Abstract
Increased activation of c-src seen in colorectal cancer is an indicator of a poor clinical prognosis, suggesting that identification of downstream effectors of c-src may lead to new avenues of therapy. Guanylyl cyclase C (GC-C) is a receptor for the gastrointestinal hormones guanylin and uroguanylin and the bacterial heat-stable enterotoxin. Though activation of GC-C by its ligands elevates intracellular cyclic GMP (cGMP) levels and inhibits cell proliferation, its persistent expression in colorectal carcinomas and occult metastases makes it a marker for malignancy. We show here that GC-C is a substrate for inhibitory phosphorylation by c-src, resulting in reduced ligand-mediated cGMP production. Consequently, active c-src in colonic cells can overcome GC-C-mediated control of the cell cycle. Furthermore, docking of the c-src SH2 domain to phosphorylated GC-C results in colocalization and further activation of c-src. We therefore propose a novel feed-forward mechanism of activation of c-src that is induced by cross talk between a receptor GC and a tyrosine kinase. Our findings have important implications in understanding the molecular mechanisms involved in the progression and treatment of colorectal cancer.
Colorectal carcinoma is one of the most common forms of cancer seen in the developed world (10). The incidence in developing countries appears to be somewhat lower, but with high rates of mortality similar to those seen in developed countries (43). Treatment usually involves surgery or palliative chemotherapy, and recent research focuses on investigating molecular signatures associated with colorectal tumorigenesis as well as means of early detection in order to avoid high and rapid fatalities (61). Increased activity and expression of the tyrosine kinase c-src is frequently seen in colorectal cancer (5, 12), and activation of c-src in early stages of neoplastic transformation (32) is an indicator of a poor clinical prognosis (26). In general, c-src activity is as much as 16-fold higher in cancerous cells than in the adjacent colonic mucosa (12). Information on potential molecular mechanisms for the role of c-src in colonic tumorigenesis includes evidence that c-src activity increases during mitosis of human colon carcinoma cells (39), and increased c-src activity promotes cell motility and invasion during intestinal cell migration (34).
A large number of substrates for c-src have been identified in different cell types, and a consensus site for tyrosine phosphorylation by c-src has been defined (50). Prediction of these sites in proteins present in colorectal carcinoma cells may assist in identifying novel targets for c-src action. The modular structure of c-src and other members of the src family kinases (SFKs), consisting of the SH4, unique, SH3, SH2, and kinase domains, allows these kinases to interact with a diverse group of proteins, creating a highly complex signal transduction network (48, 52). Consequently, SFKs have been demonstrated to be key downstream elements in signaling pathways emerging from cell surface receptors (52).
Guanylyl cyclase C (GC-C) plays an important role in maintaining fluid ion homeostasis (54) and genomic integrity in intestinal cells (29) and serves as the receptor for the diarrheagenic heat-stable enterotoxin (ST) as well as endogenous ligands guanylin and uroguanylin (57). Binding of ST to the receptor leads to increased intracellular cyclic GMP (cGMP) accumulation and activation of the cystic fibrosis transmembrane conductance regulator, resulting in fluid and ion efflux that manifests as traveler's diarrhea (45, 54). Recently it has been noted that regions of the world with the highest incidence of enterotoxigenic Escherichia coli-associated diarrhea exhibit the lowest incidence of colon cancer (43). In this context, studies have shown that activation of GC-C by its ligands inhibits human colonic cell proliferation and adenoma formation in mice, demonstrating a critical role for GC-C in inhibiting colon cancer initiation and progression (29, 33). For example, uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon carcinoma cells via cGMP produced by GC-C (46). Interestingly, expression of the endogenous paracrine hormones for GC-C, guanylin and uroguanylin, is lost at the initiation of transformation, and therefore this reduced signaling via GC-C may allow cells to proliferate more rapidly, thereby leading to carcinogenesis (14).
Expression of GC-C is largely restricted to the luminal side of intestinal cells in humans, and consequently, monitoring GC-C expression in circulating tumor cells in the peripheral blood of patients has been suggested to be a good marker for early detection of the primary tumor and/or metastasis of colon cancer (11, 28, 36). The molecular mechanisms by which GC-C activity is regulated during the initiation and progression of carcinogenesis remain undefined, and studies to investigate whether signaling events prevalent in colorectal cancer can modulate GC-C function have not been performed. Human colorectal carcinoma cell lines express GC-C and can therefore be used to study these molecular aspects of GC-C regulation and downstream signaling events (3, 4, 22).
Phosphorylation is a rapid and reversible form of covalent modification frequently found in signaling systems. In our earlier studies, we have coexpressed domains of GC-C with the tyrosine kinase EphB1/Elk and observed tyrosine phosphorylation of GC-C (7). We report here that GC-C is a substrate for c-src tyrosine kinase and identify Tyr820 as the site for phosphorylation in GC-C. Following phosphorylation, pTyr820 serves as a site for interaction with the SH2 domain of c-src, resulting in further activation of c-src. Most importantly, tyrosine phosphorylation of GC-C inhibits cGMP production and prevents the cytostatic effects of GC-C induced by ligand interaction. Our studies therefore show the existence of a new signal transduction cross talk between c-src and GC-C in colonic cells, resulting in a feed-forward mechanism to further activate c-src, with important implications in cancer cell proliferation and disease progression.
MATERIALS AND METHODS
Culture and maintenance of cell lines.
The T84, Caco2, and HT29 cell lines (3) were obtained from ATCC (Manassas, VA) and T84SF cells from R. Alessandro (University of Palermo, Italy) (1). Caco2 and HT29 cells were maintained in Dulbecco modified Eagle medium (Sigma) containing 10% fetal calf serum and nonessential amino acids (Gibco), while T84 cells were cultured in Dulbecco modified Eagle medium-F12 containing 5% fetal calf serum (Sigma).
Rat ligated ileal loop assay.
Animal experiments were performed with approval from the Institutional Animal Ethics Committee. Wistar rats (3 to 4 weeks old) were anesthetized and 2-cm ileal loops injected with either 10 μl of sterile phosphate-buffered saline (PBS) (pH 7) alone, PBS containing ST (1 nM) (19), or 8-Br-cGMP (5 mM). Pervanadate (PV) (0.5 mM), prepared just prior to use, was injected along with HgCl2 (50 μM). The abdomen was closed, and an hour later the animal was sacrificed, loops dissected, and fluid accumulation index calculated as (A − B)/B, where A is the weight of the fluid-filled loop and B the weight after removal of the fluid.
Mercuric chloride and PV treatment of cells.
HgCl2 (50 μM) was applied to cells along with PV (0.5 mM) for 30 min at 37°C. No loss of cell viability was detected. Pretreatment with 10 μM PP2 or PP3 (Calbiochem) was performed for 30 min, and monolayers were treated with 500 μM isobutylmethyl xanthine for 30 min prior to addition of ST (100 nM). Cells were incubated for 15 min, the medium was drained, the cells were lysed in 0.1 N HCl, and cGMP was measured by radioimmunoassay (3).
Knockdown of c-src by shRNA.
Short hairpin RNA (shRNA) constructs directed to c-src and cloned in the lentiviral vector pLKO.1 were obtained from Open Biosystems (catalog number RHS4533; Thermo Scientific). Lentiviruses were produced in the packaging cell line HEK293T by simultaneously transfecting the shRNA constructs along with the helper plasmids pCMV dR8.2 and pCMV VSV-G (kind gifts from Annapoorni Rangarajan, Indian Institute of Science). Control experiments were performed with an shRNA with a random sequence (Sigma). T84 cells were infected with the lentivirus in the presence of Polybrene (8 μg/ml) and subsequently reinfected 36 h after the first infection. Cells were assayed for c-src knockdown 72 h after the first infection.
Immunoprecipitation of GC-C and c-src.
Cells were lysed in modified radioimmunoprecipitation assay buffer (50 mM HEPES [pH 7], 100 mM NaCl, 1% NP-40, 0.5% deoxycholate, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 5 μg/ml soybean trypsin inhibitor, 1 mM sodium pyrophosphate, and 10 mM sodium orthovanadate). Clarified lysates were precleared with normal mouse immunoglobulin G and then allowed to interact with 10 μg/ml GCC:B10 (36) monoclonal antibody or 2 μg/ml c-src monoclonal antibody (Santa Cruz), followed by addition of protein G beads and Western blotting. For direct Western blot analysis, cell monolayers were washed with PBS and directly lysed in hot 2× sodium dodecyl sulfate (SDS) sample buffer. GCC:C8 and GCC:4D7 (25) antibodies were used at a 1:100 dilution, and c-src monoclonal antibody (Santa Cruz), phospho c-src pY416 antibody (Cell Signaling Technologies), and phosphotyrosine (pY) antibody (PT:66; Sigma-Aldrich) were all used at a 1:1,000 dilution. Blots were processed with ECL Reagent (GE Healthcare, United Kingdom) or Immobilon-P reagent (Millipore). The protein concentration was estimated by use of a modified Bradford protein assay (64).
c-src kinase assays.
The peptide KGFVEPELYEEVA (residues 812 to 823 of GC-C; Chiron Technologies, Australia) was used as the substrate for kinase assays. Immunoprecipitated c-src (from HEK293T cells overexpressing c-src) was incubated in kinase buffer (25 mM Tris-Cl [pH 7.5] containing 100 mM NaCl, 5 mM dithiothreitol, 30 mM MgCl2, 1.25 mM MnCl2, 100 μM sodium orthovanadate) in the presence of 100 μM ATP for 30 min at 30°C. Peptide was added along with ATP (25 μM) and [γ-32P]ATP (0.5 μCi, 4,000 Ci/mmol; BARC, India) and incubation continued for 30 min at 30°C. Reactions were stopped by the addition of trichloroacetic acid, products were filtered through phosphocellulose filters (Gibco BRL) and washed extensively with 0.5% o-phosphoric acid, and radioactivity was measured using a liquid scintillation counter (Perkin-Elmer Life Sciences). Nonlinear regression analysis of data was performed using GraphPad Prism 5.
In some experiments, immunoprecipitated c-src was first treated with phosphopeptide or peptide for 30 min at 4°C. Subsequently, acid-denatured enolase (1 μg), [γ-32P]ATP (2 μCi), and ATP (25 μM) were added and incubation continued for 10 min at 30°C. The reaction was terminated by the addition of 4× SDS sample buffer, and proteins were resolved by SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membrane was exposed to a phosphorimager screen and scanned using a Typhoon 9210 scanner (GE Healthcare, United Kingdom).
Characterization of mutant GC-C receptors.
The GC-CY820F, GC-CY820E, and GC-CY820L mutants were generated by site-directed mutagenesis (47) using pBluescript SK(+) GC-C as a template (49). The c-src and c-src green fluorescent protein (GFP) constructs were available as pUSE c-src (Upstate Biotechnology) and piGFP N3 c-src (a gift from J. J. Woodward, Medical University of South Carolina) (2). pEGST N2 vector was generated by replacing the enhanced-GFP-coding sequence with that of glutathione S-transferase (GST). The stop codon at the end of the GC-C-coding sequence was mutated to an alanine residue, and GC-C was subcloned in frame with the C-terminal GST tag into pEGST N2. The c-src SH2 domain was amplified by PCR from total cDNA obtained from T84 and cloned into pGEX-5X-1 (GE Healthcare) to generate a GST-c-src fusion protein. All clones were verified by DNA sequencing (Macrogen, South Korea). Proteins were expressed in HEK293T cells by transient transfection of the plasmids with polyethylenimine (PEI Polysciences Inc.). The cells were either stimulated with ST peptide or harvested for further analysis at 72 h posttransfection.
Immunofluorescence imaging.
HEK293T cells were transfected with the indicated plasmids and, at 48 h posttransfection, fixed with 3.5% paraformaldehyde-0.1% glutaraldehyde. Monolayers were washed with PBS and incubated with blocking solution (2% bovine serum albumin, 0.1% Tween 20) for 1 h. GC-C was detected using GCC:4D7 monoclonal antibody (1:100 dilution). For T84 cells, GC-C was detected using a polyclonal antibody (GCC:P1) to the extracellular domain of GC-C (37) and total c-src or active c-src detected using the c-src antibody or phospho-c-src 416 antibody, respectively. Bound antibodies were detected with appropriate fluorescent dye-conjugated secondary antibodies (Molecular Probes). Images were acquired on a Zeiss confocal microscope (LSM 510 Meta) and colocalization quantified using ImageJ software (NIH, Bethesda, MD) (9).
Surface plasmon resonance.
The carboxylated dextran surface of a CM5 sensor chip was activated by carbodiimide and succinimide chemistry, and a phosphopeptide encompassing the Tyr820 residue was coupled to the sensor by standard procedures. Analysis was performed on a BIAcore 2000 instrument (GE Healthcare, United Kingdom). Purified GST-SH2 protein was passed over the phosphopeptide surface (flow rate, 10 μl/min; 5 min) in running buffer (10 mM HEPES [pH 7.5], 150 mM NaCl, 3 mM EDTA, 0.005% [vol/vol] surfactant P20), followed by a wash with running buffer (10 μl/min, 5 min). A plot of corrected response units (cRU) versus cRU/[SH2] was generated, and the dissociation constant (Kd) was obtained from the slope of the line after nonlinear regression analysis (GraphPad Prism 5).
GST-SH2 pulldown assay.
T84 or HEK293T cells were lysed in modified radioimmunoprecipitation assay buffer, lysate diluted 1:5 in interaction buffer (50 mM HEPES [pH 7], 100 mM NaCl, 1% Triton X-100, 1 mM dithiothreitol, 5 mM EDTA, 10 mM sodium orthovanadate, 10 mM NaF, 10 mM sodium pyrophosphate, and protease inhibitors), and mixed with GST or GST-SH2 bound to beads for 4 h at 4°C. Beads were washed with interaction buffer and bound proteins detected by Western blot analysis. For peptide competition experiments, KGFVEPEL(pY)EEVA phosphopeptide or KGFVEPELYEEVA peptide was added to lysates at a final concentration of ∼10 μM, following which the lysate was allowed to interact with GST-SH2 beads.
Monitoring of cell proliferation and cytostasis following activation of GC-C.
Exponentially growing T84 or T84SF cells in 96-well plates were starved in medium without glutamine for 48 h. ST (100 nM) along with PV and HgCl2 was then applied in serum-containing medium for 24 h. [methyl-3H]thymidine incorporation into DNA was quantified as described previously (40).
Monolayers of T84 or T84SF cells after 48 h of starvation were induced to proliferate by the addition of serum-containing medium in the presence or absence of ST (100 nM), PP2 (10 μM), or PP2 and ST. Following treatment for 24 h, the cells were trypsinized, washed with PBS, and incubated with DNA stain (1 μg/ml propidium iodide, 10 μg/ml RNase A) overnight at 37°C. Cells diluted in PBS were subjected to flow cytometry (FACSCalibur; BD Biosciences), and data were analyzed using the WinMDI 2.9 software (Scripps Research Institute, La Jolla, CA).
Statistical analysis.
All data were analyzed using the paired Student's t test (GraphPad Prisms).
RESULTS
Tyrosine phosphorylation of GC-C by SFKs inhibits ST-mediated activity.
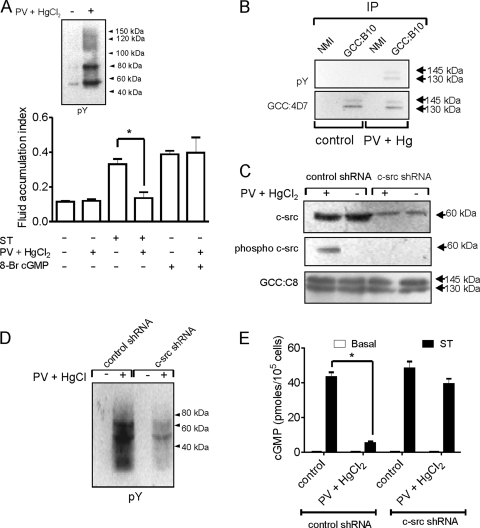
ST-mediated activation of GC-C leads to massive fluid efflux from intestinal cells, resulting in watery diarrhea in humans (45). Injection of rat intestinal loops with PV (a tyrosine phosphatase inhibitor [20]) and HgCl2 (a known activator of c-src-like tyrosine kinases [44]) led to a significant increase in tyrosine phosphorylation of cellular proteins (Fig. 1A, inset). Fluid accumulation in ligated rat intestinal loops (20, 44) was markedly reduced when ST was injected along with PV and HgCl2 (Fig. 1A). However, 8-Br-cGMP induced fluid accumulation even when injected along with HgCl2 and PV, indicating that the effect of increased cellular tyrosine phosphorylation was upstream of cGMP production, perhaps at the level of receptor GC-C (Fig. 1A). Indeed, anti-pY Western blots indicated increased tyrosine phosphorylation of the differentially glycosylated forms of GC-C on PV and HgCl2 treatment (Fig. 1B).
FIG. 1.
Regulation of GC-C activity by tyrosine phosphorylation in intestinal cells (A) Fluid accumulation was monitored in ligated ileal loop assays following injection of ST (100 nM) or 8-Br-cGMP (5 mM) in the presence or absence of HgCl2 (50 μM) and PV (0.5 mM). The experiment was repeated twice with two loops per treatment, and the values shown represent the mean ± standard error of the mean (*, P < 0.05). Inset, pY Western blot with mucosal scrapings from rat intestinal loops with or without treatment with PV and HgCl2. (B) Mucosal scrapings were prepared from treated and untreated intestinal loops as indicated, and solubilized proteins were allowed to interact with normal mouse immunoglobulin G (NMI) or GCC:B10 monoclonal antibody. Immunoprecipitates (IP) were subjected to Western blot analysis with monoclonal antibody GCC:4D7 and pY antibodies. (C) T84 cells were infected with lentiviruses encoding control or c-src shRNA. Subsequently cells were treated with HgCl2 and PV and harvested, and Western blotting was performed with total c-src, phospho-c-src, and GCC:C8 antibodies. The data shown are representative of experiments repeated thrice. (D) Lysates were prepared from T84 cells infected with lentivirus encoding either control or c-src shRNA following treatment with PV and HgCl2 or medium alone. Lysates were subjected to Western blotting with pY antibodies. The data shown are representative of experiments repeated thrice. (E) T84 cells were infected with lentivirus encoding control or c-src shRNA. Cells were then treated as indicated, following which ST (100 nM) was added and intracellular cGMP produced measured by radioimmunoassay. Values shown represent the mean ± standard error of the mean of duplicate determinations in experiments repeated thrice (*, P < 0.01).
The T84 human colorectal carcinoma cell line mimics many of the properties of the mature intestinal cell, and we used this cell line to explore the molecular mechanisms of tyrosine kinase-mediated regulation of GC-C. Given the importance of c-src tyrosine kinase activity in colon cancer cells, we specifically knocked down c-src expression in T84 cells by lentivirus-mediated transduction of c-src shRNA (Fig. 1C). The low levels of phosphorylated SFKs in c-src knockdown cells treated with HgCl2 and PV indicated that c-src was the major tyrosine kinase that was activated (Fig. 1C). Moreover, tyrosine phosphorylation of cellular proteins was also reduced following HgCl2 and PV treatment (Fig. 1D). PV and HgCl2 treatment resulted in a marked reduction in ST-mediated cGMP production in control shRNA-treated cells (Fig. 1E). Importantly, c-src-depleted cells showed a robust ST-mediated cGMP production following PV and HgCl2 treatment (Fig. 1E), indicating that the activation of c-src specifically regulated cGMP production by GC-C.
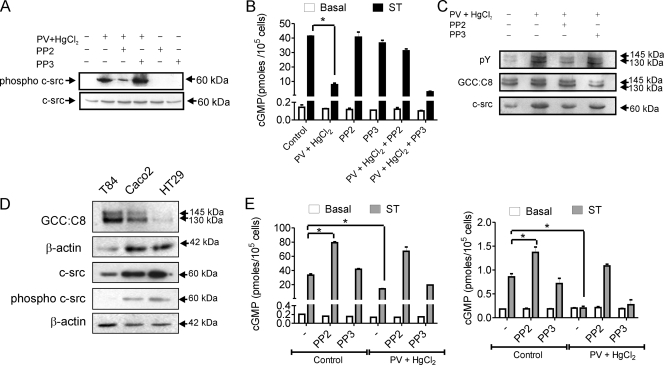
We mimicked these results by chemical knockdown of c-src using the SFK-specific inhibitor PP2 or its inactive analog PP3. We confirmed that the increased activation of c-src in T84 cells on treatment with PV and HgCl2 was reduced in the presence of PP2 but not PP3 (Fig. 2A). This reflected closely what was seen in the c-src shRNA experiments (Fig. 1C). PV and HgCl2 treatment in the presence of PP2 did not result in a reduction in ST-induced cGMP production by GC-C in comparison with untreated cells or cells treated with PP3 (Fig. 2B). The presence of active c-src in cells was correlated with an increase in tyrosine-phosphorylated GC-C (Fig. 2C), as was seen earlier in intestinal cells (Fig. 1B). Interestingly, c-src coimmunoprecipitated with GC-C, with a significant increase in the amounts of associated c-src under conditions where phosphorylated levels of GC-C were higher (Fig. 2C). This observation has important implications, as will be discussed below.
FIG. 2.
Activation of c-src in intestinal cells regulates ligand-mediated cGMP production by GC-C, (A) T84 cells were treated as indicated and lysates prepared from cells subjected to Western blot analysis to monitor levels of active c-src (phospho-c-src antibody) and total c-src. The data shown are representative of experiments repeated thrice. (B) T84 cells were treated with HgCl2 and PV following PP2 or PP3 pretreatment for 30 min. cGMP production on addition of ST (100 nM) was measured by radioimmunoassay. Values shown represent the mean ± standard error of the mean of duplicate determinations in experiments repeated thrice (*, P < 0.01). (C) GC-C was immunoprecipitated from T84 cells following treatments as indicated. Immune complexes were subjected to Western blotting with pY and GCC:C8 monoclonal antibodies. To monitor coimmunoprecipitation of c-src, the blot was stripped and then probed with c-src monoclonal antibody. (D) Western blots with lysates from T84, Caco2, and HT29 cells with GCC:C8 monoclonal antibody. The amount of T84 lysate taken was 4 times lower (as was seen with the β-actin loading control) than that of Caco2 and HT29 because of the high expression of GC-C in T84 cells. For Western blotting performed with phospho-c-src and total c-src, whole-cell lysate protein (50 μg) from the three cell lines was used, with β-actin indicating equal loading of the protein. (E) Caco2 (left panel) or HT29 (right panel) cells were treated as indicated and cGMP production on addition of ST (100 nM) measured by radioimmunoassay. Values shown represent the mean ± standard error of the mean of duplicate determinations in experiments repeated thrice (*, P < 0.05).
In order to confirm that active c-src could specifically modulate GC-C activity in different colorectal carcinoma cell lines, we made use of Caco2 and HT29 cells. Levels of GC-C varied in the cell lines, and levels of total c-src and active c-src were higher in Caco2 and HT29 cells (Fig. 2D), as reported earlier (17). Inhibiting the basal activity of c-src in Caco2 and HT29 cells by PP2 treatment led to greater ST-mediated cGMP production by GC-C, in comparison to cells treated with PP3 (Fig. 2E). Treatment of Caco2 and HT29 cells with PV and HgCl2 reduced ST-stimulated cGMP production, as was seen in T84 cells, and this effect was reversed in the presence of PP2. Therefore, the levels of active c-src in intestinal cells could regulate cGMP production by GC-C, perhaps by direct c-src-mediated phosphorylation of GC-C.
c-src phosphorylates GC-C at Tyr820 and inhibits ST-mediated GC activity.
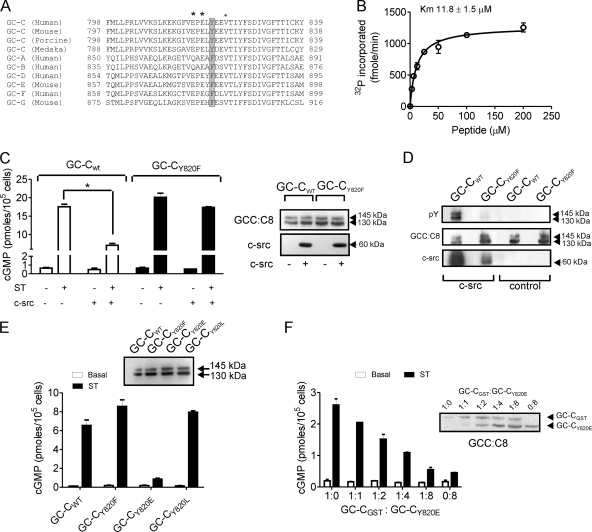
Using predictive algorithms (Scansite 2:0 and NetphosK) (8, 38), the tyrosine at position 820 was identified as a strong consensus site for phosphorylation by c-src (Fig. 3A). Tyr820, positioned N terminal to the GC domain, was found only in GC-C despite the extensive conservation in this region of other amino acids across receptor GCs (Fig. 3A). A peptide encompassing amino acids around Tyr820 served as a substrate for c-src with a Km (11.8 ± 1.5 μM), comparable to those of other substrates of SFKs (Fig. 3B) (58). To investigate the significance of phosphorylation at the Tyr820 residue in GC-C, we generated a GC-CY820F mutant receptor. Coexpression of c-src led to a reduction in ST-mediated cGMP production by wild-type GC-C (GC-CWT) but not by the mutant receptor (Fig. 3C). The lack of inhibition of cGMP production by GC-CY820F was correlated with decreased tyrosine phosphorylation of GC-CY820F in comparison to GC-CWT (Fig. 3D). Interestingly, significantly larger amounts of c-src coimmunoprecipitated with GC-CWT than with GC-CY820F, which indicates a tight association of the two proteins that is dependent on phosphorylation of Tyr820.
FIG. 3.
Identification of Tyr820 in GC-C as the site for c-src phosphorylation. (A) Amino acid sequence alignment of a region of human GC-C encompassing the Tyr820 residue along with orthologs of GC-C and other receptor GCs. GC-G and GC-E are pseudogenes in human, and therefore the mouse sequences have been used for alignment. *, acidic residues; •, hydrophobic residues downstream of Tyr820. (B) In vitro kinase assay performed with a peptide encompassing the Tyr820 residue and c-src. Values show the mean ± standard error of the mean of duplicate determinations of experiments repeated thrice. (C) HEK293T cells were cotransfected with the indicated plasmids, and following addition of ST (100 nM), the cGMP produced was measured. Values represent the mean ± standard error of the mean of duplicate determinations with each experiment repeated thrice (*, P < 0.01). Right panel, Western blotting performed with GCC:C8 and c-src monoclonal antibodies using total cell lysates. (D) GC-C was immunoprecipitated with GCC:B10 antibody from cells transfected with the indicated plasmids. Immunoprecipitates were subjected to Western blotting with pY, GCC:C8, and c-src monoclonal antibodies. (E) HEK293T cells were transfected with the indicated plasmids. Cells were treated with ST, and the cGMP produced was measured. Values represent the mean ± standard error of the mean of duplicate determinations of experiments repeated thrice (*, P < 0.01). (F) A plasmid encoding GC-CGST was transfected along with various amounts of the GC-CY820E plasmid, and cells were stimulated with ST at 48 h posttransfection. The inset shows a Western blot using GCC:C8 monoclonal antibody to indicate levels of GC-CGST and GC-CY820E in the transfected cells. Values show the mean ± standard error of the mean of duplicate measurements made with experiments repeated thrice.
We generated a phosphomimetic GC-CY820E mutant and the GC-CY820L mutant, where the large hydrophobic tyrosine residue was replaced by a smaller hydrophobic residue. While the GC-CY820L receptor showed ST-mediated cGMP production similar to that seen with GC-CWT, the GC-CY820E mutant receptor showed no cGMP production (Fig. 3E). ST binding to GC-CY820E was not compromised (data not shown), indicating that the introduction of a negative charge at position 820 abrogated cGMP production by inactivating the GC domain. Indeed, GC-CY820E had little in vitro GC activity in assays performed with Mn-GTP as the substrate (data not shown). However, GC-CY820E was able to heterodimerize with the wild-type receptor (Fig. 3F), indicating that the low GC activity was not due to lack of dimer formation by GC-C but was perhaps due to incorrect alignment of the two GC catalytic domains (60), as a result of the charge introduced in the molecule. Therefore, a single tyrosine residue in GC-C is a site for inhibitory phosphorylation by c-src, showing, for the first time, signal transduction cross talk between c-src and a receptor GC.
Phosphorylation of Y820 in GC-C generates a docking site for the c-src SH2 domain.
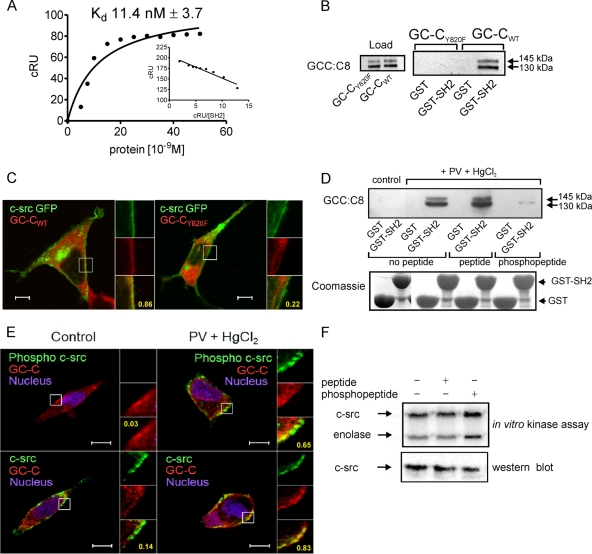
As a consequence of phosphorylation of GC-C at Tyr820 by c-src, a strong consensus site for binding of the SH2 domain of c-src is created, where charged amino acids downstream of the phosphorylated tyrosine residue, as well as a hydrophobic residue at the +3 position, facilitate interaction with the c-src SH2 domain (50). Surface plasmon resonance analysis showed that a GC-C peptide encompassing the phosphorylated Tyr820 residue bound the c-src SH2 domain with high affinity (∼11 nM) (Fig. 4A). Nonphosphorylated peptide showed no binding to the c-src SH2 domain (data not shown). To confirm that the Tyr820 position was essential for SH2 domain interaction, we performed pulldown experiments with GC-CWT and GC-CY820F receptors coexpressed with c-src in HEK293T cells. GC-CWT was able to interact specifically with the GST-SH2 domain, while a marked reduction in pulldown of the GC-CY820F receptor was observed (Fig. 4B). This demonstrated that phosphorylation at Tyr820 enabled interaction between the c-src SH2 domain and GC-C, explaining the earlier observations that in HEK293T cells, more c-src coimmunoprecipitated with GC-CWT than with GC-CY820F (Fig. 3D). The small amount of c-src that coimmunoprecipitated with GC-CY820F indicates that c-src associates with GC-C independently of an SH2 domain interaction with residues around the Tyr820 residue.
FIG. 4.
Interaction of GC-C with c-src via the SH2 domain contributes to colocalization of c-src and GC-C in the cell. (A) Surface plasmon resonance analysis of c-src SH2 binding to GC-C Y820 phosphopeptide. Inset, linear transformation of data. The data shown are from single sensogram with the experiment repeated twice. (B) GST-SH2 pulldown experiments were performed with lysates prepared from HEK293T cells transfected with either GC-CWT or GC-CY820F plasmid, along with a c-src plasmid. Proteins bound to the glutathione beads were analyzed by Western blotting with GCC:C8 monoclonal antibody. (C) Localization of GC-CWT or GC-CY820F with c-src GFP in HEK293T cells was observed using GCC:4D7 monoclonal antibody. Scale bar, 10 μm. Numbers indicate Pearson's coefficient for the region indicated in the box. The data shown are representative of experiments repeated thrice. (D) T84 cell lysates were allowed to interact with GST-SH2 in the presence of a peptide encompassing the Tyr820 residue or a peptide including a pY residue. Upper panel, Western blotting performed with GCC:C8 monoclonal antibody of proteins bound to beads. Lower panel, Coomassie blue-stained gel of GST and GST-SH2 proteins taken for pulldown experiments. (E) Immunofluorescence imaging was performed with T84 cells with phospho-c-src antibody and GCC:4D7 monoclonal antibody, or with c-src monoclonal antibody and a rabbit polyclonal antibody to GC-C, followed by incubation with anti-mouse Cy5 antibody and anti-rabbit Alexa 488 antibodies. Scale bar, 10 μm. Numbers indicate Pearson's coefficient for the region indicated in the box. The data shown are representative of experiments repeated thrice. (F) In vitro c-src kinase assay performed with c-src immunoprecipitated from HEK293T cells in the presence or absence of peptide or phosphopeptide. The lower panel shows a Western blot to detect the amount of c-src present in the immune complex used for the kinase assay.
Coimmunoprecipitation of two proteins might indicate their colocalization within a cell. GC-C is not only localized in the plasma membrane but also found in the endoplasmic reticulum and Golgi complex, where it is glycosylated (21). Significant colocalization of c-src-GFP with GC-CWT was seen in HEK293T cells, with the majority of colocalization found in the cell membrane and in the perinuclear region (Fig. 4C). Most interestingly, despite the expression of both GC-CY820F and c-src-GFP in similar regions of the cell, only a few colocalized spots were seen, indicating that colocalization was dependent on the phosphorylation of GC-C and subsequent association through the SH2 domain of c-src.
We validated these findings with T84 cells, where GST-SH2 pulldown experiments confirmed that GC-C specifically interacted with the c-src SH2 domain only when the cells were pretreated with both PV and HgCl2 (Fig. 4D). Moreover, the phosphopeptide encompassing the Tyr820 phosphorylation site was able to specifically compete out the interaction between GC-C and the c-src SH2 domain in the GST-SH2 pulldown assay (Fig. 4D). Enhanced colocalization of c-src with GC-C in the plasma membrane was seen on activation of SFKs in T84 cells (Fig. 4E), which correlated with the significant increase in coimmunoprecipitation of c-src with GC-C seen earlier (Fig. 2C).
As previously reported, conjugation of the SH2 domain of c-src with phosphopeptides results in activation of c-src (48, 52, 62). We performed c-src immune complex kinase assays in the presence or absence of the GC-C phosphopeptide or a peptide encompassing the Tyr820 residue. As shown in Fig. 4F, incubation of c-src with the phosphopeptide led not only to activation of c-src but also to phosphorylation of the substrate enolase to an extent greater than that seen on incubation with the peptide alone. Therefore, association of c-src with phosphorylated GC-C results in further activation of c-src, generating a feed-forward loop of autoactivation.
Activation of c-src can alleviate GC-C-mediated cell cycle arrest in T84 cells.
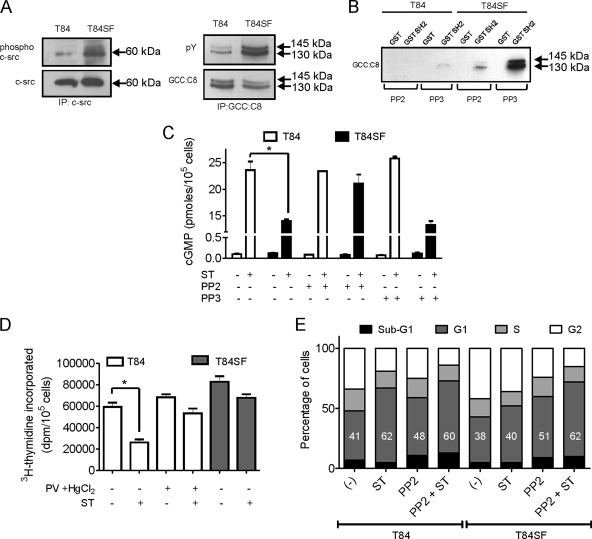
We now investigated the consequences of this GC-C and c-src cross talk for cellular processes that are regulated by GC-C and critical to intestinal cell proliferation. Signaling mediated via GC-C and its ligands results in slowing of the cell cycle and reduced cell proliferation, with both effects being cGMP dependent (41). The regulation of GC-C by c-src phosphorylation suggests that in colon carcinoma cells, where c-src activity is higher, the cell cycle would progress despite ligand binding to GC-C, since lower levels of cGMP would be produced by tyrosine-phosphorylated GC-C. The T84SF cell line is derived from T84 cells and is more invasive, with increased rates of proliferation in comparison to the parent T84 cell line (1). We were able to demonstrate a higher activity of c-src in T84SF cells than in T84 cells (Fig. 5A), with equivalent levels of total c-src being present in both cell lines. Increased overall cellular tyrosine phosphorylation (data not shown), as well as that of GC-C, was seen in T84SF cells in comparison to T84 cells (Fig. 5A). Pulldown experiments performed with lysates from T84SF cells showed a larger amount of GC-C that interacted with GST-SH2 domain protein than that seen with T84 cells in the presence of PP3, while this interaction was markedly reduced on PP2 treatment of T84SF cells (Fig. 5B). Based on our earlier observations (Fig. 2 and 3), we concluded that in T84SF cells, c-src mediates increased tyrosine phosphorylation of GC-C on the Tyr820 residue. Indeed, reduced cGMP production in response to ST was seen in T84SF cells, which was rescued by treating T84SF cells with PP2 (Fig. 5C).
FIG. 5.
Active c-src prevents GC-C-mediated cell cycle arrest in T84 cells. (A) c-src and GC-C were immunoprecipitated from T84 and T84SF cells with c-src and GCC:B10 monoclonal antibodies, respectively. Western blotting was performed on immune complexes using phospho-c-src and total c-src antibodies (left panel) and pY and GCC:C8 monoclonal antibodies (right panel). The data shown are representative of experiments repeated twice. (B) GST-SH2 pulldown experiments were performed with lysates from T84 or T84SF cells treated with either PP2 or PP3. GC-C bound to the beads was detected by Western blotting using GCC:C8 monoclonal antibody. (C) T84 and T84SF cells were treated with ST (100 nM) with or without PP2 or PP3 pretreatment, and cGMP produced was measured by radioimmunoassay. Values represent the mean ± standard error of the mean of duplicate determinations with experiments repeated thrice (*, P < 0.05). (D) [methyl-3H]thymidine incorporation was measured in T84 cells pretreated with or without PV and HgCl2 and T84SF cells after 24 h of ST (100 nM) treatment. Values represent the mean ± standard error of the mean of duplicate determinations with experiments repeated thrice (*, P < 0.05). (E) T84 and T84SF cells arrested in the G1 phase were treated with medium containing serum in the presence or absence of ST, with or without PP2; 24 h later, the cells were stained with propidium iodide and analyzed by flow cytometry. Numbers in bars indicate the percentage of cells in the G1 phase, and the data shown are representative data from four independent experiments (*, P < 0.05).
We then determined if c-src-mediated regulation of GC-C activity alters cell cycle progression and proliferation in T84 and T84SF cells. GC-C agonists suppress intestinal tumorigenesis by restricting colonic cell proliferation (41). As seen in Fig. 5D, treatment with ST led to a significant reduction in cell proliferation in T84 cells while a slight inhibition was observed in T84SF cells. ST application to T84 cells treated with PV and HgCl2 resulted in only a marginal decrease in cell proliferation, indicating an attenuation of the antiproliferative response mediated by GC-C activation. These observations are explained by the fact that lower ligand-mediated cGMP production is seen in cells containing tyrosine-phosphorylated GC-C (Fig. 5C).
ST has been shown to induce cytostasis in T84 cells by delaying cell cycle progression. As this effect is again mediated through cGMP, we compared the cytostatic effects of ST in T84 and T84SF cells by flow cytometry. PP2 treatment alone in both cell lines increased the percentage of cells in sub-G1, indicating the importance of c-src activity in these cells in allowing cell cycle progression and cell survival. ST treatment of T84 cells, either alone or in the presence of PP2, reduced the rate at which T84 cells exited from G1 arrest to similar extents (Fig. 5E). However, treatment of T84SF cells with ST alone did not increase the percentage of cells in G1 arrest, but PP2 treatment followed by ST arrested cells in the G1 phase, to an extent similar to that seen with T84 cells. This clearly indicates an important role of SFKs in preventing ST-induced cell cytostasis in T84SF cells.
A model for GC-C and c-src cross talk.
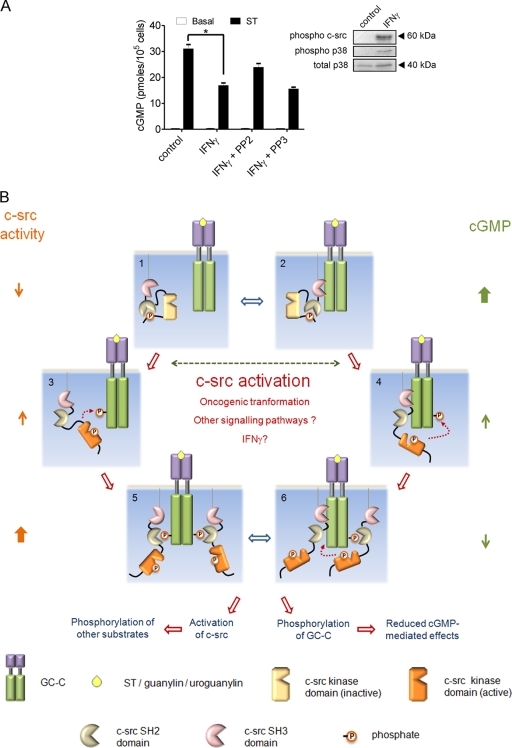
In intestinal cells, c-src activity is enhanced by the cytokine gamma interferon (IFN-γ) (53), and, based on our earlier results, this activation should inhibit GC-C activity. We detected activation of c-src and p38 mitogen-activated protein kinase after exposure of T84 cells to IFN-γ (Fig. 6A), as has been reported previously (53). Moreover, ST addition to IFN-γ-treated cells resulted in reduced cGMP production, and this effect was not seen in cells pretreated with PP2. We propose that this cross talk between IFN-γ and GC-C via c-src in intestinal cells may be one of the host defense mechanisms initiated in response to enterotoxigenic E. coli infection, since the presence of pathogenic E. coli in the intestine would induce the production of IFN-γ, thereby attenuating the watery diarrhea induced by ST peptides via cGMP.
FIG. 6.
GC-C and c-src cross talk in the intestinal cell. (A) T84 cells were treated with IFN-γ (100 ng/ml) for 24 h. Cells were then either lysed for Western blot analysis with the indicated antibodies or treated with ST for 15 min, following which cGMP was measured by radioimmunoassay. Values shown represent the mean ± standard error of the mean for duplicate determinations for assays repeated thrice (*, P < 0.05). (B) Model for GC-C and c-src cross talk. In a normal intestinal cell, c-src is inactive (1) and/or associated with GC-C via its SH3 domain (2). Under these conditions, most GC-C molecules are unphosphorylated, and on binding ligand (either ST/guanylin/uroguanylin) they produce large amounts of cGMP. Activating mutants of c-src, dysregulated cellular signaling pathways, or ligands (e.g., IFN-γ) contribute to enhanced activation of c-src in the colonic cell. GC-C is now tyrosine phosphorylated at Tyr820 by either free c-src (3) or associated c-src (4). This phosphorylation reduces cGMP production by GC-C. Phosphorylated GC-C can now recruit more c-src via the SH2 domain and, in turn, further phosphorylate GC-C (5 and 6). Binding of c-src to GC-C might also prevent subsequent dephosphorylation of GC-C by cellular phosphatases (5). With an increase in the number of GC-C molecules that are tyrosine phosphorylated, ligand-mediated cGMP production reduces drastically, effectively abrogating the antiproliferative actions of the ligands of GC-C. The c-src activated by interaction with GC-C can now phosphorylate a number of cellular substrates and modulate cell division, migration, and invasiveness.
Our results provide an interesting model for the actions of c-src and GC-C and can be summarized as follows (Fig. 6B). The differentiated enterocyte, in contrast to the crypt cell, has low levels of cytoskeleton-associated c-src (13), and therefore GC-C remains largely unphosphorylated, as a result of this low c-src activity and/or due to the actions of tyrosine phosphatases. In cells with low c-src activity, c-src may stay transiently associated with GC-C, through low-affinity SH3 domain interactions, and be poised to phosphorylate GC-C. Unphosphorylated GC-C remains responsive to its ligands, thereby regulating ion secretion and cell proliferation mediated by cGMP. Following accumulation of genetic mutations or epigenetic events that transform colonic epithelial cells into colon carcinoma cells, levels of active c-src in the cell are increased. This results in the phosphorylation of GC-C at Tyr820 and inhibition of its GC activity, thereby making the cells less responsive to guanylin or uroguanylin as well as the bacterially produced ST peptide. Moreover, c-src can now tightly associate with GC-C by interaction with the SH2 domain and can further phosphorylate additional GC-C molecules in its vicinity (present in the membrane or in the oligomer of GC-C), resulting in further inactivation of the pool of GC-C in the cell. In addition, conjugation of the SH2 domain of c-src with the peptide region around the Tyr820 residue in GC-C can now sustain the tyrosine kinase activity of c-src. This therefore represents a feed-forward mechanism by c-src for its own activation. Interestingly, association of GC-C with c-src via the SH2 domain will protect the Tyr820 residue from dephosphorylation by tyrosine phosphates, thereby prolonging the duration of GC-C inactivation.
Therefore, in conclusion, we show that as a consequence of cross talk of GC-C with c-src tyrosine kinase, tyrosine phosphorylation regulates the ability of GC-C to induce antiproliferative effects in colonic epithelia. Since activation of c-src is one of the hallmarks of intestinal neoplasia, phosphorylation of GC-C by active c-src may be one way in which the cytostatic effects of GC-C agonists (guanylin and uroguanylin) in the intestine are bypassed in spite of the high expression of GC-C, thereby leading to cancer.
DISCUSSION
We have described here in molecular detail a signaling pathway involving a receptor GC and c-src tyrosine kinase, which can regulate colon cancer cell proliferation. The sequence of GC-C around Tyr820 allows for efficient phosphorylation by c-src, and indeed, kinase assays indicate that the Km for the GC-C peptide for c-src kinase is one of the lowest reported to date (58). Moreover, following phosphorylation of Tyr820, an efficient docking site for the SH2 domain of c-src is generated, thereby stabilizing the interaction of c-src with GC-C. The current study also describes direct tyrosine phosphorylation of a receptor GC, which results in heterologous desensitization of the receptor.
Actions of GC-C in intestinal cells, which include fluid accumulation and cell cytostasis, are regulated by the activity of c-src, which in turn controls the status of GC-C phosphorylation. T84 cells also express c-yes, a related SFK (data not shown), but c-src knockdown, either by shRNA or with a specific inhibitor (PP2), reduced tyrosine phosphorylation of GC-C. This is interesting since increased c-src activity is almost always correlated with colon cancer progression, and predictive analysis indicated that the strongest consensus for tyrosine phosphorylation at Tyr820 was for c-src, and not c-yes, phosphorylation (12, 38). Since levels of phosphorylation of GC-C in T84 cells with basal c-src activity are low (as seen in SH2 pulldown experiments and pY blots), it is possible that tyrosine phosphatases are highly active in intestinal cells, thereby maintaining GC-C in an unphosphorylated, and therefore active, state (6).
The phosphorylated tyrosine residue in GC-C presents a docking site for interaction with the c-src SH2 domain, and we show here that this interaction results in colocalization with GC-C. Importantly, a small amount of colocalization of c-src with GC-C was seen in control T84 cells (Fig. 4E), as well as in HEK293T cells expressing GC-CY820F and c-src (Fig. 4C). This interaction may be mediated through the SH3 domain of c-src. We therefore propose that GC-C and c-src are associated to some extent in cells where they are coexpressed, with c-src poised to phosphorylate GC-C and thereby inhibit its activity. Moreover, this phosphorylation would subsequently stabilize the interaction of GC-C with c-src via the SH2 domain, leading to increased c-src activation.
The model presented here for GC-C is similar to a concept proposed recently regarding the mechanism of c-src kinase-dependent signaling by receptors that do not themselves possess tyrosine kinase activity (15). The model proposed that basal c-src activity in a cell results in phosphorylation of a monomeric receptor and a low level of association of c-src through its SH2 domain as a consequence of this phosphorylation. GC-C is known to exist as a preformed dimer/trimer in cells (55, 56) even in the absence of ligand, perhaps allowing a more rapid regulation by and of c-src that is ligand independent.
There are only a few reports that indicate signal transduction cross talk between GCs and either serine/threonine kinases or tyrosine kinases. GC-C is phosphorylated on a serine residue in the C-terminal tail of the receptor by protein kinase C, and this phosphorylation results in potentiation of GC activity (59). Guo et al. reported direct communication between receptor GC-A and the Rac-p21-activated kinase signaling pathway through allosteric activation of GC-A by autophosphorylated Rac-p21-activated kinase (23). No data on the regulation of GC-C were presented in these studies. Another study has shown that reactive oxygen species induce the tyrosine phosphorylation of the NO-sensitive soluble GC through direct phosphorylation of the enzyme, which also results in association of c-src with soluble GC (35). In that study, the tyrosine residue that was phosphorylated was present in the NO-binding regulatory domain of soluble GC and not near the catalytic domain, as we see in GC-C. Moreover, a twofold activation of GC activity was reported in those studies, in comparison to the almost complete inhibition that we see in the case of GC-C. Clearly, different GCs have developed their own independent mechanisms of regulation.
Could c-src and GC-C cross talk regulate normal intestinal physiology? There are some instances where the activation of c-src has been shown to be critical for promoting survival of intestinal cells in response to tumor necrosis factor alpha in vitro and in vivo (63), as well as regulating intestinal cell migration in wound healing and maintenance of the gastrointestinal tract (18). Moreover, a transient increase in the activity of c-src is essential to delay the anoikis, or detachment-induced apoptosis, in intestinal epithelial cells (30). Since GC-C is activated by its endogenous ligands continuously in the gastrointestinal tract, the increased c-src activity that is necessary for intestinal cell survival could now downregulate cGMP production by GC-C induced by uroguanylin and guanylin, thereby alleviating the cell cycle arrest seen on GC-C activation.
Chloride secretion, another downstream action of GC-C, is also regulated by c-src in the intestine. In T84 cells, it has been shown that carbachol-induced chloride ion secretion is inhibited following transactivation of the epidermal growth factor receptor (27). PP2 attenuated this carbachol-stimulated epidermal growth factor receptor and extracellular signal-regulated kinase phosphorylation, thereby potentiating chloride secretory responses to carbachol. Active c-src therefore not only would inhibit chloride ion secretion induced by carbachol but also would attenuate cGMP-mediated CFTR activation by GC-C, providing yet another method whereby these two pathways could influence each other in the normal intestinal cell.
Finally, while we have shown that the activation of c-src by IFN-γ modulates GC-C activity, there are other mechanisms by which pathogens regulate the activity of SFKs in intestinal cells. For example, invasion of intestinal cells by Shigella flexneri, the causative agent of bacillary dysentery, is mediated by tyrosine phosphorylation of cortactin by c-src (16). Under these conditions, the activity of GC-C would be attenuated not only by increased c-src activity but perhaps also by the redistribution of GC-C in cellular microcompartments during invasion, as a result of the close association of GC-C with cytoskeletal structures in intestinal cells (24, 31). Such studies, to the best of our knowledge, have not been performed to date.
The data in Fig. 5D show that ST has a marginal effect in reducing cell proliferation even in cells with high c-src activity. This observation could be because a fraction of GC-C remains unphosphorylated on Tyr820 in these cells, thereby resulting in low, but significant, cGMP production as is seen following PV and HgCl2 treatment (Fig. 2B). Alternatively, ST could induce cellular cytostasis by a pathway independent of cGMP production, which may therefore be c-src independent. Similarly, the slight reduction in cGMP production that is still seen in T84 cells treated with IFN-γ and PP2 (Fig. 6A) could be explained by the presence of a few molecules of GC-C that remained phosphorylated on Tyr820 despite PP2 treatment. IFN-γ treatment may also reduce cGMP production by a pathway independent of direct phosphorylation of GC-C by c-src. Finally, since PP2 treatment of both T84 and T84SF cells results in an increase in the number of cells arrested at the G1 phase (Fig. 5E), the importance of c-src activity in ensuring normal cell cycle progression is evident, occurring by a pathway that is independent of GC-C. Therefore, GC-C and c-src can influence each other's activities by the mechanisms shown in this study but can also regulate intestinal cell proliferation by independent mechanisms.
It has been proposed that analogs of the guanylin/uroguanylin/ST peptides could be used to inhibit cell proliferation in colon cancer (42). From our results, it appears that this strategy would be maximally effective in tumors with low c-src activity. Moreover, treatment of tumors with a c-src inhibitor (26, 51) along with the GC-C-activating peptides may prove to be an interesting therapy in the future for treatment of colon cell proliferation. In conclusion, tyrosine phosphorylation of GC-C is an important means of regulating the activity of this receptor. We have described a new target for c-src action, and this cross talk of c-src and GC-C may be of vital importance in the initiation and progression of colorectal cancer.
Acknowledgments
We thank Vani R. Iyer for technical assistance and members of the laboratory for useful discussions. We acknowledge the fluorescence-activated cell sorting and confocal microscopy facilities in the Division of Biological Sciences, Indian Institute of Science.
N.B. and T.N.V. obtained Junior and Senior Research Fellowships from the Council of Scientific and Industrial Research, Government of India. Support from the Departments of Biotechnology, Science, and Technology and the Indian Council of Scientific Research, Government of India, is acknowledged.
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Alessandro, R., A. M. Flugy, D. Russo, G. Stassi, A. De Leo, C. Corrado, G. Alaimo, and G. De Leo. 2005. Identification and phenotypic characterization of a subpopulation of T84 human colon cancer cells, after selection on activated endothelial cells. J. Cell Physiol. 203:261-272. [DOI] [PubMed] [Google Scholar]
- 2.Anders, D. L., T. Blevins, G. Sutton, L. J. Chandler, and J. J. Woodward. 1999. Effects of c-Src tyrosine kinase on ethanol sensitivity of recombinant NMDA receptors expressed in HEK 293 cells. Alcohol Clin. Exp. Res. 23:357-362. [PubMed] [Google Scholar]
- 3.Bakre, M. M., Y. Ghanekar, and S. S. Visweswariah. 2000. Homologous desensitization of the human guanylate cyclase C receptor. Cell-specific regulation of catalytic activity. Eur. J. Biochem. 267:179-187. [DOI] [PubMed] [Google Scholar]
- 4.Bakre, M. M., and S. S. Visweswariah. 1997. Dual regulation of heat-stable enterotoxin-mediated cGMP accumulation in T84 cells by receptor desensitization and increased phosphodiesterase activity. FEBS Lett. 408:345-349. [DOI] [PubMed] [Google Scholar]
- 5.Banker, N., B. M. Evers, M. R. Hellmich, and C. M. Townsend, Jr. 1996. The role of Src family kinases in the normal and neoplastic gastrointestinal tract. Surg. Oncol. 5:201-210. [DOI] [PubMed] [Google Scholar]
- 6.Bessette, D. C., D. Qiu, and C. J. Pallen. 2008. PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev. 27:231-252. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari, R., R. Mathew, K. Vijayachandra, and S. Visweswariah. 2000. Tyrosine phosphorylation of the human guanylyl cyclase C receptor. J. Biosci. 25:339-346. [DOI] [PubMed] [Google Scholar]
- 8.Blom, N., S. Gammeltoft, and S. Brunak. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351-1362. [DOI] [PubMed] [Google Scholar]
- 9.Bolte, S., and F. P. Cordelieres. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224:213-232. [DOI] [PubMed] [Google Scholar]
- 10.Burkitt, D. P. 1993. Epidemiology of cancer of the colon and rectum. Dis. Colon Rectum. 36:1071-1082. [DOI] [PubMed] [Google Scholar]
- 11.Carrithers, S. L., M. T. Barber, S. Biswas, S. J. Parkinson, P. K. Park, S. D. Goldstein, and S. A. Waldman. 1996. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc. Natl. Acad. Sci. USA 93:14827-14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright, C. A., M. P. Kamps, A. I. Meisler, J. M. Pipas, and W. Eckhart. 1989. pp60c-src activation in human colon carcinoma. J. Clin. Investig. 83:2025-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright, C. A., S. Mamajiwalla, S. A. Skolnick, W. Eckhart, and D. R. Burgess. 1993. Intestinal crypt cells contain higher levels of cytoskeletal-associated pp60c-src protein tyrosine kinase activity than do differentiated enterocytes. Oncogene 8:1033-1039. [PubMed] [Google Scholar]
- 14.Cohen, M. B., J. A. Hawkins, and D. P. Witte. 1998. Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab. Investig. 78:101-108. [PubMed] [Google Scholar]
- 15.Cooper, J. A., and H. Qian. 2008. A mechanism for SRC kinase-dependent signaling by noncatalytic receptors. Biochemistry 47:5681-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehio, C., M. C. Prevost, and P. J. Sansonetti. 1995. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J. 14:2471-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSeau, V., N. Rosen, and J. B. Bolen. 1987. Analysis of pp60c-src tyrosine kinase activity and phosphotyrosyl phosphatase activity in human colon carcinoma and normal human colon mucosal cells. J. Cell Biochem. 35:113-128. [DOI] [PubMed] [Google Scholar]
- 18.Dise, R. S., M. R. Frey, R. H. Whitehead, and D. B. Polk. 2008. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G276-G285. [DOI] [PubMed] [Google Scholar]
- 19.Dwarakanath, P., S. S. Visweswariah, Y. V. Subrahmanyam, G. Shanthi, H. M. Jagannatha, and T. S. Balganesh. 1989. Cloning and hyperexpression of a gene encoding the heat-stable toxin of Escherichia coli. Gene 81:219-226. [DOI] [PubMed] [Google Scholar]
- 20.Fantus, I. G., S. Kadota, G. Deragon, B. Foster, and B. I. Posner. 1989. Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry 28:8864-8871. [DOI] [PubMed] [Google Scholar]
- 21.Ghanekar, Y., A. Chandrashaker, U. Tatu, and S. S. Visweswariah. 2004. Glycosylation of the receptor guanylate cyclase C: role in ligand binding and catalytic activity. Biochem. J. 379:653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghanekar, Y., A. Chandrashaker, and S. S. Visweswariah. 2003. Cellular refractoriness to the heat-stable enterotoxin peptide is associated with alterations in levels of the differentially glycosylated forms of guanylyl cyclase C. Eur. J. Biochem. 270:3848-3857. [DOI] [PubMed] [Google Scholar]
- 23.Guo, D., Y. C. Tan, D. Wang, K. S. Madhusoodanan, Y. Zheng, T. Maack, J. J. Zhang, and X. Y. Huang. 2007. A Rac-cGMP signaling pathway. Cell 128:341-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakki, S., M. Crane, M. Hugues, P. O'Hanley, and S. A. Waldman. 1993. Solubilization and characterization of functionally coupled Escherichia coli heat-stable toxin receptors and particulate guanylate cyclase associated with the cytoskeleton compartment of intestinal membranes. Int. J. Biochem. 25:557-566. [DOI] [PubMed] [Google Scholar]
- 25.Jaleel, M., S. Saha, A. R. Shenoy, and S. S. Visweswariah. 2006. The kinase homology domain of receptor guanylyl cyclase C: ATP binding and identification of an adenine nucleotide sensitive site. Biochemistry 45:1888-1898. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, F. M., and G. E. Gallick. 2007. SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents Med. Chem. 7:651-659. [DOI] [PubMed] [Google Scholar]
- 27.Keely, S. J., S. O. Calandrella, and K. E. Barrett. 2000. Carbachol-stimulated transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T(84) cells is mediated by intracellular Ca(2+), PYK-2, and p60(src). J. Biol. Chem. 275:12619-12625. [DOI] [PubMed] [Google Scholar]
- 28.Krause, W. J., G. L. Cullingford, R. H. Freeman, S. L. Eber, K. C. Richardson, K. F. Fok, M. G. Currie, and L. R. Forte. 1994. Distribution of heat-stable enterotoxin/guanylin receptors in the intestinal tract of man and other mammals. J. Anat. 184:407-417. [PMC free article] [PubMed] [Google Scholar]
- 29.Li, P., S. Schulz, A. Bombonati, J. P. Palazzo, T. M. Hyslop, Y. Xu, A. A. Baran, L. D. Siracusa, G. M. Pitari, and S. A. Waldman. 2007. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology 133:599-607. [DOI] [PubMed] [Google Scholar]
- 30.Loza-Coll, M. A., S. Perera, W. Shi, and J. Filmus. 2005. A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene 24:1727-1737. [DOI] [PubMed] [Google Scholar]
- 31.Lubbe, W. J., D. S. Zuzga, Z. Zhou, W. Fu, J. Pelta-Heller, R. J. Muschel, S. A. Waldman, and G. M. Pitari. 2009. Guanylyl cyclase C prevents colon cancer metastasis by regulating tumor epithelial cell matrix metalloproteinase-9. Cancer Res. 69:3529-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundy, J., J. Chen, P. Wang, F. Fromowitz, A. Schuss, S. Lynch, J. Brugge, and M. V. Viola. 1988. Phenotypic and genetic alterations in pre-cancerous cells in the colon. Anticancer Res. 8:1005-1013. [PubMed] [Google Scholar]
- 33.Mann, E. A., K. A. Steinbrecher, C. Stroup, D. P. Witte, M. B. Cohen, and R. A. Giannella. 2005. Lack of guanylyl cyclase C, the receptor for Escherichia coli heat-stable enterotoxin, results in reduced polyp formation and increased apoptosis in the multiple intestinal neoplasia (Min) mouse model. Int. J. Cancer 116:500-505. [DOI] [PubMed] [Google Scholar]
- 34.Mathew, S., S. P. George, Y. Wang, M. R. Siddiqui, K. Srinivasan, L. Tan, and S. Khurana. 2008. Potential molecular mechanism for c-Src kinase-mediated regulation of intestinal cell migration. J. Biol. Chem. 283:22709-22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meurer, S., S. Pioch, S. Gross, and W. Muller-Esterl. 2005. Reactive oxygen species induce tyrosine phosphorylation of and Src kinase recruitment to NO-sensitive guanylyl cyclase. J. Biol. Chem. 280:33149-33156. [DOI] [PubMed] [Google Scholar]
- 36.Nandi, A., R. Bhandari, and S. S. Visweswariah. 1997. Epitope conservation and immunohistochemical localization of the guanylin/stable toxin peptide receptor, guanylyl cyclase C. J. Cell Biochem. 66:500-511. [DOI] [PubMed] [Google Scholar]
- 37.Nandi, A., R. Mathew, and S. S. Visweswariah. 1996. Expression of the extracellular domain of the human heat-stable enterotoxin receptor in Escherichia coli and generation of neutralizing antibodies. Protein Expr. Purif. 8:151-159. [DOI] [PubMed] [Google Scholar]
- 38.Obenauer, J. C., L. C. Cantley, and M. B. Yaffe. 2003. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 31:3635-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, J., and C. A. Cartwright. 1995. Src activity increases and Yes activity decreases during mitosis of human colon carcinoma cells. Mol. Cell. Biol. 15:2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitari, G. M., R. I. Baksh, D. M. Harris, P. Li, S. Kazerounian, and S. A. Waldman. 2005. Interruption of homologous desensitization in cyclic guanosine 3′,5′-monophosphate signaling restores colon cancer cytostasis by bacterial enterotoxins. Cancer Res. 65:11129-11135. [DOI] [PubMed] [Google Scholar]
- 41.Pitari, G. M., M. D. Di Guglielmo, J. Park, S. Schulz, and S. A. Waldman. 2001. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc. Natl. Acad. Sci. USA 98:7846-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitari, G. M., P. Li, J. E. Lin, D. Zuzga, A. V. Gibbons, A. E. Snook, S. Schulz, and S. A. Waldman. 2007. The paracrine hormone hypothesis of colorectal cancer. Clin. Pharmacol. Ther. 82:441-447. [DOI] [PubMed] [Google Scholar]
- 43.Pitari, G. M., L. V. Zingman, D. M. Hodgson, A. E. Alekseev, S. Kazerounian, M. Bienengraeber, G. Hajnoczky, A. Terzic, and S. A. Waldman. 2003. Bacterial enterotoxins are associated with resistance to colon cancer. Proc. Natl. Acad. Sci. USA 100:2695-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pu, M. Y., A. A. Akhand, M. Kato, T. Koike, M. Hamaguchi, H. Suzuki, and I. Nakashima. 1996. Mercuric chloride mediates a protein sulfhydryl modification-based pathway of signal transduction for activating Src kinase which is independent of the phosphorylation/dephosphorylation of a carboxyl terminal tyrosine. J. Cell Biochem. 63:104-114. [DOI] [PubMed] [Google Scholar]
- 45.Schulz, S., C. K. Green, P. S. Yuen, and D. L. Garbers. 1990. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 63:941-948. [DOI] [PubMed] [Google Scholar]
- 46.Shailubhai, K., H. H. Yu, K. Karunanandaa, J. Y. Wang, S. L. Eber, Y. Wang, N. S. Joo, H. D. Kim, B. W. Miedema, S. Z. Abbas, S. S. Boddupalli, M. G. Currie, and L. R. Forte. 2000. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 60:5151-5157. [PubMed] [Google Scholar]
- 47.Shenoy, A. R., and S. S. Visweswariah. 2003. Site-directed mutagenesis using a single mutagenic oligonucleotide and DpnI digestion of template DNA. Anal. Biochem. 319:335-336. [DOI] [PubMed] [Google Scholar]
- 48.Sicheri, F., I. Moarefi, and J. Kuriyan. 1997. Crystal structure of the Src family tyrosine kinase Hck. Nature 385:602-609. [DOI] [PubMed] [Google Scholar]
- 49.Singh, S., G. Singh, J. M. Heim, and R. Gerzer. 1991. Isolation and expression of a guanylate cyclase-coupled heat stable enterotoxin receptor cDNA from a human colonic cell line. Biochem. Biophys. Res. Commun. 179:1455-1463. [DOI] [PubMed] [Google Scholar]
- 50.Songyang, Z., and L. C. Cantley. 1995. Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem. Sci. 20:470-475. [DOI] [PubMed] [Google Scholar]
- 51.Summy, J. M., J. G. Trevino, D. P. Lesslie, C. H. Baker, W. C. Shakespeare, Y. Wang, R. Sundaramoorthi, C. A. Metcalf, 3rd, J. A. Keats, T. K. Sawyer, and G. E. Gallick. 2005. AP23846, a novel and highly potent Src family kinase inhibitor, reduces vascular endothelial growth factor and interleukin-8 expression in human solid tumor cell lines and abrogates downstream angiogenic processes. Mol. Cancer Ther. 4:1900-1911. [DOI] [PubMed] [Google Scholar]
- 52.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 53.Uribe, J. M., D. F. McCole, and K. E. Barrett. 2002. Interferon-gamma activates EGF receptor and increases TGF-alpha in T84 cells: implications for chloride secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G923-G931. [DOI] [PubMed] [Google Scholar]
- 54.Vaandrager, A. B. 2002. Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol. Cell Biochem. 230:73-83. [PubMed] [Google Scholar]
- 55.Vaandrager, A. B., E. van der Wiel, M. L. Hom, L. H. Luthjens, and H. R. de Jonge. 1994. Heat-stable enterotoxin receptor/guanylyl cyclase C is an oligomer consisting of functionally distinct subunits, which are non- covalently linked in the intestine. J. Biol. Chem. 269:16409-16415. [PubMed] [Google Scholar]
- 56.Vijayachandra, K., M. Guruprasad, R. Bhandari, U. H. Manjunath, B. P. Somesh, N. Srinivasan, K. Suguna, and S. S. Visweswariah. 2000. Biochemical characterization of the intracellular domain of the human guanylyl cyclase C receptor provides evidence for a catalytically active homotrimer. Biochemistry 39:16075-16083. [DOI] [PubMed] [Google Scholar]
- 57.Visweswariah, S. S. 2006. Guanylyl cyclase receptor C. UCSD-Nat. Mol. Pages. doi: 10.1038/mp.a000121.01. [DOI]
- 58.Vojtechova, M., Z. Tuhackova, J. Hlavacek, J. Velek, and V. Sovova. 2004. The v-Src and c-Src tyrosine kinases immunoprecipitated from Rous sarcoma virus-transformed cells display different peptide substrate specificities. Arch. Biochem. Biophys. 421:277-282. [DOI] [PubMed] [Google Scholar]
- 59.Wada, A., M. Hasegawa, K. Matsumoto, T. Niidome, Y. Kawano, Y. Hidaka, P. I. Padilla, H. Kurazono, Y. Shimonishi, and T. Hirayama. 1996. The significance of Ser1029 of the heat-stable enterotoxin receptor (STaR): relation of STa-mediated guanylyl cyclase activation and signaling by phorbol myristate acetate. FEBS Lett. 384:75-77. [DOI] [PubMed] [Google Scholar]
- 60.Winger, J. A., E. R. Derbyshire, M. H. Lamers, M. A. Marletta, and J. Kuriyan. 2008. The crystal structure of the catalytic domain of a eukaryotic guanylate cyclase. BMC Struct. Biol. 8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolpin, B. M., and R. J. Mayer. 2008. Systemic treatment of colorectal cancer. Gastroenterology 134:1296-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu, W., A. Doshi, M. Lei, M. J. Eck, and S. C. Harrison. 1999. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell 3:629-638. [DOI] [PubMed] [Google Scholar]
- 63.Yamaoka, T., F. Yan, H. Cao, S. S. Hobbs, R. S. Dise, W. Tong, and D. B. Polk. 2008. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc. Natl. Acad. Sci. USA 105:11772-11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zor, T., and Z. Selinger. 1996. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal. Biochem. 236:302-308. [DOI] [PubMed] [Google Scholar]