Abstract
The phosphine-mediated [3 + 3] annulations of aziridines and allenes are experimentally simple reactions, run under very mild conditions, for the preparation of highly functionalized tetrahydropyridines in yields of up to 98% and trans/cis ratios of up to 97:3. In addition to steps that are typical of nucleophilic phosphine-catalyzed reactions of allenoates, the mechanism of this new reaction features apparent nucleophilic aromatic substitution and concomitant desulfonylation, hitherto unknown processes during phosphine-promoted cycloaddition reactions. Notably, these reactions are the first reported examples of aziridines as reaction partners in nucleophilic phosphine-catalyzed transformations.
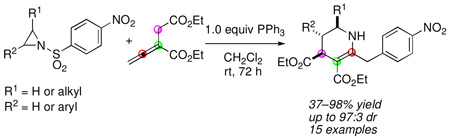
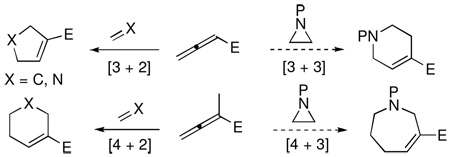
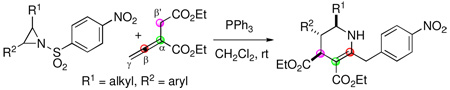
Intermolecular cycloadditions are powerful reactions for synthesizing carbo- and heterocycles from simpler starting materials.1 The development of many transition metal- and organomolecule-promoted cycloadditions has expanded the scope of starting materials that can be engaged in such transformations.2 Nucleophilic phosphine catalysis is now established as a reliable platform for cycloadditions employing activated allenes as one of the reaction partners.3 In phosphine-promoted processes, allenes typically react as three- and four-carbon synthons in [3 + 2] and [4 + 2] additions, respectively, with alkenes or imines.4 Based on this behavior, we pondered the possibility of employing aziridines as a three-atom components in [4 + 3] and [3 + 3] annulations with allenoates (Eq 1).
 |
(1) |
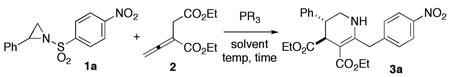
While [4 + 2] and [3 + 2] cycloadditions are used widely in organic synthesis, only a limited number of [3 + 3] additions have been reported to date.5 Aziridines contain one of the most valuable three-membered ring systems in modern synthetic chemistry; they are extremely versatile synthetic building blocks.6 Although formal [3 + 3] cycloadditions of aziridines with Pdtrimethylenemethane (TMM) species have been used to furnish piperidines,5b the phosphonium enolate zwitterionic intermediate7 has not been employed previously for coupling with aziridines. Herein, we describe the development of a new phosphine-promoted [3 + 3] annulation of aziridines with allenoates to afford highly functionalized tetrahydropyridines under simple and mild conditions (Eq 2).
 |
(2) |
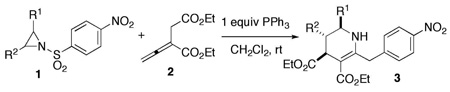
Initially, we examined the reaction of N-nosylaziridine 1a and diethyl 2-vinylidenesuccinate (2) with PBu3 (20 mol%) at room temperature (Table 1, entry 1).8 Although the aziridine was consumed completely within 24 h, we obtained no product from its coupling with the allenoate. Given that the aziridine ring can be opened directly through nucleophilic attack,9 we employed the weaker nucleophile PPh3 to take advantage of its more discerning reactivity.10 To our delight, we isolated the [3 + 3] adduct 3a in modest yield and excellent diastereoselectivity (trans/cis, 10:1; entry 2).11 Surprisingly, the three carbon atoms constituting the tetrahydropyridine 3a were the α, β, and β´ carbon atoms of the starting allenoate rather than the α, β, and γ carbon atoms encountered in well-established phosphine-mediated [3 + 2] annulations.4 Moreover, the p-nitrobenzene ring was attached to the γ carbon atom of the starting allenoate, with an apparent loss of SO2 (vide infra). The reaction yield improved significantly, without erosion of the diastereoselectivity, after increasing the amount of PPh3 (entry 3). Although NMR spectroscopy revealed that some free phosphine remained after the reaction, we added 1 equiv of phosphine to expedite the reaction (entry 4); more than 1 equiv of PPh3 did not improve the reaction efficiency (entry 5). The reaction was best run in CH2Cl2 (entry 6) and at room temperature; the product decomposed at elevated temperatures (entry 7). Other tertiary phosphines did not facilitate the reaction as well as PPh3 did (entries 8–11).
Table 1.
Phosphine-Mediated [3 + 3] Aziridine/Allene Annulationa
 | ||||
|---|---|---|---|---|
| entry | PR3 | mol% | yield (%)b | dr (trans/cis)c |
| 1 | PBu3 | 20 | 0 | N/A |
| 2 | PPh3 | 20 | 15 | 10:1 |
| 3 | PPh3 | 50 | 37 | 9:1 |
| 4 | PPh3 | 100 | 73 | 9:1 |
| 5 | PPh3 | 200 | 61 | 9:1 |
| 6d | PPh3 | 100 | 63 | 9:1 |
| 7e | PPh3 | 100 | 48 | 9:1 |
| 8 | EtPPh2 | 100 | 2 | – |
| 9 | Et2PPh | 100 | 0 | N/A |
| 10 | P(NMe2)3 | 100 | 0 | N/A |
| 11 | P(OEt)3 | 100 | 0 | N/A |
All reactions were performed using 0. 1 mmol of 1a and 4.8 equiv of 2 in CH2Cl2 at rt for 72 h, unless otherwise specified.
Isolated yield after chromatographic purification.
Diastereoisomeric ratio determined through HPLC (internal standard: 2-bromopyridine).
1,2-Dichloroethane as solvent; other common organic solvents provided isolated product yields of less than 10%.
40 °C, 48 h.
We examined a range of aziridine derivatives for their [3 + 3] annulations under the optimized reaction conditions (Table 2). Aryl-substituted aziridines underwent the reaction in good to excellent yield with good 1,2-trans-diastereoselectivity; phenyl groups featuring electron-withdrawing or -donating substituents at the ortho, meta, and para positions worked well (entries 1–11), as did a naphthyl group (entry 12). Interestingly, the alkyl-substituted aziridine 1n provided a different regioisomeric tetrahydropyridine with diminished diastereoselectivity, favoring the formation of the 1,3-cis-product 3n (entry 13).11 Whereas aryl-substituted C–N bonds of aziridines are polarized for nucleophilic fission, alkyl-substituted carbon atoms block direct nucleophilic attack. The unsubstituted aziridine substrate provided a poorer yield (entry 14), presumably because it is more susceptible to phosphine-mediated direct ring opening, leading to undesired side products.9
Table 2.
Syntheses of Tetrahydropyridinesa
 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | product | yield (%)b |
dr (trans/cis)c |
| 1 | H (1b) | 2-MeC6H4 | 3b | 88 | 90:10 |
| 2 | H (1c) | 3-MeC6H4 | 3c | 82 | 86:14 |
| 3 | H (1d) | 4-MeC6H4 | 3d | 64 | 89:11 |
| 4 | H (1e) | 2,4-Me2C6H3 | 3e | 82 | 81:19 |
| 5 | H (1f) | 2,5-Me2C6H3 | 3f | 98 | 92:8 |
| 6 | H (1g) | 4-FC6H4 | 3g | 76 | 88:12 |
| 7 | H (1h) | 2-ClC6H4 | 3h | 46 | 97:3 |
| 8 | H (1i) | 3-ClC6H4 | 3i | 86 | 83:17 |
| 9 | H (1j) | 4-ClC6H4 | 3j | 84 | 90:10 |
| 10 | H (1k) | 3-BrC6H4 | 3k | 58 | 85:15 |
| 11 | H (1l) | 4-BrC6H4 | 3l | 75 | 88:12 |
| 12 | H (1m) | 2-naphthyl | 3m | 58 | 85:15 |
| 13 | Me (1n) | H | 3n | 66 | 41:59 |
| 14 | H (1o) | H | 3o | 37 | N/A |
All reactions were performed using 0.1 mmol of the aziridine and 4.8 equiv of the allenoate.
Isolated yields.
Diastereoisomeric ratio determined using HPLC (internal standard: 2-bromopyridine).
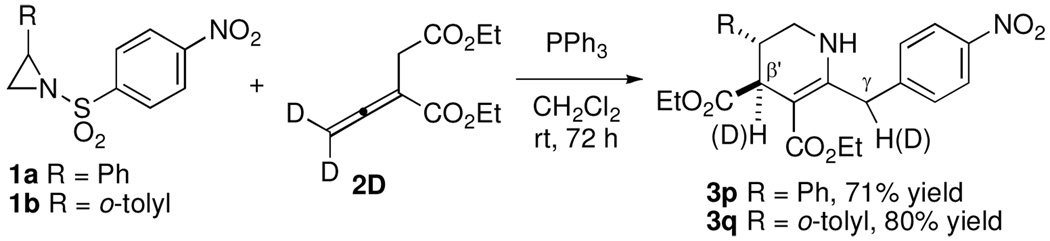
To better understand the mechanisms of these intriguing processes, we subjected the deuterium-labeled allenoate 2D (92% D) to [3 + 3] annulations with the aziridines 1a and 1b (Scheme 1). We obtained the tetrahydropyridines 3p and 3q in yields comparable with those of the non-deuterated allenoate 2, but with 19 and 25% deuterium at the γ carbon atoms of 3p and 3q, respectively, and 27% deuterium at their β´ carbon atoms. The loss of deuterium content at the γ carbon atom and its incorporation at the β´ carbon atom suggests the formation of intermediates featuring carbanions located at both carbon atom centers. The decrease in the overall deuterium content was due to the presence of adventitious water, which facilitated the intermolecular proton transfer processes.12
Scheme 1.
Deuterium Labeling Experiments
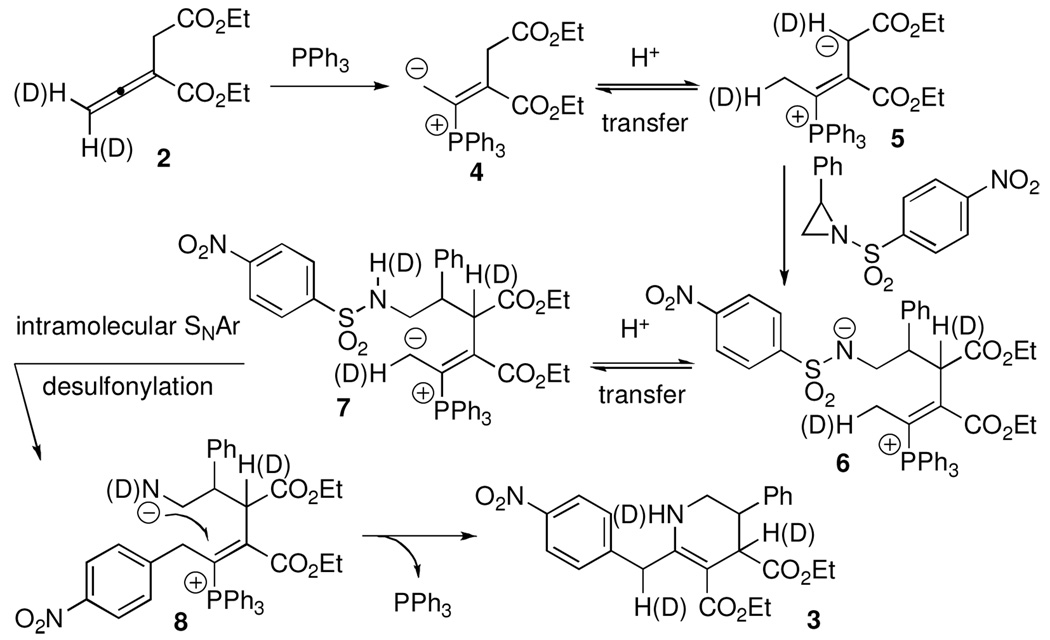
Based on these observations, Scheme 2 presents our suggested reaction mechanism. Addition of PPh3 to the allenoate forms the intermediate 4, which undergoes proton transfer to give the vinylogous ylide 5.4k Aziridine ring opening occurs at the β´ carbon atom to furnish the intermediate 6.13 Sulfonamide/dienolate equilibrium4i provides the intermediate 7, which undergoes intramolecular14 nucleophilic aromatic substitution and concomitant desulfonylation.15 Subsequent conjugate addition and β-elimination of PPh3 generates the tetrahydropyridine product 3.16
Scheme 2.
Suggested Mechanism for the Formation of Tetrahydropyridine 3
In summary, we have developed a phosphine-mediated [3 + 3] cycloaddition annulation manifold for allenes, incorporating, for the first time, aziridine derivatives as reaction partners. The reaction is operationally simple and produces highly functionalized tetrahydropyridines in good to excellent yield with high levels of diastereoselectivity. The allenoate provides its α, β, and β´ carbon atoms in the [3 + 3] cycloaddition—a new mode of reactivity for this versatile class of molecules. The mechanism includes apparent intramolecular nucleophilic aromatic substitution and extrusion of SO2; this unprecedented behavior expands the reaction repertoire of nucleophilic phosphine catalysis. We are currently exploring the further applications of azidirines in nucleophilic phosphine catalysis.
Supplementary Material
Representative experimental procedures and spectral data for all new compounds (PDF). Crystallographic data for 3a and 3n (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This study was funded by the NIH (R01GM071779 and P41GM081282). We thank Dr. Saeed Khan for performing the crystallographic analyses.
References
- 1.The Chemical Abstracts Service (CAS) database lists 16 books and 1918 reviews on cycloaddition. For a classical review, see: Padwa A. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 4. New York: Pergamon; 1991. p. 1069.
- 2.(a) Li JJ, Gribble GW. Palladium in Heterocyclic Chemistry. New York: Pergamon; 2000. [Google Scholar]; (b) Barluenga J, Santamaria J, Tomas M. Chem. Rev. 2004;104:2259. doi: 10.1021/cr0306079. [DOI] [PubMed] [Google Scholar]; (c) Nakamura I, Yamamoto Y. Chem. Rev. 2004;104:2127. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]
- 3.Reviews: Lu X, Zhang C, Xu Z. Acc. Chem. Res. 2001;34:535. doi: 10.1021/ar000253x. Valentine DH, Hillhouse JH. Synthesis. 2003:317. Methot JL, Roush WR. Adv. Synth. Catal. 2004;346:1035. Lu X, Du Y, Lu C. Pure Appl. Chem. 2005;77:1985. Nair V, Menon RS, Sreekanth AR, Abhilash N, Biji AT. Acc. Chem. Res. 2006;39:520. doi: 10.1021/ar0502026. Denmark SE, Beutner GL. Angew. Chem., Int. Ed. 2008;47:1560. doi: 10.1002/anie.200604943. Ye L-W, Zhou J, Tang Y. Chem. Soc. Rev. 2008;37:1140. doi: 10.1039/b717758e.
- 4.For representative examples of [3 + 2] cycloadditions, see: Zhang C, Lu X. J. Org. Chem. 1995;60:2906. Zhu G, Chen Z, Jiang Q, Xiao D, Cao P, Zhang X. J. Am. Chem. Soc. 1997;119:3836. Wang J-C, Krische MJ. Angew. Chem., Int. Ed. 2003;42:5855. doi: 10.1002/anie.200352218. Zhu X-F, Henry CE, Kwon O. Tetrahedron. 2005;61:6276. Wilson JE, Fu GC. Angew. Chem., Int. Ed. 2006;45:1426. doi: 10.1002/anie.200503312. Cowen BJ, Miller SJ. J. Am. Chem. Soc. 2007;129:10988. doi: 10.1021/ja0734243. Fang Y-Q, Jacobsen EN. J. Am. Chem. Soc. 2008;130:5660. doi: 10.1021/ja801344w. Voituriez A, Panossian A, Fleury-Bregeot N, Retailleau P, Marinetti A. J. Am. Chem. Soc. 2008;130:14030. doi: 10.1021/ja806060a. For representative examples of [4 + 2] cycloadditions, see: Zhu X-F, Lan J, Kwon O. J. Am. Chem. Soc. 2003;125:4716. doi: 10.1021/ja0344009. Wurz RP, Fu GC. J. Am. Chem. Soc. 2005;127:12234. doi: 10.1021/ja053277d. Tran YS, Kwon O. J. Am. Chem. Soc. 2007;129:12632. doi: 10.1021/ja0752181.
- 5.The CAS database contains 39,204 references relating to cycloadditions, but only 93 of them relate to [3 + 3] cycloadditions. For reviews on formal [3 + 3] cycloadditions, see: Hsung RP, Kurdyumov AV, Sydorenko N. Eur. J. Org. Chem. 2005:23. and references therein. Harrity JPA, Provoost O. Org. Biomol. Chem. 2005;3:1349. doi: 10.1039/b502349c. For recent examples, see: Young IS, Kerr MA. Angew. Chem., Int. Ed. 2003;42:3023. doi: 10.1002/anie.200351573. Sibi MP, Ma Z, Jasperse CP. J. Am. Chem. Soc. 2005;127:5764. doi: 10.1021/ja0421497. Movassaghi M, Chen B. Angew. Chem., Int. Ed. 2007;46:565. doi: 10.1002/anie.200603302. Chan A, Scheidt KA. J. Am. Chem. Soc. 2007;129:5334. doi: 10.1021/ja0709167. Shintani R, Park S, Duan W-L, Hayashi T. Angew. Chem., Int. Ed. 2007;46:5901. doi: 10.1002/anie.200701529.
- 6.For recent leading reviews on the synthesis and application of aziridines, see: Müller P, Fruit C. Chem. Rev. 2003;103:2905. doi: 10.1021/cr020043t. Hu XE. Tetrahedron. 2004;60:2701. Tang Y, Ye S, Sun X-L. Synlett. 2005:2720. Watson IDG, Yu L, Yudin AK. Acc. Chem. Res. 2006;39:194. doi: 10.1021/ar050038m. Singh GS, D’hooghe M, De Kimpe N. Chem. Rev. 2007;107:2080. doi: 10.1021/cr0680033.
- 7.Zhu X-F, Henry CE, Kwon O. J. Am. Chem. Soc. 2007;129:6722. doi: 10.1021/ja071990s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.N-Tosylaziridine was unreactive under the reaction conditions; N-(onosyl) aziridine led to a complex mixture of products.
- 9.Hou XL, Wu J, Fan RH, Ding CH, Luo ZB, Dai LX. Synlett. 2006:181. and references therein. [Google Scholar]
- 10.Henry CE, Kwon O. Org. Lett. 2007;9:3069. doi: 10.1021/ol071181d. [DOI] [PubMed] [Google Scholar]
- 11.The structures of 3a and 3n were established unequivocally through X-ray crystallographic analyses. See the Supporting Information for details.
- 12.(a) Xia Y, Liang Y, Chen Y, Wang M, Jiao L, Huang F, Liu S, Li Y, Yu Z-X. J. Am. Chem. Soc. 2007;129:3470. doi: 10.1021/ja068215h. [DOI] [PubMed] [Google Scholar]; (b) Mercier E, Fonovic B, Henry C, Kwon O, Dudding T. Tetrahedron Lett. 2007;48:3617. [Google Scholar]; (c) Ref. 4g
- 13.When we employed an enantiomerically pure aziridine, we obtained an optically pure tetrahydropyridine product. See the Supporting Information for details.
- 14.We obtained no crossover product when we reacted the allenaote 2 with a 1:1 mixture of a doubly (deuterium) labeled aziridine and a regular aziridine under the optimized reaction conditions. See the Supporting Information for details.
- 15.(a) Naito T, Dohmori R, Nagase O. J. Pharm. Soc. Jpn. 1954;74:593. [Google Scholar]; (b) Fukuyama T, Jow C-K, Cheung M. Tetrahedron Lett. 1995;36:6373. [Google Scholar]; (c) Meng Q, Thibblin A. J. Am. Chem. Soc. 1997;119:1224. [Google Scholar]; (d) Wilson MW, Ault-Justus SE, Hodges JC, Rubin JR. Tetrahedron. 1999;55:1647. [Google Scholar]
- 16.We suspect that the deuterium atom on the enamine nitrogen atom is exchanged to a hydrogen atom in the solvent used for NMR spectroscopy; see the Supporting Information for the results of an enamine H/D exchange experiment. Another possible mechanism explaining the loss of the enamine deuterium atom is enamine/imine tautomerization. The solid state structure of 3a reveals that the ring C(sp2)–N and C=C bond distances were 1.343 and 1.378 Å, respectively; i.e., they deviate from typical values, indicating a potential enamine/imine mixture. Typical C(sp2)–N, C=N, C=C, and C(sp3)–C(sp2) bond distances are 1.416, 1.279, 1.322, and 1.507 Å, respectively; see: Lide DR, editor. CRC Handbook of Chemistry and Physics. 78th ed. Boca Raton, NY: CRC Press; 1997. Section 9.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative experimental procedures and spectral data for all new compounds (PDF). Crystallographic data for 3a and 3n (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.