Abstract
A Trypanosoma brucei TbGPI12 null mutant that is unable to express cell surface procyclins and free glycosylphosphatidylinositols (GPI) revealed that these are not the only surface coat molecules of the procyclic life cycle stage. Here, we show that non-GPI-anchored procyclins are N-glycosylated, accumulate in the lysosome, and appear as proteolytic fragments in the medium. We also show, using lectin agglutination and galactose oxidase-NaB3H4 labeling, that the cell surface of the TbGPI12 null parasites contains glycoconjugates that terminate in sialic acid linked to galactose. Following desialylation, a high-apparent-molecular-weight glycoconjugate fraction was purified by ricin affinity chromatography and gel filtration and shown to contain mannose, galactose, N-acetylglucosamine, and fucose. The latter has not been previously reported in T. brucei glycoproteins. A proteomic analysis of this fraction revealed a mixture of polytopic transmembrane proteins, including P-type ATPase and vacuolar proton-translocating pyrophosphatase. Immunolocalization studies showed that both could be labeled on the surfaces of wild-type and TbGPI12 null cells. Neither galactose oxidase-NaB3H4 labeling of the non-GPI-anchored surface glycoconjugates nor immunogold labeling of the P-type ATPase was affected by the presence of procyclins in the wild-type cells, suggesting that the procyclins do not, by themselves, form a macromolecular barrier.
The tsetse fly-transmitted protozoan parasite Trypanosoma brucei causes human sleeping sickness and the cattle disease Nagana in sub-Saharan Africa. The organism undergoes a complex life cycle between the mammalian host and the insect, tsetse, vector. The bloodstream form of the parasite expresses a dense monolayer of glycosylphosphatidylinositol (GPI)- anchored variant surface glycoprotein dimers and avoids specific immune responses through antigenic variation (32, 47). Following ingestion in a blood meal, the parasites differentiate into procyclic-form parasites that colonize the tsetse midgut. The procyclic trypanosomes express a radically different cell surface coat that includes about 3 × 106 procyclin glycoproteins (28, 36, 37) and about 1 × 106 poly-N-acetyllactosamine-containing free GPIs (19, 29, 39, 55). The procyclins are polyanionic, rod-like (38, 50) proteins encoded by procyclin genes. In T. brucei strain 427, used in this study, the parasites contain (per diploid genome) two copies of the GPEET1 gene, encoding 6 Gly-Pro-Glu-Glu-Thr repeats; one copy each of the EP1-1 and EP1-2 genes, encoding EP1 procyclins with 30 and 25 Glu-Pro repeats, respectively; two copies of the EP2-1 gene, encoding EP2 procyclin with 25 Glu-Pro repeats; and two copies of the EP3-1 gene, encoding EP3 procyclin with 22 Glu-Pro repeats (1). The EP1 and EP3 procyclins contain a single N-glycosylation site, occupied exclusively by a conventional Man5GlcNAc2 oligosaccharide, at the N-terminal side of the Glu-Pro repeat domain (1, 50). Whereas neither EP2 nor GPEET procyclin is N-glycosylated, GPEET1 procyclin is phosphorylated on six out of seven Thr residues (25). In culture, the procyclin expression profile depends on the carbon source (56) and metabolic state of the cells (27), and in the tsetse fly, there appears to be a program of procyclin expression such that GPEET procyclin is expressed early, giving way to EP1 and EP3 procyclin expression (2, 54). GPEET and EP procyclins contain similar GPI membrane anchors. These are based on the ubiquitous ethanolamine-P-6Manα1-2Manα1-6Manα1-4GlcNα1-6PI core (where, in this case, the PI lipid is a 2-O-acyl-myo-inositol-1-P-sn-2-lyso-1-O-acylglycerol structure [50]), but they also contain the largest and most complex known GPI side chains. These side chains are large poly-disperse-branched poly-N-acetyllactosamine structures (with an average of about 8 to 12 repeats, depending on the preparation) that can terminate with α2- and α3-linked sialic acid residues (9, 50). Sialic acid is transferred from serum sialoglycoconjugates to terminal β-galactosidase residues by the action of a cell surface GPI-anchored trans-sialidase enzyme (7, 26, 34). The trans-sialylation of surface components plays a role in the successful colonization of the tsetse fly (29). In vivo, the N termini of the procyclins are removed by tsetse fly gut proteases (2), though the role of this event is unclear (20) and it is thought that the underlying (protease-resistant) anionic repeat units and associated GPI anchor side chains might protect the parasite from the approach of tsetse fly gut hydrolases (2).
The cell surface architecture of procyclic trypanosomes has been manipulated by the gene knockout of the procyclin genes themselves (55, 57), by galactose starvation (39), and by the knockout or knockdown of genes encoding enzymes of the GPI biosynthetic pathway, i.e., TbGPI10, TbGPI8, and TbGPI12 (11, 19, 29, 30). The procyclin TbGPI10 and TbGPI8 knockouts all resulted in parasites devoid of GPI-anchored procyclins, but this was compensated for by an upregulation in free GPI expression. However, the TbGPI12 null mutants that cannot synthesize GPI structures beyond GlcNAc-PI, revealed the presence of previously unidentified non-GPI-anchored surface coat components. In this paper, we characterize the fate of non-GPI-anchored procyclin protein and characterize the non-GPI-anchored surface coat components.
MATERIALS AND METHODS
Cultivation of procyclic-form trypanosomes.
Procyclic T. brucei strain 427 clone 29.13 cells, referred to as wild-type cells, were cultured at 28°C in SDM-79 in the presence of 15% fetal bovine serum, 2 mM Glutamax (Invitrogen), and 7.5 mg/liter hemin (5) containing 15 μg/ml G418 and 50 μg/ml hygromycin. The creation of the TbGPI12 null cells and the culture conditions were described previously (11). Large cultures of the TbGPI12 null cells were prepared as described above using roller bottles and gently rotated for 2 to 3 days.
Biosynthetic labeling of TbGPI12 null cells with radioactive sugars and [14C]proline.
Washed wild-type and TbGPI12 null cells (5 ml at 107 cells/ml) were labeled with [3H]glucosamine, [3H]mannose, and [3H]glucose in glucose-free SDM-79 containing 2% dialyzed fetal bovine serum. For the [14C]proline labeling, SDM-79 was depleted of l-proline and hydroxyproline. The labeling was performed using 50 μCi/ml of sugar radioisotopes for 3 h, or using 10 μCi/ml of [14C]proline for 2 and 20 h, at 28°C. The labeled cells were washed in ice-cold phosphate-buffered saline (PBS), resuspended in 15 μl PBS, and boiled with an equal volume of 2× concentrated sodium dodecyl sulfate (SDS)-sample buffer containing 0.2 M dithiothreitol (DTT). The [14C]proline-labeling culture supernatant was centrifuged, filtered (0.2-μm pore size), and immunoprecipitated for 2 h at 4°C with 5 μg anti-EP procyclin antibody (Cedarlane Laboratories, Canada) and 20 μg protein G-agarose (Pierce). The beads were washed in PBS containing 0.1% SDS and 1% Triton X-100 (TX-100), boiled in SDS-sample buffer containing 0.1 M DTT, and run on 12% or 4 to 12% Nupage/MOPS (morpholinepropanesulfonic acid) gels (Invitrogen). For the experiment for which the results are shown in Fig. 3D, total cells labeled with [3H]mannose were boiled in SDS-sample buffer with 0.1 M DTT, diluted to 0.03% SDS with 1% TX-100 in 20 mM Tris-HCl (pH 7.2) and 0.15 M NaCl, and pulled down with 30 μl ricin-agarose (Vector Labs) for 2 h at 4°C. The beads were washed in 0.03% SDS and 1% TX-100 buffer, boiled in SDS-sample buffer with 0.1 M DTT, and applied to a 4 to 12% Nupage gel. The gels were impregnated with En3Hance (Perkin-Elmer), dried, and taken for fluorography at −80°C with an intensifying screen.
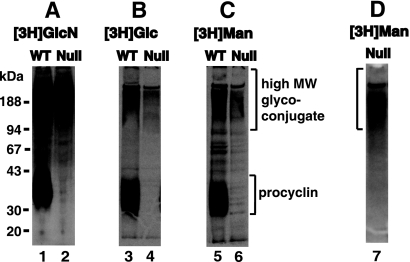
FIG. 3.
Radiolabeling of a high-apparent-MW glycoconjugate fraction with 3H-monosaccharides. Wild-type (WT) and TbGPI12 null (Null) procyclic trypanosomes were biosynthetically labeled with [3H]glucosamine ([3H]GlcN; panel A, lanes 1 and 2), [3H]glucose ([3H]Glc; panel B, lanes 3 and 4) and [3H]mannose ([3H]Man; panel C, lanes 5 and 6), as indicated. Cell lysates were analyzed by SDS-PAGE and fluorography. The lysates of the [3H]mannose-labeled TbGPI12 null cells were also subjected to a pulldown on ricin-agarose (panel D, lane 7). The positions of the MW markers are shown on the left.
Galactose oxidase-sodium borotritide labeling of live procyclic cells.
Procyclic cells (2 × 107) were washed, resuspended in 1 ml SDM-79 minus fetal bovine serum containing 1 mM nonradioactive NaBH4 (to block reducible sites), and incubated with 5 mU/ml of Clostridium perfringens neuraminidase (Sigma) for 30 min at 28°C. The cells were washed and resuspended in PBS and incubated with 1.5 U/ml of Dactylium dendroides galactose oxidase (Sigma) (previously heated for 30 min at 50°C to destroy protease contaminant activity) for 30 min at 37°C. The cells were washed, resuspended in 0.5 ml PBS, and labeled for 30 min at 28°C with 2 μl of 0.4 Ci/ml NaB[3H]4 (Perkin Elmer) dissolved in 0.1 M NaOH. The cell pellet was resuspended in 1 mM freshly prepared nonradioactive NaBH4 in PBS, washed three times in PBS, boiled in SDS-sample buffer containing DTT, loaded onto 4 to 12% Nupage/MOPS gels (Invitrogen), and processed for fluorography as described above.
Lectin agglutination experiments.
Wild-type and TbGPI12 null cells were washed, resuspended at 2 ×107 cells/ml in SDM-79 medium minus fetal bovine serum, and incubated for 1 h at 28°C with an equal volume of 0.2 μg/ml lectin (Vector Labs, United Kingdom) before examination by light microscopy under phase contrast. Lectin final concentrations of 0, 1, 2.5, and 10 μg/ml were also tested (data not shown). Pretreatment with 0.2 mU of Clostridium perfringens neuraminidase (Roche) was performed for 30 min at 28°C in SDM-79 medium minus fetal bovine serum.
High-MW glycoconjugate purification by ricin-agarose and gel filtration.
TbGPI12 null cells (5 liters at about 4 × 107 cells/ml) were harvested by centrifugation at 600 × g for 10 min at 4°C, washed with PBS, and hypotonically lysed in water containing 0.1 mM TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone), 2 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1 μg/ml aprotinin. The cell ghosts were recovered by centrifugation at 13,000 × g for 10 min at 4°C and washed three times with 100 ml ice-cold 20 mM Tris-HCl (pH 6.8), 0.15 M NaCl. The final cell ghost pellet was extracted with SDS-urea and purified on ricin-agarose beads (RCA120-agarose; Vector Labs, United Kingdom) as described previously (3). The beads (10-ml packed volume) were packed into a column, washed with 100 ml wash buffer (0.06% SDS, 0.8% n-octylglucoside, 0.1 M DTT, 20 mM Tris-HCl [pH 6.8], 0.2 M NaCl, 0.05% sodium azide), and eluted with 17 ml of 30 mg/ml of each galactose and lactose in wash buffer at 4°C. The beads were further eluted at 50°C with 10 ml 1% SDS, 0.1 M DTT, 20 mM Tris-HCl (pH 6.8), 0.2 M NaCl, 30 mg/ml galactose, and 30 mg/ml lactose and at 100°C with 10 ml of the same buffer. The combined eluates were diluted to a final concentration of 0.1% SDS, concentrated to 1 ml using a Vivaspin 20 concentrator (Sartorius) and loaded onto a Superose 6B gel filtration column (1 by 30 cm; GE Healthcare) at 0.5 ml/min using 20 mM Tris-HCl (pH 6.8) containing 0.15 M NaCl, 0.1% (wt/vol) SDS, 10 mM DTT. Fractions (0.5 ml) were collected, applied on 4 to 12% Nupage/MOPS gels, and stained or blotted to nitrocellulose as described below. Superose 6B molecular weight (MW) calibration curves were performed using 0.5 mg of each blue dextran, thyroglobulin, apoferritin, β-amylase, and bovine serum albumin (BSA).
Proteomics.
An aliquot of fraction 18 from Superose chromatography was reduced and alkylated with iodoacetamide in SDS-sample buffer, run on 4 to 12% Nupage/MOPS gels, and stained with colloidal Coomassie blue (Simply Blue; Invitrogen). The center of the band was excised and subjected to in-gel digestion with trypsin. The resulting peptides were analyzed, in the Dundee University Fingerprints Proteomics Facility, by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on a Thermo Finnegan Orbitrap instrument equipped with a Dionex 3000 nano-LC. The data were processed by Mascot.
Immunofluorescence and transmission electron microscopy.
T. brucei procyclic-form cultures (2 × 107 cells/ml) were harvested by centrifugation at 600 × g, washed in ice-cold PBS, fixed on coverslips with 4% paraformaldehyde in PBS for 15 min at room temperature (RT), permeabilized in 0.05% TX-100 in PBS, and blocked with 1% (vol/vol) fish-skin gelatin (Sigma) in PBS. For the experiment for which the results are shown in Fig. 1, the samples were double labeled for 1 h at RT with 1:2,000 mouse monoclonal anti-EP procyclin (Cedarlane, Canada) and 1:250 rabbit polyclonal anti-trypanopain (kind gift of Jay Bangs, Madison, WI). Subsequently, they were washed in fish-skin gelatin in PBS, incubated for 1 h at RT with a mix of 1:500 of anti-mouse Alexa Fluor 468 (green) and 1:500 anti-rabbit Alexa Fluor 588 (red) (Molecular Probes). The coverslips were further washed and mounted onto slides using Hydromount (National Diagnostics) containing 2.5% (wt/vol) Dabco (Sigma) and visualized with a Zeiss 510 microscope. For the experiment for which the results are shown in Fig. 7A, the coverslips containing fixed wild-type and TbGPI12 null procyclic cells were incubated with 1:400 rabbit polyclonal anti-P-type ATPase (kind gift of Roberto Docampo, University of Georgia). For the experiment for which the results are shown in Fig. 7B, live parasites were incubated for 1 h at 4°C with 1:400 rabbit polyclonal anti-vacuolar-type proton pyrophosphatase (VHPPase) (a kind gift of Norbert Bakalara, ENSCM, France) or 1:400 rabbit polyclonal anti-VHPPase peptides 324 and 326 raised against Arabidopsis thaliana VHPPase putative hydrophilic loops IV and XII, respectively (kind gift of Philip Rea, University of Pennsylvania, Philadelphia, PA). Subsequently, the parasites were washed and fixed on coverslips with 4% paraformaldehyde in PBS. The secondary antibody was 1:500 anti-rabbit Alexa Fluor 468, which was mounted and visualized as described above.
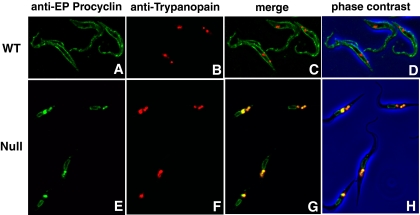
FIG. 1.
Procyclin localizes predominantly to the lysosomes in TbGPI12 null cells. Immunofluorescence microscopy of wild-type (WT) and TbGPI12 null (Null) procyclic trypanosomes stained with anti-EP procyclin (panels A and E; the TbGPI12 null mutant and its wild-type parent cell line express almost exclusively EP procyclins [25]) and anti-trypanopain (panels B and F) antibodies, as indicated. The merged images (panels C and G) and merged images together with the corresponding phase-contrast images (panels D and H) are also shown. Trypanopain is a lysosomal marker (51).
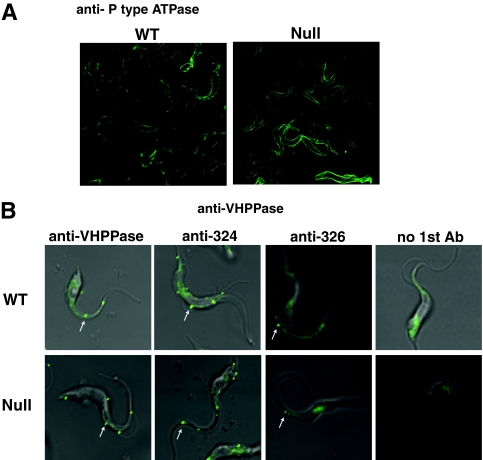
FIG. 7.
Localization of P-type ATPase and VHPPase in wild-type and TbGPI12 null procyclic cells by immunofluorescence microscopy. (A) Fixed and permeabilized cells were stained with rabbit anti-P-type ATPase antibodies followed by fluorescently labeled secondary antibodies, revealing cell surface labeling in both cases. (B) Live cells were incubated with no primary antibody (no 1st Ab; right panels), antibodies raised to a fragment of recombinant VHPPase antibodies (left panels), or one of two anti-VHPPase synthetic peptide antibodies (middle panels); fixed; and then stained with fluorescently labeled secondary antibodies. Punctate staining of surface structures is indicated by white arrows.
For the transmission electron microscopy for which the results are shown in Fig. 8, in vivo single labeling was performed prior to fixation and cryosectioning. Wild-type and TbGPI12 null procyclic cells (107 parasites in 0.5 ml SDM-79 plus 15% fetal bovine serum) were incubated for 1 h at 4°C with 1:10 rabbit polyclonal anti-P-type ATPase. The cells were washed with cold medium, resuspended, and fixed for 15 min in 4% (wt/vol) paraformaldehyde in PBS. The cells were washed in PBS and incubated with 1:50 protein A-10-nm gold (BBInternational, Cardiff, United Kingdom) for 30 min at 28°C. The cells were washed and resuspended in 0.5 ml PBS and fixed by adding 0.5 ml of 5% (vol/vol) glutaraldehyde containing 2% (wt/vol) tannic acid (Sigma) in 0.2 M cacodylate-HCl (pH 6.9). The cells were washed in 0.1 M cacodylate-HCl (pH 6.9), fixed for 1 h in 1% OsO4 containing 1.5% sodium ferrocyanide in cacodylate buffer, dehydrated in ethanol, and embedded in Durcupan overnight. Ultrathin sections were stained with 3% aqueous uranyl acetate, followed by Reynolds lead citrate, and imaged on an FEI (Eindhoven, The Netherlands) Tecnai 12 transmission electron microscope. The images collected on digital imaging plates were scanned on a Ditabis micron scanner (Pforzheim, Germany).
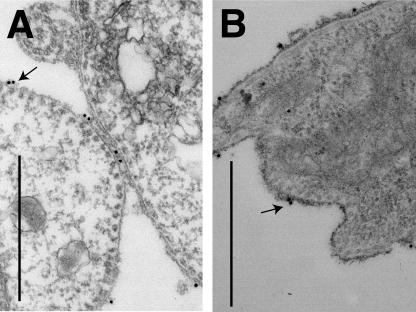
FIG. 8.
Localization of P-type ATPase on live wild-type and TbGPI12 null procyclic cells by immunoelectron microscopy. Live wild-type (A) and TbGPI12 null (B) cells were labeled with rabbit anti-P-type ATPase antibodies and fixed. Anti-P-type ATPase antibodies were revealed with 10-nm gold particles conjugated to protein A. The images were magnified ×26,000, and the black bars correspond to 1 μm in panels A and B. Black arrows indicate representative sites of surface labeling.
Reducing SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
Superose 6B fractions were run on reducing 4 to 12% Nupage/MOPS gels and either stained with Coomassie blue (Simply Blue; Invitrogen) or periodate-Schiff reagent (Sigma) or, alternatively, transferred onto nitrocellulose and blocked with blocking buffer (0.5% [wt/vol] BSA, 0.05% [wt/vol] NP-40 [Igepal; Sigma], 50 mM Tris-HCl [pH 7.4]). In the experiment for which the results are shown in Fig. 5, nitrocellulose was probed with 1:2,000 biotinylated ricin, followed by washes in blocking buffer, incubation with 1:10,000 Extravidin horseradish peroxidase (Sigma), and detection with ECL reagents (GE Healthcare). In the experiment for which the results are depicted in Fig. 6, nitrocellulose was probed with 1:400 anti-P-type ATPase or 1:400 anti-VHPPase (a kind gift from Norbert Bakalara) diluted in blocking buffer. After washing in blocking buffer, the blots were incubated with 1:5,000 goat anti-rabbit antibodies conjugated to horseradish peroxidase (Stratech, United Kingdom) and detected by ECL.
FIG. 5.
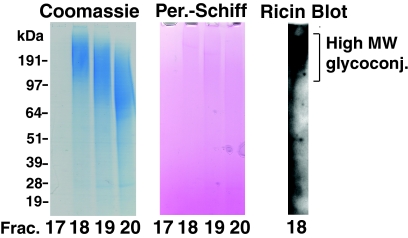
Purification of the high-MW glycoconjugate fraction. After ricin affinity chromatography, the majority of the high-MW glycoconjugate fraction was eluted in fractions 18 to 20 from a Superose 6B gel filtration column. Aliquots of fractions 17 to 20, corresponding to molecules with a predicted molecular mass of 6 MDa to 600 kDa, were analyzed by SDS-PAGE and stained for protein with Coomassie blue or for carbohydrate by periodate-Schiff (Per.-Schiff). A sample of fraction 18 was also subjected to ricin blotting. The positions of the MW markers are shown on the left.
FIG. 6.
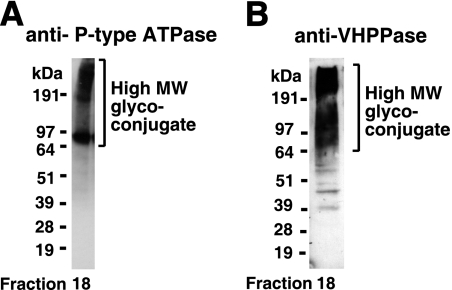
Identification of P-type ATPase and VHPPase in the high-MW glycoconjugate fraction. (A) Western blot of fraction 18 from gel filtration with anti-P-type ATPase antibodies. (B) Western blot of fraction 18 from gel filtration with anti-VHPPase antibodies.
Sugar composition analysis by GC-MS.
Superose 6 fraction 18 was desalted by diafiltration using a Microcon 30 (Amicon) concentrator. Aliquots were subsequently mixed with 2 nmol of a scyllo-inositol internal standard and subjected to acid methanolysis, re-N-acetylation, and trimethylsilylation, and the derivatives were analyzed and quantified by GC-MS, as described previously (3, 8). The monosaccharide quantifications were compared to total protein measured by the bicinchoninic acid method (Pierce) using BSA as the standard.
RESULTS
Fate of non-GPI-anchored procyclin in TbGPI12 null cells.
Procyclin was localized by immunofluorescence microscopy using anti-EP procyclin antibodies in wild-type (Fig. 1A) and TbGPI12 null cells (Fig. 1E). The former gave the expected cell surface labeling, whereas the latter produced a weak perinuclear pattern together with a strong punctate stain between the nucleus and the flagellar pocket, reminiscent of a lysosomal location. To assess this, the cells were also stained with antibodies to a lysosomal marker protein, trypanopain (33, 51). In the wild-type cells, there was no significant colocalization (Fig. 1B to D), whereas in the TbGPI12 null cells, there was considerable colocalization of the procyclin and trypanopain signals (Fig. 1F to H). These data suggest that, in the absence of GPI anchoring, the procyclins are found partly in the endoplasmic reticulum (ER) and mostly in the lysosomes.
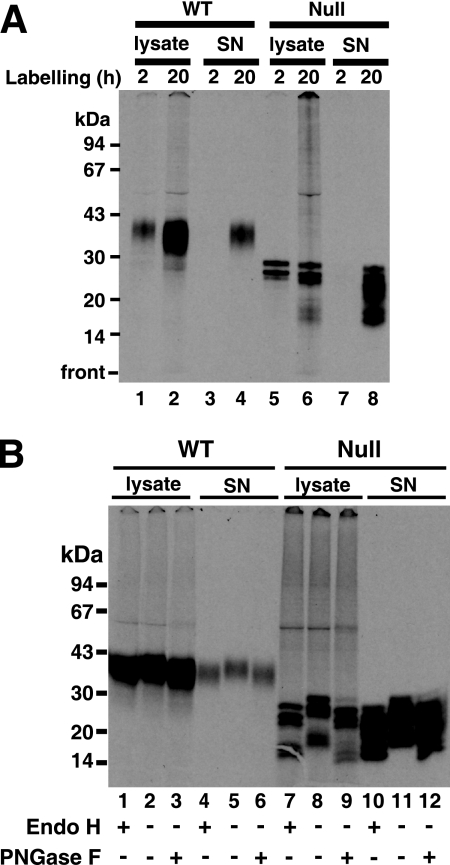
To further investigate the fate of procyclin, wild-type and TbGPI12 null cells were metabolically labeled with [14C]proline for 2 h and 20 h at 28°C. Subsequently, cell lysates and their respective culture supernatants were immunoprecipitated with anti-EP procyclin antibodies and protein G-agarose. The immunoprecipitates were analyzed by liquid scintillation counting (Table 1) and by SDS-PAGE and fluorography (Fig. 2). The wild-type cells showed the usual diffuse band (apparent molecular mass, 32 to 45 kDa) of fully processed procyclin at both 2 and 20 h of labeling (Fig. 2A, lanes 1 and 2), and a small proportion (16%) (Table 1) of the label at 20 h was found in the supernatant (Fig. 2A, lane 4).
TABLE 1.
Distribution of cellular and shed or secreted [14C]proline-labeled EP procyclin in wild-type and TbGPI12 null cells
| Labeling (h) | WT cpm (% total cpm)
|
TbGPI12 null cpm (% total cpm)
|
||
|---|---|---|---|---|
| Cell lysate | Culture supernatant | Cell lysate | Culture supernatant | |
| 2 | 14,000 (98) | 300 (2) | 18,000 (97) | 500 (3) |
| 20 | 89,000 (84) | 17,000 (16) | 105,000 (45) | 130,000 (55) |
a Wild-type (WT) and TbGPI12 null procyclic trypanosomes were labeled with [14C]proline for 2 or 20 h, and their cell lysates and corresponding culture supernatants were immunoprecipitated with anti-EP procyclin antibodies. Aliquots were taken for liquid scintillation counting.
FIG. 2.
Procyclin is N-glycosylated, proteolytically processed, and secreted from TbGPI12 null cells. (A) Wild-type (WT) cells (lanes 1 to 4) and TbGPI12 null (Null) cells (lanes 5 to 8) were labeled with [14C]proline for 2 and 20 h, and their corresponding cell lysates and culture supernatants (SN) were immunoprecipitated with anti-EP antibody and analyzed by SDS-PAGE and fluorography. (B) Samples from the 20-h labeling were digested with and without endo H and PNGase F, as indicated, and analyzed by SDS-PAGE and fluorography. The positions of the MW markers are shown on the left.
The TbGPI12 null cell lysates showed labeled bands of apparent molecular masses of 29, 27, and 26 kDa (Fig. 2A, lanes 5 and 6), and by 20 h, 55% (Table 1) of the label was found in the culture supernatant in the form of multiple bands, ranging in apparent molecular masses from 27 to 17 kDa. The digestion of the 20-h samples with endoglycosidase H (endo H) and peptide N-glycosidase F (PNGase F) showed that every band was susceptible to both enzymes (Fig. 2B). From these data, we can conclude that all of the procyclin fragments observed in the TbGPI12 null sample are N-glycosylated with oligomannose structures.
Additional [14C]proline-labeling experiments using the cysteine protease inhibitor FMK24, known to inhibit the lysosomal cysteine proteases of T. brucei (51), were performed. The presence of this compound reduced the formation of low-MW procyclin fragments in the TbGPI12 null cells (see Fig. S1 in the supplemental material), supporting the view that procyclin degradation occurs, at least in part, in the lysosome.
Biosynthetic labeling of TbGPI12 null cells with radioactive sugars.
Previously, we reported that the TbGPI12 null surface coat could be stained with ruthenium red, a glycocalyx dye, indicating that procyclic trypanosomes have non-GPI-anchored carbohydrate-containing surface coat molecules in addition to GPI-anchored procyclins (11). To further characterize the nature of these other surface coat molecules, we labeled wild-type and TbGPI12 null cells with [3H]mannose and [3H]glucose and analyzed the labeled samples by SDS-PAGE and fluorography. These data are shown alongside our previously reported [3H]glucosamine labeling (Fig. 3A). In all cases, we detected a smear of radiolabeled molecules that was present in both the wild-type and TbGPI12 null cells (Fig. 3, lanes 1 to 6). We refer to this labeled material that runs with an apparent molecular mass from 90 kDa to >200 kDa (high-MW glycoconjugate). This material was also labeled with [3H]serine and [35S]methionine, indicating it contains protein or peptide components (data not shown). In the wild-type cells, all of the sugars also labeled the procyclins, as expected (Fig. 3, lanes 1, 3, and 5).
Interestingly, whereas the cell-associated N-glycosylated [3H]proline-labeled 29-, 27-, and 26-kDa procyclin fragments are clearly visible after 2 h of labeling (Fig. 2A, lane 5), there is only a very weak signal in those fragments during the 3-h [3H]mannose-labeling experiment (Fig. 3C, lane 6). We investigated the relative efficiencies of [3H]mannose incorporation into the GPI anchor and the N-linked oligosaccharide of procyclin by repeating the 3-h [3H]mannose-labeling experiment with wild-type cells and analyzing the procyclin by SDS-PAGE before and after treatment with PNGase F. The shift in apparent MW confirmed the removal of the N-linked glycan, and the subsequent in-gel base digestion, neutralization, and scintillation counting revealed that 70% of the radioactivity was associated with the GPI anchor, despite it containing only three Man residues compared with five in the N-glycan. The relatively low efficiency of radiolabeling of the N-glycan versus the GPI anchor presumably represents a much longer half-life for steady-state labeling of the Man9GlcNAc2-PP-Dol N-glycan precursor than that for the GPI anchor precursor in wild-type cells. This, together with the observed proteolytic turnover of the non-GPI-anchored procyclin in the TbGPI12 null mutant, provides an explanation for the aforementioned discrepancy.
To confirm that the [3H]mannose label in the high-MW glycoconjugate fraction seen in Fig. 3C was associated with ricin-binding glycoproteins (see below), we also performed a pulldown from the [3H]mannose-labeled TbGPI12 null mutant total cell lysate with ricin-agarose. Subsequent SDS-PAGE and fluorography revealed that the radiolabeled material pulled down by the ricin lectin contained the high-MW glycoconjugate (Fig. 3D, lane 7).
In a separate experiment, TbGPI12 null cells labeled with [3H]mannose were extracted overnight with chloroform-methanol water (1:2:0.8, vol/vol), and the insoluble pellet extracted with 9% butan-1-ol in water. The latter fraction and the final pellet solubilized in SDS-sample buffer were analyzed by SDS-PAGE and fluorography. No radiolabeled products were found in the 9% butan-1-ol extract, whereas several labeled bands were observed in the final pellet solubilized in SDS-sample buffer (data not shown), indicating that, like wild-type procyclic-form cells, the TbGPI12 null mutant cells do not express any lipophosphoglycan-like molecules (9).
Cell agglutination with lectins.
To provide direct evidence for the exposure of carbohydrates on the surface, live wild-type and procyclin-less TbGPI12 null cells were washed and assayed for agglutination by various lectins (Table 2). Wheat germ agglutinin (WGA) and concanavalin A both agglutinated wild-type and TbGPI12 null cells and, at higher concentrations, caused cell death. However, ricin (RCA120) had no effect on either cell type unless they were pretreated with Clostridium perfringens neuraminidase, after which it caused efficient agglutination but not cell death. The WGA lectin has dual specificity and recognizes both terminal sialic acid and GlcNAc residues. In this case, WGA only agglutinated cells before treatment with neuraminidase. Furthermore, the terminal GlcNAc-specific Griffonia simplicifolia lectin II failed to agglutinate the cells before or after neuraminidase treatment. Taken together, these results suggest that both wild-type and TbGPI12 null cells express terminal sialic acid residues linked to underlying galactose residues on their cell surface. The absence of significant agglutination by tomato lectin suggests an absence of surface-linear poly-N-acetyllactosamine structures.
TABLE 2.
Agglutination of wild-type (WT) and TbGPI12 null cells by lectinsa
| Lectin | Lectin specificity | Neuraminidase pretreatment | Degree of agglutination for indicated cells
|
|
|---|---|---|---|---|
| WT | TbGPI12 null | |||
| WGA | GlcNAc/sialic acid | No | +++ | +++ |
| GlcNAc/sialic acid | Yes | − | − | |
| Ricin (RCA 120) | Terminal Gal or GalNAc | No | − | − |
| Terminal Gal or GalNAc | Yes | +++ | +++ | |
| Concanavalin A | α-Mannose | No | +++ | +++ |
| GSL II | α- or β-GlcNAc | No | − | − |
| α- or β-GlcNAc | Yes | − | − | |
| Tomato lectin | (GlcNAc)2 to (GlcNAc)4 | No | +/− | +/− |
Cells, with or without pretreatment with neuraminidase, were incubated with WGA, ricin, concanavalin A, Griffonia simplicifolia lectin II (GSL II), and tomato lectin. The degrees of cell agglutination, from none (−) to completely (+++) agglutinated, were scored by light microscopy. Similar results were obtained when lectins were used at 0.1, 1, 2.5, and 10 μg/ml. Control cells without lectin were always viable and nonagglutinated.
Surface labeling with galactose oxidase-sodium borotritide.
To check that the high-MW glycoconjugate(s) contains subterminal galactose residues by an independent method, and to assess the accessibility of those galactose residues on the high-MW glycoconjugate(s) in the presence and absence of the procyclin coat, we performed cell surface labeling of C. perfringens neuraminidase treated with live wild-type and TbGPI12 null cells by the galactose oxidase-NaB[3H]4 method (10). A slight modification of the standard method was developed in these studies, namely, the prereduction of the living cells with nonradioactive 1 mM NaBH4 under physiological conditions prior to washing and galactose oxidase-NaB[3H]4 labeling. We found that this modification greatly reduced nonspecific radiolabeling, and we would recommend it to others planning to use this cell surface-labeling method.
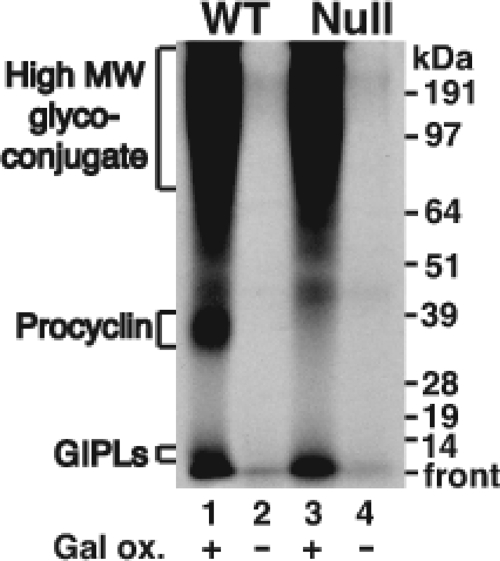
Using this method, the procyclins, as well as the free GPIs, of wild-type cells were labeled after (but not before) galactose oxidase treatment (Fig. 4, lanes 1 and 2), consistent with their known structures (reviewed in reference 14). However, the high-MW glycoconjugates were even more efficiently labeled than the procyclins (Fig. 4, lane 1), suggesting that they contain significantly more terminal galactose residues (after neuraminidase treatment) than the procyclins and/or that they are preferentially reactive with galactose oxidase. Most significantly, the labeling of the high-MW glycoconjugate(s) was the same in the wild-type cells and in the TbGPI12 null mutants (Fig. 4, compare lanes 1 and 3), suggesting that on live cells, the procyclin coat has no effect on the accessibility of the 68-kDa galactose oxidase enzyme to the high-MW glycoconjugate(s).
FIG. 4.
Cell surface labeling of the high-apparent-MW glycoconjugate fraction on neuraminidase-treated wild-type (WT) and TbGPI12 null (Null) procyclic cells with galactose oxidase and NaB[3H]4. The wild-type (lanes 1 and 2) and TbGPI12 null (lanes 3 and 4) procyclic cells were treated (+) or not treated (−) with galactose oxidase (Gal ox.), as indicated, prior to reduction with NaB[3H]4. After washing, the cells were lysed and analyzed by SDS-PAGE and fluorography. The positions of the MW markers are shown on the right. GIPLs, free GPIs, or glycoinositolphospholipids.
Together with the lectin data, the surface labeling results support a model in which the high-MW glycoconjugate(s) terminate in sialic acid-galactose motifs in both wild-type and TbGPI12 null cells and that these molecules coexist with procyclins and free GPIs in the wild-type cells.
Purification of the high-MW glycoconjugates by ricin affinity chromatography and gel filtration.
TbGPI12 null cells from 5 liters of culture were extensively washed in PBS under conditions that allow the complete removal of cell surface sialic acid through the combined activities of endogenous cell surface neuraminidase and trans-sialidase (the latter works as an α2- and α3-specific neuraminidase in the absence of serum sialyl donor glycoproteins). The cells were then subjected to hypotonic lysis, and the washed cell ghosts were solubilized with an SDS, urea and DTT-containing buffer. The clarified lysate was diluted with n-octylglucoside buffer and applied to ricin-agarose beads as described previously (3). The beads were washed extensively to remove nonspecifically bound proteins and eluted first with lactose and galactose and second with 1% SDS to recover all adsorbed material. The combined eluates were concentrated and applied to a Superose 6 gel filtration column equilibrated in buffer containing 0.1% SDS and 10 mM DTT. Fractions 18 to 20 (corresponding to proteins with a molecular mass of >600 kDa) were subjected to SDS-PAGE and shown to contain high-MW glycoconjugate(s) that could be stained for protein with colloidal Coomassie blue and for carbohydrate with periodate-Schiff and, after transfer to nitrocellulose, could be blotted with ricin (Fig. 5). If 30 mg/ml galactose and lactose were added prior to ricin-agarose pulldown, no comparable material was isolated (data not shown).
Carbohydrate composition of the high-MW glycoconjugate fraction.
Aliquots of pooled fractions 18 to 20 from the Superose 6 fractionation were subjected to protein quantification and to quantitative carbohydrate composition analysis by GC-MS (Table 3). From these data, we estimate that about 7% of the fraction is carbohydrate, by mass. The monosaccharides present are mannose, galactose, and N-acetylglucosamine (typical of T. brucei glycoproteins) and fucose. Fucose has not been previously described in any glycoconjugate of T. brucei.
TABLE 3.
Monosaccharide composition of the high-MW glycoconjugate fractiona
| Monosaccharideb | Amt of protein (nmol/mg)c | % by wt |
|---|---|---|
| Mannose | 175 ± 25 | 2.8 |
| Galactose | 162 ± 19 | 2.6 |
| Fucose | 83 ± 16 | 1.2 |
| N-Acetylglucosamine | Present but not quantitatedd | N/Ae |
Pooled material (fractions 18 to 20) from the gel filtration step of the purification was analyzed for protein content by colorimetric assay and monosaccharide content by GC-MS.
Glucose and a trace of xylose were also detected, but these are common contaminants of biological preparations and have been omitted from the table.
Means and standard deviations of the results from triplicate analyses.
The N-acetylglucosamine derivatives were positively identified by retention time and mass spectrum, but quantification of these derivatives is unreliable.
N/A, not applicable.
Characterization of components of the high-MW glycoconjugate fraction.
The colloidal Coomassie blue-stained smear in fraction 18 (Fig. 5) was excised from the gel, digested with trypsin, and submitted to LC-MS/MS for proteomic identification (Table 4). The majority of the hits were putative integral membrane proteins that span the bilayer several times, and the putative single-pass transmembrane procyclic-form specific antigen PSSA-2 (15) was noticeably absent (see Discussion). Some of the identified multispanning membrane protein hits are known or assumed to be plasma membrane proteins, like the TbN10 nucleoside transporter (46), the THT2A glucose transporter (4), and the recently described TbPAD2 putative carboxylate transporter (6). To validate other hits as surface molecules, we acquired available antibodies to two components, P-type ATPase and VHPPase. The antibody to the P-type ATPase was raised against a T. cruzi sequence that has 80% identity and good immunological cross-reactivity to the T. brucei homologue (21, 22). Fraction 18 from the Superose 6 fractionation was tested by Western blotting with antiproton ATPase (Fig. 6A). A band with an apparent MW similar to that predicted for the T. brucei P-type ATPase was observed (at about 100 kDa) together with a >200-kDa smear. A similar pattern of staining has been reported previously for this protein (21). We used the same antibodies to localize the T. brucei P-type ATPase in fixed and permeabilized wild-type and TbGPI12 null cells by immunofluorescence microscopy (Fig. 7A). In both cases, a strong cell surface label was apparent, in agreement with the results presented in reference 21.
TABLE 4.
Proteins identified in the high-MW glycoconjugate fractiona
| Protein | Gene no. | Mascot scorec | No. of matched peptides | Molecular mass (kDa)d | No. of transmembrane domainse | No. of N-glycosylation sitesf |
|---|---|---|---|---|---|---|
| Tubulinb | Tb927.1.2330 | 1171 | 38 | 50 | 0 | N/A |
| VHPPase | Tb927.4.4380 | 812 | 22 | 86 | 14 | 3 |
| Hypothetical protein | Tb927.4.2530 | 640 | 13 | 17 | 4 | 1 |
| TbPAD2, putative carboxylate transporter | Tb927.7.5940g | 435 | 18 | 67 | 14 | 2 |
| Triosephosphate-isomerase | Tb11.02.3210 | 297 | 6 | 27 | 0 | N/A |
| P-type proton-ATPase (TbHA1 and/or TbHA2) | Tb10.389.1180 and/or Tb10.389.1170 | 269 | 11 | 100 | 7 | 1 |
| Adenosine nucleoside transporter (P1); TbNT10 | Tb09.160.5480 | 213 | 6 | 51 | 10 | 1 |
| Oxoglutarate-malate carrier protein | Tb10.389.0690 | 210 | 6 | 33 | 4 | 1 |
| Copper-transporting ATPase-like protein | Tb11.47.0023 | 197 | 4 | 102 | 10 | 5 |
| ADP-ATP translocase protein | Tb10.61.1830 | 187 | 9 | 34 | 3 | 2 |
| PGPA multidrug resistance protein A | Tb927.8.2160 | 171 | 7 | 175 | 11 | 7 |
| Glucose transporter (THT2A) | Tb10.6k15.2020 | 169 | 3 | 57 | 12 | 2 |
| PGPA multidrug resistance protein E | Tb927.4.4490 | 163 | 5 | 194 | 8 | 9 |
Proteins identified by LC-MS/MS in fraction 18 by gel filtration of the high-MW glycoconjugate fraction. N/A, not applicable.
Proteins in italics are major cytosolic proteins that are unlikely to be glycosylated and are most likely contaminants.
The proteins are ordered by Mascot score, which to a first approximation, tends to correlate with relative protein abundance.
Predicted molecular mass from the predicted coding sequence.
Number of predicted transmembrane domains according to TMHMM2 (16).
Number of predicted N-glycosylation sites according to NetNGlyc.
Tb927.7.5940 belongs to a family of eight closely related sequences. However, three peptides unique to Tb927.7.5490 were observed (TFTGLGAAIVGSIQLAFFSK, LCMGYFEVWSQK, and AEDRVPITLSMFVPSVCIITMLTLFLTLPK), suggesting that this is the main form of the family detected in this experiment.
Antibodies raised to a recombinant segment of the T. brucei VHPPase (18) also recognized a high-MW smear in fraction 18 from the Superose 6 fractionation (Fig. 6B). These same antibodies, and two anti-synthetic peptide antibodies made for regions of plant VHPPase that have almost complete sequence identity with the T. brucei homologue (41), were used to label live cells and fixed and permeabilized cells. As expected, the former showed intense staining of intracellular structures (data not shown), consistent with previous reports of its localization in acidocalcisomes (18). However, the antibodies also labeled discrete cell surface patches on intact, live wild-type and TbGPI12 null cells (Fig. 7B). The appearance of the VHPPase in discrete patches on the surface of live parasites may be due to capping induced by the antibodies prior to fixation. The localization of VHPPase to both the plasma membrane and the acidocalcisome has been previously reported for T. cruzi (24, 43).
Attempts to observe the localization of VHPPase by immunoelectron microscopy were not successful. However, to further analyze the cell surface P-type ATPase, live wild-type and TbGPI12 null cells were incubated with rabbit anti-P-type ATPase, washed, fixed with paraformaldehyde, and then labeled by incubation with protein A conjugated to 10-nm gold particles. Specific and similar densities of the cell surface labeling of P-type ATPase were seen for both cell types (Fig. 8).
DISCUSSION
We previously described the lack of procyclins on the surface of the T. brucei procyclic-form TbGPI12 null mutant (11). Here, we have more thoroughly monitored the fate of the procyclin protein in these cells. The absence of a GPI anchor does not appear to affect the processing of the single N-glycosylation sequon in EP procyclins in the ER, such that all cell-associated and secreted procyclin fragments appear to be N-glycosylated, like wild-type procyclin, with endo H-sensitive oligomannose glycans. Further, since the antibody used to precipitate the procyclin fragments recognizes the EP repeat domain that is immediately preceded by the N-glycosylation site, we suggest that the majority of the procyclin fragments found in the cells and the supernatant are generated by the proteolysis of the N-terminal domain of the procyclin molecules. In summary, in the absence of a GPI anchor addition, the procyclins appear to be normally N-glycosylated in the ER, transported to the lysosomal compartment, proteolytically degraded in the N-terminal domain, and secreted from the cells into the culture supernatant. The appearance of proteolytically degraded procyclin in the culture supernatant of TbGPI10 null mutants has also been described (30). These data suggest that proteins can traffic from the lysosome to the surrounding medium in procyclic-form T. brucei, presumably via the flagellar pocket. We also noted the shedding of fully processed wild-type procyclin into the culture supernatant of procyclic cells. However, the amount of shedding was low and the slow release of GPI-anchored proteins containing two acyl chains from the surface of T. brucei has been previously noted (42).
The ability to radiolabel a high-MW glycoconjugate fraction with glucosamine, mannose, and glucose (though mannose can be metabolized into fucose, and glucose can be metabolized into several other sugars by trypanosomes [52]) is consistent with the ability to agglutinate both wild-type and surface procyclin-less TbGPI12 null mutant cells with lectins. The pattern of lectin agglutination (i.e., agglutination by WGA only prior to desialylation and by ricin only after desialylation) is consistent with the presence of glycans that terminate in galactose residues capped with sialic acid. This model is consistent with the ability to surface label the high-MW material of desialylated parasites using galactose oxidase and NaB[3H]4 reduction. Significantly, the galactose oxidase-NaB[3H]4 labeling of the high-MW glycoconjugate(s) on living procyclic cells was unaffected by the presence (in wild-type cells) or absence (in TbGPI12 null mutants) of the GPI-anchored procyclin coat. Similarly, we found that the presence or absence of the procyclins had no effect on our ability to label cell surface P-type ATPase molecules on live cells with specific antibodies. These data strongly suggest that the procyclin coat does not prevent the approach of proteins of 68 kDa (galactose oxidase) or 150 kDa (immunoglobulin G) to non-GPI-anchored cell surface coat components on live cells.
Ricin affinity purification of the high-MW glycoconjugate was performed from the TbGPI12 null cells, thus obviating the need to purify it away from the procyclins and free GPIs present in the wild-type cells. Compositional analysis confirmed the glycoprotein nature of this material, and the presence of galactose is consistent with ricin binding. Fucose was also found in the preparation. This sugar has been found previously in gp72 of T. cruzi (8, 12), a homologue of the T. brucei Fla-1 and Fla-2 flagellar adhesion zone glycoproteins (17, 31), but has not been previously reported in any T. brucei glycoconjugate. However, the sugar nucleotide GDP-fucose was recently identified in bloodstream and procyclic-form T. brucei and shown to be essential for both life cycle stages (52, 53).
The proteomic analysis of the high-MW glycoconjugate revealed multiple known and putative multispanning transmembrane proteins with a variety of predicted monomeric molecular masses, ranging from 17 to 194 kDa. The elution of this material by gel filtration, in the presence of SDS, in fractions predicted to contain molecules in excess of 600 kDa and the appearance of this material on SDS-PAGE as a smear ranging from 65 kDa to the top of the gel suggest an unusual physical state. However, multispanning membrane proteins can undergo aggregation under denaturing conditions (40, 45). We therefore checked to see whether the appearance by SDS-PAGE of the galactose oxidase-NaB[3H]4-labeled high-MW glycoconjugate(s) changes when we extracted the cells at 100°C, 50°C, or 37°C and whether or not the presence of 4 M urea had any effect (see Fig. S2 in the supplemental material). The results indicate that, in the absence of urea, temperature has no effect on the extraction efficiency or appearance of the labeled products on SDS-PAGE, whereas lower temperatures were less efficient in the presence of urea, and that at 100°C, 4 M urea assisted in the dissociation of the very high-apparent-MW material that ran at the top of the gel into material that entered the gel. We conclude that the appearance of the high-MW glycoconjugate material on the SDS-PAGE gels is due in part to glycosylation but that cross-linking and/or temperature- and urea-insensitive aggregation of the components may also be a factor. It may be significant that no single-pass transmembrane proteins, like PSSA-2 (15), were identified in the high-MW glycoconjugate fraction, suggesting that these might not be modified with ricin-binding glycans and/or do not coaggregate with the aforementioned multispanning membrane proteins.
The presence of N-acetylglucosamine and mannose in the sugar composition of the high-MW glycoconjugate material is suggestive of protein N-glycosylation. However, despite numerous attempts, we were unable to show convincing effects using PNGase F to de-N-glycosylate the glycoproteins. This could be due to difficulties in the enzyme reaching the glycosylation sites because of an unusual cross-linked/aggregated physical state and/or the presence of α1- to α3-linked fucose attached to the chitobiosyl core of the glycans, which is known to render N-glycans resistant to PNGase F (49). In addition, it is possible that the material contains O-linked oligosaccharides, as found in T. cruzi mucins (35, 44), and/or phosphate-linked oligosaccharides, as found in leishmania (13), T. cruzi (12, 23), and Trypanosoma congolense (48). A detailed analysis of the carbohydrate moieties in the high-MW glycoconjugate fraction will be undertaken but is beyond the scope of this report.
Taking two examples of the transmembrane proteins purified by ricin affinity chromatography for which antibody reagents are available, the P-type ATPase and the VHPPase, we were able to confirm previous reports that the P-type ATPase is expressed on the surface of procyclic-form T. brucei cells (22) and that, like in T. cruzi (43), VHPPase is present both in the plasma and the membrane of the acidocalcisome in procyclic-form T. brucei. The presence of VHPPase in these two locations suggests that the biogenesis of the plasma membrane and acidocalcisome membrane may share common origins.
In summary, the high-MW glycoconjugate and residual surface coat of procyclic-form T. brucei, identified by deleting all surface GPI-anchored molecules in the TbGPI12 null mutant (11), appears to be made up predominantly of multispanning transmembrane proteins, several of which are proton and nutrient transporters. These appear to be significantly glycosylated with galactose, mannose, N-acetylglucosamine, and fucose-containing glycans that normally terminate with sialic acid linked to galactose. We suggest that this group of transporters, and possibly other as-yet-unidentified molecules, exists alongside the procyclins in wild-type cells and that this network of molecules is not sterically protected from the approach of macromolecules by the procyclin coat. On the other hand, the combination of the procyclin coat and the glycosylated transporter-containing molecules may well play a physical role in protecting the plasma membrane, as well as a role in maintaining intracellular pH and transporting nutrients and terminal metabolites.
Supplementary Material
Acknowledgments
This work was supported by a Wellcome Trust program grant to M.A.J.F. (085622) and a Wellcome Trust strategic award (083481).
We thank Roberto Docampo, Philip Rea, Norbert Bakalara, and Jay Bangs for kindly providing antibodies and Alvaro Acosta Serrano for helpful discussions.
Footnotes
Published ahead of print on 24 July 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Acosta-Serrano, A., R. N. Cole, A. Mehlert, M. G. Lee, M. A. Ferguson, and P. T. Englund. 1999. The procyclin repertoire of Trypanosoma brucei. Identification and structural characterization of the Glu-Pro-rich polypeptides. J. Biol. Chem. 274:29763-29771. [DOI] [PubMed] [Google Scholar]
- 2.Acosta-Serrano, A., E. Vassella, M. Liniger, C. Kunz Renggli, R. Brun, I. Roditi, and P. T. Englund. 2001. The surface coat of procyclic Trypanosoma brucei: programmed expression and proteolytic cleavage of procyclin in the tsetse fly. Proc. Natl. Acad. Sci. USA 98:1513-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atrih, A., J. M. Richardson, A. R. Prescott, and M. A. Ferguson. 2005. Trypanosoma brucei glycoproteins contain novel giant poly-N-acetyllactosamine carbohydrate chains. J. Biol. Chem. 280:865-871. [DOI] [PubMed] [Google Scholar]
- 4.Bringaud, F., and T. Baltz. 1993. Differential regulation of two distinct families of glucose transporter genes in Trypanosoma brucei. Mol. Cell. Biol. 13:1146-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun, R., and Schonenberger. 1979. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 36:289-292. [PubMed] [Google Scholar]
- 6.Dean, S., R. Marchetti, K. Kirk, and K. R. Matthews. 2009. A surface transporter family conveys the trypanosome differentiation signal. Nature 459:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstler, M., G. Reuter, and R. Schauer. 1993. The developmentally regulated trans-sialidase from Trypanosoma brucei sialylates the procyclic acidic repetitive protein. Mol. Biochem. Parasitol. 61:1-13. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson, M. A., A. K. Allen, and D. Snary. 1983. Studies on the structure of a phosphoglycoprotein from the parasitic protozoan Trypanosoma cruzi. Biochem. J. 213:313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson, M. A., P. Murray, H. Rutherford, and M. J. McConville. 1993. A simple purification of procyclic acidic repetitive protein and demonstration of a sialylated glycosyl-phosphatidylinositol membrane anchor. Biochem. J. 291:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahmberg, C. G., and S. I. Hakomori. 1973. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J. Biol. Chem. 248:4311-4317. [PubMed] [Google Scholar]
- 11.Guther, M. L., S. Lee, L. Tetley, A. Acosta-Serrano, and M. A. Ferguson. 2006. GPI-anchored proteins and free GPI glycolipids of procyclic form Trypanosoma brucei are nonessential for growth, are required for colonization of the tsetse fly, and are not the only components of the surface coat. Mol. Biol. Cell 17:5265-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes, P. A., M. A. Ferguson, and G. A. Cross. 1996. Structural characterization of novel oligosaccharides of cell-surface glycoproteins of Trypanosoma cruzi. Glycobiology 6:869-878. [DOI] [PubMed] [Google Scholar]
- 13.Ilg, T., P. Overath, M. A. Ferguson, T. Rutherford, D. G. Campbell, and M. J. McConville. 1994. O- and N-glycosylation of the Leishmania mexicana-secreted acid phosphatase. Characterization of a new class of phosphoserine-linked glycans. J. Biol. Chem. 269:24073-24081. [PubMed] [Google Scholar]
- 14.Izquierdo, L., M. Nakanishi, A. Mehlert, G. Machray, G. J. Barton, and M. A. Ferguson. 2009. Identification of a glycosylphosphatidylinositol anchor-modifying beta1-3 N-acetylglucosaminyl transferase in Trypanosoma brucei. Mol. Microbiol. 71:478-491. [DOI] [PubMed] [Google Scholar]
- 15.Jackson, D. G., D. K. Smith, C. Luo, and J. F. Elliott. 1993. Cloning of a novel surface antigen from the insect stages of Trypanosoma brucei by expression in COS cells. J. Biol. Chem. 268:1894-1900. [PubMed] [Google Scholar]
- 16.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 17.LaCount, D. J., B. Barrett, and J. E. Donelson. 2002. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J. Biol. Chem. 277:17580-17588. [DOI] [PubMed] [Google Scholar]
- 18.Lemercier, G., S. Dutoya, S. Luo, F. A. Ruiz, C. O. Rodrigues, T. Baltz, R. Docampo, and N. Bakalara. 2002. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J. Biol. Chem. 277:37369-37376. [DOI] [PubMed] [Google Scholar]
- 19.Lillico, S., M. C. Field, P. Blundell, G. H. Coombs, and J. C. Mottram. 2003. Essential roles for GPI-anchored proteins in African trypanosomes revealed using mutants deficient in GPI8. Mol. Biol. Cell 14:1182-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liniger, M., S. Urwyler, E. Studer, M. Oberle, C. K. Renggli, and I. Roditi. 2004. Role of the N-terminal domains of EP and GPEET procyclins in membrane targeting and the establishment of midgut infections by Trypanosoma brucei. Mol. Biochem. Parasitol. 137:247-251. [DOI] [PubMed] [Google Scholar]
- 21.Luo, S., J. Fang, and R. Docampo. 2006. Molecular characterization of Trypanosoma brucei P-type H+-ATPases. J. Biol. Chem. 281:21963-21973. [DOI] [PubMed] [Google Scholar]
- 22.Luo, S., D. A. Scott, and R. Docampo. 2002. Trypanosoma cruzi H+-ATPase 1 (TcHA1) and 2 (TcHA2) genes complement yeast mutants defective in H+ pumps and encode plasma membrane P-type H+-ATPases with different enzymatic properties. J. Biol. Chem. 277:44497-44506. [DOI] [PubMed] [Google Scholar]
- 23.Macrae, J. I., A. Acosta-Serrano, N. A. Morrice, A. Mehlert, and M. A. Ferguson. 2005. Structural characterization of NETNES, a novel glycoconjugate in Trypanosoma cruzi epimastigotes. J. Biol. Chem. 280:12201-12211. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, R., Y. Wang, G. Benaim, M. Benchimol, W. de Souza, D. A. Scott, and R. Docampo. 2002. A proton pumping pyrophosphatase in the Golgi apparatus and plasma membrane vesicles of Trypanosoma cruzi. Mol. Biochem. Parasitol. 120:205-213. [DOI] [PubMed] [Google Scholar]
- 25.Mehlert, A., A. Treumann, and M. A. Ferguson. 1999. Trypanosoma brucei GPEET-PARP is phosphorylated on six out of seven threonine residues. Mol. Biochem. Parasitol. 98:291-296. [DOI] [PubMed] [Google Scholar]
- 26.Montagna, G., M. L. Cremona, G. Paris, M. F. Amaya, A. Buschiazzo, P. M. Alzari, and A. C. Frasch. 2002. The trans-sialidase from the African trypanosome Trypanosoma brucei. Eur. J. Biochem. 269:2941-2950. [DOI] [PubMed] [Google Scholar]
- 27.Morris, J. C., Z. Wang, M. E. Drew, and P. T. Englund. 2002. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. EMBO J. 21:4429-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mowatt, M. R., and C. E. Clayton. 1987. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol. Cell. Biol. 7:2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagamune, K., A. Acosta-Serrano, H. Uemura, R. Brun, C. Kunz-Renggli, Y. Maeda, M. A. Ferguson, and T. Kinoshita. 2004. Surface sialic acids taken from the host allow trypanosome survival in tsetse fly vectors. J. Exp. Med. 199:1445-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagamune, K., T. Nozaki, Y. Maeda, K. Ohishi, T. Fukuma, T. Hara, R. T. Schwarz, C. Sutterlin, R. Brun, H. Riezman, and T. Kinoshita. 2000. Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 97:10336-10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nozaki, T., P. A. Haynes, and G. A. Cross. 1996. Characterization of the Trypanosoma brucei homologue of a Trypanosoma cruzi flagellum-adhesion glycoprotein. Mol. Biochem. Parasitol. 82:245-255. [DOI] [PubMed] [Google Scholar]
- 32.Pays, E., L. Vanhamme, and D. Perez-Morga. 2004. Antigenic variation in Trypanosoma brucei: facts, challenges and mysteries. Curr. Opin. Microbiol. 7:369-374. [DOI] [PubMed] [Google Scholar]
- 33.Peck, R. F., A. M. Shiflett, K. J. Schwartz, A. McCann, S. L. Hajduk, and J. D. Bangs. 2008. The LAMP-like protein p67 plays an essential role in the lysosome of African trypanosomes. Mol. Microbiol. 68:933-946. [DOI] [PubMed] [Google Scholar]
- 34.Pontes de Carvalho, L. C., S. Tomlinson, F. Vandekerckhove, E. J. Bienen, A. B. Clarkson, M. S. Jiang, G. W. Hart, and V. Nussenzweig. 1993. Characterization of a novel trans-sialidase of Trypanosoma brucei procyclic trypomastigotes and identification of procyclin as the main sialic acid acceptor. J. Exp. Med. 177:465-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Previato, J. O., C. Jones, L. P. Goncalves, R. Wait, L. R. Travassos, and L. Mendonca-Previato. 1994. O-glycosidically linked N-acetylglucosamine-bound oligosaccharides from glycoproteins of Trypanosoma cruzi. Biochem. J. 301:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson, J. P., R. P. Beecroft, D. L. Tolson, M. K. Liu, and T. W. Pearson. 1988. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol. Biochem. Parasitol. 31:203-216. [DOI] [PubMed] [Google Scholar]
- 37.Roditi, I., M. Carrington, and M. Turner. 1987. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature 325:272-274. [DOI] [PubMed] [Google Scholar]
- 38.Roditi, I., H. Schwarz, T. W. Pearson, R. P. Beecroft, M. K. Liu, J. P. Richardson, H. J. Buhring, J. Pleiss, R. Bulow, R. O. Williams, et al. 1989. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J. Cell Biol. 108:737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roper, J. R., M. L. Guther, J. I. Macrae, A. R. Prescott, I. Hallyburton, A. Acosta-Serrano, and M. A. Ferguson. 2005. The suppression of galactose metabolism in procyclic form Trypanosoma brucei causes cessation of cell growth and alters procyclin glycoprotein structure and copy number. J. Biol. Chem. 280:19728-19736. [DOI] [PubMed] [Google Scholar]
- 40.Sagne, C., M. F. Isambert, J. P. Henry, and B. Gasnier. 1996. SDS-resistant aggregation of membrane proteins: application to the purification of the vesicular monoamine transporter. Biochem. J. 316:825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarafian, V., Y. Kim, R. J. Poole, and P. A. Rea. 1992. Molecular cloning and sequence of cDNA encoding the pyrophosphate-energized vacuolar membrane proton pump of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 89:1775-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz, K. J., R. F. Peck, N. N. Tazeh, and J. D. Bangs. 2005. GPI valence and the fate of secretory membrane proteins in African trypanosomes. J. Cell Sci. 118:5499-5511. [DOI] [PubMed] [Google Scholar]
- 43.Scott, D. A., W. de Souza, M. Benchimol, L. Zhong, H. G. Lu, S. N. Moreno, and R. Docampo. 1998. Presence of a plant-like proton-pumping pyrophosphatase in acidocalcisomes of Trypanosoma cruzi. J. Biol. Chem. 273:22151-22158. [DOI] [PubMed] [Google Scholar]
- 44.Serrano, A. A., S. Schenkman, N. Yoshida, A. Mehlert, J. M. Richardson, and M. A. Ferguson. 1995. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J. Biol. Chem. 270:27244-27253. [DOI] [PubMed] [Google Scholar]
- 45.Soulie, S., J. V. Moller, P. Falson, and M. le Maire. 1996. Urea reduces the aggregation of membrane proteins on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 236:363-364. [DOI] [PubMed] [Google Scholar]
- 46.Spoerri, I., R. Chadwick, C. K. Renggli, K. Matthews, I. Roditi, and G. Burkard. 2007. Role of the stage-regulated nucleoside transporter TbNT10 in differentiation and adenosine uptake in Trypanosoma brucei. Mol. Biochem. Parasitol. 154:110-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor, J. E., and G. Rudenko. 2006. Switching trypanosome coats: what's in the wardrobe? Trends Genet. 22:614-620. [DOI] [PubMed] [Google Scholar]
- 48.Thomson, L. M., D. J. Lamont, A. Mehlert, J. D. Barry, and M. A. Ferguson. 2002. Partial structure of glutamic acid and alanine-rich protein, a major surface glycoprotein of the insect stages of Trypanosoma congolense. J. Biol. Chem. 277:48899-48904. [DOI] [PubMed] [Google Scholar]
- 49.Tretter, V., F. Altmann, and L. Marz. 1991. Peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase F cannot release glycans with fucose attached alpha 1-3 to the asparagine-linked N-acetylglucosamine residue. Eur. J. Biochem. 199:647-652. [DOI] [PubMed] [Google Scholar]
- 50.Treumann, A., N. Zitzmann, A. Hulsmeier, A. R. Prescott, A. Almond, J. Sheehan, and M. A. Ferguson. 1997. Structural characterisation of two forms of procyclic acidic repetitive protein expressed by procyclic forms of Trypanosoma brucei. J. Mol. Biol. 269:529-547. [DOI] [PubMed] [Google Scholar]
- 51.Triggs, V. P., and J. D. Bangs. 2003. Glycosylphosphatidylinositol-dependent protein trafficking in bloodstream stage Trypanosoma brucei. Eukaryot. Cell 2:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turnock, D. C., and M. A. Ferguson. 2007. Sugar nucleotide pools of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Eukaryot. Cell 6:1450-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turnock, D. C., L. Izquierdo, and M. A. Ferguson. 2007. The de novo synthesis of GDP-fucose is essential for flagellar adhesion and cell growth in Trypanosoma brucei. J. Biol. Chem. 282:28853-28863. [DOI] [PubMed] [Google Scholar]
- 54.Vassella, E., A. Acosta-Serrano, E. Studer, S. H. Lee, P. T. Englund, and I. Roditi. 2001. Multiple procyclin isoforms are expressed differentially during the development of insect forms of Trypanosoma brucei. J. Mol. Biol. 312:597-607. [DOI] [PubMed] [Google Scholar]
- 55.Vassella, E., P. Butikofer, M. Engstler, J. Jelk, and I. Roditi. 2003. Procyclin null mutants of Trypanosoma brucei express free glycosylphosphatidylinositols on their surface. Mol. Biol. Cell 14:1308-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vassella, E., J. V. Den Abbeele, P. Butikofer, C. K. Renggli, A. Furger, R. Brun, and I. Roditi. 2000. A major surface glycoprotein of Trypanosoma brucei is expressed transiently during development and can be regulated posttranscriptionally by glycerol or hypoxia. Genes Dev. 14:615-626. [PMC free article] [PubMed] [Google Scholar]
- 57.Vassella, E., M. Oberle, S. Urwyler, C. K. Renggli, E. Studer, A. Hemphill, C. Fragoso, P. Butikofer, R. Brun, and I. Roditi. 2009. Major surface glycoproteins of insect forms of Trypanosoma brucei are not essential for cyclical transmission by tsetse. PLoS ONE 4:e4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.