Abstract
Nitric oxide (NO) plays major roles during development and in adult organisms. We examined the temporal and spatial patterns of nitric oxide synthase (NOS) appearance in the embryonic lobster brain to localize sources of NO activity; potential NO targets were identified by defining the distribution of NO-induced cGMP. Staining patterns are compared with NOS and cyclic 3,5 guanosine monophosphate (cGMP) distribution in adult lobster brains. Manipulation of NO levels influences olfactory glomerular formation and stabilization, as well as levels of neurogenesis among the olfactory projection neurons. In the first 2 days following ablation of the lateral antennular flagella in juvenile lobsters, a wave of increased NOS immunoreactivity and a reduction in neurogenesis occur. These studies implicate nitric oxide as a developmental architect and also support a role for this molecule in the neural response to injury in the olfactory pathway.
Keywords: nitric oxide, cGMP, bromodeoxyuridine, BrdU, serotonin, olfaction, neurogenesis, adult neurogenesis
INTRODUCTION
Nitric oxide (NO) is a diffusible gaseous signaling molecule that is produced by nitric oxide synthase (NOS) during the conversion of L-arginine to citrulline. Once present, NO is membrane-permeable and in many systems effects change by activating soluble guanylate cyclase in target cells, resulting in the production of cyclic 3,5 guanosine monophosphate (cGMP). cGMP then activates a variety of downstream pathways by means of actions, for example, on phosphodiesterases or protein kinases, ultimately evoking cellular responses. There are also cGMP-independent pathways of NO action, such as when NO interacts with metal complexes or oxygen species. However, in the nervous system of both vertebrate and invertebrate species, the cGMP-dependent actions appear to be the most prevalent.
NO has been implicated in many physiological processes (e.g., vasodilation, muscle contractility, neurotransmission) in a variety of organisms. The functions of NO are particularly diverse in the nervous system, but perhaps most notable is the association of NO with dynamic processes such as neuronal migration, differentiation, and synapse formation during development (Truman et al., 1996; Kuzin et al., 2000; Gibbs, 2003; Bicker, 2005), and neuronal plasticity, synaptic remodeling, and sensory processing in the mature nervous system (Chen et al., 2004; Collmann et al., 2004; Sunico et al., 2005; Moreno-Lopez and Gonzalez-Forero, 2006). NOS is concentrated in the primary olfactory centers of vertebrate and invertebrate species (Broillet and Firestein, 1996; Gelperin et al., 1996; Fuji et al., 2002), and there is strong physiological evidence for a role of NO in odor processing (Collmann et al., 2004). Nitric oxide mechanisms also appear to be important during both developmental and adult neurogenesis, where NO can act as an antiproliferative agent in some systems (Moreno-Lopez et al., 2004; Matarredona et al., 2005; Ciani et al., 2006; Romero-Grimaldi et al., 2006; Torroglosa et al., 2007), while promoting neurogenesis in others (Zhang et al., 2001; Lu et al., 2003; Cayre et al., 2005). These dual roles may be explained by the levels of NO and the timing of synthesis (Cardenas et al., 2005).
The life-long addition of new neurons has been found in the olfactory pathway of vertebrate and invertebrate organisms (Kempermann, 2005), and of particular interest to our work, in the brains of lobsters and other crustaceans (Harzsch and Dawirs, 1996; Schmidt, 1997; Harzsch et al., 1999; Beltz and Sandeman, 2003). Neurogenesis in the crustacean brain is influenced by a variety of endogenous and exogenous factors (Beltz and Sandeman, 2003; Sullivan et al., 2007), including serotonin (Benton and Beltz, 2001a; Beltz et al., 2001). In addition to stimulating neurogenesis, serotonin promotes the survival and differentiation of new projection neurons in the olfactory pathway of lobsters (Sullivan et al., 2000; Beltz et al., 2001; Benton and Beltz, 2001a). A role for NO in regulating neurogenesis was suggested by preliminary studies that demonstrated the coexistence of NO with serotonin in identified neurons and synaptic regions in the lobster brain, and its presence in regions where neurons continue to proliferate throughout life (Benton and Beltz, 2001b; Beltz and Sandeman, 2003). In addition, several studies in vertebrate systems suggest an interrelationship between serotonin and NO levels (Kaehler et al., 1999; Sinner et al., 2001; Ramos et al., 2002; Tagliaferro et al., 2003).
Other neuronal functions for NO in decapod crustaceans have also been identified, including network partitioning and motor pattern selection in the stomatogastric nervous system (Scholz et al., 2001; Christie, 2003; Stein et al., 2005) and retrograde signaling in the heart (Scholz et al., 2002; Goy, 2005). A role in central nervous system development is suggested by a large number of NO-sensitive neurons found in lobster larvae, and by subsequent changes in the expression of NOS and of NO sensitivity (Scholz et al., 1998). Scholz et al. concluded that the NO/cGMP pathway participates in the developmental maturation of neural circuits in the accessory lobes, higher order processing centers in the olfactory pathway. Studies in adult crayfish suggest that, as in insects and mammals, NOS is strongly expressed in the olfactory centers of the brain (Johansson and Carlberg, 1994; Johansson and Mellon, 1998).
Our primary interest is in the development and maturation of olfactory centers in the crustacean brain, and in the life-long production of neurons in the olfactory pathway. Because NO appears to be important in mechanisms underlying both olfaction and neurogenesis in a variety of species, we investigated the role of this molecule in the olfactory system in the American lobster, Homarus americanus. The goal of the present study is to describe NO-associated pathways in the embryonic and adult crustacean brain, and to define potential roles for NO. We have used immunocytochemical techniques to identify and describe putative sources of NO (NOS immunoreactivity) in the embryonic and adult brain. The NO donor sodium nitroprusside (SNP) in combination with isobutylmethylxanthine (IBMX; which blocks cGMP turnover by phosphodiesterases) were used to increase cGMP levels in NO-targets, and cGMP was then labeled immunocytochemically. To reveal possible functional roles of NO in the brain, levels of NO were manipulated using the NO donor S-Nitroso-N-acetyl-D,L-penicillamine (SNAP) and the NOS inhibitor NG-Nitro-L-arginine (LNAA) at various times during embryonic development; structural and chemical changes in the lobster brain were then documented. We also asked whether the rate of neurogenesis in the embryonic brain is influenced by NO levels. Finally, the lateral flagella of the antennules of juvenile lobsters containing the olfactory receptor neurons were ablated, and changes in NOS expression in the olfactory pathway were documented, as were levels of neurogenesis among the olfactory projection neurons.
The results of this study provide an overall view of the changing location of NOS during embryonic development and its final distribution in the adult brain, as well as identifying presumptive target areas where cGMP was up-regulated in response to increased levels of NO. Our data suggest a role for NO in (1) establishing the structural integrity of olfactory glomeruli during development, (2) synaptogenesis in the accessory lobe, (3) the regulation of neurogenesis, and (4) the neural response to injury.
RESULTS
NOS and cGMP Localization Studies
Distribution and sequence of appearance of NOS in the embryonic and adult brain
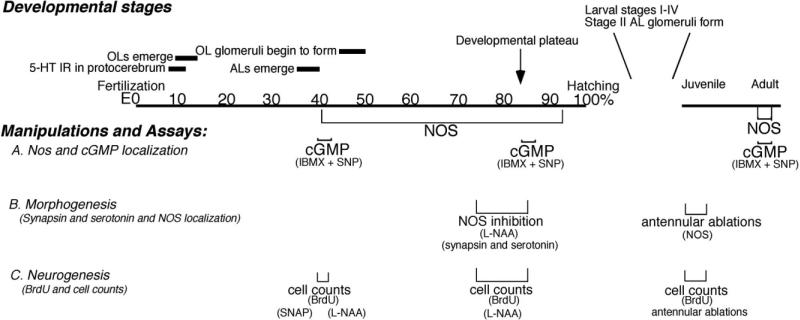
We defined the temporal and spatial patterns of expression of NOS in the embryonic lobster brain by assessing the distribution of NOS immunoreactivity at several developmental stages, beginning at E40% (where E0% is the time of fertilization, and E100% is hatching) and continuing until just before hatching (Table 1). These localization patterns can be correlated with specific developmental characteristics in the brain (Fig. 1). At E40%, for example, the anlagen of the accessory lobes (ALs) have just emerged; the olfactory lobes (OLs), whose primordia first appeared at E10–E15%, are well established and the OL glomeruli begin to form at E45% (Helluy and Beltz, 1991; Beltz et al., 1992; Helluy et al., 1993). Midembryonic life is characterized by the elaboration and growth of various brain structures, until ∼E85% when the animal enters a developmental plateau (Helluy and Beltz, 1991). Throughout this period, the eyes and body do not grow even when animals are reared at constant temperature, and neurogenesis undergoes a precipitous decline as the neuroblasts die (Harzsch et al., 1999; Beltz and Sandeman, 2003). While brain growth then continues after hatching due to the elaboration of fibers and addition of glia, neurogenesis resumes only in restricted areas in the olfactory (deutocerebral cell clusters 9 and 10) and optic pathways (Harzsch et al., 1999; Sullivan and Beltz, 2005).
TABLE 1.
Localization of NOS in the Brains of Adult and Embryonic Lobstersa
| Stage of development → | E40% | E60% | E68% | E75% | E85% | E95% | Adult |
|---|---|---|---|---|---|---|---|
| Protocerebrum | |||||||
| Frontal eye | − | − | − | − | − | − | − |
| Cluster 6 cells | − | + − | + − | + | 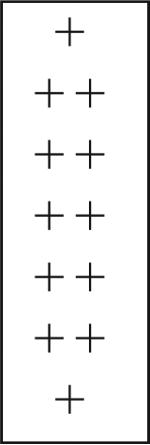 |
+ | + − |
| AMPN/ lateral cluster | + − | − | + − | + | − | − | |
| PB | + − | + − | + − | + − | − | + − | |
| CB | − | + − | + − | + − | − | + − | |
| Medial longitudinal fibers | − | + − | + − | + − | − | − | |
| Cluster 8 cells | − | − | + − | + | + | − | |
| Cluster 9 cells | − | − | − | − | + | − | |
| Deutocerebrum | |||||||
| DC interneurons | − | − | − | − | − | + | 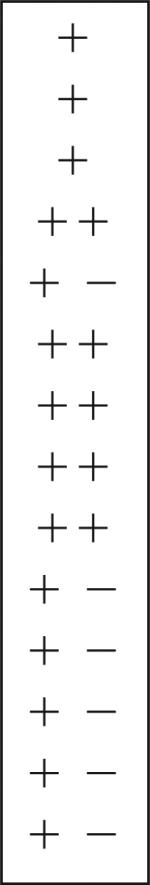 |
| DCN | − | − | − | − | − | − | |
| DC | − | − | − | − | − | − | |
| OGTN | − | − | + − | + |  |
− | |
| OL glomeruli | + − | + − | + − | + − | − | ||
| AL glomeruli | − | − | − | − | − | − | |
| Fibers entering AL | − | − | + − | + |  |
+ − | |
| Fibers in OL | + − | + − | + | + | + − | ||
| Cluster 10 cells | − | + − | + | + | + − | ||
| OGT punctate staining | − | − | − | − | − | ||
| DGN axon | − | − | + | − | − | ||
| DGN soma | − | − | + − | + | + | ||
| Large cells in cluster 11 | − | − | + | ++ | + | ||
| LAN | − | − | − | − | − | − | |
| Tritocerebrum | |||||||
| Cluster 15/16 | − | + | + | ++ |  |
+ | + − |
| Cluster 17 | + − | ++ | + | ++ | ++ | − | |
| AnN II | − | − | − | − | + | − | |
| Fibers / cells in the CEG | − | − | − | − | + − | ||
| n = 6 | n = 5 | n = 6 | n = 6 | n = 6 | n = 2 | n = 4 |
E is percentage embryonic development where 0% is the time of fertilization and 100% is hatching. Three regions within the brain are distinguished: protocerebrum (anterior regions), deutocerebrum (midbrain regions), and tritocerebrum (posterior regions). The intensity of NOS labeling in neuropil structures, cell clusters, and individual cells and fibers is subjectively rated on a relative scale: (−) = no labeling observed; (+ −) = weak and variable; (+) = consistent and strong; (++) = intense. Embryonic NOS labeling reaches it widest distribution throughout the brain and most intense level at E85% (boxed scores), whereas NOS is found primarily in the olfactory pathway (boxed scores) in the adult brain. The number of brains assessed per stage is indicated at the bottom of each column. For abbreviations, see list.
Fig. 1.
A time line of brain development in Homarus, emphasizing the developmental sequence in the olfactory pathway. Numbers indicate percentage development, from fertilization (0%) to hatching (100% development); juvenile and adult stages complete the range. Three assays related to the nitric oxide (NO) pathway were used: immunocytochemical localization of nitric oxide synthase (NOS) and cGMP (A); immunocytochemical labeling for synapsin and serotonin to monitor morphogenic changes (B); 5-bromo-2′-deoxyuridine (BrdU) labeling to assess the numbers of cells in S phase (C). To accomplish these studies, NO and cyclic 3,5 guanosine monophosphate (cGMP) levels also were manipulated using pharmacological agents (sodium nitroprusside [SNP], S-Nitroso-N-acetyl-D,L-penicillamine [SNAP], NG-Nitro-L-arginine [L-NAA], isobutylmethylxanthine [IBMX]) and by antennular ablations. The timing of the immunocytochemical and pharmacological experiments are noted below the timeline at the developmental stage when they were performed.
In the embryonic brain, NOS immunoreactivity is found in the cytoplasm of neuronal cell bodies, as well as in fiber tracts and neuropil (synaptic) regions. As embryogenesis progresses, NOS immunoreactivity gradually becomes more intense and the sites of labeling more widespread, eventually including structures throughout the brain by the embryonic plateau (∼E85%; Table 1). At E40%, NOS immunoreactivity is most pronounced in protocerebral and tritocerebral regions of the brain, although the intensity of labeling is relatively faint and variable (Table 1). In the tritocerebrum, two pairs of cell bodies in clusters 15/16 label consistently, but are less intense than at later stages. In the protocerebrum, punctate labeling is found in the central body and protocerebral bridge, as well as the anterior and posterior median protocerebral neuropils (AMPN, PMPN). In contrast, there is an absence of staining in clusters 6, 8, and 9, the frontal (naupliar) eye (Eloffson, 2006), and in longitudinal medial fiber tracts. However, by E70–E75%, NOS immunoreactivity is much more extensive and culminates in maximal labeling at E85%; at that time, intensely labeled structures include fibers in clusters 6 (Table 1; Fig. 2A,B,E) and 8, neuropil in the lateral lobes of the PMPN (Fig. 2G), and longitudinal tracts that extend on the dorsal surface of the brain from the protocerebrum to the esophageal connectives (Fig. 2F). In the tritocerebrum, neurons in clusters 15/16 and 17 label lightly by E40%; the consistency and intensity of staining increases in these regions as development progresses (Table 1). In cell clusters 15/16 at E85%, several large cells label, and some of these appear as pairs within the cluster (Fig. 2F, arrowheads). At least one fiber emerging from CL 15/16 consistently labels and projects into the OL (Fig. 2G, arrow), and ascending NOS-immunoreactive fibers from these clusters also innervate the OGTN. There is a distinct decrease in NOS labeling in most brain areas, with the exception of the tritocerebrum, during the period just before hatching. Notably, NOS labeling is absent throughout embryonic development in the lateral antennular and antenna II neuropils (LAN, AnN II) and in the emerging ALs (Table 1).
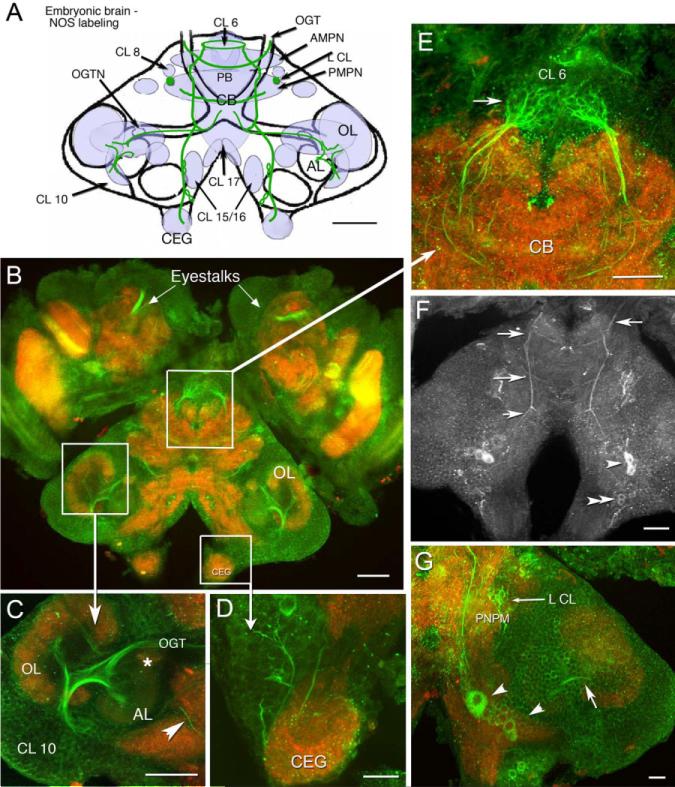
Fig. 2.
Distribution of NOS immunoreactivity in the embryonic brain at E85%. In all figures, preparations labeled with a single antibody are shown in black and white images; preparations labeled with multiple antibodies where more than one color channel is displayed, are illustrated in color. B–G: Stacked confocal images of whole-mounted brains that were double-labeled with anti-NOS antibody (green) and anti-synapsin antibody (red). F: NOS immunostaining alone. A: Schematic brain diagram, compressed in the dorsal–ventral plane, indicates neuropil regions and cell clusters that label for NOS (light blue shading) with major NOS-labeled tracts (green lines). Three horizontal sections at different depths in the brain illustrate details on the dorsal surface (F), more ventrally (G), and mid-level in the brain (B). B: Mid-level horizontal section of the brain, lateral protocerebrum (eyestalks), and circumesophageal ganglia (CEGs) show the extent of NOS labeling at E85%. C: In the lateral deutocerebrum, labeling is seen in fibers of the CL 10 cells that innervate the OL and AL and project to the protocerebrum in the OGT. A NOS-immunoreactive fiber (arrowhead) from cells in CL 15/16 (not shown) is also found in this region. The asterisk denotes the OGTN. D: An enlarged view of the CEG; cells and fibers descend from CL 15/16 into these ganglia. E: Ascending fibers form dense fine branches that innervate CL 6 (arrow). The base of the frontal eye is also labeled (arrowhead). F: On the dorsal surface of the brain, longitudinal, medial fibers extend across the entire brain with ladder-like branches at intervals (arrows). These branches project to the PB, AMPN, PMPN, and anteriorly to the esophagus. Several of the longitudinal processes appear to emerge from cell bodies in CL 15/16. The bilateral pairs of very dorsal cells (CL 15/16; arrowheads) label throughout embryonic and larval (Scholz et al., 1998) life. G: In this ventral section, large cells are seen in CL 15/16 (large arrowhead), some of which send fibers to the OL (arrow). The small arrow points to NOS immunoreactive fibers in L CL. For abbreviations, see list. Scale bars = 100 μm in A–C, 50 μm in D,E, 100 μm in F,50 μm in G.
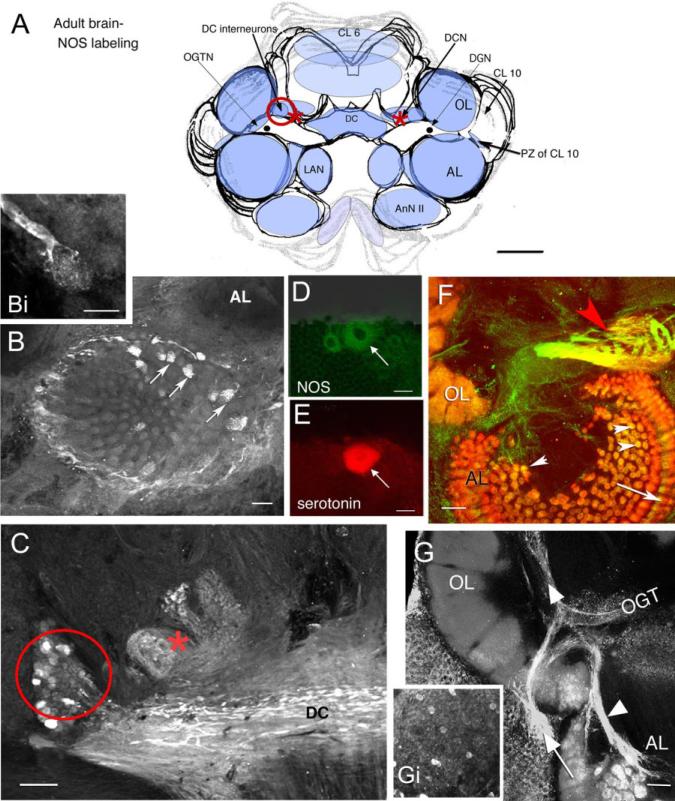
NOS immunoreactivity in most deutocerebral regions in the embryo appears later relative to protocerebral and tritocerebral areas, although it is in the deutocerebrum that the most intense staining persists during adult life (Table 1; Fig. 3). Immunoreactivity in the deutocerebrum becomes consistent and intense during the developmental plateau (Table 1, E85%). At that time, labeling is observed in tracts, cells, and synaptic regions associated with olfactory processing (i.e., the olfactory globular tract [OGT]; Fig. 2C) and its neuropil (the OGTN; Fig. 2C, asterisk), fibers and glomeruli primarily in the cortex of the OL (Fig. 2B,C), and fibers emerging from cell cluster 10 (Fig. 2C). By adult life, staining in these areas is intense and includes labeling in the DC interneurons of cluster 11 (Fig. 3A,C, red circle), DCN, especially the anterolateral half (Fig. 3C, red asterisk) and DC (Fig. 3A,C). Individual fibers from the DC project into the AL and innervate single AL glomeruli (Fig. 3B,Bi). The dorsal giant neuron (DGN), a serotonergic neuron (Fig. 3E) that innervates both the OLs and ALs, also labels for NOS (Fig. 3D); however, while staining for serotonin is consistently strong, NOS labeling is highly variable in this neuron. Whether this variability is associated with time of day, neuronal activity, or behavior is not known. Glomeruli in the accessory lobe, which do not label during embryonic life, stain intensely in the adult brain (Fig. 3B arrows, F arrowheads). The intensity of staining is not evenly distributed throughout the AL, but is often confined to a subset of glomeruli that can be clustered (Fig. 3B) or distributed throughout the AL. The bases of the cylindrical AL cortical glomeruli (Helluy et al., 1996) label consistently (Fig. 3F, arrow). As in the E85% embryos, the primary neurites of olfactory projection neurons (cluster 10; Fig. 3G, arrow) and their branches in the OLs and ALs (Fig. 3G, arrowhead) are intensely labeled. The antenna II neuropil and LAN also contain faint NOS immunoreactivity in the adult brain.
Fig. 3.
Confocal images of vibratome sections showing NOS, serotonin, and synapsin immunoreactivity in the adult Homarus brain. A: Schematic diagram indicates neuropil regions and cell clusters that label for NOS (light blue) with most intense NOS-labeled regions in a darker blue. The red circle (DC neurons: also in C), asterisk (DCN; also in C), and the proliferation zone (PZ) of CL 10 in the diagram indicate brain regions that correspond to labeled tissue shown in the images. B,Bi: A small number of AL glomeruli show NOS immunoreactivity (arrows). Higher magnification of a NOS-labeled process from a DC neuron ending in a glomerulus is shown in Bi. C: The DC fiber tract, the DC cell bodies (in red circle), and the lateral region of the DCN (asterisk) label for NOS. D,E: In a double-labeled section, NOS (D, green) and serotonin (E, red) colocalize in the DGN (arrows in D,E). F: The morphological characteristics of the OGTN (red arrowhead) are highlighted when colabeled for NOS (green) and synapsin (red). The OGTN (red arrowhead; double-labeled, yellow), AL glomeruli (white arrow heads), and base of the AL cortex (arrow) are labeled in this image. G: Fibers from the CL 10 neurons in the proliferation zone (arrow) innervating the OL and AL (arrowheads) and scattered cell bodies throughout CL 10 (Gi) are labeled. For abbreviations, see list. Scale bars = 3 mm in A; 100 μm in B,Bi,C,F,G, 50 μm in D,E.
Distribution of cGMP-like immunoreactivity
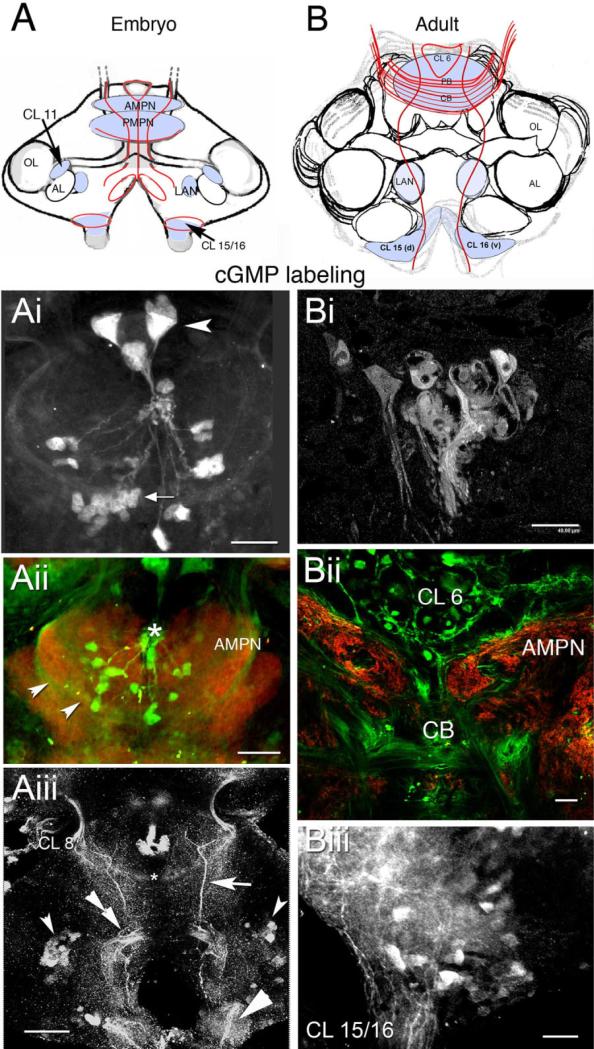
In an effort to visualize cGMP-dependent targets of the NOS-immunoreactive cells, antibodies were used to localize cGMP immunocytochemically. However, the only sites labeled for cGMP in the embryonic brain are a subset of cells that compose the frontal eye (Fig. 4Ai) in the protocerebrum. To increase the likelihood of identifying cGMP-dependent NO targets in embryos, levels of NO and cGMP were up-regulated in vitro using IBMX and SNP. When IBMX and SNP are combined in the incubation medium to maximize cGMP levels, cell bodies in clusters 11 and 15/16 (Fig. 4Aiii) label, as well as several fiber tracts: (1) a commissure caudal to the PMPN that projects between the eyestalks (Fig. 4Aiii, asterisk); (2) fibers from the eyestalk that end in cell cluster 8 (Fig. 4Aiii); (3) a tract that arborizes within the CB (seen also in the adult brain, Fig. 4Bii); (4) a set of cGMP-labeled fibers extending medially between cluster 6 and tritocerebral cell clusters 15/16 (Fig. 4Aiii, arrow); (5) a fan-shaped collection of labeled fibers in the deutocerebrum, just medial to cluster 11 (Fig. 4Aiii, double arrow); elements from this region project in the connectives to the commissural ganglia (Fig. 4Aiii; also in adults see Biii). The AMPN, PMPN, and LAN also label for cGMP in the embryonic brain. There is no cGMP labeling in the olfactory and accessory lobes, DC, and OGT in embryos.
Fig. 4.
Localization of cyclic 3,5 guanosine monophosphate (cGMP) in the embryonic (E85%) and adult brain when NO is up-regulated with SNP and IBMX. cGMP antibody is revealed in green in the double-labeled images. The colabel, synapsin (red), delineates brain structures. A,B: Schematic diagrams of embryonic (A) and adult (B) stages indicating neuropil regions and cell clusters that label for NOS (light blue). The red lines in A and B represent longitudinal fibers and fibers that cross the brain at the level of the PB and CB. Some fibers lie in the protocerebral tracts. Ai: By E85%, three components of the frontal eye (arrowhead) label. A second cluster of smaller photoreceptor cells also label (arrow). Aii: cGMP antibody photoreceptor cells and AMPN (punctate labeling, arrowheads). The asterisk marks the base of the naupliar eye. Aiii: Medial tracts (arrow) in the embryo (in the same location as NOS-labeled fibers at this same stage), as well as a commissure that projects to the eyestalks (asterisk), label for cGMP. Also stained are cells in CL 11 (small bilateral arrowheads) and 15/16 (large arrowhead). The double arrowhead marks the deutocerebral fan-shaped collection of fibers whose origin has not been identified. Bi: In the adult brain, photoreceptor cells label in the protocerebrum; the three large components of the frontal eye do not. Bii: In fibers in the CB, AMPN and CL 6 label. Biii: Labeled cells in CL 15/16 have fibers that extend into the connectives. For abbreviations, see list. Scale bars = 80 μm in Ai,Aiii, 50 μm in Aii, 40 μm in Bi, 50 μm in Bii,Biii.
There is no cGMP labeling in the adult brain in the absence of SNP and IBMX. However, when adult brains are incubated in these pharmacological agents, cGMP labeling is found in what appear to be (1) derivatives of the frontal eye (Fig. 4Bi), (2) AMPN (Fig. 4Bii), (3) PMPN, (4) LAN, (5) fibers projecting between cluster 6 and the tritocerebrum, and (6) cell bodies in clusters 15/16 (Fig. 4Biii). Cells in cluster 11 that labeled in embryonic brains do not label in the adult, suggesting that this staining is related to a developmental function.
Developmental Manipulations
From our localization studies, we know that NOS is found in the output pathway of those cluster 10 neurons that reside closest to the accessory lobe. This particular area of cluster 10 houses the proliferation zone where new cluster 10 neurons are born throughout life (Harzsch et al., 1999). NOS also is found in OL and AL glomeruli at specific times in development and in the midbrains of adult lobsters, suggesting that NOS plays a role in the developing olfactory pathway. We therefore tested the influence of embryonic NO levels on neurogenesis and glomerular formation and stabilization. Specifically, we asked whether the rate of neurogenesis responds differently to NO manipulations at various stages of embryonic development. Changes in NO levels were timed to coincide with particular developmental events in the lobster brain, such as the period when olfactory glomeruli form, or the early growth of the AL (see Fig. 1; Helluy et al., 1993). The production of new neurons was assayed using 5-bromo-2′-deoxyuridine (BrdU) labeling methods. Morphological changes in the brain were assessed using immunocytochemical labeling for synapsin and/or serotonin.
Regulation of NO levels and neurogenesis
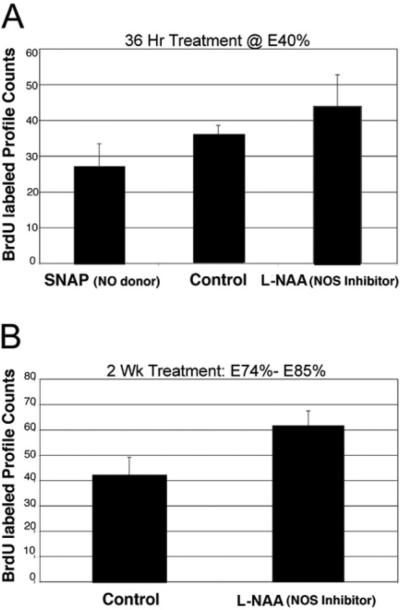
SNAP and L-NAA were used to upand down-regulate NO levels, respectively, at several embryonic stages and corresponding levels of neurogenesis assessed in cluster 10 during those periods. Incubation in SNAP for 3 days at E40% decreases neurogenesis in cluster 10 relative to control embryos incubated in saline alone; LNAA treatment for 3 days increases the rate of neurogenesis (Fig. 5A). This finding was also true in older embryos incubated in L-NAA for 2 weeks at E75–E85%, where L-NAA treatment caused an increase in the numbers of BrdU-labeled cells in cluster 10 (Fig. 5B), again indicating that NO is an inhibitor of neurogenesis in this system.
Fig. 5.
Manipulation of NO levels influences embryonic neurogenesis in cluster 10. A: Counts of 5-bromo-2′-deoxyuridine (BrdU)-labeled CL 10 cells for embryos at E40% incubated in SNAP and those incubated in L-NAA for 36 hr are compared with BrdU-labeled cell counts from embryos incubated in untreated lobster saline. Analysis of variance analysis of data and further t-tests reveal a significant difference among the three groups (n = 6 embryos/group; P < 0.05). B: Treatment of lobster embryos with L-NAA for 2 weeks beginning at stage E75%, resulted in a 33% increase in BrdU-labeled soma counts compared with embryos incubated in untreated saline. Student t-test reveals a significant difference between the two groups (n = 9 embryos/group; P < 0.001). For abbreviations, see list.
Longer incubations in these compounds were attempted to examine the influence of NO on neurogenesis during specific developmental events. For instance, embryos were incubated in SNAP and L-NAA from E45–E70%, which includes the period when olfactory glomeruli begin to form. The question here was whether the formation of the olfactory glomeruli would be delayed, advanced, or disrupted in any way by changes in NO levels. However, the embryos were particularly sensitive to prolonged treatment with SNAP and L-NAA during this early period, and animals began to deteriorate and die within a few days of the beginning of treatment, even when SNAP and L-NAA levels were reduced.
Influence of NO levels on morphogenesis in the olfactory pathway
When NO levels are manipulated during E75–E85%, there are several major morphological changes in the brain, mostly in the deutocerebrum. Down-regulation of NO with L-NAA results in clear and significant changes in the OLs and ALs: the olfactory glomeruli, which begin to develop at E40% and increase in number until the end of larval life (Helluy et al., 1996), lose their characteristic form (Fig. 6). We used antibodies raised against synapsin, a protein found in synapses that is important in the process of transmitter mobilization (Rosahl et al., 1995), to explore the effect of NO down-regulation on the morphogenesis of the crustacean olfactory glomeruli because we suspect that the period of synaptogenesis coincides with glomerular formation (Oland and Tolbert, 1996; see also Fig. 7).
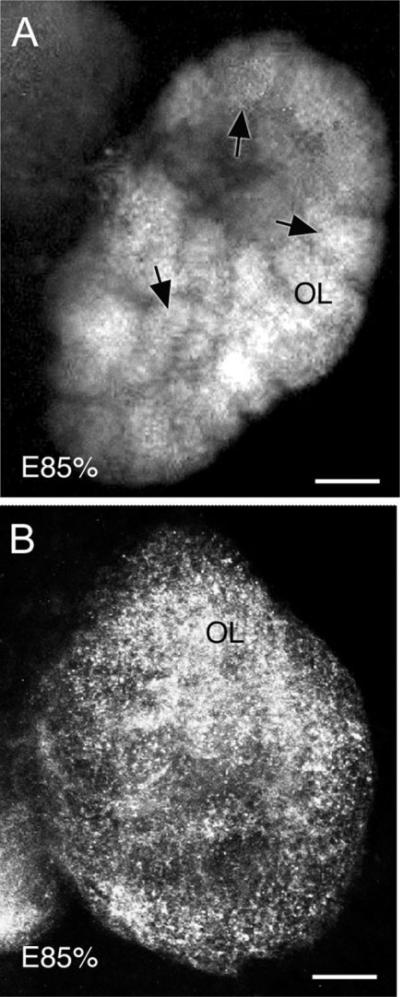
Fig. 6.
Stacked confocal images show that OL glomeruli, which had begun to form ∼E45%, are deconstructed following long-term NO inhibition during embryogenesis. Embryos at E75% were treated with L-NAA in saline for 2 weeks and dissections were then completed at E85%, followed by immunocytochemistry for synapsin. A: Control embryos (E85%) labeled for synapsin show the morphology of typical OL glomeruli (arrows) at this stage. B: L-NAA treatment of lobster embryos results in a marked decrease or complete loss of OL glomerular structure. Synapsin labeling is punctate and diffuse. For abbreviations, see list. Scale bars = 100 μm.
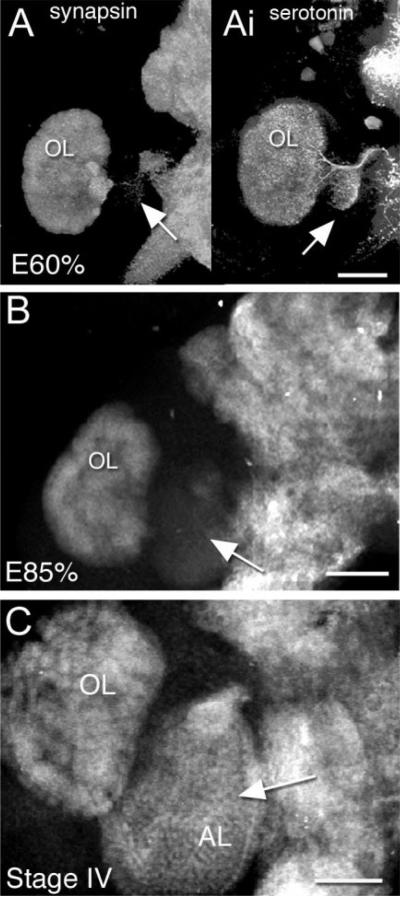
Fig. 7.
Stacked confocal images illustrate the developmental sequence of immunocytochemical labeling for synapsin in the accessory lobe during normal development (late embryonic through larval life shown). A: At E60%, there is no staining for synapsin in the AL (arrow), although the OL shows distinct labeling for this protein; however, labeling with antiserotonin antibody clearly outlines the morphology of both the OL and AL (Ai; arrow). B: At E85%, there is faint background-level labeling for synapsin (arrow) in the AL. The outline of the AL is difficult to see and glomerular structures are not present. C: The AL in a postembryonic stage IV lobster is labeled for synapsin (arrow) and shows the glomerular morphology that is typical at this stage. Scale bars = 50 μm in A,C, 200 μm in B.
The lobster olfactory lobes first label for synapsin during the mid-embryonic period when glomeruli begin to appear (E40–E45%; Helluy et al., 1996). However, when L-NAA treatment is applied at E75–E85%, the tissue condensations associated with glomeruli in the olfactory lobes disappear, although an evenly distributed punctate synapsin labeling persists throughout the OL (Fig. 6B).
Glomerular formation in the accessory lobes occurs much later than in the olfactory lobes, during larval, not embryonic life (Helluy et al., 1996). Nevertheless, as in the olfactory lobe, the onset of synapsin labeling in the accessory lobes also coincides with the first evidence of glomerular formation during the transition from the first to the second larval stage, and is intense by the fourth postembryonic stage (Fig. 7A–D). Unexpectedly, however, ALs in embryos treated with L-NAA at E85% to reduce NO levels exhibit precocious and intense labeling for synapsin (Fig. 8Bii).
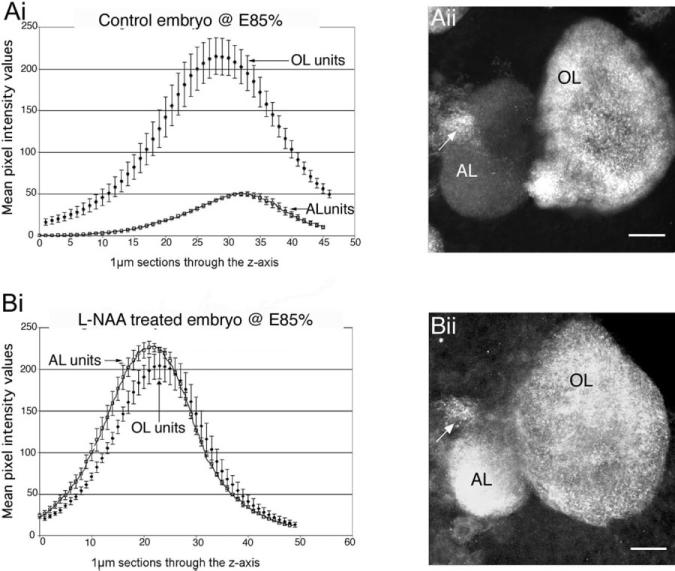
Fig. 8.
Aii: Synapsin labeling is not present in the accessory lobes in control embryos (see also Fig. 7A). Bii: However, after L-NAA treatment (down-regulation of NO) of E85% embryos, there is intense synapsin labeling in the AL. Ai, Bi: Graphs show the relative intensity levels of synapsin labeling in the OL and AL in embryonic brains at E85%; Control, n = 10 (Ai), NOS-inhibited, n = 10 (BI). Bi: Semiquantitative measurements demonstrate that the intensity of synapsin staining has indeed increased following L-NAA treatment and is comparable to the intensity of labeling in the OL. Each graph represents the average mean intensity values of the two lobes ± the SD through the z-axis of a stacked confocal image. Designated regions of interest in the confocal stacked images of the control (Ai) and NOS-inhibited (Bi) brains were analyzed with Leica software to produce the data for the graphs. The arrows point to the OGTN, which labels in both conditions. For abbreviations, see list.
Synapsin labeling in the OLs and ALs of untreated E85% embryos shows that labeling in the OLs is normally approximately 3 times more intense than in the ALs (Fig. 8Ai). Following a 2-week L-NAA treatment (E75–E85%), the intensity of synapsin labeling in the AL is similar to, or even greater, than that in the OL (Fig. 8Bi). It appears that lowered NO levels accelerate some aspects of accessory lobe development, but not glomerular formation.
Down-regulation of NO with L-NAA treatment for 2 weeks (at E75–E85% ), also increases the intensity of serotonin immunolabeling in the DGN, and increases the numbers of serotonin-immunoreactive cells around the DGN in cell cluster 11. These cells have been noted in earlier studies (Benton and Beltz, 2001a), but do not label reliably for serotonin in lobsters reared in laboratory conditions. Serotonin labeling within the olfactory and accessory lobes, however, decreases in intensity after L-NAA treatment.
NO and Response to Injury
Antennule ablation: increases in NOS labeling in the OGT
The data reported above have resulted from pharmacological treatments of lobster embryos with NOS inhibitors and NO donors. How might such changes in NO levels occur in the normal events and physiological changes during a lobster's life? One insight into how NO levels may be altered in vivo comes from studies where NOS labeling in the brain was assessed over a month-long period following unilateral antennule ablation. Damage or loss of the antennules, which contain the cell bodies and axons of the primary olfactory receptor neurons, can occur under natural conditions in these animals, and it is known that following antennule ablation in juvenile crayfish, the initial response is a reduction in the volume of the ipsilateral olfactory lobe due to the death of olfactory afferents. This size reduction is also associated with an increase in the numbers of apoptotic profiles among the local (cluster 11) and projection (cluster 10) neurons, resulting in first an overall decrease in numbers of interneurons in the olfactory pathway, and then later in time an increase that re-establishes the original number of interneurons (Sandeman et al., 1998).
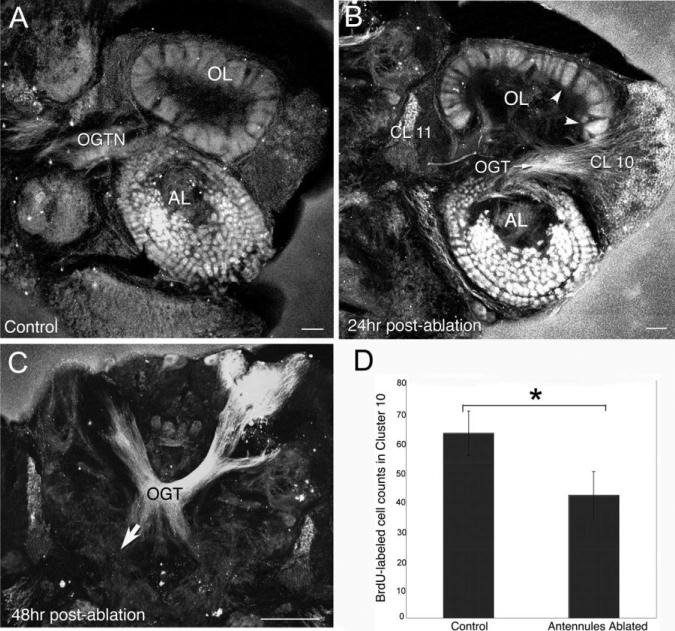
We labeled NOS in the brains of juvenile lobsters at intervals following unilateral antennule ablation. Four hours after ablation, the OGT and cells in clusters 9, 10, and 11 exhibit an increase in the intensity of NOS labeling; 24 hr after ablation, the general level of staining for NOS is even more intense, such that on the ablated side the AL and cluster 10 neurons are uniformly labeled (Fig. 9B). NOS labeling in the OGT in unablated juvenile animals is only detectable as it emerges from cluster 10 (as also is seen in the brains of embryos and adults; Figs. 2C, 3G), but is not visible in the more medial parts of the brain (Table 1). However, at 48 hr after unilateral ablation the OGT labels on both sides of the brain, consistent with the bilateral projection of the cluster 10 axons in this tract (Fig. 9C, arrow).
Fig. 9.
Levels of NOS in the olfactory pathway of juvenile lobsters increase after antennular ablation, while neurogenesis in cluster 10 decreases. A: Intense NOS labeling is found in the AL and OGTN in brains of juvenile lobsters with intact antennules. B: Twenty-four hours following unilateral ablation of the lateral antennular flagellum, intense NOS immunoreactivity is observed in the AL, OL, cell bodies and fibers of CL 10 neurons, and some cell bodies in cluster 11. C: By 48 hr following ablation, NOS staining has extended bilaterally in the OGT (arrow). D: In experiments where the lateral flagella of both antennules were ablated, corresponding changes in 5-bromo-2′-deoxyuridine (BrdU) incorporation in CL 10 were documented at 7 days after ablation. These counts revealed a 30% decrease in BrdU-labeled cells in the antennular-ablated lobsters compared with the unablated controls. Student t-tests reveal significant differences (P =< 0.0001) between the control and antennular-ablated groups. For abbreviations, see list. Scale bars = 100 μm in A,B; 50 μm in C.
Antennular ablation influences levels of neurogenesis in juvenile lobsters
Our studies in embryos where NO levels were manipulated and BrdU incorporation in cluster 10 cells was assessed, demonstrated that increased levels of NO result in decreased levels of neurogenesis (Fig. 5). Therefore, the increase in NOS labeling in the olfactory pathway following antennular ablation would be expected to correlate with decreased levels of neurogenesis among the cluster 10 neurons. To test this possibility, we ablated the lateral flagella of both antennules in juvenile lobsters and 1 week later incubated these animals in BrdU. We found a significant decrease (P < 0.0001) in the numbers of BrdU-labeled profiles in the cluster 10 proliferation zones of the antennuleablated animals. This result is consistent with the idea that NOS levels increase after antennular ablation in the olfactory pathway, and, either directly or indirectly, suppress neurogenesis among the olfactory projection neurons.
DISCUSSION
NOS and cGMP Localization
The distribution of NOS immunoreactivity in the lobster brain is distinctive for each embryonic stage that has been examined. The sequence of labeling and its spatial distribution suggest a requirement for NO early in development in the protocerebrum and tritocerebrum, and in the deutocerebrum later in embryonic development. Although intense NOS labeling in the deutocerebrum is a relatively late embryonic event, it is in these areas that NOS persists throughout juvenile and adult life. The intense staining of deutocerebral regions associated with the olfactory pathway is consistent with reports that NOS is concentrated in the primary olfactory centers in many species (Broillet and Firestein, 1996; Gelperin et al., 1996).
The onset of intense and widespread labeling in the brain at ∼E85% coincides with the embryonic developmental plateau (Helluy and Beltz, 1991). This period is characterized by a lack of growth in both the eye index and the cephalothoracic length, and is equivalent to stage D0 of the metanaupliar molt cycle that occurs during embryonic life. Molt cycles in postembryonic crustaceans are under hormonal (ecdysteroid) control, and it is likely that embryonic molts are regulated in a similar way. NO levels in the embryo may, therefore, be regulated by circulating hormones that coordinate the expansion of NOS expression with the ongoing molt cycle and impending eclosion. Evidence from insect systems also suggests that ecdysteroids are involved in regulating neurogenesis by means of an NO signaling pathway (Champlin and Truman, 2000).
NOS is not found in the accessory lobe, a higher order processing area in the deutocerebrum, until postembryonic stages. The accessory lobes undergo a pronounced increase in size during larval and early postlarval development (Helluy et al., 1995). In adult lobsters and crayfish, the ALs are composed of discrete glomeruli that are segregated into a cortex that is primarily concerned with integrating olfactory inputs, and a medullary region that processes visual and mechanosensory information (Sandeman et al., 1995; Sullivan and Beltz, 2005). In contrast to the embryonic formation of glomeruli in the olfactory lobes, glomerular formation in the accessory lobes is delayed until mid-larval life (Helluy et al., 1993, 1995). We have proposed (Helluy et al., 1996) that glomerular formation in the accessory lobes may be contingent on patterns of activity in visual, mechanosensory, and olfactory interneurons projecting to this area. Such activity may depend upon primary sensory processing areas as they respond to the first environmental input after hatching. If so, the coincidence of NOS labeling in the postembryonic accessory lobe glomeruli (Scholz et al., 1998) may be related to the relationships between these inputs and the formation and maturation of the accessory lobe glomeruli. Intense labeling of a selection of accessory lobe glomeruli is a feature of the adult brain, although which glomeruli and how many stain is highly variable. There appear to be at least three sources of NOS found in the glomeruli (1) DC neurons that project to accessory lobe glomeruli; (2) fibers of olfactory projection neurons, which are intensely labeled as they emerge from cluster 10 and project into the AL; and (3) the DGNs, which project ipsilaterally to the OLs and ALs and have fibers that innervate each and every glomerulus in these two regions (Sandeman and Sandeman, 1987; Benton and Beltz, 2001a). We have found that serotonin and NOS are colocalized in the DGNs. However, while the serotonin labeling is routinely intense, NOS labeling is highly variable, suggesting an intermittent expression of this enzyme.
The presence of cGMP labeling following treatment with an NO donor and IBMX suggests that, in many parts of the lobster brain, NO acts by means of a cGMP-mediated pathway. However in the olfactory pathway, the absence of cGMP labeling in the olfactory and accessory lobes suggests that NO action in these areas is accomplished by means of a different signaling pathway. In Manduca sexta, NO is found in the olfactory receptor axons that innervate the primary olfactory area, the antennal lobes (Gibson and Nighorn, 2000; Gibson et al., 2001). However, as in lobsters, cGMP production in moths is not induced in the antennal lobes by NO donors in combination with IBMX. Evidence in M. sexta suggests that soluble guanylate cyclase does not serve as the NO receptor in the antennal lobes, but that ADP-ribosylation may serve as the effector pathway instead (Gibson et al., 2001). Alternative downstream effectors of NO signaling in the lobster olfactory pathway have not yet been explored.
These studies, in combination with those of Scholz et al. (1998) on lobster larvae, suggest at least three specific roles for NO in the lobster brain: (1) interacting with the serotonergic system, (2) regulating levels of neurogenesis, and (3) directing specific morphogenetic changes. In addition, we would propose that NO has more general developmental roles, because NOS is localized in different brain regions at different developmental stages, and that the intensity of labeling waxes and wanes from one period to another.
Links between NO, Serotonin, and Neurogenesis
The increases in soma labeling intensities and altered morphologies of serotonergic cells following NOS down-regulation by L-NAA, as well as the colocalization of NO with serotonin in the DGNs, suggest that the nitrergic and serotonergic systems in the lobster brain are linked. A close relationship between these two systems has been noted before by Gibson et al. (2001), who observed that dendrites of an identified serotonergic neuron grew beyond their normal range following a reduction in NO levels. NO also regulates the release of serotonin from the hypothalamus in rats (Kaehler et al., 1999). Interactions in the opposite direction also have been documented by Ramos et al. (2002) and Tagliaferro et al. (2003), who found that serotonin depletion caused alterations in the nitrergic system in rats. Evidence from several systems, therefore, supports the conclusion that there is a strong interaction between nitrergic and serotonergic systems, and that this relationship may be bidirectional.
As a result of the apparent interdependence of the nitrergic and serotonergic systems, it is difficult to separate the direct effects of NO from potential indirect influences of NO by means of a serotonin-mediated pathway. For example, we believe that the DGN is one source of serotonin that stimulates neurogenesis (Beltz et al., 2001; Sullivan et al., 2007). We also have demonstrated in the present study that decreased levels of NO in embryos are associated with increased neurogenesis. Decreased levels of NO (through NOS inhibition) causes a significant increase in serotonin labeling in the soma of the DGN. Therefore, is the increased level of neurogenesis seen after NOS inhibition due to a direct effect of NO on the machinery producing new neurons, or is this effect due to an increase in serotonin levels that are responsible for regulating neurogenesis? If NO and serotonin act independently to alter neurogenesis, these two neuroactive compounds may provide a push–pull mechanism for up- and down-regulating the speed of the cell cycle and consequent neuronal production. Regardless of the specific mechanism, the colocalization of NOS and serotonin in the DGN leads to the possibility that the DGN can alter the rate of neurogenesis, depending upon the relative concentrations of the two substances and the timing of their release.
In contrast to our data that support a role for NO in suppressing neurogenesis, Cayre et al. (2005) have found that NO has a stimulatory effect on mushroom body neuroblast proliferation. Furthermore, they show that neural activity regulates NO production, as does environmental enrichment. Their data, therefore, also suggest a key role for NO in neuronal proliferation, but the direction of the influence is opposite to what has been observed in lobsters and most vertebrates, where increases in NO tend to suppress neuronal proliferation (Matarredona et al., 2005; Ciani et al., 2006; Romero-Grimaldi et al., 2006).
NO and Morphogenesis
Two observations suggest that nitric oxide is involved in morphogenesis in the deutocerebrum, and specifically in the olfactory pathway, during embryonic life. Down-regulating NO in mid- to late embryonic life results in a dissolution of the olfactory glomeruli that had begun to form at E45%. Concomitantly, synapsin labeling, which normally would not occur until larval life in the accessory lobes, appears precociously in these regions. Both results suggest that NO may be involved in the formation of glomeruli and synapses, and in the coordination of these two processes. These data are reminiscent of the studies of Gibson et al. (2001), where NOS is expressed in the axons of the olfactory receptor neurons projecting to all antennal lobe glomeruli. In M. sexta, normal glomerularization depends upon the in-growth of the olfactory receptor axons that form protoglomeruli, an event that is followed by migration of glia that encircle the protoglomeruli. When NO levels are reduced using a competitive inhibitor of NOS (L-NAME) during the period of active in-growth of the sensory axons to the antennal lobes, glomerular development is abnormal; this finding appears to be due to the failure of neuropil-associated glial cells in the antennal lobe to migrate, suggesting that NO in the receptor cells triggers glial cell migration. NO also appears to limit the arborization of serotonergic neurons in the antennal neuropil (Gibson et al., 2001).
Our finding that NOS inhibition during mid- to late embryonic life results in the dissolution of emerging olfactory lobe glomeruli suggests once again that NO is involved in signaling that underlies morphogenesis in these primary olfactory processing areas, although the cellular mechanisms underlying this effect are not known. It is possible that NO regulation of serotonin levels and arborization of serotonergic cells may contribute to these influences. However, in prior studies where serotonin levels were reduced for extended periods in lobster embryos, olfactory glomeruli formed at the expected time and appeared to be normal for that developmental stage in terms of number, size, and general organization as assessed at the light-microscopic level (Benton et al., 1997). It follows, therefore, that the change in glomerular morphology induced by L-NAA treatment in embryos is the result of the alteration in NO levels directly, or indirectly by downstream effectors, but is not mediated by a serotonergic pathway.
The appearance of strong synapsin labeling in the accessory lobe following NOS inhibition also suggests an intimate connection between NO and synaptogenesis. During the normal development of the lobster, the late embryonic period is characterized by a developmental plateau during which growth ceases. The plateau period can be of variable length, and it is thought that environmental stimuli are responsible for triggering the end of the plateau period and resumption of growth and development. During this plateau, neurogenesis slows or stops (Harzsch et al., 1999) and does not resume until after hatching and then only in restricted regions in the brain. That NOS expression intensifies during the plateau period suggests functions for NOS in the maturation of circuits that is presumably occurring during this time. That NOS inhibition accelerates the timing of synapsin immunoreactivity in the accessory lobe indicates that normal NOS signaling may suppress aspects of synaptic development, which normally occur during larval life and are coordinated with the formation of glomeruli in this area.
Overall, the influences of NOS on glomerular development in the olfactory lobe, synapsin expression in the accessory lobe and neurogenesis suggest that NO is important in coordinating these processes. Normal levels of NO in the deutocerebrum during the late embryonic plateau period presumably suppress neurogenesis as well as synapsin appearance in the accessory lobes, while permitting or promoting the development of the olfactory glomeruli. These types of influences for NO fit into an existing large literature of similar developmental influences in a wide range of organisms.
EXPERIMENTAL PROCEDURES
Animals
Lobster eggs (Homarus americanus) were obtained from the New England Aquarium Lobster Rearing Facility (Boston, MA), adult lobsters from a local fish market. At Wellesley College, lobsters of all stages were maintained in recirculating artificial sea-water at 14°C in a 12/12 light/dark cycle. Throughout these studies, embryos were staged using the Perkins eye index and other morphological criteria, where E0% is the time of fertilization and E100% is hatching (Helluy and Beltz, 1991).
Immunocytochemical Protocols
Brains and nerve cords were dissected in cold lobster saline (462 mM NaCl, 15.96 mM KCl, 26 mM CaCl2, 8 mM MgClβ, 11.11 mM glucose, and 10 mM Hepes, pH 7.4). Preparations were fixed in either 4% paraformaldeyhyde (PFA) for 12−24 hr, or for NOS labeling in ice-cold 90% methanol/10% formalin fixative (Sigma) for 15 min (similar to Ott and Elphick, 2002). After fixation and then rinsing in 0.1 M phosphate buffer + 0.3% Triton (PBTx), standard immunocytochemical methods were used to localize several antigens. Brains from adult lobsters were processed through the same steps as embryonic and juvenile tissue whole-mounts, except that after fixation and rinsing they were embedded in 6% Noble Agar and 100 μm sections produced by a Vibratome.
Primary antibodies diluted in PBTx were applied to tissues for a minimum of 16 hr at 4°C. Preparations were double-labeled with (a to c): a. Rabbit anti-uNOS (1:200; universal NOS, Affinity BioReagents, Golden, CO; No. PA1−039) and mouse anti-synapsin (1: 50, anti-Drosophila synapsin, SYNORF1, provided by E. Buchner); b. Rabbit anti-cGMP (1:400; provided by J. De Vente, EURON, Maastricht, The Netherlands) and mouse anti-synapsin (1:50; anti-Drosophila synapsin); c. Rabbit anti-serotonin (1:1,000; ImmunoStar, Hudson, WI; No. 20080) and anti-BrdU (1:50; Becton Dickinson, Biosciences Pharmingen, San Jose, CA; No. 347580 for the ablation studies). After primary antibody incubations and rinses in PBTx, the appropriate secondary antibodies (combinations of goat anti-rabbit and goat anti-mouse antibodies conjugated to Alexa 488 or Alex 594 fluorophors diluted 1:50 in PBTx; Molecular Probes) were applied overnight at 4°C. Subsequently, brains were rinsed in phosphate buffer, mounted with Gelmount (Biomeda), and viewed by laser scanning confocal microscopy.
cGMP
To localize NO-sensitive cGMP sites in tissues, dissected brains were incubated in lobster saline with the NO donor SNP (3 × 10−2 M, Axxora LLC San Diego, CA; No. ALX-400−001) and an inhibitor of phosphodiesterases, IBMX (5 × 10−4 M, Sigma; No.17018) or IBMX alone at 10°C for 15 min. The reaction was stopped by placing the tissue in 4% PFA. Tissues were subsequently processed immunocytochemically using rabbit anti-cGMP (de Vente et al., 1987; Scholz et al., 1998). In controls, SNP and IBMX were omitted from the incubation medium.
NOS localization experiments were conducted at six time points during embryonic development (E40%, E60%, E68%, E75%, E85%, and E95%; see n values on Table 1) and in the adult brain. cGMP was evaluated at E40% and E85% and in the adult brain.
Regulation of Endogenous NO Levels to Test Neurogenesis
Three groups of embryos at E40% were treated for 36 hr with: (1) the NOS inhibitor L-NAA (@ 1×−5 M in lobster saline, Axxora, NG-Nitro-L-arginine ALX-105−001; final n = ≥ 6/assay); (2) the NO-donor S-Nitroso-N-acetyl-D,L-penicillamine (SNAP @ 1 × 10−6 M in lobster saline, Axxora, No. ALX-420−003; final n = ≥ 6/assay); or (3) served as controls and were incubated in lobster saline alone (n = ≥ 6/assay). Treatments were refreshed twice daily to compensate for the half-life properties of the inhibitors and donors when in solution. At the end of the treatment periods embryos were staged, dissected, and processed immunocytochemically as described for colocalization or BrdU labeling.
A second NO regulation experiment that used more advanced embryos (E75%) also was conducted. Treatments with L-NAA or SNAP continued for 2 weeks with final dissections at E85%. Controls were incubated in lobster saline. Although this experiment also tested several different concentrations of SNAP, the embryos treated with this NO-donor experienced high mortality levels; hence, this treatment was discontinued, and data are not included.
BrdU Methods
Cells in S phase were identified by in vivo incorporation of the substitute nucleoside BrdU (Sigma; No.B5002). Eggs (@E40% or E85%) were incubated in saline, or saline with SNAP (10−6M in lobster saline, 14°C) or L-NAA (10−5 M in lobster saline, 14°C) for 36 hr or 2 weeks, respectively. Brains were then dissected, rinsed in phosphate buffer, treated with 2 N HCl, rinsed with PBTx, and incubated for 2.5 hr in mouse anti-BrdU antibody conjugated to Alexa 488 (1:20; Invitrogen Carlsbad, CA, No. A21303; n = 9). Brains were mounted as previously described.
BrdU-labeled specimens were assessed using a Leica TCS SP confocal microscope. Optical sections were taken at intervals of 0.5 μm or 1 μm and saved as three-dimensional stacks. BrdU-labeled cell profiles in cluster 10 in each optical section in the stacked series were traced onto a transparent sheet attached to the monitor and then counted. Data are presented as mean counts ± SD. Comparisons between control and treatment groups were performed by using Student's t-test or an analysis of variance as appropriate. A value of P < 0.05 was considered statistically significant.
Semiquantitative Assessment of Synapsin Levels
To define the magnitude of change in synapsin labeling in the olfactory (OL) and accessory (AL) lobes in response to NOS inhibition, the intensity of synapsin labeling in these regions was assessed semiquantitatively. Whole-mounted brains from control and L-NAA–treated embryos (E75–E85%; see above) that had been labeled immunocytochemically for synapsin were scanned with a Leica TCS SP confocal microscope after setting the laser intensities to fixed levels, and the images were analyzed using Leica software (Heidelberg, GmbH). The brightness of regions of interest (ROI), defined as 10-μm circles throughout the OLs and ALs, were determined using the stack profile analysis tool. Four ROIs per lobe were assigned to the most densely labeled areas of both the olfactory and accessory lobes. After the analysis tool was applied, one curve per ROI through the stack of images for each of the ROIs was displayed in a two-dimensional graph showing the median intensity of each ROI throughout the stacked image. The calculated values range from 0 to 255, corresponding to the intensity distribution statistics of the confocal image for every pixel in the ROI. The data also were imported to Excel software, where the mean average intensity for the ALs and the OLs in all brains analyzed were computed with standard deviations and graphed over the z-axis, which was defined by 1-μm sections throughout the depth of each OL and AL (see Fig. 8).
Antennular Ablations and BrdU Assays
To test whether NOS localization is altered by damage, the lateral antennular flagella of juvenile lobsters (seventh stage) were ablated unilaterally, and bilaterally to assess levels of BrdU incorporation in cluster 10. Brains were dissected and assayed immunocytochemically for NOS at 4, 24, and 48 hr after unilateral ablation. To examine levels of neurogenesis in cluster 10, lobsters were incubated in BrdU (2 mg/ml sea water; n = 5) for 4 hr, 7 days after the ablations. Unablated animals of the same size were used as controls (n = 5). Standard immunocytochemical methods were used for the detection of the BrdU (see above).
ACKNOWLEDGMENTS
We dedicate this paper to the memory of Stephen Benton, whose creativity and dedication to science continue to inspire us. We thank J. De Vente and E. Buchner for kindly providing antibodies; M. Goy and J. Sullivan for helpful discussions; Y. Kim, C. Kirkhart, Rosa Lafer-Sosa, and L. Murphy for piloting the antennular ablation experiments; M. Tlusty and A. Kim of the New England Aquarium for lobster rearing; and P. Carey and V. Quinan for technical assistance.
Grant sponsor: NIH; Grant number: 1R01 MH67157; Grant sponsor: NSF; Grant number: IBN 0344448; Grant sponsor: The Maren Foundation; Grant sponsor: Mount Desert Island Biological Laboratory.
ABBREVIATIONS
- AL
accessory lobe
- AMPN
anterior medial protocerebral neuropil
- AnNII
antenna II neuropil
- CB
central body
- CEG
circumesophageal (commissural) ganglion
- CL
cell cluster: 6 8 9 10 15/16 and 17
- DC
deutocerebral commissure
- DCN
deutocerebral commissure neuropil
- DGN
dorsal giant neuron
- LAN
lateral antennular neuropil
- L CL
lateral cluster
- OGT
olfactory globular tract
- OGTN
olfactory globular tract neuropil
- OL
olfactory lobe
- PB
protocerebral bridge
- PMPN
posterior medial protocerebral neuropil
- PZ of CL 10
proliferation zone of cluster 10
REFERENCES
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arth Struct Dev. 2003;32:39–60. doi: 10.1016/S1467-8039(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Helluy SM, Ruchhoeft ML, Gammill LS. Aspects of the embryology and neural development of the American lobster. J Exp Zool. 1992;261:288–297. doi: 10.1002/jez.1402610308. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transient uptake of serotonin by newborn olfactory projection neurons may mediate their survival. Proc Natl Acad Sci U S A. 2001;98:12730–12735. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton J, Beltz BS. Effects of embryonic serotonin depletion on olfactory interneurons in lobsters. J Neurobiol. 2001a;46:193–205. doi: 10.1002/1097-4695(20010215)46:3<193::aid-neu1002>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Benton J, Beltz BS. Serotonin, nitric oxide and neuronal proliferation in the olfactory pathway in lobsters. Soc Neurosci Abstr. 2001b;622:20. [Google Scholar]
- Benton J, Helluy S, Huber R, Beltz B. Serotonin depletion by 5,7-dihydroxytryptamine alters deutocerebral development in the lobster. J Neurobiol. 1997;33:357–373. doi: 10.1002/(sici)1097-4695(199710)33:4<357::aid-neu2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bicker G. STOP and GO with NO: nitric oxide as a regulator of cell motility in simple brains. Bioessays. 2005;27:495–505. doi: 10.1002/bies.20221. [DOI] [PubMed] [Google Scholar]
- Broillet MC, Firestein S. Gaseous second messengers in vertebrate olfaction. J Neurobiol. 1996;30:49–57. doi: 10.1002/(SICI)1097-4695(199605)30:1<49::AID-NEU5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Moro MA, Hurtado O, Leza JC, Lizasoain I. Dual role of nitric oxide in adult neurogenesis. Brain Res Brain Res Rev. 2005;50:1–6. doi: 10.1016/j.brainresrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Holstein GR, Martinelli GP, Forni C, Nicolas S, Aouane A, Strambi C, Strambi A. A role for nitric oxide in sensory-induced neurogenesis in an adult insect brain. Eur J Neurosci. 2005;21:2893–3902. doi: 10.1111/j.1460-9568.2005.04153.x. [DOI] [PubMed] [Google Scholar]
- Champlin DT, Truman JW. Ecdysteroid coordinates optic lobe neurogenesis via a nitric oxide signaling pathway. Development. 2000;127:3543–3551. doi: 10.1242/dev.127.16.3543. [DOI] [PubMed] [Google Scholar]
- Chen J, Tu Y, Moon C, Matarazzo V, Palmer AM, Ronnett GV. The localization of neuronal nitric oxide synthase may influence its role in neuronal precursor proliferation and synaptic maintenance. Dev Biol. 2004;269:165–182. doi: 10.1016/j.ydbio.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Christie AE, Edwards JM, Cherny E, Clason TA, Graubard K. Immunocytochemical evidence for nitric oxide- and carbon monoxide-producing neurons in the stomatogastric nervous system of the crayfish Cherax quadricarinatus. J Comp Neurol. 2003;467:293–306. doi: 10.1002/cne.10926. [DOI] [PubMed] [Google Scholar]
- Ciani E, Calvanese V, Crochemore C, Bartesaghi R, Contestabile A. Proliferation of cerebellar precursor cells is negatively regulated by nitric oxide in newborn rat. J Cell Sci. 2006;119:3161–3170. doi: 10.1242/jcs.03042. [DOI] [PubMed] [Google Scholar]
- Collmann C, Carlsson MA, Hansson BS, Nighorn A. Odorant-evoked nitric oxide signals in the antennal lobe of Manduca sexta. J Neurosci. 2004;24:6070–6077. doi: 10.1523/JNEUROSCI.0710-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vente J, Steinbusch HWM, Schipper J. A new approach to immunocytochemistry of 3′,5′-cyclic guanosine monophosphate: preparation, specificity, and initial application of a new antiserum against formaldehyde-fixed 3′,5′-cyclic guanosine monophosphate. Neuroscience. 1987;22:361–373. doi: 10.1016/0306-4522(87)90226-0. [DOI] [PubMed] [Google Scholar]
- Elofsson R. The frontal eyes of crustaceans. Arth Struct Dev. 2006;35:275–291. doi: 10.1016/j.asd.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Fuji S, Aonuma H, Ito I, Gelperin A, Ito E. The nitric oxide/cyclic GMP pathway in the olfactory processing system of the terrestrial slug Limax marginatus. Zoolog Sci. 2002;19:15–26. doi: 10.2108/zsj.19.15. [DOI] [PubMed] [Google Scholar]
- Gelperin A, Kleinfeld D, Denk W, Cooke IR. Oscillations and gaseous oxides in invertebrate olfaction. J Neurobiol. 1996;30:110–122. doi: 10.1002/(SICI)1097-4695(199605)30:1<110::AID-NEU10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gibbs SM. Regulation of neuronal proliferation and differentiation by nitric oxide. Mol Neurobiol. 2003;27:107–120. doi: 10.1385/MN:27:2:107. [DOI] [PubMed] [Google Scholar]
- Gibson NJ, Nighorn A. Expression of nitric oxide synthase and soluble guanylyl cyclase in the developing olfactory system of Manduca sexta. J Comp Neurol. 2000;422:191–205. doi: 10.1002/(sici)1096-9861(20000626)422:2<191::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Gibson NJ, Rossler W, Nighorn AJ, Oland LA, Hildebrand JG, Tolbert LP. Neuron-glia communication via nitric oxide is essential in establishing antennal-lobe structure in Manduca sexta. Dev Biol. 2001;240:326–339. doi: 10.1006/dbio.2001.0463. [DOI] [PubMed] [Google Scholar]
- Goy MF. Nitric oxide: an inhibitory retrograde modulator in the crustacean heart. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:151–163. doi: 10.1016/j.cbpb.2005.05.050. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Dawirs RR. Neurogenesis in the developing crab brain: postembryonic generation of neurons persists beyond metamorphosis. J Neurobiol. 1996;29:384–398. doi: 10.1002/(SICI)1097-4695(199603)29:3<384::AID-NEU9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the crustacean deutocerebrum. J Neurosci. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helluy SM, Beltz BS. Embryonic development of the American lobster (Homarus americanus): quantitative staging and characterization of an embryonic molt cycle. Biol Bull. 1991;180:355–371. doi: 10.2307/1542337. [DOI] [PubMed] [Google Scholar]
- Helluy SM, Sandeman RE, Beltz BS, Sandeman DC. Comparative brain ontogeny of the crayfish and clawed lobster: implications of direct and larval development. J Comp Neurol. 1993;335:343–354. doi: 10.1002/cne.903350305. [DOI] [PubMed] [Google Scholar]
- Helluy S, Ruchhoeft M, Beltz B. Development of the olfactory and accessory lobes in the American lobster: an allometric analysis and its implications for the deutocerebral structure of decapods. J Comp Neurol. 1995;358:1–13. doi: 10.1002/cne.903570308. [DOI] [PubMed] [Google Scholar]
- Helluy S, Benton J, Ruchhoeft M, Langworthy K, Beltz B. Glomerular formation in the developing olfactory and accessory lobes of the American lobster: stabilization of numbers and increase in size after metamorphosis. J Neurobiol. 1996;29:459–472. doi: 10.1002/(SICI)1097-4695(199604)29:4<459::AID-NEU4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Johansson KU, Carlberg M. NADPH-diaphorase histochemistry and nitric oxide synthase activity in deutocerebrum of the crayfish, Pacifastacus leniusculus (Crustacea, Decapoda). Brain Res. 1994;649:36–42. doi: 10.1016/0006-8993(94)91046-4. [DOI] [PubMed] [Google Scholar]
- Johansson KU, Mellon D., Jr Nitric oxide as a putative messenger molecule in the crayfish olfactory midbrain. Brain Res. 1998;807:237–242. doi: 10.1016/s0006-8993(98)00826-9. [DOI] [PubMed] [Google Scholar]
- Kaehler ST, Singewald N, Sinner C, Philippu A. Nitric oxide modulates the release of serotonin in the rat hypothalamus. Brain Res. 1999;835:346–349. doi: 10.1016/s0006-8993(99)01599-1. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Adult neurogenesis: stem cells and neuronal development in the adult brain. Oxford University Press; New York: 2005. [Google Scholar]
- Kuzin B, Regulski M, Stasiv Y, Scheinker V, Tully T, Enikolopov G. Nitric oxide interacts with the retinoblastoma pathway to control eye development in Drosophila. Curr Biol. 2000;10:459–462. doi: 10.1016/s0960-9822(00)00443-7. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Zhang R, Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg. 2003;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Role of nitric oxide in subventricular zone neurogenesis. Brain Res Brain Res Rev. 2005;49:355–366. doi: 10.1016/j.brainresrev.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez B, Gonzalez-Forero D. Nitric oxide and synaptic dynamics in the adult brain: physiopathological aspects. Rev Neurosci. 2006;17:309–357. doi: 10.1515/revneuro.2006.17.3.309. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Multiple factors shape development of olfactory glomeruli: insights from an insect model system. J Neurobiol. 1996;30:92–109. doi: 10.1002/(SICI)1097-4695(199605)30:1<92::AID-NEU9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ott SR, Elphick MR. Nitric oxide synthase histochemistry in insect nervous systems: methanol/formalin fixation reveals the neuroarchitecture of formaldehyde-sensitive NADPH diaphorase in the cockroach Periplaneta americana. J Comp Neurol. 2002;448:165–185. doi: 10.1002/cne.10235. [DOI] [PubMed] [Google Scholar]
- Ramos AJ, Tagliaferro P, Lopez-Costa JJ, Lopez EM, Pecci Saavedra J, Brusco A. Neuronal and inducible nitric oxide synthase immunoreactivity following serotonin depletion. Brain Res. 2002;958:112–121. doi: 10.1016/s0006-8993(02)03489-3. [DOI] [PubMed] [Google Scholar]
- Romero-Grimaldi C, Gheusi G, Lledo P-M, Estrada C. Chronic inhibition of nitric oxide synthesis enhances both subventricular zone neurogenesis and olfactory learning in adult mice. Eur J Neurosci. 2006;24:2461–2470. doi: 10.1111/j.1460-9568.2006.05127.x. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Sandeman DC. Serotonin-like immunoreactivity of giant olfactory neurons in the crayfish brain. Brain Res. 1987;403:371–374. doi: 10.1016/0006-8993(87)90078-3. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biol Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Beltz B, Sandeman RE. Crayfish brain interneurons that converge with serotonin giant cells in the accessory lobe glomeruli. J Comp Neurol. 1995;352:263–279. doi: 10.1002/cne.903520209. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Clarke D, Sandeman DC, Manly M. Growth-related and antennular amputation-induced changes in the olfactory centers of crayfish brain. J Neurosci. 1998;18:6195–6206. doi: 10.1523/JNEUROSCI.18-16-06195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus maenas. Brain Res. 1997;762:131–143. doi: 10.1016/s0006-8993(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Scholz NL, Chang ES, Graubard K, Truman JW. The NO/cGMP pathway and the development of neural networks in postembryonic lobsters. J Neurobiol. 1998;34:208–226. doi: 10.1002/(sici)1097-4695(19980215)34:3<208::aid-neu2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Scholz NL, de Vente J, Truman JW, Graubard K. Neural network partitioning by NO and cGMP. J Neurosci. 2001;21:1610–1618. doi: 10.1523/JNEUROSCI.21-05-01610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz NL, Labenia JS, de Vente J, Graubard K, Goy MF. Expression of nitric oxide synthase and nitric oxide-sensitive guanylate cyclase in the crustacean cardiac ganglion. J Comp Neurol. 2002;454:158–167. doi: 10.1002/cne.10442. [DOI] [PubMed] [Google Scholar]
- Sinner C, Kaehler ST, Philippu A, Singewald N. Role of nitric oxide in the stress-induced release of serotonin in the locus coeruleus. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:105–109. doi: 10.1007/s002100100428. [DOI] [PubMed] [Google Scholar]
- Stein W, Eberle CC, Hedrich UB. Motor pattern selection by nitric oxide in the stomatogastric nervous system of the crab. Eur J Neurosci. 2005;21:2767–2781. doi: 10.1111/j.1460-9568.2005.04117.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J Neurobiol. 2005;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Beltz BS. Serotonin depletion in vivo inhibits the branching of olfactory projection neurons in the lobster deutocerebrum. J Neurosci. 2000;20:7716–7721. doi: 10.1523/JNEUROSCI.20-20-07716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Benton JL, Beltz BS. Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons. J Mol Histol. 2007 doi: 10.1007/s10735-007-9112-7. [Epub ahead of print, PMID: 17624620] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunico CR, Portillo F, Gonzalez-Forero D, Moreno-Lopez B. Nitric oxide-directed synaptic remodeling in the adult mammalian CNS. J Neurosci. 2005;25:1448–1458. doi: 10.1523/JNEUROSCI.4600-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Ramos AJ, Lopez-Costa JJ, Lopez EM, Brusco A. Changes in the postnatal development on nitric oxide system induced by serotonin depletion. Brain Res Dev Brain Res. 2003;146:39–49. doi: 10.1016/j.devbrainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- Truman JW, De Vente J, Ball EE. Nitric oxide-sensitive guanylate cyclase activity is associated with the maturational phase of neuronal development in insects. Development. 1996;122:3949–3958. doi: 10.1242/dev.122.12.3949. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50:602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]