasts.
Figure 3.
Fibroblasts from MsrA−/− mice are more sensitive to oxidative stress. A) Skin-derived fibroblasts from MsrA−/− mice are more sensitive to cell death caused by paraquat (PQ), hydrogen peroxide (PX), t-butyl hydrogen peroxide (TBH), and sodium hypochlorite (NaOCl). B) Skin-derived fibroblasts from MsrA−/− mice show no difference in sensitivity to cell death caused by cadmium, UV-C light, or methyl methanesulfonate (MMS). Bar graphs represent mean ± se LD50 of fibroblast cultures for the number of independent cell lines shown (n). *P < 0.05; Student’s t test.
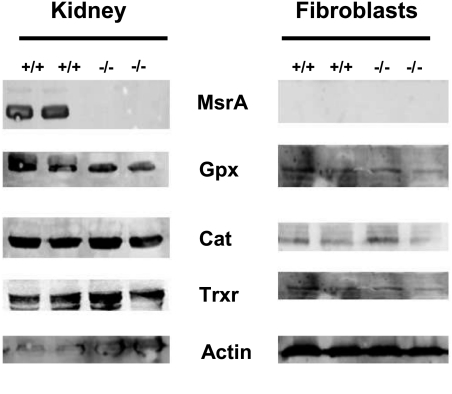
We then performed Western blots on samples from kidney, liver, and fibroblast cell lines to test whether the differences observed in their oxidative stress resistance was related to changes in other antioxidant-related proteins. We found that both MsrA−/− and control mice, and cells from those mice, expressed similar levels of glutathione peroxidase, catalase, and thioredoxin reductase, suggesting that the lack of MsrA does not cause compensatory up or down-regulation of other antioxidant enzymes (Fig. 4).
Figure 4.
MsrA−/− and control mice do not differ in the expression of several other antioxidant enzymes. Representative Western blots for glutathione peroxidase (Gpx), catalase (Cat), and thioredoxin reductase (Trxr) protein in extracts from kidney, and cultured fibroblasts from MsrA−/− and control mice. Equal amounts of total protein were loaded in each lane. No differences were detectable between MsrA−/− and control mice. A similar lack of difference was found for liver homogenates (not shown).
Lack of MsrA does not diminish Rota-rod performance
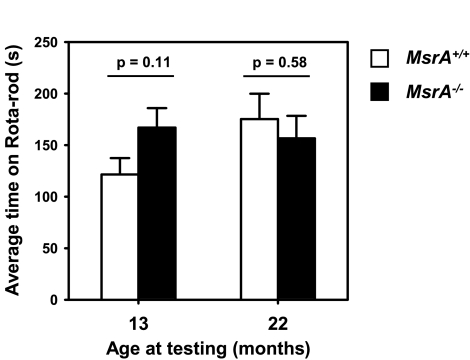
MsrA−/− mice have been reported to exhibit ataxia at an early age of 6 mo, though this was a relatively subjective measurement of whether mice displayed tiptoe walking (8). In the San Antonio animal facility and during our entire survival study, we did not observe any obvious motor function differences between MsrA−/− or control mice. Similarly, in the Bethesda facility, no abnormal gait was observed in a cohort of 24 MsrA−/− mice observed to at least age 21 mo (12 female and 12 male). To objectively test whether MsrA−/− mice show age-related diminution of motor function, we measured the Rota-rod performance of animals while they were relatively young (∼13 mo) and then again when the animals were older (∼22 mo). Rota-rod performance measures the ability of a mouse to remain on a slowly accelerating rod and is a very sensitive quantitative, objective, and reproducible measure of functional motor coordination and thus is a good indicator of neuromuscular function (35). For each age, the latency to fall from the Rota-rod was tested over 4 trials on a single day with 20 min between each trial; data are presented as the average latency to fall for each animal over the 4 trials. This testing was performed following an initial learning protocol consisting of a similar 4-trial exposure to the Rota-rod performed on the prior day. We found no significant difference between MsrA−/− and MsrA+/+ mice in their ability to remain on the Rota-rod, though MsrA−/− mice did tend to have a slightly higher average time than control mice (Fig. 5). The same mice were retested at an older age (22 mo) following the same protocol as described above, and again, we found no difference between MsrA−/− and MsrA+/+ mice. Because body size can significantly confound tests of animal behavior, such as the Rota-rod, we weighed the MsrA−/− and MsrA+/+ mice from 3 to ∼24 mo of age; we found no significant difference in size at either 13 mo (26.2±0.6 vs. 27.8±0.4 g for MsrA−/− and MsrA+/+ mice, respectively) or at 22 mo (27.8±0.5 vs. 28.7±0.4 g). Thus, these data indicate that the loss of MsrA had no effect, either positive or negative, on neuromuscular function of adult mice as measured by Rota-rod performance through nearly 2 yr of age.
Figure 5.
MsrA−/− and control mice do not differ in their ability to perform Rota-rod challenge. For each figure, 8 male MsrA−/− mice (solid bars) and 8 male MsrA+/+ mice (open bars) were assayed for their ability to remain on the accelerating Rota-rod. Bars represent average ± se latency to fall time (y-axis) for each genotype. Data represent performance measurements in mice assayed at age 13 mo (left) or 22 mo (right).
No difference in life span between MsrA−/− and WT mice
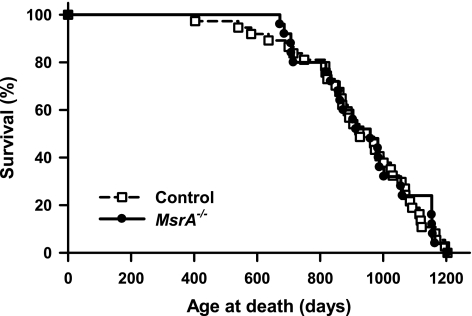
It was previously reported that the life span of MsrA−/− mice was ∼40% shorter than control mice, with 40–50% of MsrA−/− dying before 1 yr of age (8). In our lifespan study, control mice consisted of both MsrA+/+ and MsrA+/− mice because we found no difference in survival between these two groups (data not shown). Further, Moskovitz et al. (8) also found no difference in life span between MsrA+/+ and MsrA+/− mice. Fig. 6 shows the survival curves of male MsrA−/− and control mice. We found no difference in the survival of control and MsrA−/− mice as measured by Kaplan-Meier log-rank test (P=0.84). Table 1 gives the survival data obtained from the survival curves; the data show no significant difference between MsrA−/− and control mice in any survival parameter, including 25th, median, 75th, and 90th percentile survival. Further, we found no difference in mean or maximum survival between the two groups.
Figure 6.
MsrA−/− and control mice do not differ in life span. Each circle represents time of death of a single animal; control animals are represented by dashed line with open squares; MsrA−/− mice are represented by solid line with closed circles. For survival, control group consists of 37 male mice of both MsrA+/+ and MsrA+/− genotypes; MsrA−/− group consists of 25 male mice.
TABLE 1.
Lifespan parameters from control and MsrA−/− male mice
| Parameter | Control | MsrA−/− | Significance (P) |
|---|---|---|---|
| Sample size (n) | 37 | 25 | |
| Survival | |||
| 25% | 823 (714–887) | 833 (708–904) | 0.85 |
| 50% | 926 (868–1030) | 959 (858–1056) | 0.85 |
| 75% | 1070 (988–1115) | 1061 (983–1105) | 0.99 |
| 90% | 1140 (1092–1204) | 1157 (1105–1203) | 0.65 |
| Mean | 925 (893–956) | 939 (906–972) | 0.64 |
| Maximum | 1204 | 1203 |
Data were obtained from the survival curves presented in Figure 6. Statistical analyses are described in Materials and Methods. Survival data represent days with 95% confidence intervals.
DISCUSSION
Organisms have evolved a complex antioxidant system that can reduce the accumulation of oxidative damage to cellular macromolecules (2). MsrA is an important component of the antioxidant system that contributes to the maintenance of protein homeostasis by repairing oxidized methionine residues in proteins, thereby maintaining protein structure and function (3, 36). Because of their high concentrations in proteins and high sensitivity to oxidation, methionine residues have been proposed to act as a free-radical sink that protects proteins from damage to critical amino acids (15, 16). Thus, MsrA may contribute to the overall protection of proteins against oxidation by maintaining a low level of protein-bound and free oxidized methionine residues, and thereby preserve the free-radical sink.
In this study, we found that the mice whose MsrA was knocked out had increased sensitivity to oxidative challenge by paraquat. Although MsrA is not expressed in fibroblasts, cells cultured from MsrA−/− mice were more sensitive to several oxidizing agents. This suggests that disruption of MsrA leads to epigenetic changes in the animal’s fibroblasts, which render them sensitive to oxidative stress. A similar scenario has been presented for the stress-resistant properties of fibroblasts from long-lived hypopituitary dwarf mice; these mice are long lived because of mutations in genes encoding transcription factors responsible for the development of the pituitary, yet skin-derived fibroblasts from these animals are resistant to multiple forms of stress (22, 28). Thus, subtle changes in the antioxidant status of adult animals may be passed on to cells derived from these animals yet maintained in culture for extended periods of time. Because we also found no significant differences in other antioxidant enzymes, such as glutathione peroxidase, catalase, and thioredoxin reductase, it appears that the difference in stress resistance is likely due to unidentified factors secondary to the lack of MsrA.
Also, in this study, we found no evidence that MsrA−/− mice show diminished neuromuscular function relative to control mice. Moskovitz et al. (8) initially reported that MsrA−/− mice exhibited ataxia (tiptoe walking) beginning at a relatively young age, 6 mo. Further studies have suggested that the lack of MsrA in mice can increase hippocampal degeneration (18), diminish learning capability, and reduce spontaneous locomotion (37). We observed no evidence of tiptoe walking at any age in the MsrA−/− mice in either of our colonies, even in MsrA−/− mice >30 mo of age. In addition, we observed no difference between MsrA−/− and control mice in their performance on the Rota-rod, further suggesting that the lack of MsrA did not have a negative effect on neuromuscular function, even when the mice were relatively old, 22 mo of age. It is unclear why our findings are in such sharp contrast to previous reports on the locomotion and behavior of this particular mutation. It may be that Rota-rod performance measures a particular neuromuscular function that does not accurately reflect the particular functions measured in past reports. Mice lacking an isoform of MsrB (MsrB1) have also been reported to have a normal gait pattern (38), suggesting that the lack of methionine sulfoxide reductases alone may not be the ultimate cause of the originally reported ataxia.
Despite their sensitivity to oxidative stress, we found that mutant mice lacking MsrA did not differ from control mice in their longevity. This finding is in sharp contrast to the findings of Moskovitz et al. (8), who reported that the life span of MsrA−/− mice was ∼40% shorter than that of control mice and that none of the MsrA−/− mice lived longer than ∼18 mo. However, several aspects of the previous investigators’ experimental design might significantly contribute to the differences between the studies. First, the study by Moskovitz et al. (8) used a relatively small number of mice (a total of 17 MsrA−/−, 14 MsrA+/+, and 8 MsrA+/− mice). Using larger sample sizes reduces the effects of uncontrolled variables, such as maternal- or paternal-specific effects on life span (39). Perhaps the most important issue with the previous study is that the control mice in that study were relatively short lived; e.g., the mean life span of MsrA+/+ and MsrA+/− mice was ∼680 d (8). In our study, the mean life span of control mice was 925 d, or ∼35% longer than the control mice in the study by Moskovitz et al. (8). The lifespan parameters in this current study are in line with other survival studies performed with C57BL/6 mice in our laboratory (20, 21) and with other studies performed using either 129/SvJ or C57BL/6 mice under contemporary pathogen-free, barrier conditions (40). Both the current study and the previous study by Moskovitz et al. (8) utilized mice of the same genetic background (a mixed strain of 129/SvJ and C57BL/6). In addition, it was recently reported that mice lacking MsrB1, an isoform of MsrB, showed no difference in the life span through 20 mo (38), suggesting again that methionine sulfoxide reductases may not be critical determinants of longevity under careful animal husbandry. By maximizing the life span of the mice, the effect of genotype/environment interactions on life span is minimized; i.e., one has a more accurate measure of the effect of the genetic manipulation on aging.
Alternatively, it could be argued that MsrA should be expected to have little effect on mammalian life span. A large number of antioxidants and oxidation repair systems have developed in higher eukaryotes during evolution in response to an environment with relatively high oxygen content. MsrA represents a very specialized oxidation repair protein that only plays one part (30). Because of the many backup and redundant antioxidant systems, researchers have argued that alterations in single, or even multiple, members of these protective mechanisms have little effect on mammalian life span (2, 20, 41).
More generally, our results add to a growing number of reports suggesting that oxidative stress resistance may not be an absolute correlate of retarded aging in mice. Mice generated to express reduced levels of Mn-superoxide dismutase (Sod2+/−) show high levels of oxidative damage to nucleic acids in many tissues and are sensitive to paraquat toxicity. However, the life span of these mice is no different than that of mice expressing normal levels of Sod2 (20). Similarly, Ran et al. (21) showed that mice with decreased levels of glutathione peroxidase 4 (Gpx4+/−) did not show a decrease in life span, even though they were sensitive to oxidative stress. Conversely, mice lacking the receptor for growth hormone (GHRKO) live ∼40% longer than wild-type controls, yet GHRKO males are sensitive to paraquat, while GHRKO females show no difference from wild-type controls (42). Similarly, mice generated to overexpress α-MUPA (urokinase-type plasminogen activator) live ∼20% longer than controls, yet show sensitivity to paraquat treatment (43, 44).
In summary, our findings show that the lack of MsrA in mice increases the sensitivity of the mice and cells from the mice to oxidative stress but has no effect on the life span of mice. These findings highlight the importance of replicating of lifespan studies and performing lifespan experiments under conditions that maximize animal health and life span when determining the effect of genetic manipulations of aging.
Acknowledgments
We thank Jay Cox, Marian Sabia, and Quy Fung for assistance in animal care. We also thank Dr. James Harper for statistical analysis assistance and intellectual discourse. This study was supported by National Institutes of Health (NIH) training grant T32 AG021890-05 (A.B.S.), NIH MERIT grant R37AG026557 (A.R.), the San Antonio Nathan Shock Center for Excellence in the Basic Biology of Aging (A.R.), and the Intramural Research Program of the National Heart, Lung, and Blood Institute (G.K., H.Z., and R.L.L.).
References
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Stadtman E R, Van Remmen H, Richardson A, Wehr N B, Levine R L. Methionine oxidation and aging. Biochim Biophys Acta. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Stadtman E R. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Rahman M A, Strassman J, Yancey S O, Kushner S R, Brot N, Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Berlett B S, Poston J M, Stadtman E R. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci. 1997;94:9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarow M, Hawse J R, Cowell T L, Benhamid S, Pizarro G S, Reddy V N, Hetmancik J F. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci U S A. 2004;101:9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams W M, Requena J, Berlett B S, Stadtman E R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M A, Lee W, Cowell T L, Wells T M, Weissbach H, Kantorow M. Silencing of the methionine sulfoxide reductase A gene results in loss of mitochondrial membrane potential and increased ROS production in human lens cells. Exp Eye Res. 2006;83:1281–1286. doi: 10.1016/j.exer.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J W, Gordiyenko N V, Marchetti M, Tserentsoodol N, Sagher D, Alam S, Weissbach H, Kantorow M, Rodriguez I R. Gene structure, localization and role in oxidative stress of methionine sulfoxide reductase A (MSRA) in the monkey retina. Exp Eye Res. 2006;82:816–827. doi: 10.1016/j.exer.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Flescher E, Berlett B S, Poston J M, Stadtman E R. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci U S A. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Tang X D, Chen M L, Joiner M L, Sun G, Brot N, Weissbach H, Heinemann S H, Iverson L, Wu C F, Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot C R, Petropoulos I, Perichon M, Moreau M, Nizard C, Friguet B. Overexpression of MsrA protects WI-38 SV40 human fibroblasts against H2O2-mediated oxidative stress. Free Radic Biol Med. 2005;39:1332–1341. doi: 10.1016/j.freeradbiomed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Prentice H M, Moench I A, Rickaway Z T, Dougherty C J, Webster K A, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem Biophys Res Commun. 2008;366:775–778. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- Levine R L, Mosoni L, Berlett B S, Stadtman E R. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Levine R L. Methionine in proteins defends against oxidative stress. FASEB J. 2008;23:464–472. doi: 10.1096/fj.08-118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc A, Gasch A P, Rutherford J C, Kim H Y, Gladyshev V N. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc Natl Acad Sci U S A. 2004;101:7999–8004. doi: 10.1073/pnas.0307929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Oien D, Ersen F, Moskovitz J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp Brain Res. 2007;180:765–774. doi: 10.1007/s00221-007-0903-6. [DOI] [PubMed] [Google Scholar]
- Erickson J R, Joiner M l, Guan X, Kutschke W, Yang J, Oddis C V, Bartlett R K, Lowe J S, O'Donnell S E, Aykin-Burns N, Zimmerman M C, Zimmerman K, Ham A J, Weiss R M, Spitz D R, Shea M A, Colbran R J, Mohler P J, Anderson M E. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe S R, Alderson N L, Baynes J W, Epstein C J, Huang T T, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Ran Q, Liang H, Ikeno Y, Qi W, Prolla T A, Roberts L J, II, Wolf N, VanRemmen H, Richardson A. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- Salmon A B, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller R A. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Harper J M, Salmon A B, Chang Y, Bonkowski M, Bartke A, Miller R A. Stress resistance and aging: Influence of genes and nutrition. Mech Ageing Dev. 2006;127:687–694. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M D, Young J I, Yuva-Paylor L A, Spencer C M, Antalffy B A, Noebels J L, Armstrong D L, Paylor R, Zoghbi H Y. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Pletcher S D, Khazaeli A A, Curtsinger J W. Why do life spans differ? Partitioning mean longevity differences in terms of age-specific mortality parameters. J Gerontol A Biol Sci Med Sci. 2000;55:B381–B389. doi: 10.1093/gerona/55.8.b381. [DOI] [PubMed] [Google Scholar]
- Wang C, Li Q, Redden D T, Weindruch R, Allison D B. Statistical methods for testing effects on “maximum lifespan.”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Bus J S, Cagen S Z, Olgaard M, Gibson J E. A mechanism of paraquat toxicity in mice and rats. Toxicol Appl Pharmacol. 1976;35:501–513. doi: 10.1016/0041-008x(76)90073-9. [DOI] [PubMed] [Google Scholar]
- Murakami S, Salmon A, Miller R A. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Cocheme H M, Murphy M P. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J M C. Oxford, UK: Oxford University Press; Free Radicals in Biology and Medicine. 1989 [Google Scholar]
- Khor H K, Fisher M T, Schöneich C. Potential role of methionine sulfoxide in the inactivation of the chaperone GroEL by hypochlorous acid (HOCl) and peroxynitrite (ONOO-) J Biol Chem. 2004;279:19486–19493. doi: 10.1074/jbc.M310045200. [DOI] [PubMed] [Google Scholar]
- Leiser S F, Salmon A B, Miller R A. Correlated resistance to glucose deprivation and cytotoxic agents in fibroblast cell lines from long-lived pituitary dwarf mice. Mech Ageing Dev. 2006;127:821–829. doi: 10.1016/j.mad.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Harper J M, Salmon A B, Leiser S F, Galecki A T, Miller R A. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A B, Sadighi Akha A, Buffenstein R, Miller R A. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, UV light, and ER stress. J Gerontol A Biol Sci Med Sci. 2008;63:232–241. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D C, Campbell C A, Stretton J L, Mackay K B. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2066. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Stadtman E R, Moskovitz J, Berlett B S, Levine R L. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem. 2002;234–235:3–9. [PubMed] [Google Scholar]
- Oien D B, Osterhaus G L, Latif S A, Pinkston J W, Fulks J, Johnson M, Fowler S C, Moskovitz J. MsrA knockout mouse exhibits abnormal behavior and brain dopamine levels. Free Radic Biol Med. 2008;45:193–200. doi: 10.1016/j.freeradbiomed.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomenko D E, Novoselov S V, Natarajan S K, Lee B C, Koc A, Carlson B A, Lee T H, Kim H Y, Hatfield D L, Gladyshev V N. Methionine-R-sulfoxide reductase 1 (MsrB1) knockout mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form. J Biol Chem. 2008;284:5986–5993. doi: 10.1074/jbc.M805770200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest N K, Mackowiak B, Promislow D E L. The role of parental age effects on the evolution of aging. Evolution. 2002;56:927–935. doi: 10.1111/j.0014-3820.2002.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Yuan R, Paigen B. Longitudinal analysis of lifespan in 32 laboratory mouse strains. [Online] Jackson Laboratories, http://agingmice.jax.org/data/projectprogress.html. 2008 [Google Scholar]
- Perez V, Van Remmen H, Bokov A, Epstein C, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck S J, Aaron J M, Wright C, Kopchick J J, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res. 2002;34:481–486. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- Miskin R, Masos T. Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. J Gerontol A Biol Sci Med Sci. 1997;52A:B118–B124. doi: 10.1093/gerona/52a.2.b118. [DOI] [PubMed] [Google Scholar]
- Tirosh O, Pardo M, Schwartz B, Miskin R. Long-lived αMUPA transgenic mice show reduced SOD2 expression, enhanced apoptosis and reduced susceptibility to the carcinogen dimethylhydrazine. Mech Ageing Dev. 2005;126:1262–1273. doi: 10.1016/j.mad.2005.07.003. [DOI] [PubMed] [Google Scholar]