Abstract
The human cytomegalovirus (HCMV)-encoded G-protein-coupled receptor (GPCR) US28 is a potent activator of a number of signaling pathways in HCMV-infected cells. The intracellular carboxy-terminal domain of US28 contains residues critical for the regulation of US28 signaling in heterologous expression systems; however, the role that this domain plays during HCMV infection remains unknown. For this study, we constructed an HCMV recombinant virus encoding a carboxy-terminal domain truncation mutant of US28, FLAG-US28/1-314, to investigate the role that this domain plays in US28 signaling. We demonstrate that US28/1-314 exhibits a more potent phospholipase C-β (PLC-β) signal than does wild-type US28, indicating that the carboxy-terminal domain plays an important role in regulating agonist-independent signaling in infected cells. Moreover, HMCV-infected cells expressing the US28/1-314 mutant exhibit a prolonged calcium signal in response to CCL5, indicating that the US28 carboxy-terminal domain also regulates agonist-dependent signaling. Finally, while the chemokine CX3CL1 behaves as an inverse agonist or inhibitor of constitutive US28 signaling to PLC-β, we demonstrate that CX3CL1 functions as an agonist with regard to US28-stimulated calcium release. This study is the first to demonstrate that the carboxy terminus of US28 controls US28 signaling in the context of HCMV infection and indicates that chemokines such as CX3CL1 can decrease constitutive US28 signals and yet simultaneously promote nonconstitutive US28 signals.
Cytomegaloviruses (CMVs) belong to the Herpesviridae and are characterized by their ability to replicate in a large number of cell types in vivo and establish a life-long persistent infection in their host (39, 43). The prototypic member of this family, human CMV (HCMV), is present in a latent or persistent form in ∼75% of the human population (24). Healthy individuals harboring HCMV are typically asymptomatic, and yet the virus is the leading infectious cause of birth defects, causes significant morbidity in immunocompromised patients, and has been implicated as a cofactor in the progression of cardiovascular disease (5, 17, 30, 46). The gene products required for viral replication and promotion of disease in vivo, as well as their mechanism of action, remain largely unknown.
The genomes of all of the CMVs sequenced to date contain one or more open reading frames with significant homology to the chemokine family of G-protein-coupled receptors (GPCRs) (1, 10, 23, 32, 37, 42). For example, HCMV encodes four GPCR homologues in its genome termed US27, US28, UL33, and UL78, while murine CMV (MCMV) encodes two GPCR homologues termed M33 and M78 (10, 15, 32, 42). The best studied of the CMV GPCRs are US28 from HCMV and M33 from MCMV (2, 6, 33, 36, 47, 54). Although neither of these genes is required for replication of their respective viruses in cell culture, both of these genes appear to play important roles during viral infection in vivo (3, 47, 50). US28 activity is central to HCMV-directed chemotaxis of vascular smooth muscle cells since HCMV mutants with US28 deleted fail to promote cellular migration in vitro when tested in modified Boyden chamber assays (35, 47, 48). M33 and its signaling activity is involved in replication in sites of persistence in vivo since MCMV recombinants with M33 deleted are not detected in the salivary glands of mice after intraperitoneal or intraglandular infection (9, 12). Taken together, these studies suggest that CMV GPCRs such as M33 and US28 play important roles in pathogenesis in vivo by promoting viral dissemination and/or inducing virus-associated pathologies, including the acceleration of atherosclerosis.
US28 is closely related to the receptors for β-chemokines, such as CCR5, and accordingly binds with low nanomolar affinity to several chemokines, including CCL5 (RANTES), CCL2 (MCP-1), and CX3CL1 (Fractalkine) (7, 8, 15, 25, 27, 42). Although US28 binds to these ligands, the receptor exhibits high levels of agonist-independent signaling activity and agonist-independent endocytosis (6, 13). A combination of both agonist-independent and agonist-dependent signaling activity appears to be important for the biologic effects of US28, including smooth muscle cell migration (6, 47, 54). Agonist-independent signaling activity results in the constitutive activation of phospholipase C-β (PLC-β) and a number of transcription factors (6, 34, 38, 44, 54). In contrast, US28-mediated release of intracellular calcium and activation of the small G-protein Rho only occur after chemokine binding, suggesting that the interaction of ligands with US28 does in fact induce a conformational change in the receptor (2, 15, 35, 48). In addition, the CC and CX3C chemokines appear to have differential effects on US28 signaling. For example, CCL5 has been shown to behave as both a neutral agonist, having no measurable effect on PLC-β signaling or transcription factor activation, and as a positive agonist leading to the release of intracellular calcium (2, 15, 38). On the other hand, CX3CL1 has been suggested to behave as an inverse agonist, since it significantly blocks the ability of US28 to promote PLC-β activity (6-8, 25, 54). Surprisingly, the effect of CX3CL1 on US28-directed calcium release has not been reported. The mechanisms involved in the regulation of agonist-dependent and agonist-independent signaling by US28 remain poorly understood.
The cytoplasmic carboxy-terminal region of US28 has been identified as an important regulator of US28 signaling (36, 40, 53). This 40-amino-acid carboxy-terminal region contains several clusters of Ser/Thr residues that are constitutively phosphorylated by GPCR kinases (GRKs) and are responsible for recruitment of the β-arrestin proteins (36, 40). Phosphorylation by GRKs and recruitment of β-arrestin proteins represent key steps in the desensitization or downregulation of GPCR-mediated signal transduction and is a process utilized by virtually all cellular GPCRs to control the magnitude and duration of signaling (28, 29, 31, 41, 45). It is therefore not surprising that US28 mutants deleted for the carboxy-terminus regulatory domain demonstrate increased constitutive signaling activity in heterologous expression experiments (36, 53). Interestingly, these studies also demonstrate that deletion of the carboxy-terminal region confers onto US28 the ability to respond in a positive manner to CX3CL1 stimulation (53). Thus, at least in heterologous systems, it appears that the carboxy terminus of wild-type US28 (US28/WT) functions to “camouflage” agonism, leading to the speculation that chemokines such as CX3CL1 may act as true agonists in certain scenarios, including that exhibited in HCMV-infected cells (53). Although it is clear that the carboxy-terminal domain plays an important role in the regulation of US28-dependent signal transduction, the mechanism by which this occurs is not clear. The carboxy-terminal domain may stimulate receptor endocytosis, such that deletion of this important domain results in increased cell surface expression of the receptor and a concomitant increase in signal transduction or agonist responsiveness (40, 53). Alternatively, the carboxy-terminal domain may be responsible for receptor desensitization (or uncoupling of receptor from cognate G protein), such that deletion of this domain results in an increase in the magnitude and duration of the US28 signal (36).
In the present study, we have utilized a recombinant HCMV expressing a carboxy-terminal mutant of US28, US28/1-314, to study the role of the US28 carboxy terminus in regulating signaling during HCMV infection. We present evidence in HCMV-infected cells that US28/1-314 exhibits increased constitutive agonist-independent signaling to PLC-β and exhibits extended Ca2+ signaling in response to CCL5. Our results suggest that the carboxy terminus of US28 controls signaling by regulating desensitization and not by destabilizing cell surface expression. Surprisingly, while CX3CL1 can function as an inverse agonist to dampen constitutive US28 signaling to PLC-β, we demonstrate for the first time that CX3CL1 can also function as an agonist to promote Ca2+ signaling from both US28/WT and US28/1-314. Thus, CX3CL1 appears to induce a US28 conformation that simultaneously inhibits or promotes signaling depending on the readout. These findings mechanistically define the role of the carboxy terminus during US28 signaling in infected cells and provide a new understanding of the role that “agonists” and “inverse agonists” play in regulating US28 signaling in a physiological setting.
MATERIALS AND METHODS
Cell culture and virus propagation.
Human foreskin fibroblasts (HFFs) were purchased from the American Type Culture Collection and maintained in Dulbecco modified Eagle medium (Mediatech) supplemented with 10% Fetal Clone III serum (HyClone) and 1% penicillin-streptomycin (Mediatech). Cells were grown at 37°C in a humidified atmosphere of 95% air-5% CO2, split 1:2 twice weekly, and used between passages 3 and 20. For propagation of HCMV, cells were infected at a multiplicity of infection (MOI) of 0.01 and fed every 3 days until the cytopathic effect (CPE) reached 100%. Cell culture supernatant containing virus was harvested, snap-frozen, and stored at −80°C until use.
Construction of the US28/1-314 mutant.
Bacterial artificial chromosomes (BACs) containing either the wild-type VR1814 strain of HCMV (FIX-BAC) or a mutant in which the US28 open reading frame was replaced by a Kanr/LacZ cassette (FIX-BAC-ΔUS28) have been previously described (16, 49). Lambda red recombination was utilized to construct a mutant of US28 termed US28/1-314 from which amino acids 315 to 354 have been deleted. Briefly, a cassette containing a FLAG epitope sequence and US28 amino acids 1 to 314 was PCR amplified and recombined into the US28 locus of FIX-BAC-ΔUS28 deleting the Kanr/LacZ cassette. Recombinants were selected by Kan sensitivity and white color when plated on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-containing media. FIX-BAC DNA was isolated from Escherichia coli by using a standard miniprep procedure and amplified by PCR to detect recombination. Two independent recombinant BACs were made for each mutant used in the present study. Recombination at the US28 locus was verified by PCR of BAC DNA isolated from E. coli using a standard miniprep and amplified using primer sets with homology external to the site of recombination at the US28 locus, within the FLAG sequence, or the UL146 gene. The sequences of the US28/1-314 recombinants including the US28 locus and regions surrounding the recombination site were confirmed by automated ABI DNA sequencing. For reconstitution of recombinant viruses, 2 × 105 MRC-5 cells were plated in six-well plates and transfected with 2 μg of FIX-BAC DNAs by using either Superfect (Qiagen) or Transit IT (Mirus) lipid transfection reagents according to the manufacturer's protocol. At 10 days posttransfection, MRC-5 cells were transferred to T-25 flasks and fed with fresh medium every 3 to 4 days. After the appearance of virus-associated CPEs, infected MRC-5 cells were mixed with uninfected HFFs and cultured until the CPE reached 100%. Supernatants containing recombinant viruses were used for the generation of virus stocks.
Inositol phosphate accumulation.
HFFs were seeded in 12-well plates at a density of 1.5 × 105 cells per well and either mock infected or infected with HCMV recombinants at an MOI of 0.03, 0.1, or, 0.3. At 24 h postinfection, medium containing virus was removed and replaced with serum-free modified Eagle medium (Mediatech) containing 2 μCi of [3H]myoinositol (Perkin-Elmer Life Sciences)/ml. At 48 h postinfection, the medium was removed and replaced with serum-free medium supplemented with 20 mM LiCl for 3 h. For samples to be stimulated with 10 nM CCL5 (Peprotech), 10 nM CX3CL1 (Peprotech), or costimulated with 10 nM CX3CL1 and 50 nM CCL5, the ligand was added to the appropriate concentration at the time the medium was replaced with serum-free medium supplemented with LiCl. Reactions were stopped by aspirating the medium, adding 1 ml of 0.4 M perchloric acid, and cooling undisturbed at 4°C for 5 min. Then, 800 μl of supernatant was neutralized with 400 μl of 0.72 M KOH-0.6 M KHCO3 and subjected to centrifugation. Next, 1 ml of supernatant was diluted with 3 ml of distilled H2O and applied to freshly prepared Dowex columns (AG1-X8; Bio-Rad). Columns were washed two times with distilled H2O, total inositol phosphates were eluted with 4.0 ml of 0.1 M formic acid-1 M ammonium formate, and eluates containing accumulated inositol phosphates were counted in a liquid scintillation counter. Then, 50 μl of neutralized supernatant was counted in a liquid scintillation counter to measure the total incorporated [3H]myoinositol. The data are expressed as the accumulated inositol phosphate divided by the total incorporated [3H]myoinositol.
Immunoprecipitation and Western blotting.
HFFs were seeded in 100-mm dishes at a density of 2.0 × 106 cells per plate and either mock infected or infected with HCMV recombinants at an MOI of 3. At 48 h postinfection, cells were lysed in radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris, 5 mM EDTA, 0.1% sodium dodecyl sulfate, 1.0% sodium deoxycholate, 1.0% Triton X-100, and Complete protease inhibitors [Roche]). A small sample of the clarified lysate was saved as whole-cell extracts, and the remainder was incubated with anti-FLAG M2-agarose beads (Sigma) to immunoprecipitate FLAG-US28 proteins. Beads were washed twice with lysis buffer and eluted using 50 μl of Laemmli sample buffer. Whole-cell extracts or FLAG immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to Western blotting with antibodies directed against the FLAG epitope (sc-805; Santa Cruz); HCMV IE proteins (MAB810, Chemicon) or HCMV pUL44 (kindly provided by John D. Shanley; University of Connecticut). Reactive proteins were detected by using the appropriate secondary antibodies in an enhanced chemiluminescence system (Amersham Biosciences).
Radioligand binding and endocytosis assays.
HFFs were seeded in 12-well plates at a density of 1.5 × 105 cells per well and either mock infected or infected with HCMV recombinants at an MOI of 3. At 48 h postinfection, cells were preincubated in the absence or presence of 14 nM unlabeled CCL5 for 15 min in ice-cold binding buffer (50 mM HEPES, 1 mM CaCl2, 5 mM MgCl2, and 0.5% bovine serum albumin). [125I]CCL5 (Perkin-Elmer Life Sciences) was then added to a final concentration of 28 pM, followed by incubation for 3 h at 4°C. Cells were washed three times in ice-cold binding buffer supplemented with 500 mM NaCl and lysed in 500 mM NaOH, and specific binding was evaluated by using a liquid scintillation counter. To measure US28 endocytosis, cells were labeled as described above and warmed by the addition of Dulbecco modified Eagle medium at 37°C for various time points. At each time point, cells were either washed in ice-cold binding buffer (normal wash to determine total cell-associated [125I]CCL5) or washed in ice-cold binding buffer adjusted to pH 3.0 (acid wash to determine internalized [125I]CCL5). Washed cells were lysed in 500 mM NaOH, and binding was evaluated by using a liquid scintillation counter. The percentage of internalized activity was determined by dividing the acid-resistant ligand binding by the total cell-associated ligand binding.
FACS analysis of cell surface expression.
HFFs were seeded in 12-well plates at a density of 1.5 × 105 cells per well and either mock infected or infected with HCMV recombinants at an MOI of 3. At 48 h postinfection, cells were dislodged by trypsinization, washed twice in ice-cold phosphate-buffered saline (PBS), and stained for 2 h at 4°C in M2-biotin (Sigma) diluted 1:100 in fluorescence-activated cell sorting (FACS) staining buffer (PBS supplemented with 0.5% bovine serum albumin). Cells were washed two times with ice-cold PBS and stained for 2 h at 4°C in streptavidin-PE (BD biosciences) diluted 1:100 in FACS staining buffer. Cells were washed twice with ice-cold PBS and analyzed by using a FACSCalibur flow cytometer (BD Biosciences).
Measurement of intracellular calcium.
HFFs were infected with HCMV ΔUS28, FLAG-US28/WT, or FLAG-US28/1-314 at an MOI of 3 for 48 h and then loaded with Fluo4-AM (Molecular Probes) for 60 min at 37°C in the absence or presence of 20 μM 2APB (Calbiochem) or 2 μM U73122 (Cayman Chemicals). Pertussis toxin (PTx) was used at a concentration of 200 ng/ml to treat infected cells 24 h prior to loading (List Biological Laboratories). After basal levels of fluorescence were attained for 20 s, cells were stimulated 10 nM CCL5/RANTES or 10 nM CX3CL1 Fractalkine (Peprotech), and the fluorescence was measured by using an excitation wavelength of 488 nm and an emission wavelength of 505 to 515 nm every 3 s for 180 to 300 s using a FlexStation II analyzer (Molecular Devices). The data are representative of at least three experiments performed in duplicate.
RESULTS
Construction of an HCMV mutant expressing the US28/1-314 protein.
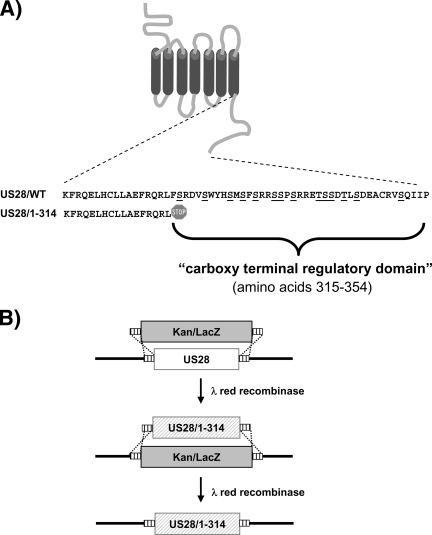
The HCMV US28 protein contains a 40-amino-acid carboxy-terminal regulatory domain that is extremely rich in serine and threonine residues typical of many cellular GPCRs (Fig. 1A). A number of studies have utilized heterologous expression systems to ascribe a variety of roles for this regulatory domain, including phosphorylation-dependent desensitization, endocytosis, and agonist responsiveness (36, 40, 53). In order to evaluate the role of the carboxy terminus in regulating US28 signaling during HCMV infection, we constructed a viral recombinant, based on the VR1814 (FIX-BAC) HCMV strain (16), encoding a US28 protein lacking this carboxy-terminal domain. This mutant, termed US28/1-314, was constructed by lambda red recombination using a two-step process in which the US28 gene was first replaced with a Kanr/LacZ cassette that was subsequently replaced with a FLAG-tagged allele of US28 encoding amino acids 1 to 314 (Fig. 1B). This second step, in which we replaced the Kanr/LacZ cassette with the US28/1-314 allele, was monitored by the generation of white colonies and proper recombination was determined by PCR (Fig. 2A). Recombination into the appropriate locus and integrity of the mutant BAC genome was confirmed by restriction digestion and Southern blotting with a US28 specific probe (see Fig. S1 in the supplemental material). The entire sequence of the US28 gene in the new recombinant was confirmed by PCR, and the US28/1-314 FIX-BAC was reconstituted into infectious virus as previously described (49). Virus stocks were titered on HFFs using a standard plaque assay, and it was determined that the US28/1-314 HCMV-FIX replicated with similar kinetics and reached a similar titer as the parental HCMV-FIX (2.3 × 106 for the parental virus and 1.8 × 106 for the US28/1-314 virus). This new viral recombinant will be used in parallel with the previously described ΔUS28 and US28/WT HCMV-FIX based viruses to ascertain the role that the C terminus plays in the regulation of US28 signaling (49).
FIG. 1.
Strategy for construction of the HCMV recombinant FLAG-US28/1-314 in HCMV FIX-BAC. (A) Schematic of US28 depicting the Ser/Thr-rich carboxy-terminal regulatory region. Amino acids 315 to 354 that are deleted in the US28/1-314 mutant are expanded underneath the diagram. (B) Schematic representation of the recombination strategy used to manipulate US28 and construct the US28/1-314 mutant.
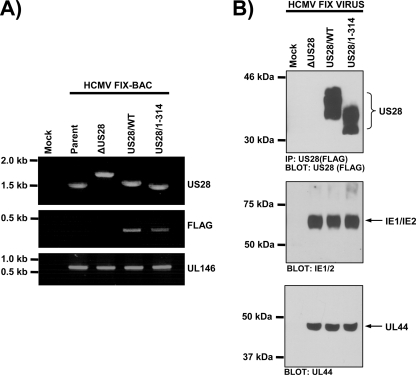
FIG. 2.
Construction and analyses of the FLAG-US28/1-314 HCMV recombinant in infected cells. (A) FIX-BAC DNAs isolated from E. coli were used as templates in all PCRs. Primers with homology to 5′ and 3′ sequences flanking the US28 coding region were used to PCR amplify the US28 locus (upper panel). The primer with homology to the 5′ flanking sequence was used in combination with a FLAG-specific primer to verify the addition of an N-terminal FLAG epitope (middle panel). Amplification of the UL146 gene serves as a positive PCR control (lower panel). (B) Immunoprecipitation followed by Western blotting with FLAG-specific antibodies was performed to detect expression of the various forms of FLAG-US28 encoded by HCMV ΔUS28, HCMV FLAG-US28/WT, and HCMV FLAG-US28/1-314 viruses at 48 h postinfection (upper panel). Whole-cell lysates from the same samples were subjected to Western blotting with an α-IE1/IE2 or α-UL44 antibodies (lower panels). The results shown are representative of six independent experiments.
Biochemical characterization of the US28/1-314 protein.
Using the recombinant US28/1-314 virus, we characterized the expression, chemokine binding, and endocytic properties of the US28/1-314 protein in comparison to US28/WT in HCMV-infected cells. In some heterologous transfection experiments, it has been shown that deletion of the US28 carboxy-terminal region or removal of the Ser/Thr phosphorylation sites results in a US28 molecule that accumulates to much higher levels on the cell surface due to a severe defect in endocytosis (40, 53). The incorporation of the N-terminal FLAG epitope into the US28 protein allows us to assess expression levels of wild-type and mutant US28 proteins by Western blot and flow cytometry. Human fibroblasts were mock infected or infected with ΔUS28, US28/WT, or US28/1-314 HCMV-FIX viruses at an MOI of 3, and the US28 expression was analyzed at 48 h postinfection by using an immunoprecipitation-Western blotting approach (49). US28/1-314 is expressed at similar, albeit slightly lower levels (83% ± 9% compared to US28/WT as determined by densitometric quantitation of Western blots, n = 6), indicating that deletion of the carboxy terminus does not drastically affect protein stability (Fig. 2B, upper panel). As expected, US28/1-314 runs with a lower apparent molecular weight, reflecting the deletion of 40 amino acids from the carboxy terminus. As a control, we analyzed IE1/IE2 and UL44 expression to ensure that we achieved similar infections with each recombinant virus (Fig. 2B, lower panels).
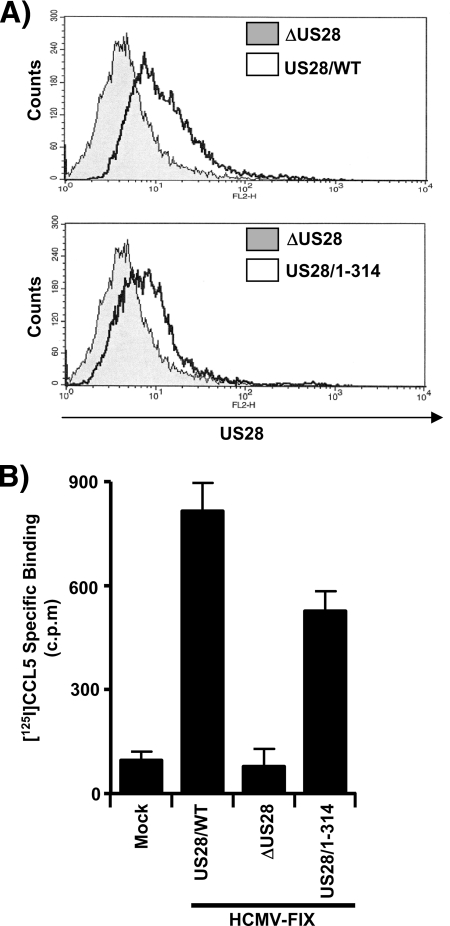
We next wanted to assess the cell surface expression patterns of US28/WT and US28/1-314 proteins. We used flow cytometry with an antibody directed against the extracellular amino-terminal FLAG epitope present on the US28 proteins to assess the cell surface expression (Fig. 3A). Interestingly, in HCMV-infected fibroblasts, the US28/1-314 protein does not accumulate to higher levels on the cell surface compared to US28/WT and in fact exhibits slightly lower levels of cell surface expression (82% ± 11% compared to US28/WT as determined by quantitation of the flow cytometry data, n = 6). This may possibly be reflective of its slight reduction in overall expression (Fig. 2B and 3A).
FIG. 3.
US28/WT and US28/1-314 proteins exhibit similar cell surface expression and ligand-binding activity in infected cells. (A) HFFs were infected with HCMV ΔUS28 or HCMV FLAG-US28/WT (top panel) and with HCMVΔUS28 or HCMV FLAG-US28/1-314 (bottom panel) viruses for 48 h. Surface expression of FLAG-US28 on infected cells was detected by staining with FLAG-specific M2-biotin, followed by streptavidin-PE and analyzed by FACS. The histograms shown are representative of six independent experiments performed in duplicate. (B) Infected HFFs were incubated with 28 pM [125I]CCL5 in the absence or presence of 14 nM unlabeled RANTES to discriminate between specific and nonspecific binding. The data shown represent specific binding of [125I]CCL5, as assessed by liquid scintillation chromatography, and are derived from six independent experiments performed in duplicate and represent the means ± the standard errors of the mean (SEM).
The relative abilities of US28/WT and US28/1-314 to bind to C-C chemokines during HCMV infection of HFFs were then assessed using [125I]CCL5/RANTES binding experiments. Uninfected cells and cells infected with the ΔUS28 virus exhibited negligible levels of [125I]CCL5/RANTES binding, whereas cells infected with the US28/WT and US28/1-314 viruses strongly bound CCL5/RANTES (Fig. 3B). US28/1-314 does exhibit a modest reduction in the level of CCL5/RANTES binding (527 ± 58 cpm for US28/1-314 versus 815 ± 82 cpm for US28/WT) consistent with the slight reduction in cell surface expression.
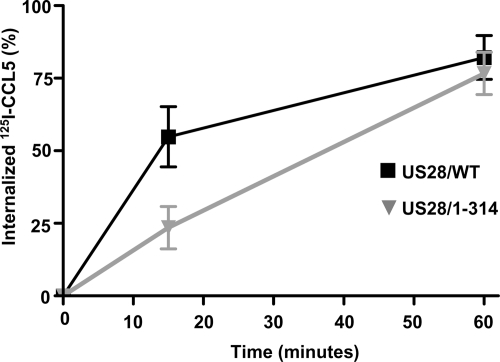
As a final step in the biochemical characterization of the US28/1-314 protein in HCMV-infected cells, we measured the relative abilities of the US28/WT and US28/1-314 proteins to undergo endocytosis. To measure US28 endocytosis, cells were infected with the US28/WT and US28/1-314 viruses, labeled with the US28 ligand [125I]CCL5 at 4°C and warmed to 37° for various times to allow for receptor internalization (Fig. 4). US28/1-314 was somewhat impaired in its ability to internalize when measured at 15 min (24% ± 7% or 1.6% per minute for US28/1-314 versus 55% ± 11% or 3.7% per minute for US28/WT) but exhibited similar levels of internalization in comparison to US28/WT when measured at 60 min.
FIG. 4.
Endocytic properties of US28/WT and US28/1-314 proteins in HCMV-infected cells. HFFs were infected with the HCMV FLAG-US28/WT or HCMV FLAG-US28/1-314 viruses for 48 h. Infected cells were then labeled at 4°C with [125I]CCL5, washed, and then warmed to 37°C for various time to allow for endocytosis of the US28 proteins. Internalized ligand was assessed by acid washing to remove surface-bound ligand and is presented as the percentage of total ligand bound. The data shown are derived from six independent experiments performed in duplicate and represent the means ± the SEM.
Taken together, our data in infected cells differs somewhat from studies in transfected cells which demonstrated that deletion of the carboxy terminus or replacement of carboxy-terminal serines with alanines led to a severe defect in endocytosis and a subsequent accumulation of high levels of the mutant US28 protein on the cell surface compared to wild-type US28 (40, 53). Although we do observe a somewhat delayed level of endocytosis with the US28/1-314 mutant, both US28/WT and US28/1-314 exhibit similar patterns of endocytosis by 60 min and US28/1-314 does not accumulate to high levels on the cell surface compared to US28/WT. The reason for this difference at this point is unclear but may be due to slight differences in the design of the US28 mutants utilized in the various studies or due to altered regulation of US28 in transfection experiments.
Deletion of the carboxy-terminal regulatory domain results in higher levels of constitutive US28 signaling to PLC-β.
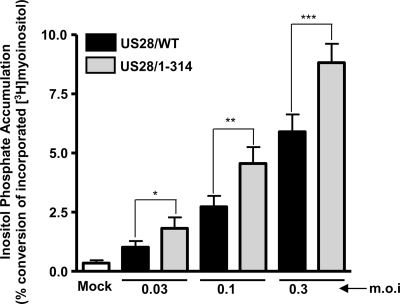
Next, we assessed the constitutive signaling properties of US28/1-314 in infected cells by measuring agonist-independent activation of PLC-β. The assay we utilized measures the accumulation of total inositol phosphates (InsP), which occurs as a result of activation of the PLC-β enzyme and was confirmed to be PLC specific by using the pharmacological inhibitor U73122 (data not shown). HFFs were either mock infected or infected with increasing doses (MOI = 0.03 to 0.3) of the US28/WT or US28/1-314 viruses. The MOIs utilized were designed to be submaximal to reduce the possibility of saturating the signal, which could occur at MOIs greater than 1. Cells infected with US28/1-314 HCMV consistently exhibited higher levels of agonist-independent PLC-β signaling compared to cells infected with US28/WT HCMV (Fig. 5). This increased signaling potential of US28/1-314 over US28/WT was observed at each MOI tested (P < 0.05 at all MOIs tested). These data confirm results obtained in transfected cells, in which US28/1-314 exhibits higher signaling potential than US28/WT (36). Since the US28/1-314 protein is expressed on the cell surface at levels similar to US28/WT (Fig. 3), these results suggest that the increased signaling of US28/1-314 is a result of a lack of regulation by the cellular desensitization machinery and not due to an increased presence of the mutant protein on the cell surface.
FIG. 5.
US28/1-314 exhibits increased levels of constitutive PLC-β signaling in HCMV-infected cells. HFFs were infected with increasing MOIs (0.03 to 0.3 PFU/cell) using HCMV FLAG-US28/WT or HCMV FLAG-US28/1-314 viruses. Medium containing 1 μCi of [3H]myoinositol/ml was added at 24 h postinfection, and the accumulated inositol phosphates were determined at 48 h postinfection by using anion-exchange chromatography. The data shown are derived from 10 independent experiments performed in duplicate and represent the means ± the SEM.
CCL5-dependent Ca2+ signaling through US28/1-314 is not rapidly desensitized like US28/WT.
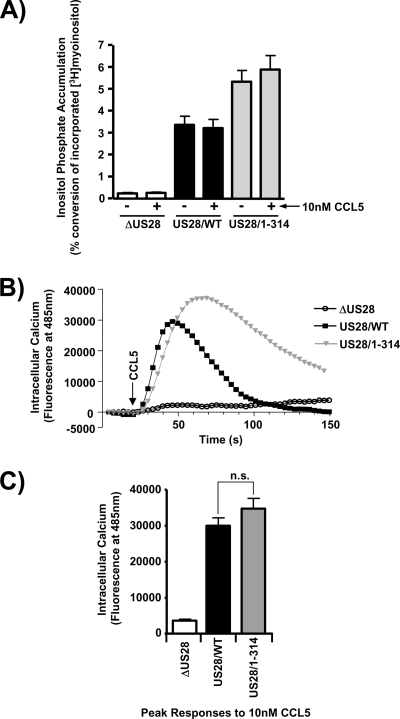
The PLC-β biochemical assay utilized to compare the constitutive agonist-independent signaling of US28/1-314 and US28/WT is useful to crudely compare US28/1-314 and US28/WT signaling activity, but it does provide an insight into the mechanism involved in the increased signaling exhibited by the US28/1-314 mutant since it measures US28 signaling activity over a extended time frame (3 h in this case). Since US28 has been shown to acutely induce calcium release from intracellular stores in the presence of the chemokine CCL5, we sought to compare the signaling activity of US28/WT and US28/1-314 with respect to CCL5-induced calcium release. As previously reported, CCL5 did not affect the constitutive PLC-β signaling activity (6, 36, 38), although, in agreement with our earlier studies, the US28/1-314 mutant did exhibit increased PLC-β activity (Fig. 6A) (36). We then assessed the acute effects of CCL5/RANTES on calcium release mediated by either US28/1-314 or US28/WT. Stimulation of US28/WT with CCL5/RANTES resulted in an acute stimulation of calcium release that returned to baseline by 80 s, a finding reflective of desensitization by the cellular machinery (Fig. 6B). Interestingly, stimulation of US28/1-314 with CCL5/RANTES resulted in an extended stimulation of calcium release that retained 40% of the maximal signal out to 130 s. The release of intracellular calcium eventually returned to baseline at ∼200 s (see Fig. S2 in the supplemental material). Although we observed an extension in time of the calcium signal with the US28/1-314 mutant, the absolute magnitude of the calcium signal was slightly increased but not statistically significant (Fig. 6C). Cells infected with ΔUS28 HCMV exhibited no increase in calcium in the presence of CCL5/RANTES, indicating that the observed effects are indeed specific to US28. We also utilized a CCL5/RANTES binding mutant of US28 (US28/ΔN) to confirm that the effects of RANTES are due to stimulation of US28 (see Fig. S2 in the supplemental material). These results indicate that agonist-dependent US28 signaling is desensitized by the cellular machinery in HCMV-infected cells and that the US28 carboxy terminus is required for this activity.
FIG. 6.
US28/1-314 exhibits prolonged levels of calcium signaling in response to CCL5/RANTES. (A) HFFs were infected with HCMV ΔUS28, HCMV FLAG-US28/WT, or HCMV FLAG-US28/1-314 viruses at an MOI of 0.1. Medium containing 1μCi of [3H]myoinositol/ml was added 24 h postinfection. At 45 h postinfection, the cells were left untreated or were stimulated with 10 nM CCL5 for 3 h in the presence of LiCl. Accumulated inositol phosphates were determined at 48 h postinfection by using anion-exchange chromatography as described above. The data shown are derived from seven independent experiments performed in duplicate and represent the means ± the SEM. (B) HFFs were infected with the above-described viruses at an MOI of 3. At 48 h postinfection, the effects of CCL5 was measured by labeling cells with Fluo-4 AM and analyzing calcium signaling after addition of 10 nM CCL5 using a FlexStation II fluorometer. The calcium traces are representative of at least three independent experiments performed in duplicate. (C) The peak calcium response after the addition of CCL5 is displayed graphically. The data shown are derived from eight independent experiments performed in duplicate and represent the means ± the SEM. n.s., not significant.
CX3CL1/Fractalkine functions as an “inverse agonist” to block constitutive US28-mediated PLC-β signaling but as an “agonist” to promote US28-mediated calcium release.
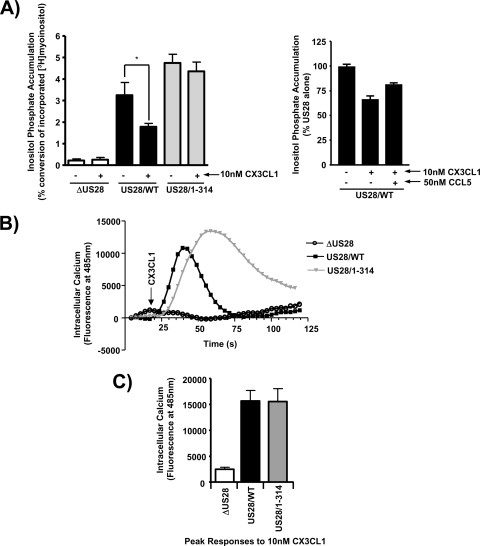
CX3CL1 has been demonstrated to function as an “inverse agonist” to partially block constitutive US28 signaling to PLC-β, but its effect on US28-mediated calcium release has not been reported (6, 7). Moreover, Waldhoer et al. reported that, in transfection studies, the deletion of the US28 carboxy-terminal regulatory domain enabled CX3CL1 to function not as an “inverse agonist” but as an agonist to enhance US28 signaling through PLC-β (53). Based on these interesting properties of CX3CL1 and the carboxy-terminal regulatory domain in transfected cells, we sought to analyze the effects of CX3CL1 on US28/WT and US28/1-314 signaling in HCMV-infected cells. HFFs were infected with US28/WT HCMV or US28/1-314 HCMV, and the PLC-β signaling activity was measured. Consistent with the reports of CX3CL1 behaving as an inverse agonist, US28/WT HCMV-infected cells treated with 10 nM CX3CL1 exhibited a decrease in PLC-β signaling activity (Fig. 7A, left panel) (6, 8, 25, 54). The “inverse agonist” effect of CX3CL1 can begin to be titrated out in the presence of excess CCL5, suggesting that the net effect on signaling in the presence of both chemokines would be an intermediate level between the chemokines alone (Fig. 7A, right panel). Unlike Waldhoer et al., who utilized a slightly different US28 carboxy-terminal mutant, we did not observe any agonistic properties of CX3CL1 toward US28/1-314 stimulated PLC-β activity (53). However, treatment of cells expressing US28/1-314 with 10 nM CX3CL1 were refractory to the effects of CX3CL1 as US28/1-314 stimulated PLC-β activity was the same as in untreated cells (Fig. 7A, left panel). Thus, the elements in US28 controlling the “inverse agonist” effect of CX3CL1 are contained within the carboxy terminus.
FIG. 7.
CX3CL1/Fractalkine exhibits both agonist and inverse agonist properties toward US28 in HCMV-infected cells. (A) HFFs were infected with HCMV ΔUS28, HCMV FLAG-US28/WT, or HCMV FLAG-US28/1-314 virus at an MOI of 0.1. Medium containing 1 μCi of [3H]myoinositol/ml was added 24 h postinfection. At 45 h postinfection, the cells were left untreated or stimulated with 10 nM CX3CL1 for 3 h in the presence of LiCl (left panel). HFFs similarly infected with FLAG-US28/WT HCMV were incubated with 10 nM CX3CL1 in the presence or absence of 50 nM CCL5 for 3 h in the presence of LiCl (right panel). Accumulated inositol phosphates were determined at 48 h postinfection by using anion-exchange chromatography as described above. The data shown are derived from at least four independent experiments performed in duplicate and represent the means ± the SEM. (B) HFFs were infected with the above-described viruses at an MOI of 3. At 48 h postinfection, the effects of CX3CL1 were measured by labeling cells with Fluo-4 AM and analyzing calcium signaling after addition of 10 nM CX3CL1 using a FlexStation II fluorometer. The calcium traces are representative of at least three independent experiments performed in duplicate. (C) The peak calcium response after the addition of CX3CL1 is displayed graphically. The data shown are derived from eight independent experiments performed in duplicate and represent the means ± the SEM.
We next sought to determine whether CX3CL1 could affect US28 stimulated calcium release. Interestingly, whereas CX3CL1 functions as an “inverse agonist” with regard to PLC-β activity (Fig. 7A), it functions as an agonist to promote the ability of US28/WT to drive calcium release (Fig. 7B). The half-maximal effective concentration for CX3CL1 in this assay is ∼2 nM, whereas 10 nM produces a maximal calcium response (see Fig. S3 in the supplemental material). Similar to the effects of CCL5, CX3CL1-stimulated calcium release was rapid and returned to baseline by ∼60 s. When the US28/1-314 mutant was assayed for its ability to stimulate calcium release in response to CX3CL1, we again observed an extended calcium signal in comparison to US28/WT that still retained 40% of the maximal activity at ∼100 s (Fig. 7B). CX3CL1 appears to be a partial agonist in comparison to CCL5 since the maximal change in intracellular calcium stimulated by CX3CL1 was 15,000 fluorescence units compared to 31,000 fluorescence units for CCL5 (Fig. 6C and 7C). Independent experiments directly comparing the efficacy of CX3CL1 and CCL5 confirmed the conclusion that CX3CL1 functions as a partial agonist compared to CCL5 in calcium assays (data not shown). The apparent “inverse agonist” effect of CX3CL1 with respect to PLC-β signaling activity (which is measured over a 3-h time period) is therefore probably not due to a conformational change in US28 that uncouples US28 from its cognate G-protein but rather to the ability of CX3CL1 to cause US28 internalization and degradation, as has been reported by Marsh and coworkers (13). Finally, the data indicate that the carboxy terminus controls the effects of CX3CL1 on both agonist-independent and agonist-dependent signaling by US28.
CCL5/RANTES and CX3CL1/Fractalkine binding to US28 stimulates calcium release via a pathway involving Gαq and PLC-β.
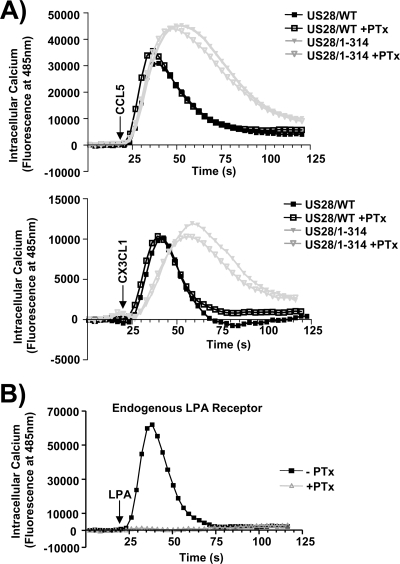
Although there is good evidence supporting the fact that US28 utilizes Gαq proteins to stimulate PLC-β in an agonist-independent manner, it remains unclear whether US28 agonists such as CCL5 and CX3CL1 cause a switch in G-protein usage from Gαq to Gαi, which then drives calcium release. In fact, there is some evidence suggesting that US28 stimulated calcium release may occur via Gαi proteins (2). To determine whether US28 couples to Gαq or Gαi to promote calcium release in HCMV-infected cells, we infected HFFs with US28/WT or US28/1-314 HCMVs and then analyzed the effects of CCL5 or CX3CL1 in cells that had been left untreated or treated overnight with PTx to inhibit Gαi proteins (21). US28/WT and US28/1-314 HCMV-infected cells were then stimulated with 10 nM CCL5 or CX3CL1, and the release of intracellular calcium was measured over time. Both CCL5 and CX3CL1 induced calcium release in a PTx-insensitive manner, indicating that the agonist-dependent calcium signaling utilizes Gαq proteins identical to the agonist-independent PLC-β activity (Fig. 8A). To ensure that the PTx was functioning to inhibit Gαi in our cells, we pretreated cells with PTx and then stimulated with them the Gαi-coupled agonist lysophosphatidic acid (LPA) and observed a complete block in LPA signaling, indicating that the Gαi in these cells was indeed inhibited (Fig. 8B).
FIG. 8.
CCL5 and CX3CL1 binding to US28 triggers calcium release by activating PTx-insensitive Gq proteins. (A) HFFs were infected with HCMV FLAG-US28/WT or HCMV FLAG-US28/1-314 viruses at an MOI of 3 and left either untreated (solid symbols) or treated overnight with 200 ng of PTx/ml (open symbols). At 48 h postinfection, cells were labeled with Fluo-4 AM and stimulated with 10 nM CCL5 (top panel) or 10 nM CX3CL1 (lower panel), and the calcium flux was measured by using a FlexStation II fluorometer. (B) To control for the effects of PTx, uninfected HFFs left untreated (closed symbols) or treated with 200 ng of PTx/ml (open symbols) were stimulated with 10 nM LPA, and the calcium flux was measured as described above. The calcium traces are representative of at least four independent experiments performed in duplicate.
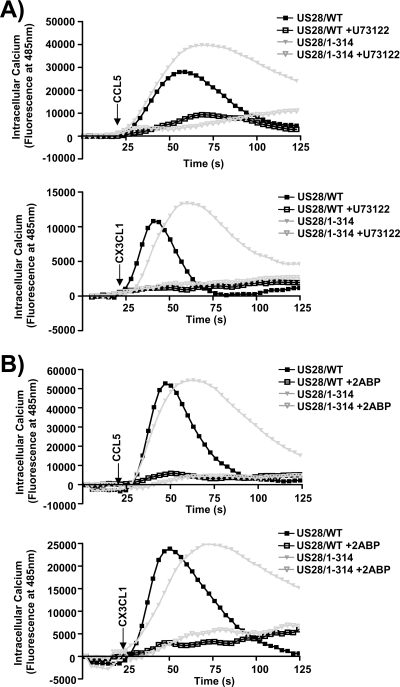
The classic pathway for Gq-coupled receptors to induce calcium release involves the sequential action of Gαq, PLC-β, inositol triphosphate (IP3), and IP3-gated calcium channels. Since US28 causes a constitutive activation of Gαq and PLC-β but does not stimulate calcium release until agonist is present, we next sought to determine whether US28 stimulation of calcium release does in fact involve PLC-β and IP3-gated calcium channels or whether US28 uses a divergent pathway. HFFs infected with US28/WT or US28/1-314 HCMVs were pretreated with the PLC-β inhibitor U73122 and then stimulated with CCL5 or CX3CL1 (Fig. 9A). Inhibition of PLC-β totally ablated the ability of CCL5 or CX3CL1 to activate US28-mediated calcium release. Similar experiments utilizing the IP3 channel inhibitor 2-amino-ethoxydiphenylborate (2-ABP) similarly demonstrated a requirement for IP3 and the IP3-gated channel in the US28 driven calcium response (Fig. 9B). Thus, CCL5 and CX3CL1 both promote calcium signaling through a seemingly identical pathway involving PLC-β/IP3. It remains unclear at this point why the agonist is required to drive calcium release if US28 can constitutively activate PLC-β and IP3. Our results suggest that the US28 agonists are important for perhaps controlling the subcellular distribution of active PLC-β/IP3 or providing a secondary signal that enables the accumulated IP3 to gate the calcium channel.
FIG. 9.
CCL5 and CX3CL1 binding to US28 triggers calcium release via a PLC-β/IP3 signaling pathway. HFFs were infected with HCMV FLAG-US28/WT or HCMV FLAG-US28/1-314 viruses at an MOI of 3. (A) At 48 h postinfection, cells were labeled with Fluo-4 AM in the absence (closed symbols) or presence (open symbols) of the PLC-β inhibitor U73122. Cells were then stimulated with 10 nM CCL5 (upper panel) or 10 nM CX3CL1 (lower panel) and analyzed by using a FlexStation II fluorometer. (B) At 48 h postinfection, cells were labeled with Fluo-4 AM in the absence (closed symbols) or presence (open symbols) of the IP3 channel blocker 2-ABP. Cells were then stimulated with 10 nM CCL5 (upper panel) or 10 nM CX3CL1 (lower panel) and analyzed by using a FlexStation II fluorometer. The calcium traces shown are representative of at least four independent experiments performed in duplicate.
DISCUSSION
Our results support a model in which US28 is targeted to the plasma membrane of HCMV-infected cells, where it productively engages Gαq and PLC-β in the absence of agonist resulting in high level accumulation of IP3. In the presence of chemokines such as CCL5/RANTES or CX3CL1/Fractalkine, US28 is further activated, allowing the Gq→PLC-β→IP3 axis to productively regulate calcium handling. The magnitude and duration of this signaling is tightly regulated by the presence of the carboxy-terminal regulatory domain (amino acids 315 to 354), which serves to attenuate US28 activity, thus preventing runaway levels of agonist-independent and agonist-dependent signaling.
There has been a significant level of interest regarding the role that the US28 carboxy terminus plays in regulating signal transduction since the carboxyl termini of most cellular GPCRs serve a critical function by regulating the intensity of the signal and enabling agonist-stimulated receptors to return to an inactive or ground-state (14, 29, 36, 40, 45, 53). We demonstrate for the first time that the US28 carboxy terminus does in fact regulate signaling in HCMV-infected cells since deletion of this domain results in an increase in the magnitude and duration of signal transduction. Although US28/1-314 does undergo a lower initial internalization rate than US28/WT in HCMV-infected cells, this difference is unlikely to contribute to the increased Ca2+ and PLC-β signaling observed with US28/1-314. The Ca2+ studies demonstrate extended signaling with US28/1-314 during the initial 120 s of the assay, during which only 3.2 and 7.4% of the US28/1-314 and US28/WT proteins are internalized. Moreover, since US28/1-314 and US28/WT exhibit similar levels of steady cell surface expression, the increased constitutive PLC-β signaling observed with the US28/1-314 in comparison to US28/WT (which is measured over a 3-h time period) is also unlikely to be affected by the difference in initial internalization rates. These findings point to the fact that the increased and prolonged signaling we observe with the US28/1-314 mutant in HCMV-infected cells is likely due to an inability of the US28/1-314 protein to interact with components of the desensitization machinery such as the GRK and β-arrestin proteins. Deletion of the carboxy terminus results in a US28 variant that is unable to recruit GRKs and β-arrestins, resulting in a receptor that is more productively coupled to G-proteins or, in other words, unable to be properly desensitized (36, 40). We have also obtained genetic support for the hypothesis that the βarrestin proteins play a critical role in regulating US28 signaling (see Fig. S4 and S5 in the supplemental material). Using wild-type mouse embryonic fibroblasts (MEFs), MEFs with Gq/11 deleted, and MEFs with β-arrestins 1 and 2 deleted, we assessed US28 signaling to PLC-β (26, 51). Interestingly, whereas this US28 signaling activity is totally dependent on the Gq proteins (see Fig. S4 in the supplemental material), US28 signaling is hyperactivated in cells with the β-arrestins genetically deleted (see Fig. S5 in the supplemental material). Taken together, the data indicate that the US28 viral GPCR utilizes the cellular desensitization machinery to regulate the intensity of signaling akin to its cellular relatives.
It is clear from our studies that the agonist-independent signaling of US28 through PLC-β does not necessarily lead to the activation of all of the classical targets of PLC-β (such as IP3-regulated calcium channel activity). The presence of agonists such as CCL5/RANTES and CX3CL1/Fractalkine then enables US28 to promote calcium release from intracellular stores. Using a combination of pharmacological inhibitors, we demonstrate that the agonist-induced US28 calcium flux in HCMV-infected cells does indeed occur via a pathway involving Gq→PLC-β→IP3 and is not the result of the agonist causing a switch in G protein coupling that could explain the requirement for the agonist. What then is the effect of the agonist? It is possible that the agonist causes an acute increase in a localized concentration of PLC-β generated IP3 that is able to gate calcium channels but is undetectable in whole-cell biochemical PLC-β activity assays (4, 11, 20). Conversely, the agonist may activate an as-yet-unknown intermediate that “sensitizes” the IP3 channels, thus allowing calcium release. In the case of cellular Gq-coupled receptors, their effects on PLC-β activity and on calcium handling are both dependent on agonist, and thus the function of this unknown intermediate in regulating calcium release may have gone undiscovered to this point. Moreover, it remains unknown at this point whether the agonist-independent US28→PLC-β activity directly leads to the activation of other PLC-β effectors (such as diacylglycerol) or whether US28 agonists are required similar to what was observed for calcium. In this regard, the US28 viral receptor provides a unique reagent to revisit this important signaling pathway between PLC-β and its effectors.
In several studies, the CX3C chemokine CX3CL1/Fractalkine has been suggested to behave as an “inverse agonist” toward US28 since it can significantly attenuate the constitutive signaling of US28 with respect to PLC-β activity (6, 8, 53). Although we observe the same results in PLC-β activity assays, we demonstrate for the first time that CX3CL1/Fractalkine can function as an agonist in promoting calcium release. Since the calcium assay measures acute signaling in real time, it is arguably a more relevant way to differentiate between agonists and inverse agonists. The PLC-β activity assay measures constitutive signaling over a 3-h period and thus would be subjected to additional regulatory events such as targeting of US28 to lysosomes, ultimately resulting in receptor degradation. Thus, over the longer time periods utilized in PLC-β assays, the inhibitory effects of molecules such as CX3CL1/Fractalkine could be on receptor turnover and not on the acute conversion of the receptor from an active to inactive conformation. In fact, CX3CL1/Fractalkine has been shown to induce the turnover of US28, thus providing a plausible explanation for the apparent activity of CX3CL1 in US28/PLC-β assays (13).
Interestingly, the carboxy-terminal regulatory domain is required for the inhibitory effects of CX3CL1/Fractalkine on US28 stimulated PLC-β activity, largely in agreement with the results observed by Waldhoer et al. (53) in transfected cells. This ability of the carboxy-terminal domain to exert the inhibitory effects of CX3CL1/Fractalkine toward US28 PLC-β activity may be due to the recruitment of sorting proteins such as GPCR-activated sorting protein or sorting nexin 1, which bind to US28 and may target it to be degraded in the lysosome (19). Thus, deletion of this domain could alter normal agonist-induced trafficking patterns and therefore would appear to be resistant to the inhibitory effects of CX3CL1. It will be interesting to assess the function of the recently described US28 small molecule inhibitor VUF2274 in its regulation of US28 induced calcium signaling and to determine whether this is influenced by the carboxy-terminal region (7, 22). Since CX3CL1/Fractalkine functions as an acute agonist for calcium but as an inhibitor of PLC-β activity, it will be interesting to determine whether the US28 inhibitor VUF2274 also functions in a similar manner. Nonetheless, it will be important to more fully examine the effects of CX3CL1 and VUF2274 on a wide variety of signaling activities prior to investigating whether these US28 “inhibitors” could be used as models for potential novel drug design (7, 22).
Our results clearly indicate that US28 can constitutively turn on PLC-β signaling activity but also indicate that this signaling is not necessarily transduced downstream to effectors such as calcium unless agonist is present. Thus, it is essential to continue to explore the potential effects of agonist on US28 activity since many of the downstream events may not occur as a result of the constitutive PLC-β activity. Hertel and Mocarski analyzed the effects of US28 on global transcriptional changes during HCMV infection and concluded that US28 itself did not contribute to the transcriptional effects promoted by HCMV (18). Although these experiments provided a real insight into many of the transcriptional changes induced by HCMV, these studies only accounted for constitutive signaling by US28 and do not take into account any effects of chemokine stimulation, which may be of central importance regarding US28 signaling activity in vivo. The US28/1-314 mutant, which fails to be rapidly desensitized, may be a useful reagent in identifying US28 regulated genes as it exhibits an expanded signaling repertoire.
Using a combination of approaches, we demonstrate that US28 is desensitized by the cellular machinery, allowing for an appropriate level of signaling activity in a physiological setting. Regulation of signaling activity is a central process in mammalian cells since it prevents aberrantly high levels of signaling that can lead to maladaptive responses in the cell exhibiting the aberrant signal (29, 45, 52, 55). This ability to undergo regulation by desensitization would be extremely important for viral receptors such as US28 that appear to exhibit high levels of constitutive or agonist-independent signaling to at least a subset of pathways. Thus, while US28 may have lost some ability to be regulated by agonist, this viral GPCR has maintained the ability to be regulated by the cellular desensitization machinery, similar to cellular GPCRs. Moreover, we demonstrate that CX3CL1/Fractalkine functions not only as an inhibitor of constitutive US28 PLC-β signaling but also as an agonist to promote calcium release after an acute stimulation. Taken together, our results provide a new insight into the regulation of US28 signaling in virus-infected cells and highlight the fact that even highly “constitutively” active GPCRs such as US28 exhibit intricate levels of regulatory control not only at the level of ligand/receptor interaction but also at the level of the receptor-effector interaction.
Supplementary Material
Acknowledgments
We thank G. Hahn for FIX-BAC DNA, B. Wanner for recombination plasmids, and J. Shanley for UL44 antibody. We thank D. McGraw for help with the FlexStation II.
This study was supported by an American Heart Association Predoctoral Fellowship 0615196B awarded to M.P.S. and by National Institutes of Health grant R01 AI058159 and March of Dimes grant 5-FY-04-17 to W.E.M.
Footnotes
Published ahead of print on 15 July 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ahuja, S. K., and P. M. Murphy. 1993. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J. Biol. Chem. 268:20691-20694. [PubMed] [Google Scholar]
- 2.Billstrom, M. A., G. L. Johnson, N. J. Avdi, and G. S. Worthen. 1998. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J. Virol. 72:5535-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodaghi, B., T. R. Jones, D. Zipeto, C. Vita, L. Sun, L. Laurent, F. Arenzana-Seisdedos, J. L. Virelizier, and S. Michelson. 1998. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J. Exp. Med. 188:855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bootman, M., E. Niggli, M. Berridge, and P. Lipp. 1997. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J. Physiol. 499(Pt. 2):307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boppana, S. B., R. F. Pass, W. J. Britt, S. Stagno, and C. A. Alford. 1992. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr. Infect. Dis. J. 11:93-99. [DOI] [PubMed] [Google Scholar]
- 6.Casarosa, P., R. A. Bakker, D. Verzijl, M. Navis, H. Timmerman, R. Leurs, and M. J. Smit. 2001. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J. Biol. Chem. 276:1133-1137. [DOI] [PubMed] [Google Scholar]
- 7.Casarosa, P., W. M. Menge, R. Minisini, C. Otto, J. van Heteren, A. Jongejan, H. Timmerman, B. Moepps, F. Kirchhoff, T. Mertens, M. J. Smit, and R. Leurs. 2003. Identification of the first nonpeptidergic inverse agonist for a constitutively active viral-encoded G protein-coupled receptor. J. Biol. Chem. 278:5172-5178. [DOI] [PubMed] [Google Scholar]
- 8.Casarosa, P., M. Waldhoer, P. J. LiWang, H. F. Vischer, T. Kledal, H. Timmerman, T. W. Schwartz, M. J. Smit, and R. Leurs. 2005. CC and CX3C chemokines differentially interact with the N terminus of the human cytomegalovirus-encoded US28 receptor. J. Biol. Chem. 280:3275-3285. [DOI] [PubMed] [Google Scholar]
- 9.Case, R., E. Sharp, T. Benned-Jensen, M. M. Rosenkilde, N. Davis-Poynter, and H. E. Farrell. 2008. Functional analysis of the murine cytomegalovirus chemokine receptor homologue M33: ablation of constitutive signaling is associated with an attenuated phenotype in vivo. J. Virol. 82:1884-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dargan, S. L., B. Schwaller, and I. Parker. 2004. Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocytes by Ca2+-binding proteins. J. Physiol. 556:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis-Poynter, N. J., D. M. Lynch, H. Vally, G. R. Shellam, W. D. Rawlinson, B. G. Barrell, and H. E. Farrell. 1997. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J. Virol. 71:1521-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraile-Ramos, A., T. N. Kledal, A. Pelchen-Matthews, K. Bowers, T. W. Schwartz, and M. Marsh. 2001. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 12:1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gainetdinov, R. R., R. T. Premont, L. M. Bohn, R. J. Lefkowitz, and M. G. Caron. 2004. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 27:107-144. [DOI] [PubMed] [Google Scholar]
- 15.Gao, J. L., and P. M. Murphy. 1994. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J. Biol. Chem. 269:28539-28542. [PubMed] [Google Scholar]
- 16.Hahn, G., H. Khan, F. Baldanti, U. H. Koszinowski, M. G. Revello, and G. Gerna. 2002. The human cytomegalovirus ribonucleotide reductase homolog UL45 is dispensable for growth in endothelial cells, as determined by a BAC-cloned clinical isolate of human cytomegalovirus with preserved wild-type characteristics. J. Virol. 76:9551-9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebart, H., and H. Einsele. 1998. Diagnosis and treatment of cytomegalovirus infection. Curr. Opin. Hematol. 5:483-487. [DOI] [PubMed] [Google Scholar]
- 18.Hertel, L., and E. S. Mocarski. 2004. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of pseudomitosis independent of US28 function. J. Virol. 78:11988-12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heydorn, A., B. P. Sondergaard, B. Ersboll, B. Holst, F. C. Nielsen, C. R. Haft, J. Whistler, and T. W. Schwartz. 2004. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J. Biol. Chem. 279:54291-54303. [DOI] [PubMed] [Google Scholar]
- 20.Horne, J. H., and T. Meyer. 1997. Elementary calcium-release units induced by inositol trisphosphate. Science 276:1690-1693. [DOI] [PubMed] [Google Scholar]
- 21.Hsia, J. A., J. Moss, E. L. Hewlett, and M. Vaughan. 1984. ADP-ribosylation of adenylate cyclase by pertussis toxin: effects on inhibitory agonist binding. J. Biol. Chem. 259:1086-1090. [PubMed] [Google Scholar]
- 22.Hulshof, J. W., P. Casarosa, W. M. Menge, L. M. Kuusisto, H. van der Goot, M. J. Smit, I. J. de Esch, and R. Leurs. 2005. Synthesis and structure-activity relationship of the first nonpeptidergic inverse agonists for the human cytomegalovirus encoded chemokine receptor US28. J. Med. Chem. 48:6461-6471. [DOI] [PubMed] [Google Scholar]
- 23.Isegawa, Y., Z. Ping, K. Nakano, N. Sugimoto, and K. Yamanishi. 1998. Human herpesvirus 6 open reading frame U12 encodes a functional beta-chemokine receptor. J. Virol. 72:6104-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna, R., and D. J. Diamond. 2006. Human cytomegalovirus vaccine: time to look for alternative options. Trends. Mol. Med. 12:26-33. [DOI] [PubMed] [Google Scholar]
- 25.Kledal, T. N., M. M. Rosenkilde, and T. W. Schwartz. 1998. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS. Lett. 441:209-214. [DOI] [PubMed] [Google Scholar]
- 26.Kohout, T. A., F. S. Lin, S. J. Perry, D. A. Conner, and R. J. Lefkowitz. 2001. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc. Natl. Acad. Sci. USA 98:1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn, D. E., C. J. Beall, and P. E. Kolattukudy. 1995. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem. Biophys. Res. Commun. 211:325-330. [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitz, R. J. 1998. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 273:18677-18680. [DOI] [PubMed] [Google Scholar]
- 29.Lin, F., and E. C. Butcher. 2008. Modeling the role of homologous receptor desensitization in cell gradient sensing. J. Immunol. 181:8335-8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljungman, P., P. Griffiths, and C. Paya. 2002. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin. Infect. Dis. 34:1094-1097. [DOI] [PubMed] [Google Scholar]
- 31.Marchese, A., C. Chen, Y. M. Kim, and J. L. Benovic. 2003. The ins and outs of G protein-coupled receptor trafficking. Trends. Biochem. Sci. 28:369-376. [DOI] [PubMed] [Google Scholar]
- 32.Margulies, B. J., H. Browne, and W. Gibson. 1996. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology 225:111-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maussang, D., D. Verzijl, M. van Walsum, R. Leurs, J. Holl, O. Pleskoff, D. Michel, G. A. van Dongen, and M. J. Smit. 2006. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 103:13068-13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean, K. A., P. J. Holst, L. Martini, T. W. Schwartz, and M. M. Rosenkilde. 2004. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology 325:241-251. [DOI] [PubMed] [Google Scholar]
- 35.Melnychuk, R. M., D. N. Streblow, P. P. Smith, A. J. Hirsch, D. Pancheva, and J. A. Nelson. 2004. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Gα12. J. Virol. 78:8382-8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, W. E., D. A. Houtz, C. D. Nelson, P. E. Kolattukudy, and R. J. Lefkowitz. 2003. G-protein-coupled receptor (GPCR) kinase phosphorylation and β-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J. Biol. Chem. 278:21663-21671. [DOI] [PubMed] [Google Scholar]
- 37.Milne, R. S., C. Mattick, L. Nicholson, P. Devaraj, A. Alcami, and U. A. Gompels. 2000. RANTES binding and downregulation by a novel human herpesvirus-6 beta chemokine receptor. J. Immunol. 164:2396-2404. [DOI] [PubMed] [Google Scholar]
- 38.Minisini, R., C. Tulone, A. Luske, D. Michel, T. Mertens, P. Gierschik, and B. Moepps. 2003. Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28. J. Virol. 77:4489-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2973. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 40.Mokros, T., A. Rehm, J. Droese, M. Oppermann, M. Lipp, and U. E. Hopken. 2002. Surface expression and endocytosis of the human cytomegalovirus-encoded chemokine receptor US28 is regulated by agonist-independent phosphorylation. J. Biol. Chem. 277:45122-45128. [DOI] [PubMed] [Google Scholar]
- 41.Moore, C. A., S. K. Milano, and J. L. Benovic. 2007. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 69:451-482. [DOI] [PubMed] [Google Scholar]
- 42.Neote, K., D. DiGregorio, J. Y. Mak, R. Horuk, and T. J. Schall. 1993. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell 72:415-425. [DOI] [PubMed] [Google Scholar]
- 43.Pass, R. F. 2001. Cytomegaloviruses, p. 2675-2706. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 44.Pleskoff, O., P. Casarosa, L. Verneuil, F. Ainoun, P. Beisser, M. Smit, R. Leurs, P. Schneider, S. Michelson, and J. C. Ameisen. 2005. The human cytomegalovirus-encoded chemokine receptor US28 induces caspase-dependent apoptosis. FEBS. J. 272:4163-4177. [DOI] [PubMed] [Google Scholar]
- 45.Premont, R. T., and R. R. Gainetdinov. 2007. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol. 69:511-534. [DOI] [PubMed] [Google Scholar]
- 46.Stagno, S., R. F. Pass, M. E. Dworsky, R. E. Henderson, E. G. Moore, P. D. Walton, and C. A. Alford. 1982. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 306:945-949. [DOI] [PubMed] [Google Scholar]
- 47.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 48.Streblow, D. N., J. Vomaske, P. Smith, R. Melnychuk, L. Hall, D. Pancheva, M. Smit, P. Casarosa, D. D. Schlaepfer, and J. A. Nelson. 2003. Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J. Biol. Chem. 278:50456-50465. [DOI] [PubMed] [Google Scholar]
- 49.Stropes, M. P., and W. E. Miller. 2008. Functional analysis of human cytomegalovirus pUS28 mutants in infected cells. J. Gen. Virol. 89:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vieira, J., T. J. Schall, L. Corey, and A. P. Geballe. 1998. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J. Virol. 72:8158-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogt, S., R. Grosse, G. Schultz, and S. Offermanns. 2003. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J. Biol. Chem. 278:28743-28749. [DOI] [PubMed] [Google Scholar]
- 52.Vroon, A., C. J. Heijnen, and A. Kavelaars. 2006. GRKs and arrestins: regulators of migration and inflammation. J. Leukoc. Biol. 80:1214-1221. [DOI] [PubMed] [Google Scholar]
- 53.Waldhoer, M., P. Casarosa, M. M. Rosenkilde, M. J. Smit, R. Leurs, J. L. Whistler, and T. W. Schwartz. 2003. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J. Biol. Chem. 278:19473-19482. [DOI] [PubMed] [Google Scholar]
- 54.Waldhoer, M., T. N. Kledal, H. Farrell, and T. W. Schwartz. 2002. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J. Virol. 76:8161-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao, R. P., W. Zhu, M. Zheng, C. Cao, Y. Zhang, E. G. Lakatta, and Q. Han. 2006. Subtype-specific α1- and β-adrenoceptor signaling in the heart. Trends Pharmacol. Sci. 27:330-337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.