Abstract
Billions of neurons are interconnected in the central nervous system (CNS). Identification of specific neuronal circuit is indispensable for understanding the relationship between structure and function in the CNS. The midbrain dopamine (DA) neuron system consists of the retrorubral area (A8), the substantia nigra (SN; A9), and the ventral tegmental area (VTA; A10). We hypothesized that genetic methods using cell-type specific promoters may offer the possibility to express tracer molecules in DA neurons to facilitate neuronal tracing. To address this, we used the 2.5 kb rat tyrosine hydroxylase (TH) promoter in adenovirus or adeno-associated virus (AAV) to express tracers specifically in DA neurons. We found that stereotaxic injection of TH promoter containing adenoviral construct resulted in cell type-specific transgene expression in the noradrenaline (NA) neurons of the locus coeruleus (LC). However, it caused a significant toxicity to DA neurons in the SN. In contrast, stereotaxic injection of TH promoter containing AAV to the SN resulted in cell type-specific transgene expression in DA neurons with no detectable toxicity. Taken together, our results demonstrate that it is possible to selectively trace DA neuronal circuits in rodent brains using the TH promoter in the context of AAV.
Keywords: dopamine neuron, tyrosine hydroxylase promoter, adeno-associated virus, tracers, neuronal circuit
The CNS is composed of billions of neurons interconnected to form intricate neuronal circuits 1, 2. Neuronal tracing has been used to identify and characterize neuronal pathways and functions in specific neuronal circuits of the complex nervous system 3. Conventional tracing methods use axonal transport of tracers, such as horseradish peroxidase (HRP) or wheat germ agglutinin (WGA), via the microtubular systems of the neurons followed by detection of tracers with immunohistochemical methods 4. However, tracers can be taken by all the neurons located at the injection sites resulting in nonspecific labeling of unrelated pathways in the conventional tracing methods. In contrast, genetic tracing methods can selectively express tracers in specific neurons using cell-type specific promoters 3,5-8.
DA neurons play a profound role in diverse brain functions such as movement, mood, attention, and visceral functions. In the ventral midbrain, DA is synthesized in the VTA and SN 9. VTA neurons project to the cortex and the ventral striatum constituting the mesolimbic system, which controls motivation, reward, and emotional behavior. Dysfunction of this system is implicated in major psychiatric disorders such as schizophrenia. SN neurons project to the striatum forming the nigrostriatal system, which regulates voluntary movement. Abnormal regulation of the nigrostriatal system leads to Parkinson's disease.
We previously developed a synthetic promoter driving transgene expression in NA neurons 10. In order to trace the neuronal circuit of midbrain DA neurons, we sought to use the TH upstream promoter. Toward this goal, we investigated the use of the 2.5 kb rat TH upstream promoter (sequence information is shown in Supplementary Table 1) as a DA neuron specific promoter for two reasons. First, this upstream promoter was found to drive cell type-specific gene expression to midbrain DA neurons using transgenic founder analysis 11. Second, due to its relatively short length, this promoter fragment could be tested in various viral vectors including AAV.
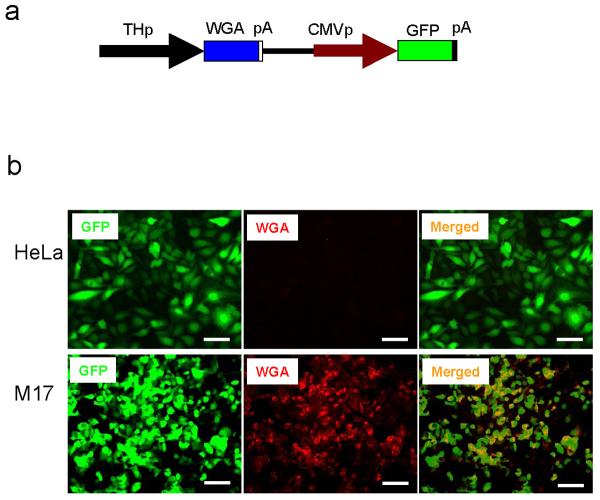
TH is expressed in catecholamine (CA) neurons of the CNS, which include DA neurons of the midbrain and NA neurons of the LC. We first tested the CA neuron cell-type specificity of the 2.5 kb rat TH promoter using non-CA (HeLa) and CA neuron cells (SK-N-BE(2)M17). As shown in Supplementary Figure 1, the great majority (>95%) of SK-N-BE(2)M17 cells are TH-positive, while HeLa cells are TH-negative. These cell lines were infected with adenovirus and its infectivity was accessed by expression of GFP. Both non-CA cells (HeLa) and CA neuron cells (SK-N-BE(2)M17) were efficiently infected with 6 MOI (Multiplicity Of Infection) of the adenoviral construct. Expression of WGA, which is under the TH promoter, was prominently detected only in SK-N-BE(2)M17 cells suggesting that the 2.5 kb rat TH promoter is specific for CA neuron cells (Figure 1).
Figure 1.
The 2.5 kb rat TH promoter expresses WGA in CA neuronal cells. (a) Schematic drawing of adenoviral vector used in the study. Abbreviations; THp (2.5 kb rat TH promoter), WGA (wheat germ agglutinin), CMVp (cytomegalovirus promoter), GFP (green fluorescence proteins), pA (poly adenylation signal). (b) HeLa and SK-N-BE(2)M17 cells were analyzed 2 days after adenovirus infection. GFP was expressed by CMV promoter. Expression of WGA was detected with goat anti-WGA specific antibody (1: 500 dilution, Vector Lab). Alexa 594 anti-goat IgG (1:200 dilution, Molecular Probes) was used as a secondary antibody. Merged images of GFP and WGA are shown. Bar; 100 μm. Recombinant adenovirus was prepared as described 20. The WGA gene, which is under 2.5 kb rat TH promoter, was cloned into the pAdTrack vector. The resulting plasmid was linearized by digesting with PmeI and transformed into E. coli BJ5183 in which an adenoviral backbone plasmid (pAdEasy-1) was introduced. The recombinant adenoviral DNA was selected and transfected into the adenovirus packaging cell line, HEK293, using lipofectamine (Invitrogen). Viruses were harvested 5 days after transfection and concentrated by CsCl density-gradient ultracentrifugation. Viral titer was determined by quantifying the number of cells showing GFP signal (expression forming units, EFU). Cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Hyclone), 100 μg/ml of streptomycin, and 100 units/ml of penicillin in a CO2 incubator.
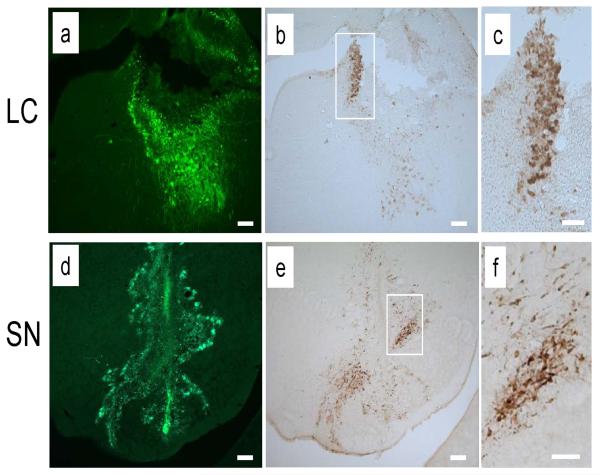
To examine whether TH promoter driven WGA is specifically expressed in NA neurons in vivo, we injected recombinant adenovirus into the LC area. GFP expression confirmed that stereotaxic injection delivered adenovirus into the LC areas (Figure 2a). Expression of WGA, which is under the control of the 2.5 kb rat TH promoter, was predominantly detected in LC (Figure 2b, c). This result confirmed the specificity of the TH promoter for NA neurons. We next delivered the adenovirus into the SN. Looking at the needle track, GFP expression was not found in the regions where adenovirus was delivered (Figure 2d, e). Instead, GFP expression was found in cells adjacent to the injection site suggesting adenovirus toxicity to midbrain DA neurons in contrast to NA neuron in the LC (Figure 2d). Though virus was delivered to a wider area, expression of WGA was mainly restricted to the SN area which was not affected by neurotoxicity (Figure 2e, f). Together, specific transgene expression in the LC and SN by stereotaxic viral injection strongly suggest the in vivo specificity of the 2.5 kb rat TH promoter for transgene expression.
Figure 2.
Adenovirus showed toxicity to midbrain DA neurons of SN. Adenovirus in Figure 1a was delivered to LC and SN of adult rat by stereotaxic injection. Three days after injection, animals were analyzed. Delivery sites of adenovirus were detected by expression of GFP (a, d). Expression of WGA was detected with WGA specific antibody (b, c, e, f; 1:1,000 dilution, Vector Lab). Magnified view of boxed area of (b) and (e) are shown in (c) and (f), respectively. Bar; 200 μm (a, b, d, e), 100 μm (c, f). Ten week-old male Sprague-Dawley rats (280-320 g, Charles River) were used. Adenovirus (1.5 μl of 8 × 1010 EFU/ml) was unilaterally injected into the SN (AP -5.8 mm relative to bregma, L -2.0 mm from midline, and DV -6.0 mm below dura) and the LC (AP -3.9 mm relative to bregma, L -1.4 mm from midline, and DV -7.2 mm below dura) using a stereotaxic instrument (Stoelting Co.) equipped with an autoinjector (Stoelting Co.). After stereotaxic injection of adenovirus, rats were perfused with 4% PFA and the brains were sectioned coronally to 40 μm thickness using a cryostat. Brain sections were mounted on poly-L-lysine-coated glass slides. Antibodies were detected using the Vectastain kit (Vector Labs) and the signal was visualized using 3,3'-diaminobenzidine (DAB). Goat anti-WGA antibody was used to detect expression of WGA. All animal procedures were performed according to experimental protocols approved by the Institutional Animal Care and Use Committee of McLean Hospital and followed the National Institutes of Health guidelines.
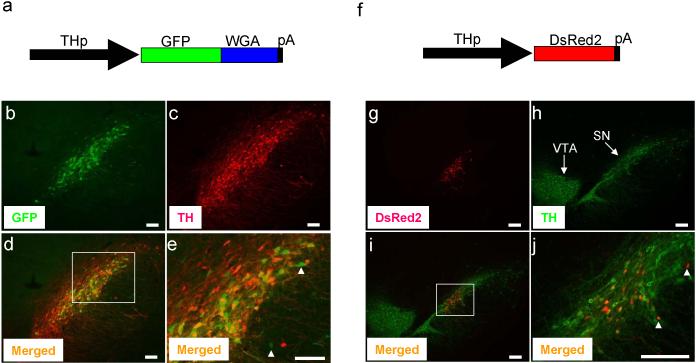
Because adenovirus showed toxicity to SN cells, we next sought to express transgenes, GFP-WGA fusion protein or DsRed2, using the same 2.5kb rat TH promoter in the context of AAV. No significant toxicity was found at the injection sites, when analyzing rodent brains at day 7 or day 17 post stereotaxic injection to the SN (Figure 3). GFP-WGA fusion protein was specifically expressed in the SN (Figure 3b). Its expression mostly overlapped with endogenous expression of TH in the SN (Figure 3d). Notably, expression of GFP-WGA was detected in a small number of TH-negative cells, (Figure 3e, arrowheads). However, it is not clear whether these cells represent non-DA cells or express very low TH levels. In any case, given that TH positive DA neurons are minor fraction of the ventral midbrain area (e.g., 5%), our results could be considered to be significantly DA cell type-specific. In a parallel experiment, we also found that expression of the same TH promoter-driven DsRed2 was mainly detected in the SN (Figure 3g), although DsRed2 expression was also detected in a small number of non-DA neurons (Figure 3j, arrowheads). Taken together, our results indicate that TH promoter will be useful for specific in vivo expression of tracer molecules in midbrain DA neurons and future tracing studies of the DA neuronal circuitry. In support of this, our preliminary analysis showed that WGA could be readily detected in the somas of remotely innervated hypothalamus areas 12, showing that tracers were retrogradely transported from DA neurons (Supplementary Figure 2).
Figure 3.
Transgenes were expressed in SN of adult rat. AAV (1.5 μl of 2-5 × 1012 genome copy/ml) which expresses GFP-WGA fusion protein (a) or DsRed2 (f) was delivered to the SN by stereotaxic injection as described in Figure 2. Seven or 17 days after injection, animals were analyzed. Expression of GFP-WGA (b) and DsRed2 (g) was detected by its fluorescence. TH expression was detected by immunostaining (c, h). Merged images of GFPWGA and TH (d) or DsRed2 and TH (i) are shown. Magnified view of boxed area of (d) and (i) are shown in (e) and (j), respectively. Expression transgenes in the non-DA cells are shown by white arrow heads. VTA and SN are shown. Bar; 100 μm (b, c, d, e), 200 μm (g, h, i, j). Goat anti-WGA antibody (1:200 dilution, Vector Lab) and rabbit anti-TH antibody (1:1,000 dilution, Pel-Freez) were used. For immunofluorescence labeling, Alexa 594 or 488 anti-rabbit or anti-goat IgG (1:200 dilution, Molecular Probes) were used as secondary antibodies. Recombinant AAV was obtained by using the AAV helper-free system according to manufacture's protocol (Stratagene). We replaced the CMV promoter of pAAV-MCS with the 2.5 kb rat TH promoter to obtain the pAAV2.5THp. The GFP-WGA fusion protein or DsRed2 were cloned in the pAAV2.5THp. This plasmid was used to transfect AAV-293 cells with pAAV-RC and pHelper using the calcium phosphate method to make recombinant viral particles. Viruses were harvested 3 days after transfection and purified using heparin-agarose column 21 and concentrated by centrifugation using a filter membrane (Amicon). One ml of viral solution was treated with proteinase K and viral DNA was recovered by phenol extraction followed by ethanol precipitation. The genome copy of viral DNA was determined by PCR.
Tracing the neuronal circuit of specific neurons is of utmost importance to understand their connectivity and function in the nervous system. In contrast to conventional tracing, genetic tracing methods can label specific neuronal subtypes because tracers are expressed by cell-type specific promoters. Indeed, use of cell-type specific promoters in transgenic mice revealed precise neuronal circuits 3, 7, 8, 13. In addition, transgenes, which are under the control of cell-type specific promoters, can be delivered to target neurons in spatiotemporal specific manners using viral vectors by stereotaxic injection 14-17. Several viral vectors have been developed to deliver transgenes in the nervous system 18. However, each viral vector having specific properties, such as cloning capacity and viral titer it is important to choose the proper viral vectors for the desired purpose. Tracing of neuronal pathways is mostly based on detection of tracers which are anterogradely or retrogradely transported in the neurons 4. Thus proper selection of cell-type specific promoters, viral vectors, and tracers is critical for efficient tracing studies of the neuronal circuitry. As an initial step to identify the neuronal circuit of midbrain DA neuron by genetic tracing, we chose the rat TH promoter as a cell-type specific promoter and adenovirus as a vehicle. Strikingly, in contrast to NA neurons in the LC, adenovirus showed significant toxicity to DA neurons in the SN (Figure 2). We next chose AAV which is known to be less toxic and offer long term expression 19. Indeed, AAV proved to be non-toxic and expression of TH promoter driven transgene was specifically detected in DA neurons of the SN although we found a small number of TH-negative cells that expressed the transgenes. This largely cell type-specific transgene expression is consistent with the previoius work which showed that the rat 2.5 kb TH promoter can drive cell type-specific transgene expression using transgenic founder analysis11. Taken together, our results suggest that the rat 2.5 kb TH promoter is relatively specific for driving transgene expression to DA neurons of the SN in vivo and may be useful for future genetic tracing experiments although its cell type-specificity was not perfect.
Supplementary Material
Acknowledgement
This work was supported by NIH grants DC006501 and MH48866, and an International Grant from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea, and the Korea Research Foundation Grant KRF-2006-214-E00037.
References
- 1.Boldogkoi Z, Sik A, Denes A, Reichart A, Toldi J, Gerendai I, et al. Novel tracing paradigms--genetically engineered herpesviruses as tools for mapping functional circuits within the CNS: present status and future prospects. Prog Neurobiol. 2004;72:417–445. doi: 10.1016/j.pneurobio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 3.Yoshihara Y. Visualizing selective neural pathways with WGA transgene: combination of neuroanatomy with gene technology. Neurosci Res. 2002;44:133–140. doi: 10.1016/s0168-0102(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 4.Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 5.Yoshihara Y, Mizuno T, Nakahira M, Kawasaki M, Watanabe Y, Kagamiyama H, et al. A genetic approach to visualization of multisynaptic neural pathways using plant lectin transgene. Neuron. 1999;22:33–41. doi: 10.1016/s0896-6273(00)80676-5. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz LF, Montmayeur JP, Echelard Y, Buck LB. A genetic approach to trace neural circuits. Proc Natl Acad Sci U S A. 1999;96:3194–3199. doi: 10.1073/pnas.96.6.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugita M, Shiba Y. Genetic tracing shows segregation of taste neuronal circuitries for bitter and sweet. Science. 2005;309:781–785. doi: 10.1126/science.1110787. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Prakash N, Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci. 2006;63:187–206. doi: 10.1007/s00018-005-5387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang DY, Carlezon WA, Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Merlie JP, Todd RD, O'Malley KL. Identification of cell type-specific promoter elements associated with the rat tyrosine hydroxylase gene using transgenic founder analysis. Brain Res Mol Brain Res. 1997;50:33–42. doi: 10.1016/s0169-328x(97)00163-0. [DOI] [PubMed] [Google Scholar]
- 12.Gerfen CR, Staines WA, Arbuthnott GW, Fibiger HC. Crossed connections of the substantia nigra in the rat. J Comp Neurol. 1982;207:283–303. doi: 10.1002/cne.902070308. [DOI] [PubMed] [Google Scholar]
- 13.Zou Z, Horowitz LF, Montmayeur JP, Snapper S, Buck LB. Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature. 2001;414:173–179. doi: 10.1038/35102506. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita N, Mizuno T, Yoshihara Y. Adenovirus-mediated WGA gene delivery for transsynaptic labeling of mouse olfactory pathways. Chem Senses. 2002;27:215–223. doi: 10.1093/chemse/27.3.215. [DOI] [PubMed] [Google Scholar]
- 15.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- 16.Sato Y, Shiraishi Y, Furuichi T. Cell specificity and efficiency of the Semliki forest virus vector-and adenovirus vector-mediated gene expression in mouse cerebellum. J Neurosci Methods. 2004;137:111–121. doi: 10.1016/j.jneumeth.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Otaki JM, Firestein S. Adenovirus-mediated gene transfer in olfactory neurons in vivo. J Neurobiol. 1996;30:521–530. doi: 10.1002/(SICI)1097-4695(199608)30:4<521::AID-NEU7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 19.Grimm D, Kleinschmidt JA. Progress in adeno-associated virus type 2 vector production: promises and prospects for clinical use. Hum Gene Ther. 1999;10:2445–2450. doi: 10.1089/10430349950016799. [DOI] [PubMed] [Google Scholar]
- 20.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auricchio A, Hildinger M, O'Connor E, Gao GP, Wilson JM. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.