Abstract
Increased expression of PSMA, a differentiation antigen with folate hydrolase activity, is an independent marker of prostate cancer progression. Mice expressing moderate levels of human PSMA in their prostate develop PIN-like lesions by 9 months. The aim of this study was to determine whether PSMA is involved in prostate carcinogenesis and progression; and if so the possible mechanism by which PSMA may exert its effects. Utilizing prostates from PSMA-transgenic mice, we developed a tissue recombinant model which exhibits small atypical glands with features of adenocarcinoma. This was not observed in tissue recombinants that were composed of prostate tissues from the wild-type siblings. Cells from PSMA-transgenic tissue recombinants have the ability to form colonies in semi-solid agar. PSMA may facilitate this phenotype by increasing the cells’ invasive ability. Ectopic PSMA expression on PC-3 cells increased cells’ invasive capacity in in vitro invasion assays, which could be competed out by folic acid. These results suggest PSMA facilitates the development of prostate cancer, and the invasive ability of these cells may be modulated by folate levels. These findings demonstrate a novel mechanism that may contribute to the known role of folate in cancer prevention, and may lead to the use of PSMA inhibitors as novel chemopreventive agents for prostate cancer. Moreover, our model should prove useful for further dissecting pathways involved in prostate carcinogenesis and progression.
Keywords: PSMA, prostate, recombination, folate, invasion
Introduction
Prostate cancer is the most commonly diagnosed cancer and second leading cause of cancer death in American men (1). When compared with all other cancers, the incidence of prostate carcinoma increases most rapidly with age (2). Successful management and elimination of recurrent and metastatic prostate cancer continues to present a major clinical challenge (3).
PSMA, a type II membrane glycoprotein, is expressed in the human prostate and at lower levels in the brain, spine, peripheral nervous system, kidney, small intestine and the salivary gland (4). PSMA is expressed in the luminal epithelial cells of normal and hyperplastic prostatic tissues, Prostatic Intraepithelial Neoplasia (PIN), and in primary and metastatic prostate cancers (5). Its expression in adenocarcinoma correlates with stage of disease and Gleason score (6, 7). PSMA expression is also higher in cancer cells from hormone-refractory patients (7, 8). Increased PSMA expression is an independent marker of recurrence (9, 10). Interestingly, endothelial cells of the neovasculature of almost all solid tumors express PSMA, but not cells in the neovasculature associated with normal tissues (11, 12, 13, 14).
PSMA has glutamate carboxypeptidase II activity that cleaves α-linked glutamate from N-acetylaspartyl glutamate (NAALADase activity) (15) and γ-linked glutamates from polyglutamated folates sequentially (folate hydrolase activity) (16, 17). Prostate cancer tissues have significantly elevated levels of PSMA enzymatic activity as compared with normal prostate and BPH tissues (18). Increase in both expression and enzymatic activity of PSMA in aggressive prostate tumors imply that PSMA provides a selective advantage for cells expressing it, thereby contributing to the development and progression of prostate cancer.
Transgenic mice have been generated that express human PSMA in their ventral, lateral and dorsal prostate lobes under the control of the human Prostate Specific Antigen (PSA) promoter and enhancer (19). The level of PSMA expressed in the prostates of the transgenic mice is comparable to that in normal human prostate tissues. The level of folate hydrolase activity in the prostate of the transgenic mice is similar to the endogenous level seen in the murine brain, and is significantly greater than in the prostates of non-transgenic mice. Characterization of PSMA transgenic mice found that their prostates started to develop PIN-like lesions as early as 21 weeks of age, with the majority (above 60%) developing PIN-like lesions by 9 months of age. Prostates of the wild-type siblings did not exhibit PIN lesions (19). The presence of PIN-like lesions in PSMA transgenic mice suggests that PSMA plays a role in the control of cell division or transformation, and as such the PSMA-transgenic mouse could be a suitable model for examining potential causes of prostate carcinogenesis. However, the length of time for PIN-like lesions to arise in the majority of animals is too long for the model to be cost-effective, and thus far, the model has not progressed from PIN lesions to cancer.
The aim of this study was to determine if PSMA is involved in prostate carcinogenesis and progression. We used the prostatic reconstruction model (20) comprising of rat urogenital mesenchyme (rUGM) and prostate tissues from PSMA-transgenic mice or their wild-type siblings grafted beneath the renal capsule of immunodeficient mouse hosts and given pharmacological doses of hormones to achieve our objective. Similar models have been used to show that the absence of Rb gene may predispose prostatic epithelial cells to carcinogenesis (21); and to drive PIN lesions in the prostates of Nkx3.1 knockout mice to a progressively severe histopathological phenotype (22). Using this model we were able to elicit carcinogenesis in a timely manner and significantly advance the understanding of the role that PSMA plays in prostate cancer.
We also explored the mechanism by which PSMA plays a role in prostate cancer. Previously, we showed that PSMA provides a growth advantage for prostate cancer cells in media containing low and physiological folate levels (23) Ghosh et al., found that PSMA-expressing PC-3 cells have reduced invasive capability in RPMI-1640 media (24). However, this media contains about 2.3µM of folic acid, which is about 100 times the level found at physiological levels. Therefore, we examined the invasive ability of PC-3 cells that do not express PSMA and those that express PSMA in physiologically relevant folate levels.
Materials and Methods
PSMA Transgenic Mouse Prostate Tissues
Animals were housed in the University of Pittsburgh at the Department of Urology. All animal experiments were approved by and followed the guidelines set out by Institutional Animal Care and Use Committee (IACUC). PSMA-transgenic mice and their wild-type siblings were kindly provided by Dr. Warren Heston (The Cleveland Clinic, Cleveland, OH). Mice (age matched, 27–33 weeks old) were killed and prostates were harvested and microdissected to separate the prostate lobes.
Tissue Separation, Recombination, and Grafting
Rat urogential sinuses were removed from 18-day embryonic fetuses of pregnant Sprague Dawley rats (Taconic) and separated into epithelial and mesenchyme components as previously described (20). Prostate lobes from PSMA-transgenic mice and wild-type siblings were cut into small (0.2–0.5 mm) pieces and combined with 250,000 rUGM cells in 40µL neutralized type I rat tail collagen gels as previously described (25). The gels of PSMA-transgenic or wild-type prostate tissue recombinants were incubated overnight in RPMI 1640 media (Cambrex Corp.) containing 5% FBS (Atlanta Biological) and 10−8M testosterone (Sigma). Tissue recombinants were grafted beneath the renal capsule of adult male nude (Nu/Nu) CD1 mice (Charles River Laboratories) as described previously (20). UGM alone was grafted to determine contamination from urogenital epithelium.
Host nude mice carrying PSMA-transgenic or wild-type prostate tissue recombinants were treated hormonally at the time of grafting by implanting subcutaneously a Silastic capsule (1.54 mm inside diameter and 3.18 mm outside diameter (Dow-Corning Co.)) tightly packed with 25 mg of testosterone propionate (T) and 2.5 mg of 17β-estradiol benzoate (E2) (Sigma). Eight weeks post-grafting, host nude mice were killed and each of tissue recombinant was carefully dissected from the host kidney, weighed, and cut into small pieces (0.5–2 mm). Tissue pieces were either paraffin embedded or recombined with fresh rUGM cells in collagen gels and then re-grafted for a further 8 weeks under the renal capsule of male nude mice. After a total of 16 weeks under the renal capsule, the tissue recombinants were recombined with fresh rUGM to again grown under the renal capsule of male nude mouse hosts for a further 8 weeks. Animal were treated hormonally with fresh T&E2 upon each sequential serial grafting.
Histopathology and Hoechst 33258 Dye Staining
Tissue sections were stained with H&E for histopathology; and Hoechst dye 33258 (Calbiochem) to confirm that the epithelium was of mouse origin and the stroma of rat origin (26). H&E and CK14 stained slides were examined blindly by a pathologist to determine the incidence of normal, hyperplasia, PIN or carcinoma. The most severe histological phenotype in each tissue recombinant was recorded. PIN-like lesions were scored blindly as 1+, 2+ or 3+ in increasing severity based on their glandular, architectural and cytological changes by a pathologist (Dr. A. Parwani).
Immunohistochemical Staining and Proliferation Index
Tissue sections were stained using the Vector® M.O.M™ Peroxidase Kit (Vector Laboratories). Non-immune mouse IgG alone served as the control. Primary antibodies include anti-PSMA (PEQ226.5, Hybritech Inc.), anti-Cytokeratin 18 (CK18, clone ks18.04, Research Diagnostics Inc.), anti-CK14 (clone LL001, Abcam), anti-E-cadherin (clone 36, BD Transduction), anti-smooth muscle α-actin (clone 1A4, Sigma), and anti-Ki-67 (clone B56, Research Diagnostics Inc.). Sections were developed with Vector® NovaRED™ Substrate Kit (Vector Laboratories). Proliferation index was compiled by manually counting total epithelial nuclei and epithelial nuclei showing positive immunoreactivity against Ki-67 in microscopic fields. Six to 15 samples were examined using two fields per sample. All photomicrographs were taken using Zeiss Axioplan 2 imaging microscope with an Axiocam and an Axio-Vision software.
Anchorage-Independent Growth
Tissue recombinants (20mg) that had been grafted under the kidney capsule for 24 weeks were digested with 108U collagenase for at least 4 hours at 37°C and seeded in 6-well plates in a double layer agar culture system. The underlay contained culture media with a final agar concentration of 0.6% (Difco Laboratories) and the overlay contained culture media with a final agar concentration of 0.3%. The dishes were incubated at 37°C in a humidified atmosphere and colonies of greater than 30 cells were counted after 21 days. Photomicrographs were captured on a Nikon T1-SM microscope.
Matrigel Invasion Assay
PC-3 cells transfected with pLNCX2-PSMA vector or pLNCX2 control vector were maintained in RPMI 1640 medium (2.3µM folic acid, referred to as “high folate” (HF)) supplemented with 10% fetal bovine serum (FBS, 1.8–2ng/mL), L-glutamine (2mM), and penicillin/streptomycin. Folate-free RPMI 1640 medium supplemented with 10% FBS and penicillin/streptomycin was referred to as ‘low folate’ (1nM folic acid, LF) medium. Cell invasion assay was performed using the BD BioCoat Matrigel Invasion Chambers (BD Biosciences) as per manufacturer’s instructions. Folate-free RPMI 1640 medium supplemented with 10% FBS, ~25nM HF medium, L-glutamine, and penicillin and streptomycin was referred to as “physiological folate” (25nM folic acid, PF) medium (27). Approximately 2.5×104 cells were seeded onto each matrigel-coated or control (uncoated) (8µm pore) inserts. The inserts contained either high folate or low folate medium without FBS whereas the wells were filled with either high folate or low folate medium containing 10% FBS so that the FBS act as the chemoattractant. The chambers were incubated for 22 hours at 37°C in a humidified atmosphere of 5% CO2. After incubation, the non-invading cells were removed from the upper surface of the membrane by scrubbing with a cotton-tipped swab. The cells that invaded into the lower compartment were stained with 0.5% Crystal Violet/20% methanol and counted. All experiments were done in triplicates. This assay was performed on two different clones of vector-only or PSMA vector transfected PC-3 cells. Data is expressed as the percent invasion through the Matrigel Matrix membrane relative to the migration through the control membrane.
Statistical Methods
Statistical analysis on wet weight and proliferation index of the tissue recombinants were done using the Student’s t-test. Wilcoxon-Mann-Whitney test was used to analyze incidence of PIN 1+, 2+ and 3+ in wild-type and PSMA-transgenic tissue recombinants. The Fisher’s exact test was used to analyze the incidence of PIN and foci with features of adenocarcinoma in wild-type and PSMA-transgenic tissue recombinants. The Jonckheere-Terpstra test was used to determine whether there is histopathological progression with serial grafting of the tissue recombinants.
Results
To test if PSMA is involved in prostate carcinogenesis, we grafted tissue recombinants consisting of PSMA-positive or non-transgenic (wild-type) prostate tissues with rUGM under the kidney capsule of host mice also implanted with Silastic tubes containing T&E2. It has previously been demonstrated that implantation of T&E2 filled silastic capsules into nude mice significantly increases their plasma testosterone levels at 1 month and estradiol levels at 2 and 4 months post implantation (28). Tissue recombinants were harvested after 8, 16 and 24 weeks under the kidney capsule. At each time point, the rUGM control grafts weighed significantly less (p<0.05) than recombinants that included PSMA-transgenic or wild-type prostate tissues (Table 1). There was no significant difference in the wet weights between PSMA-transgenic and wild-type tissue recombinants, however, the wet weights of both declined with passage (Table 1). The wild-type recombinants grafted for 8 weeks had a larger mean wet weight (103.2mg ± 5.2) than recombinants grown for 16 weeks (70.8mg ± 11.4), although this did not reach statistical significance (p>0.05). By 24 weeks, the mean wet weight (57.3mg ± 8.3) had decreased even further, resulting in a statistically significant difference in size between wild-type tissue recombinants at week 8 and week 24 (p<0.01). Similarly, for PSMA-transgenic tissue recombinants, the mean wet weight at 8 weeks (90.2mg ± 7.4) was significantly larger than the mean wet weight at 16 weeks (59.6mg ± 6.5) (p<0.01) and at 24 weeks (62.8mg ± 8.7) (p<0.05). The decline in wet weights with serial grafting may be due to the limited proliferative capacity of the majority of cells in the tissue recombinants. At no time did the tissue recombinants grow beyond the immediate area of the renal capsule and invade the host kidney.
Table 1.
Wet weights of rat UGM alone, wild-type and PSMA-transgenic prostate tissue recombinants after 8, 16 and 24 weeks of kidney grafting in intact male nude host mice treated with testosterone and estradiol. There was no difference in wet weights of wild-type and PSMA-transgenic tissue recombinants. Wet weights of both tissue recombinants declined significantly with serial grafting.
| rUGM only | Wild-type tissue recombinants | PSMA-transgenic tissue recombinants | |||
|---|---|---|---|---|---|
| 8 weeks | 19.2 ± 4 mg (11) | 103.2 ± 5.2 mg (9) | 90.2 ± 7.4 mg (20) | ||
| 16 weeks | 30.4 ± 5.2 mg (7) | 70.8 ± 11.4 mg (10) | 59.6 ± 6.5 mg (26)+ | ||
| 24 weeks | 19.7 ± 3.9 mg (8) | 57.3 ± 8.3 mg (27)+ | 62.8 ± 8.7 mg (32)* | ||
Data are reported as mean ± SE (n)
denotes p<0.01
denotes p<0.05 by Student’s t-test.
Both wild-type and PSMA-transgenic tissue recombinants formed large masses of well-differentiated prostatic glands surrounded by dense sheets of stromal cells when grown for 8, 16 and 24 weeks under the renal capsule (Figure 1A, left). As determined by Hoechst 33258 nuclear staining, the epithelial cells were of mouse origin whereas the stroma was mostly of rat origin (Figure 1A, right). Their genotypes of wild-type and PSMA-transgenic tissue recombinants were confirmed by staining with anti-human PSMA antibody (Figure 1B, top left and top right). Prostatic glands were lined by glandular epithelial cells that expressed CK18 (Figure 1B, bottom left) and surrounded by stromal cells that expressed smooth muscle α-actin (Figure 1B, bottom right).
Figure 1.
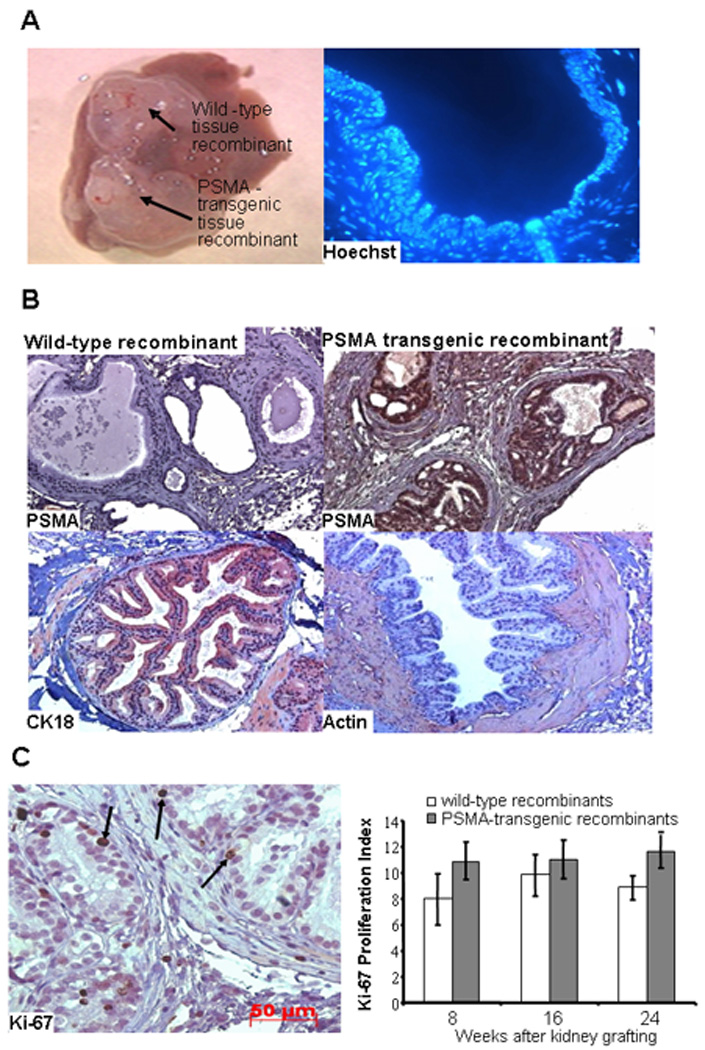
Histology and proliferation index of wild-type and PSMA-transgenic tissue recombinants. The urogenital mesenchyme microdissected from embryonic day 18 rat fetuses were combined with prostates from either wild-type or PSMA-transgenic tissues and grafted underneath the kidney capsules. A, Wild-type and PSMA-transgenic tissue recombinants harvested from a representative host mouse showing size, 8 weeks after kidney grafting (left). Hoechst 33258 staining of paraffin embedded tissue recombinants show that the epithelial cells were of mouse origin because they contained several small discrete intranuclear fluorescent bodies whereas their absence in stromal cells indicates that they are of rat origin (right). B, Immunohistochemical staining of paraffin embedded wild-type (top left) and PSMA-transgenic (top right) tissue recombinants with monoclonal anti-mouse PSMA antibody show PSMA expression only in PSMA-transgenic recombinants. Immunohistochemical staining of paraffin embedded tissue recombinants with CK-18 and smooth muscle α-actin monoclonal antibodies show CK18-expression in luminal epithelial cells (bottom left) and α-actin expression in the stromal cells surrounding glandular structures (bottom right). Photomicrographs were captured at 20× magnification. C, Immunohistochemical staining of paraffin embedded tissue recombinants with an anti-mouse Ki-67 monoclonal antibody show strong brown nuclear staining of cells undergoing proliferation. Arrows point to Ki-67-positive cells (left). The cells positive for Ki-67 were counted by monitoring at least 200 luminal epithelial cells for normal, PIN and carcinoma lesions in multiple regions of the same sample (right). There was no difference in the proliferation index of wild-type and PSMA-transgenic tissue recombinants grafted beneath the kidney capsule of intact nude mice hosts treated with T&E2 for 8, 16 and 24 weeks. Data are reported as mean ± SE (n = 7) by Student t-test. Photomicrograph was captured at 40× magnification.
We determined the proliferation index of the recombinant tissue by dividing the number of epithelial cells with Ki-67-stained nuclei by the total number of epithelial cells (Figure 1C, left). As shown in Figure 1C right, the mean proliferation index of PSMA-transgenic tissue recombinants was greater than that of wild-type tissue recombinants at week 8, 16 and 24, although this did not reach statistical significance.
Analysis of H&E and CK14-staining of PSMA-transgenic tissue recombinants revealed the presence of small atypical glands with features of adenocarcinoma at 16 and 24 weeks (see arrows in Figure 2). These recombinants also displayed hyperplastic ducts and PIN-like lesions at 8, 16 and 24 weeks (Figure 2). Ducts with nuclear stratification and/or crowding and rare CK14-expressing basal cells were classified as PIN lesions, while adenocarcinoma were defined as small atypical glands with prominent nuclei and nucleoli as well as absence of basal cells as evidenced by negative CK14-staining. PIN-like lesions and some of the small atypical glands expressed E-cadherin along adjacent epithelial cell membranes (Figure 2). However, other small atypical glands displayed abnormal and variable (weak membrane or cytoplasmic) E-cadherin staining (see arrows in Figure 2).
Figure 2.
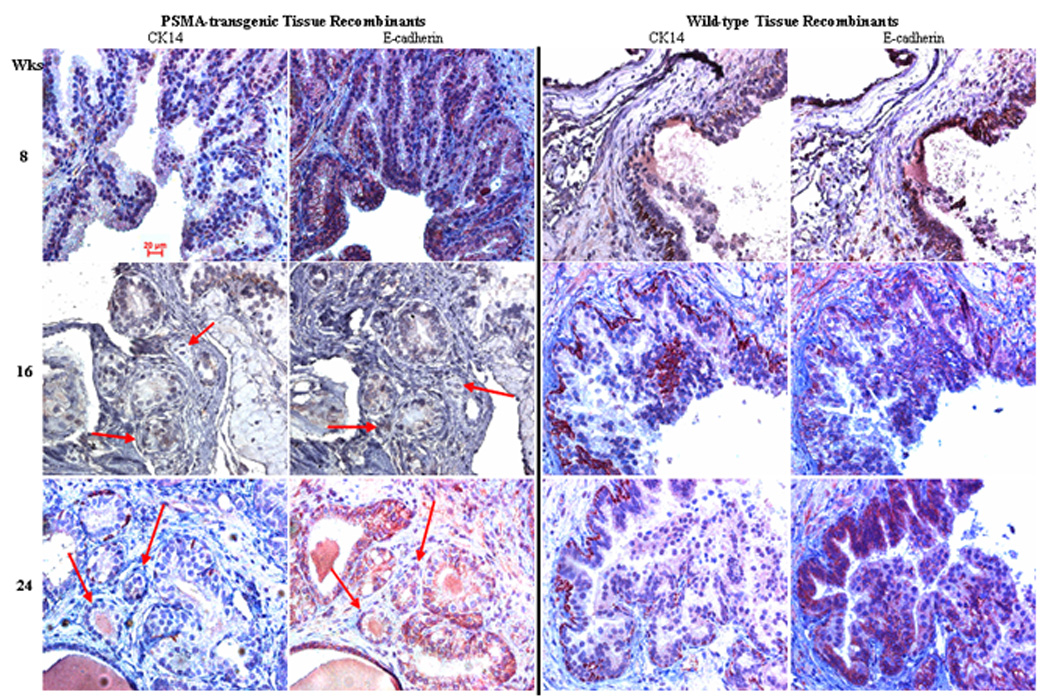
PSMA expression promotes cancer formation in mouse prostate recombinants. Paraffin embedded tissue recombinants grafted for a total of 8, 16 and 24 weeks were stained with either CK14 or E-cadherin. A pathologist (Dr. A Parwani) graded all specimens in blinded fashion. Arrows point to small atypical glands that lack CK-14 (basal cell) expression and display aberrant E-cadherin expression. These prostate glands display features of carcinoma similar to that of human prostate adenocarcinoma. Only PSMA-transgenic tissue recombinants were observed to display these small atypical glands with features of adenocarcinoma. Wild-type tissue recombinants displayed PINlike lesions that have strong CK14- and membrane E-cadherin expressions. Photomicrographs were captured at 40× magnification.
In contrast, at no point did we observe adenocarcinoma in the wild-type prostate tissue recombinants, although we did observe normal, hyperplastic and focal PIN-like glandular structures (Figure 2). Normal glands were lined by a single layer of columnar epithelial cells with a continuous layer of CK14-expressing basal epithelial cells. Hyperplastic ducts were lined by multilayered luminal epithelium with a patchy layer of basal cells. Some glands displayed features typical of human PIN, including cribriform, tufted or papillary architecture of epithelial cells with atypical and hyperchromatic nuclei, enlarged nucleoli (one or more), and mitotic bodies. CK14-staining showed that the PIN-like glands had a patchy basal cell layer (Figure 2). E-cadherin was expressed along adjacent epithelial cell membranes (Figure 2).
We next compared the histopathological features of both types of recombinants at each time point. Although a recombinant may display glands with all histological types (normal, hyperplasia, PIN and small atypical glandular foci with features of adenocarcinoma), only the most advanced histological phenotype found in each of the tissue recombinant was noted. As shown in Table 2, the majority of wild-type tissue recombinants displayed minor PIN-like lesions (PIN 1 and 2) at 8 (78%), 16 (64%) and 24 (79%) weeks. More importantly, none (0%) of the wild-type tissue recombinants displayed glandular foci with features of adenocarcinoma. In contrast, a variable number of small atypical glandular foci with features of adenocarcinoma were present in PSMA-transgenic tissue recombinants at 8 (4%), 16 (30%) and 24 (46%) weeks. This difference is statistically significant at 16 (p=0.046) and 24 (p=0.012) weeks but not at 8 (p=0.719) weeks, as determined by the Fisher’s exact test. In addition, unlike wild-type tissue recombinants, PSMA-transgenic tissue recombinants displayed a statistically significant (p=.0014) progression from PIN lesions to foci suspicious for adenocarcinoma with serial grafting, as determined by the Jonckheere-Terpstra Test. This is illustrated by a concomitant increase in the number of tissue recombinants with small atypical glands with features of adenocarcinoma (4%, 29% and 46%) and a decline in the number of recombinants with PIN lesions (96%, 71% and 50%) as their worst histology over the course of the experiment.
Table 2.
PSMA expression facilitates formation of small atypical glands with features of cancer, which increases in number with serial grafting. A pathologist determined the incidence of normal, hyperplasia, PIN and adenocarcinoma in all specimens in a blinded fashion by analyzing H&E and CK-14 staining of paraffin-embedded wild-type and PSMA-transgenic tissue recombinants. Only PSMA-transgenic tissue recombinants displayed small atypical glands with features of adenocarcinoma. The number of these small atypical glands with features of adenocarcinoma increases with serial grafting of the tissue recombinants. The Fisher’s exact test was used to analyze the incidence of PIN and foci with features of adenocarcinoma in wild-type and PSMA-transgenic tissue recombinants. The Jonckheere-Terpstra test was used to determine whether there is histopathological progression with serial grafting of the tissue recombinants.
| Epithelium | Normal | Hyperplasia | PIN | Small atypical foci with features of adenocarcinoma |
|
|---|---|---|---|---|---|
| 8 wks | Wild-type | 1/9 (11%) | 1/9 (11%) | 7/9 (78%) | 0/9 (0%) |
| PSMA+ | 0/23 (0%) | 0/23 (0%) | 22/23 (96%) | 1/23 (4%) | |
| 16 wks | Wild-type | 0/11 (0%) | 4/11 (36%) | 7/11 (64%) | 0/11 (0%) |
| PSMA+ | 0/23 (0%) | 0/23 (0%) | 16/23 (70%) | 7/23 (30%) | |
| 24 wks | Wild-type | 2/19 (10.5%) | 2/19 (10.5%) | 15/19 (79%) | 0/19 (0%) |
| PSMA+ | 0/26 (0%) | 1/26 (4%) | 13/26 (50%) | 12/26 (46%) | |
Although both wild-type and PSMA-transgenic tissue recombinants displayed PIN-like lesions, the cytological and pathological atypia of PIN-like lesions in wild-type tissue recombinants were less severe than those in PSMA-transgenic tissue recombinants. PIN-like lesions of both wild-type and PSMA-transgenic prostate tissue recombinants were scored blindly by a single pathologist. The classification of PIN lesions was based on their architectural and cytological atypia. The scale ranged from 1+ to 3+, with 3+ representing the most severe histopathology (see supplementary data). PIN-like lesions scored as 1+ (least severe) displayed prostatic hyperplasia with the glandular epithelial cells having uniform oval nuclei, some of which were slightly enlarged and/or exhibited weak hyperchromasia. Also, nucleoli were either absent or inconspicuous. PIN-like lesions with a score of 2+ (moderately severe) consisted of glands with the moderate hyperplasia, larger cells, higher nucleus to cytoplasmic ratios, more prominent nucleoli, and more pronounced hyperchromasia. Cells with an overall papillary-type architecture displayed more crowding and stratification. Occasionally, a tufted type of architecture was also noted. PIN-like lesions with a score of 3+ (most severe) were characterized by a pronounced increase in cell size, more pleomorphism, striking hyperchromasia with enlarged nucleoli, and some cells contained more than one nucleoli – a feature more commonly seen in neoplasia. There was significant architectural disorder, including the presence of papillary tufts. At low power, these foci were very prominent due to the overall dark, hyperchromatic appearance of the nuclei, giving them an overall dark blue appearance in contrast to adjacent benign glandular structures. In addition, there was significant stratification of the nuclei, giving the appearance of multi-layering.
Table 3 summarizes the incidence of 1+, 2+ and 3+ PIN-like in wild-type and PSMA-transgenic tissue recombinants. PIN-like lesions present in wild-type tissue recombinants never scored 3+ in severity. In contrast, in the PSMA-transgenic tissue recombinants, a score of 3+ was assigned to 23% (5/22), 25% (4/16) and 54% (7/13) of PIN-like lesions at 8, 16 and 24 weeks, respectively. The incidence of PIN 3+ (most severe) in PSMA-transgenic tissue recombinants is statistically significant from wild-type prostate tissue recombinants at 8 (p=0.00046), 16 (p=0.0334) and 24 (p=0.0000021) weeks as analyzed with the Wilcoxon-Mann-Whitney test statistics. In addition, serial grafting led to a statistical significant (p=0.0336) increase in the number of PSMA-transgenic tissue recombinants with PIN 3+ (most severe) as analyzed with the Jonckheere-Terpstra Test.
Table 3.
PSMA expression facilitates formation of PIN-like lesions with more severe cytological and histological features than PIN-like lesion observed in wild-type tissue recombinants. A pathologist scored PIN-like lesions 1+, 2+ and 3+ in increasing pathological severity in a blinded fashion by analyzing H&E and CK-14 staining of paraffin-embedded wild-type and PSMA-transgenic tissue recombinants. Although PIN-like lesions were observed in both wild-type and PSMA-transgenic tissue recombinants, only PSMA-transgenic tissue recombinants displayed PIN-like lesions scored 3+ (most severe) and the number of these lesions increased with serial grafting. The Wilcoxon-Mann-Whitney test was used to analyze the incidence of PIN 1+, 2+ and 3+ in wild-type and PSMA-transgenic tissue recombinants. The Jonckheere-Terpstra test was used to determine whether there is histopathological progression with serial grafting of the tissue recombinants.
| Epithelium | PIN 1+ | PIN 2+ | PIN 3+ | |
|---|---|---|---|---|
| 8 weeks | Wild-type | 5/7 (71%) | 2/7 (29%) | 0/7 (0%) |
| PSMA+ | 1/22 (4%) | 16/22 (73%) | 5/22 (23%) | |
| 16 weeks | Wild-type | 3/7 (43%) | 4/7 (57%) | 0/7 (0%) |
| PSMA+ | 4/16 (25%) | 8/16 (50%) | 4/16 (25%) | |
| 24 weeks | Wild-type | 9/15 (60%) | 6/15 (40%) | 0/15 (0%) |
| PSMA+ | 1/13 (8%) | 5/13 (38%) | 7/13 (54%) | |
A hallmark characteristic of tumor cells is their ability to grow in an anchorage-independent manner (29). As shown in Figure 3A, cells from PSMA-transgenic tissue recombinants, which had been grafted under the kidney capsule for 24 weeks, were able to grow and form an average of about 5 colonies of greater than 30 cells in semi-solid agar. In contrast, cells from wild-type tissue recombinants, which had been grafted under the kidney capsule for 24 weeks, were not able to form colonies of greater than 30 cells in semi-solid agar.
Figure 3.
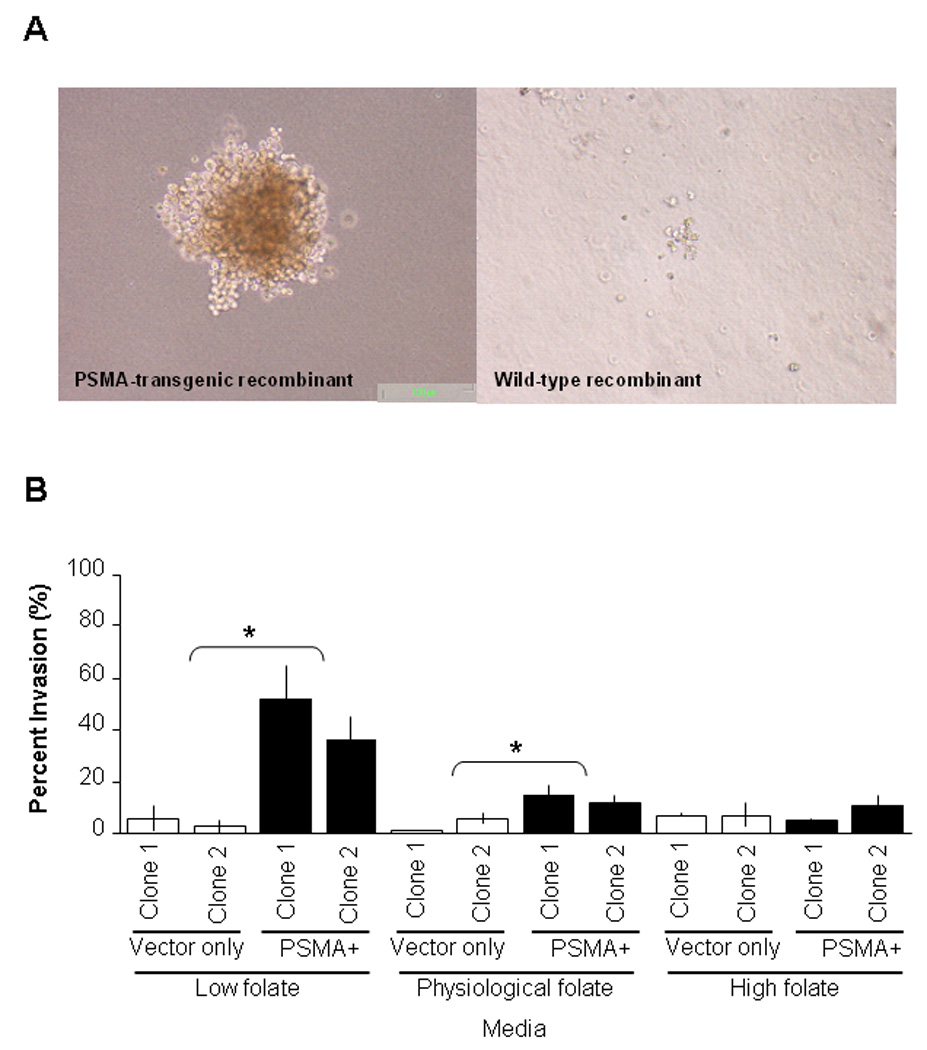
PSMA expression facilitates formation of cells with the ability to grow in an anchorage-independent manner and ectopic expression of PSMA on prostate cancer cell line increases invasion in media containing physiological level of folate. A, cells isolated from PSMA-transgenic tissue recombinants that had been grafted under the kidney capsule for 24 weeks were able to form colonies of greater than 30 cells on semi-sold agar (left). In contrast, cells isolated from wild-type tissue recombinants did not form colonies on semi-solid a gar (right). Photomicrographs were captured at 40× magnification. B. PC-3 cells expressing PSMA displayed increased invasive ability than PC-3 transfected with vector only in media containing physiological (25nM) and low (< 1nM) folate levels compared to those grown in high (normal media, 2.3µM) folate media. Transwell migration assay was performed using a Boyden chamber system. Data is reported as percent invasion through the matrigel matrix and membrane relative to the migration through the control membrane; mean ±SE (n = 3) * denotes p<0.05, by Student t-test.
We explored the mechanism by which PSMA may influence carcinogenesis by testing the invasive ability of PC-3 cells that do not express PSMA and those that express PSMA in physiologically-relevant folate levels. As shown in Figure 3B, we found that the invasive ability of PC-3 cells with ectopic expression of PSMA were not statistically significant compared to PC-3 cells that do not express PSMA in standard media which contains high (2.3µM) (percentage invasion: 5.0% ± 0.6 and 11.0% ± 3.8 versus 7.3.% ± 0.9 and 7.3% ± 4.1) folate levels. However, PSMA-expressing PC-3 cells had increased invasive ability compared to non-expressing PC-3 cells in media containing low (<1nM) (51.7% ± 13.0 and 36.0% ± 8.7 versus 6.2% ± 4.9 and 2.6% ± 3.1) and physiological folate levels (25nM) (percentage invasion: 14.6% ± 3.9 and 11.9% ± 2.5 for PSMA-expressing PC-3 cells versus 1.2% ± 0.2 and 5.8% ± 2.1 for PC-3 cells transfected with vector only) (p<0.05). Two clones of PSMA-expressing PC-3 cells and vector-transfected PC-3 cells were used to confirm the results.
Discussion
PSMA-transgenic mice provide tantalizing preliminary evidence that PSMA contributes to prostate cancer development. However, PIN-like lesions in PSMA-transgenic mice did not progress to cancer. Given that carcinogenesis requires multiple insults, these lesions may not have acquired all of the underlying molecular traits necessary for malignant transformation. Previous work has shown that the tissue recombinant model provides the additional factors needed for cancer development. This model combines hormonal induction of prostate cancer (30–33) with enhanced epithelial proliferation elicited by embryonic rUGM (34–36). We used this model to more definitively evaluate the role of PSMA in prostate carcinogenesis and progression.
Prostatic hyperplasia, PIN, and foci with features of adenocarcinoma were observed in PSMA-transgenic tissue recombinants. In contrast, PIN 2+ was the most severe lesion observed in wild-type tissue recombinants, which may be induced by 17β-estradiol. Only PSMA-transgenic tissue recombinants developed PIN with the most severe architectural and cytological atypia, PIN 3+. Serial grafting of the PSMA-transgenic, but not wild-type tissue recombinants, resulted in a statistically significant progression in the architectural and cytological severity from PIN 2+ to PIN 3+. In addition, serial grafting led to progression of the PIN-like lesions in PSMA-transgenic tissue recombinants progressed to small atypical glands with features of adenocarcinoma.
At each time point, the PSMA-transgenic tissue recombinants developed small atypical glands that appeared similar to adenocarcinoma. Such small atypical glands were absent in wild-type prostate tissue recombinants and prostate tissues from PSMA transgenic mice. These small atypical glands did not form large masses of back-to-back glands, but existed in isolation or in clusters of 2–4 glands. They exhibited features of adenocarcinoma such as nuclear pleomorphism, prominent and often multiple nucleoli, loss of CK14-expressing basal cells, and aberrant E-cadherin staining. Loss or abnormal E-cadherin expression has been associated with metastasis and poor prognosis (37). The fact that we were able to grow these small atypical cells in semisolid medium (anchorage-independent growth) provides further evidence of their tumorigenicity (38). Similar to Wang et al (21), we were able to transplant the small atypical glands. Moreover, their number increased with serial grafting, suggesting neoplastic progression with successive passage.
In this model, the level of human PSMA expressed in PSMA-transgenic prostates is comparable to that found in normal human prostate tissues. In addition, no other genetic manipulations were made that would not be present in normal human prostate tissue samples before cancer development. Therefore, formation of PIN-like lesions and foci with features of adenocarcinoma in PSMA-transgenic prostate recombinants suggests that PSMA, thought to be a cell differentiation marker and not an oncogene or cell cycle regulator, may be an initiator of prostate carcinogenesis.
Previously, Ghosh et al., found that ectopic expression of PSMA in PC-3 cells decreased their invasive capability while knockdown of PSMA expression increased the invasiveness of LNCaP cells (24). They also found that the enzymatic activity of PSMA is associated with the effect of PSMA on invasion. However, their study was conducted in RPMI-1640 media, which contains about 100 times the physiological folate level. At this concentration, the enzymatic site of PSMA, a folate hydrolase, may be competitively occupied by folic acid, thereby, masking the affects of PSMA. Therefore, we decided to look at the effects of ectopic PSMA expression on the invasive ability of PC-3 cells in RPMI-1640 media containing low (<1nM), physiological (25nM), and high (2.3µM) folate levels. In contrast to Ghosh et al., we counted the number of invading cells 22 hours instead of 48 hours after seeding (24). We found that PC-3 cells expressing PSMA have greater invasive ability than non-expressing cells in media containing low and physiological folate levels, but not in media containing high folate levels. Increased invasive ability was most prominent in RPMI-1640 media containing low (<1nM) levels of folic acid where prostate cancer cells expressing PSMA had about 10-fold greater percentage invasion than prostate cancer cells transfected with vector only. Based on the data presented here and from our previous study (23), PSMA expression may increase the invasiveness of prostate cancer cells and provide cells with a growth advantage in the low folate tumor microenvironment. The observation that PSMA expression increases invasion, at least under physiological folate conditions is not without precedent. Conway et al., found that angiogenesis is impaired in PSMA-null animals due to reduced invasion of endothelial cells through extracellular matrix (29).
In conclusion, we have shown that PSMA, expressed at a level comparable to that of normal human prostate tissue and not mutated in any way, can initiate the development of prostate cancer using a hormone-induced tissue recombination model. PSMA may facilitate carcinogenesis by enhancing the proliferative and invasive capability of prostate cancer cells. This invasive capability can be blocked by folic acid. These findings demonstrate a novel mechanism that may contribute to current knowledge about the role of folate in cancer prevention and could potentially lead to the use of PSMA inhibitors as novel chemotherapeutic agents for prostate cancer. Our laboratory is currently using a compound that can inhibit the enzymatic activity of PSMA to investigate if it contributes to the initiation of prostate carcinogenesis.
Supplementary Material
Photomicrograph of H&E-stained sections of PSMA-transgenic tissue recombinants showing histological features of 1+ (A), 2+ (B) and 3+ (C) PIN as determined by a pathologist (Dr. A. Parwani).
Acknowledgments
We thank Simon Hayward for his technical advice. We would also like to thank our laboratory technician Diana Sisca Harya.
Funding sources: This research study was supported by the Department of Defense Prostate Cancer Research Program under the award number W81XWH-05-1-0015. Views, opinions, and endorsements by the authors do not reflect those of the US Army or the Department of Defense. This work was also supported by a grant from the AFUD/AUAER Research Scholar Program and the Reston Ambassador’s Golf Classic for Prostate Cancer Research Fund. This work was also supported by NIH RO1 CA101069 (WDWH).
Footnotes
Disclosure of potential conflicts of interest: No potential conflicts of interest to disclose.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Coffey DS. Prostate cancer: an overview of an increasing dilemma. Cancer. 1993;71:880–886. doi: 10.1002/1097-0142(19930201)71:3+<880::aid-cncr2820711403>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Walczak JR, Carducci MA. Prostate cancer: a practical approach to current management of recurrent disease. Mayo Clin Proc. 2007;82:243–249. doi: 10.4065/82.2.243. [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe DS, Bacich DJ, Heston WD. Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate. 2004;58:200–210. doi: 10.1002/pros.10319. [DOI] [PubMed] [Google Scholar]
- 5.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate Specific Membrane Antigen expression in prostatic Intraepithelial Neoplasia and adneocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Su SL, Huang I-P, Fair WR, Powell CT, Heston WDW. Alternatively spliced variants of prostate-specific membrane antigen RNA ratio of expression as a potential measure of progression. Cancer Res. 1995;55:1441–1443. [PubMed] [Google Scholar]
- 7.Kawakami M, Nakayama J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997;57:2321–2324. [PubMed] [Google Scholar]
- 8.Wright GL, Jr., Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 9.Ross JS, Sheehan CE, Fisher HAG, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6462. [PubMed] [Google Scholar]
- 10.Mitsiades CS, Lembessis P, Sourla A, Milathianakis C, Tsintavis A, Koutsilieris M. Molecular staging by RT-pCR analysis for PSA and PSMA in peripheral blood and bone marrow samples is an independent predictor of time to biochemical failure following radical prostatectomy for clinically localized prostate cancer. Clin Exp Metastasis. 2004;21:495–505. doi: 10.1007/s10585-004-3217-0. [DOI] [PubMed] [Google Scholar]
- 11.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 12.Liu H, Moy P, Kim S, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–3634. [PubMed] [Google Scholar]
- 13.Chang SS, O’Keefe DS, Bacich DJ, Reuter VE, Heston WDW, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 14.Chang SS, Reuter VE, Heston WDW, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 15.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptide. Proc Natl Acad Sci USA. 1996;93:749–753. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto JT, Suffoletto BP, Berzin TM, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2:1445–1451. [PubMed] [Google Scholar]
- 17.Tiffany CW, Lapidus RG, Merion A, Calvin DC, Slusher BS. Characterization of the enzymatic activity of PSM: comparison with brain NAALADase. Prostate. 1999;39:28–35. doi: 10.1002/(sici)1097-0045(19990401)39:1<28::aid-pros5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Lapidus RG, Tiffany CW, Isaacs JT, Slusher BS. Prostate-specific membrane antigen (PSMA) enzyme activity is elevated in prostate cancer cells. Prostate. 2000;45:350–354. doi: 10.1002/1097-0045(20001201)45:4<350::aid-pros10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Bacich DJ, Harsch KM, Heston WD. Transgenic mice expressing human Prostate-Specific Membrane Antigen (PSMA) in their prostate: A potential model for prostate cancer initiation, development and therapy; Proceedings of the American Association for Cancer Research Annual Meeting; San Francisco. 2002. [Google Scholar]
- 20.Cunha GR, Donjacour AA. Mesenchymal-epithelial interactions: technical considerations. In: Coffey DS, Bruchovsky N, Gardner WA, Resnick MI, Karr JP, editors. Assessment of Current Concepts and Approaches to the Study of Prostate Cancer. New York: A.R. Liss; 1987. pp. 273–282. [Google Scholar]
- 21.Wang Y, Hayward SH, Donjacour AA, et al. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 2000;60:6008–6017. [PubMed] [Google Scholar]
- 22.Kim MJ, Bhatia-Gaur R, Banach-Petrovsky WA, et al. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- 23.Yao V, Bacich DJ. Prostate specific membrane antigen (PSMA) expression gives prostate cancer cells a growth advantage in physiologically relevant folate environment in vitro. Prostate. 2006;66:867–875. doi: 10.1002/pros.20361. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh A, Wang X, Klein E, Heston WD. Novel role of prostate-specific membrane antigen in suppressing prostate cancer invasiveness. Cancer Res. 2005;65:727–731. [PubMed] [Google Scholar]
- 25.Hallowes RC, Bone EJ, Jones W. A new dimension in the culture of human breast. In: Richards RJ, Rajan KT, editors. Tissue culture in medical research. Oxford: Pergamon Press; 1980. pp. 213–220. [Google Scholar]
- 26.Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59:7–12. doi: 10.3109/10520298409113823. [DOI] [PubMed] [Google Scholar]
- 27.Chanarin I. In: The folate in vitamins in medicine. Baker BM, Bender DA, editors. London: Heinemann Medical Books; 1980. pp. 247–314. [Google Scholar]
- 28.Ricke WA, Ishii K, Ricke EA, Wang SJ, Simko J, Wang Y, Hayward SW, Cunha GR. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;18:2123–2131. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- 29.Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang YZ, Wong YC. Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) in the development of prostate cancer. Prostate. 1998;35:165–177. doi: 10.1002/(sici)1097-0045(19980515)35:3<165::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Noble RL. Prostate cancer of the Nb rat in relation to hormones. Int Rev Exp Pathol. 1980;23:113–159. [PubMed] [Google Scholar]
- 32.Isaacs JT, Furuya Y, Berges R. The role of androgen in the regulation of programmed cell death/apoptosis in normal and malignant prostatic tissue. Semin Cancer Biol. 1994;5:391–400. [PubMed] [Google Scholar]
- 33.Hayashi N, Cunha GR, Parker M. Permissive and instructive induction of adult rodent prostatic epithelium by heterotypic urogenital sinus mesenchyme. Epithelial Cell Biol. 1993;2:66–78. [PubMed] [Google Scholar]
- 34.Triche TJ, Harkin JC. An ultrastructural study of hormonally induced squamous metaplasia in the coagulating gland of the mouse prostate. Lab Investigation. 1971;25:596–606. [PubMed] [Google Scholar]
- 35.Wang Y, Sudilovsky D, Zhang B. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 2001;61:6064–6072. [PubMed] [Google Scholar]
- 36.Cunha GR, Sekkingstad M, Meloy BA. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit, and human tissues. Differentiation (Camb.) 1983;24:174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 37.Umbas R, Isaacs WB, Bringuier PP, et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–3933. [PubMed] [Google Scholar]
- 38.Colburn NH, Bruegge WF, Bates JR, et al. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978;38:624–634. [PubMed] [Google Scholar]
- 39.Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol. 2006;26:5310–5324. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photomicrograph of H&E-stained sections of PSMA-transgenic tissue recombinants showing histological features of 1+ (A), 2+ (B) and 3+ (C) PIN as determined by a pathologist (Dr. A. Parwani).


