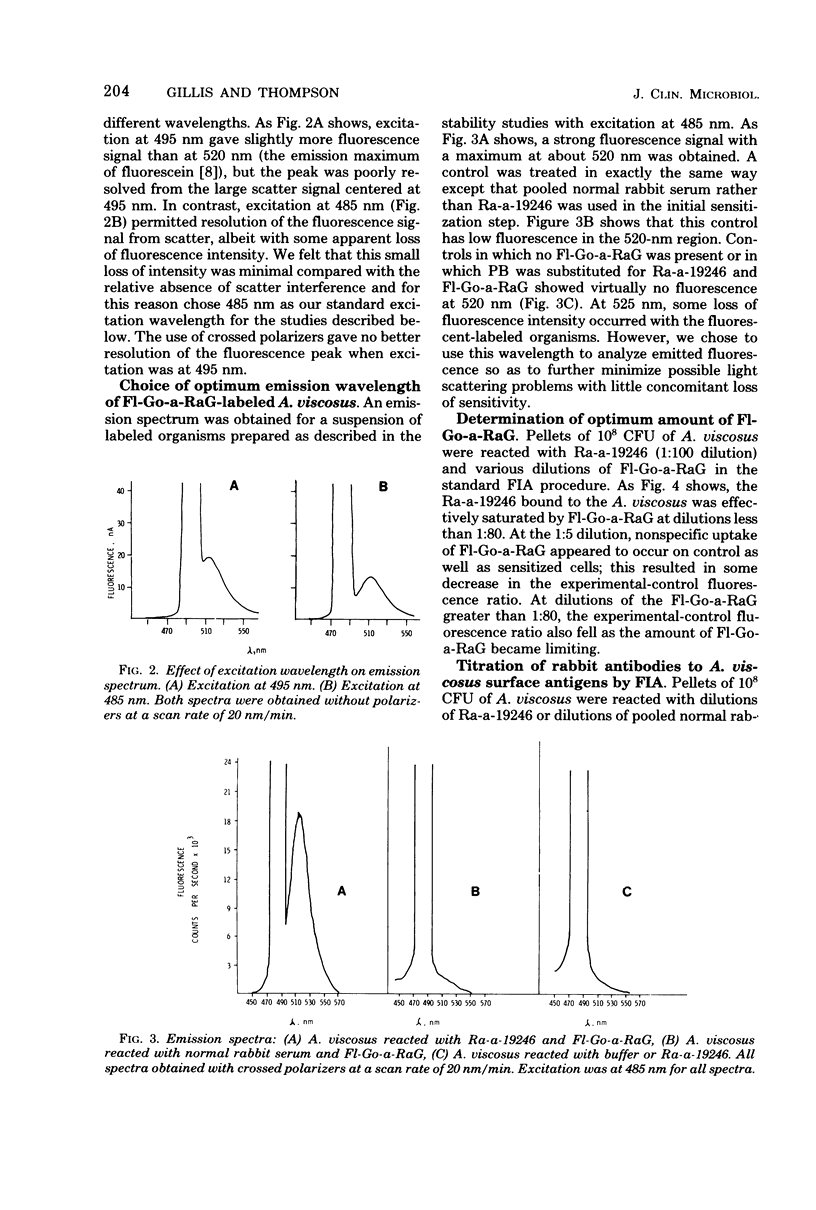
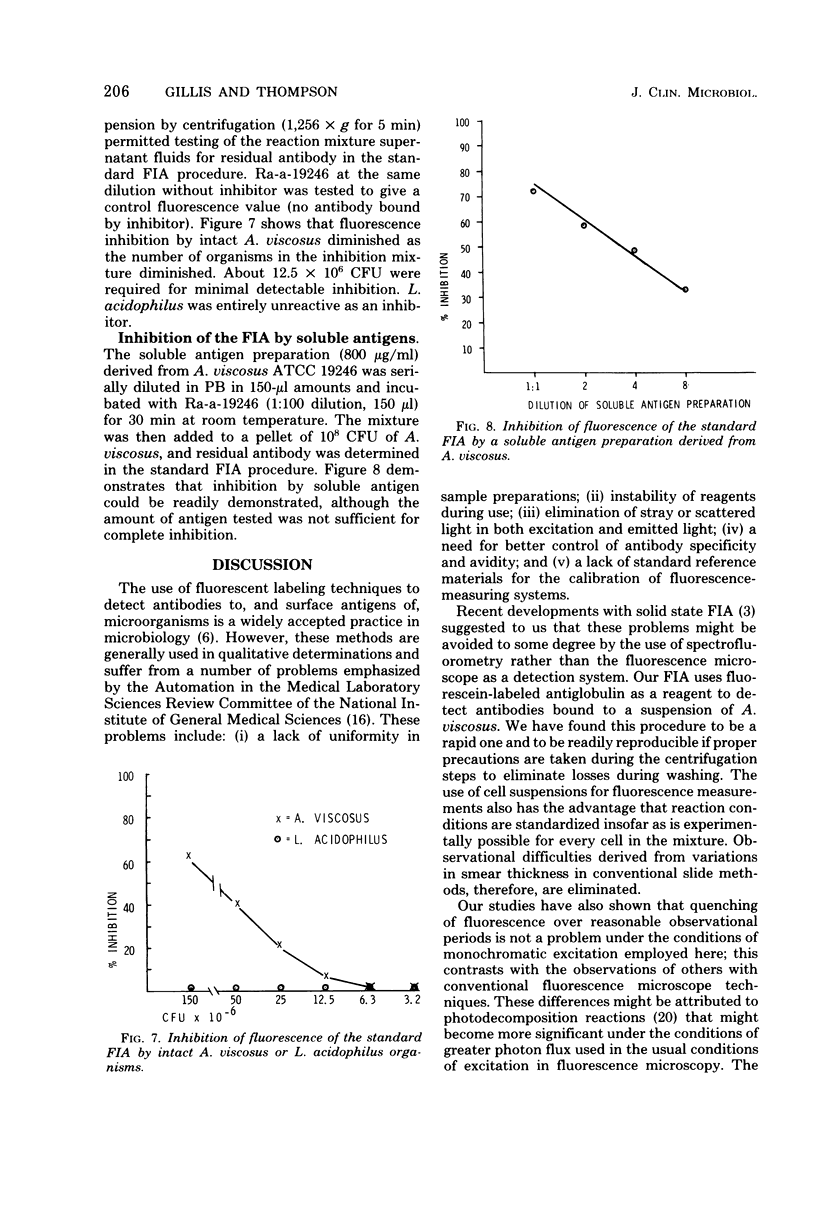
Abstract
Optimal conditions for a fluorescence immunoassay of antibodies to, and surface antigens of, Actinomyces viscosus ATCC 19246 are described. In the standard fluorescence immunoassay, 10(8) colony-forming units of A. viscosus reacted with an antibody preparation, were washed, and then were treated with an excess of fluorescein-conjugated goat anti-rabbit immunoglobulin G. After another set of washes, fluorescence was determined in a spectofluorometer; in most cases excitation was at 485 nm, with emission measured at 525 nm. These conditions minimized interference from light scatter and stray light. Under appropriate conditions, antibodies to A. viscosus could be readily determined, with the fluorescence of the specific antibody-treated cells more than five times the fluorescence of controls treated with normal rabbit serum. Organisms coated with specific antibody could be detected at levels approaching 10(5) colony-forming units per ml. The standard fluorescence immunoassay procedure was readily adapted to the measurement of either particulate or soluble surface antigens of A. viscosus by competition of the antigen with a fixed amount of antibody in the standard assay system; the competition resulted in an antigen dose-dependent inhibition of fluorescence. The fluorescent immunoassay system thus appears to be a general one that could be applied to other microbial systems as well.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloemmen F. J., Rádl J., Haaijman J. J., Van den Berg P., Schuit H. R., Hijmans W. Microfluorometric evaluation of the specificity of fluorescent antisera against mouse immunoglobulins with the defined antigen substrate spheres (DASS) system. J Immunol Methods. 1976;10(4):337–355. doi: 10.1016/0022-1759(76)90028-4. [DOI] [PubMed] [Google Scholar]
- Burgett M. W., Fairfield S. J., Monthony J. F. A solid phase fluorescent immunossay for the quantitation of the C4 component of human complement. J Immunol Methods. 1977;16(3):211–219. doi: 10.1016/0022-1759(77)90199-5. [DOI] [PubMed] [Google Scholar]
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel P. J. A quantitative immunofluorescence method based on the covalent coupling of protein to sepharose beads. J Immunol Methods. 1974 Jul;5(2):165–178. doi: 10.1016/0022-1759(74)90007-6. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Reduction of light scatter in fluorometry by the use of horizontally polarized excitation. Anal Biochem. 1966 Mar;14(3):497–499. doi: 10.1016/0003-2697(66)90295-8. [DOI] [PubMed] [Google Scholar]
- Cherry W. B., Reimer C. B. Standardization of diagnostic materials. 4. Diagnostic immunofluorescence. Bull World Health Organ. 1973 Jun;48(6):737–746. [PMC free article] [PubMed] [Google Scholar]
- Collins P. A., Gerencser M. A., Slack J. M. Enumeration and identification of Actinomycetaceae in human dental calculus using the fluorescent antibody technique. Arch Oral Biol. 1973 Feb;18(2):145–153. doi: 10.1016/0003-9969(73)90133-7. [DOI] [PubMed] [Google Scholar]
- DAVIS R. P., CANESSA-FISCHER M. SPECTROFLUOROMETRIC IDENTIFICATION OF REDUCED PYRIDINE NUCLEOTIDE IN THE INTACT ISOLATED URINARY BLADDER OF THE TOAD. Anal Biochem. 1965 Feb;10:325–343. doi: 10.1016/0003-2697(65)90273-3. [DOI] [PubMed] [Google Scholar]
- Fagraeus A., Bergquist N. R. The raison d'etre of standards in indirect immunofluorescence. Ann N Y Acad Sci. 1975 Jun 30;254:69–76. doi: 10.1111/j.1749-6632.1975.tb29158.x. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Jongsma A. P., Hijmans W., Ploem J. S. Quantitative immunofluorescence. Standardization and calibration in microfluorometry. Histochemie. 1971;25(4):329–343. doi: 10.1007/BF00278226. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Knapp W., Ploem J. S. Microfluoremetry of antigen-antibody interactions in immunofluorescence using the defined antigen substrate spheres (DASS) system. Sensitivity, specificity and variables of the method. J Immunol Methods. 1974 Aug;5(3):259–273. doi: 10.1016/0022-1759(74)90111-2. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Landfried S., Gerencser M. A. Identification of Actinomyces and related bacteria in dental calculus by the fluorescent antibody technique. J Dent Res. 1971 Jan-Feb;50(1):78–82. doi: 10.1177/00220345710500013501. [DOI] [PubMed] [Google Scholar]
- Wick G., Schauenstein K., Herzog F., Steinbatz A. Investigations of the recovery phenomenon after laser excitation in immunofluorescence. Ann N Y Acad Sci. 1975 Jun 30;254:172–174. doi: 10.1111/j.1749-6632.1975.tb29167.x. [DOI] [PubMed] [Google Scholar]


