Abstract
Mycobacterium tuberculosis remains a widespread and devastating human pathogen, whose ability to infiltrate macrophage host cells from the human immune system is an active area of investigation. We have recently reported the discovery of a novel diterpene from M. tuberculosis, edaxadiene, whose ability to arrest phagosomal maturation in isolation presumably contributes to this critical process in M. tuberculosis infections. (Mann, F. M., Xu, M., Chen, X., Fulton, D. B., Russell, D. G., and Peters, R. J. (2009) J. Am. Chem. Soc., in press). Here, we present characterization of the class II diterpene cyclase that catalyzes the committed step in edaxadiene biosynthesis, i.e. the previously identified halimadienyl-diphosphate synthase (HPS; EC 5.5.1.16). Intriguingly, our kinetic analysis suggests a potential biochemical regulatory mechanism that triggers edaxadiene production upon phagosomal engulfment. Furthermore, we report characterization of potential HPS inhibitors: specifically, two related transition state analogs (15-aza-14,15-dihydrogeranylgeranyl diphosphate (7a) and 15-aza-14,15-dihydrogeranylgeranyl thiolodiphosphate (7b)) that exhibit very tight binding. Although arguably not suitable for clinical use, these nevertheless provide a basis for pharmaceutical design against this intriguing biosynthetic pathway. Finally, we provide evidence indicating that this pathway exists only in M. tuberculosis and is not functional in the closely related Mycobacterium bovis because of an inactivating frameshift in the HPS-encoding gene. Thus, we hypothesize that the inability to produce edaxadiene may be a contributing factor in the decreased infectivity and/or virulence of M. bovis relative to M. tuberculosis in humans.
Tuberculosis is a prevalent human disease that leads to >1.5 million deaths annually. Over 98% of these fatalities are caused by infections of the eponymous microbe Mycobacterium tuberculosis (1). M. tuberculosis can be extremely infectious, with a dose of as little as a single bacterium sufficient for establishment of a potentially fatal infection (2). Intriguingly, the closely related Mycobacterium bovis appears to be less infectious in humans (3) and is a significantly less common causative agent of tuberculosis (1), despite sharing >99.9% genome sequence identity with M. tuberculosis (4).
M. tuberculosis is taken up by and resides in macrophage cells in the mammalian immune system, specifically in phagosome compartments that are arrested at an early stage of endocytic progression (2). The ability of M. tuberculosis to block such phagosomal maturation has been attributed to multiple factors. Although mycobacterial cell-surface lipids have a clear role, that of other effectors remains less definitive, as different genetic screens have indicated roles for non-overlapping sets of genes (5).
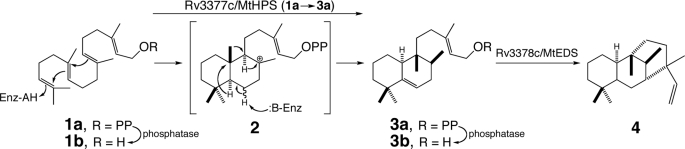
A genetic screen focused on primary effects very early in the infection process strongly implicated the product of a five-gene isoprenoid biosynthetic operon (6). In particular, inactivating transposon insertion in the two unique (presumably non-redundant) genes in the operon resulted in mutant M. tuberculosis unable to fully block phagosomal maturation. Closely following work demonstrated that the first of these, Rv3377c, encoded a class II diterpene cyclase that catalyzed bicyclization and rearrangement of (E,E,E)-geranylgeranyl diphosphate (GGPP4; 1a) to halimadienyl diphosphate (HPP; 3a) (Fig. 1) (7). The corresponding enzyme has been termed halimadienyl-diphosphate synthase (HPS; EC 5.5.1.16). Recently, we have reported that the second implicated gene, Rv3378c, encodes a subsequently acting class I diterpene cyclase that further cyclizes HPP (3a) to the novel tricyclic diterpene edaxadiene (4), which directly inhibits phagosomal maturation in vitro (8), consistent with the results of the previously reported genetic screen (6). Edaxadiene (4) then presumably contributes, at least at an early stage in the infection process, to the phagosomal arrest that provides M. tuberculosis with its host cell/compartment, with HPS catalyzing the committed step in its biosynthesis.
FIGURE 1.
Reaction catalyzed by HPS and subsequent production of edaxadiene (4). Shown is the acid-catalyzed protonation-initiated bicyclization of GGPP (1) to a copalyl diphosphate carbocation intermediate (2), the subsequent rearrangement via a series of alternating 1,2-hydride and methyl migrations to form the HPP (3a) product after terminating deprotonation, and the following separate additional cyclization of 3a to edaxadiene (4) catalyzed by Rv3378c/M. tuberculosis edaxadiene synthase (MtEDS).
Initial functional characterization of HPS was limited by enzymatic instability. Here, we report the development of a construct amendable to kinetic characterization, along with the implications of the observed striking Mg2+ cofactor inhibition effect. We further report analysis of potential inhibitors, with two transition state analogs (7a and 7b) (Fig. 2) found to exhibit high affinity. In addition, investigation of the corresponding gene in M. bovis demonstrates the presence of an inactivating frameshift, abrogating the ability of this otherwise closely related mycobacterium to produce edaxadiene (4), which we hypothesize contributes to its reduced infectivity and/or virulence in humans relative to M. tuberculosis.
FIGURE 2.
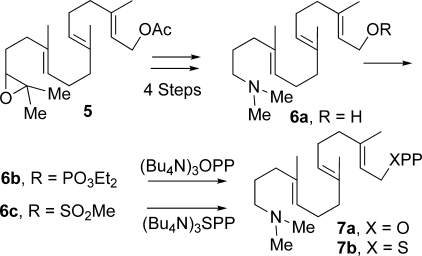
Outline of chemical syntheses of aza analog inhibitors 15-aza-GGPP (7a) and 15-aza-GGSPP (7b) from 14,15-epoxy-GGOH (5) via a common 15-aza-GGOH intermediate (6a).
EXPERIMENTAL PROCEDURES
General
Unless stated otherwise, molecular biology reagents were purchased from Invitrogen, and chemicals were from Fisher. Gas chromatography with flame ionization detection was carried out using an Agilent 6890N GC system as described previously (9) and with mass spectral detection using a Varian 3900 GC system with a Saturn 2100 ion trap mass spectrometer as described previously (10).
Cloning
HPS was cloned from genomic DNA from both M. tuberculosis strain H37Rv (11) and M. bovis strain 95-1315 (12). Both were inserted into the Gateway expression system (pENTR), verified by complete sequencing, and then transferred via directional recombination into expression vectors.
Protein Expression
MtHPS was transferred into six different expression vectors to optimize (fusion) protein expression. These vectors included pDEST14 (no tag/fusion), pDEST15 (glutathione S-transferase), pDEST17 (His6), pTH8 (thioredoxin), pTH1 (maltose-binding protein (MBP)), and pRW1 (thioredoxin-His patch), with all fusion proteins or tags expressed N-terminal to MtHPS. These vectors were individually transformed into Escherichia coli strain C41 (Lucigen Corp., Middleton, WI) and grown in liquid NZY media at 37 °C to an absorbance of 0.6–0.8 at 600 nm. The temperature was then dropped to 16 °C for 1 h, and the cells were induced with 0.5 mm isopropyl β-d-thiogalactopyranoside and cultured for an additional 16 h. Cells were removed from the medium by centrifugation and resuspended in 0.02 volume of lysis buffer (10 mm Tris-Cl, 10% glycerol, 10 mm MgCl2, and 1 mm dithiothreitol, pH 6.8). Cells were lysed by brief sonification and clarified via centrifugation as described previously (13).
Detection of Enzymatic Activity
Initial analysis of HPS activity was carried out with clarified cell extracts. Assays were conducted with an assay buffer consisting of 10 mm HEPES, pH 7.75, 10% glycerol, 1 mm MgCl2, and 10 mm KCl. To 0.9 ml of the assay buffer was added 0.1 ml of clarified lysate, and the assays were then initiated by the addition of GGPP to a final concentration of 5 μm. After incubation at 30 °C for 1 h, the substrate (GGPP, 1a) and any resulting product (HPP, 3a) were enzymatically dephosphorylated to the corresponding primary alcohols (1b and 3b, respectively) using 10 units of calf intestinal alkaline phosphatase (New England Biolabs), which was allowed to incubate for 14–16 h at 37 °C. The diterpene alcohols (1b and 3b) were then extracted from the aqueous assay buffer via three successive 1-ml co-incubations with hexanes. The hexanes were dried to completion, and 1b and 3b were brought up in 50 μl of fresh hexanes for gas chromatography-mass spectrometry analysis. The identity of 3b was established by NMR structural analysis and comparison with that reported previously (see supplemental material) (7).
Protein Purification
Clarified extracts from MBP-HPS-expressing cells were mixed with 3 ml of a slurry (50%, w/v) of amylose resin (New England Biolabs) and MBP buffer (50 mm NaHPO4, 10 mm MgCl2, and 300 mm NaCl, pH 6.8) and incubated for 2 h. The resin was successively washed three times with 15 ml of MBP buffer before eluting with MBP buffer containing 50 mm maltose. The resulting MBP-HPS was estimated to be ∼95% pure by SDS-PAGE analysis. This purified protein was dialyzed in 25-kDa molecular weight cutoff membrane (Spectrum Laboratory Products, Inc., Gardena, CA) against dialysis buffer (50 mm NaHPO4, 300 mm NaCl, 10% glycerol, 1 mm dithiothreitol, and 100 mm EDTA, pH 7.4) for 16 h and then dialyzed for two 45-min periods against dialysis buffer without EDTA. The resulting pure MBP-HPS was assayed immediately, as freezing leads to loss of ∼10% activity over the course of 1 week, although preparations stored at 4 °C for ≤24 h retain essentially full activity.
Kinetic Analyses
The enzymatic concentration and assay time were iteratively optimized for kinetic analysis with purified MBP-MtHPS, resulting in the selection of 25 nm enzyme and 1 min, respectively. These 1-ml assays were carried out much as described previously (14). Briefly, enzymatic activity was quenched via the addition of 110 μl of 20 mm N-ethylmaleimide and incubation at 75 °C for 5 min. The remaining N-ethylmaleimide was neutralized with 20 mm dithiothreitol prior to dephosphorylation and extraction of the resulting alcohols (1b and 3b), carried out as described above. All measurements for kinetic analysis were carried out via gas chromatography-flame ionization detection analysis of the fractional conversion of substrate to product much as described previously (9), with duplicate assays run for each reported data point. Divalent cation dependence was measured by replacing the 1 mm MgCl2 in the assay buffer with a 0.1, 1, or 10 mm concentration of various divalent cation salts with 5 μm substrate (GGPP, 1a). More detailed analysis was carried out for the optimal Mg2+ using concentrations ranging from 0.001 to 10 mm. Assays were then carried out at the optimal 0.1 mm Mg2+ concentration using GGPP (1a) concentrations ranging from 1 to 100 μm. All reported data points are averages from duplicate assays, with the error bars corresponding to the corresponding S.D. Kinetic data were fit using KaleidaGraph (Synergy Software) to the standard substrate inhibition equation: v = kcat[E][S]/(Km + [S](1 + ([S]/Ki))).
Synthesis of Aza Analog Inhibitors (7a and 7b)
15-Aza-GGOH (6a) was prepared in four steps from 14,15-epoxy-GGOH (5) (9) as described previously (15): epoxide hydrolysis to the 14,15-diol, periodate cleavage, reductive amination with Me2NH (NaBH3CN, MeOH), and acetate hydrolysis. Conversion to the mixed monophosphate 6b (ClP(O)(OEt)2, Pyr, CH2Cl2, 0 °C) and displacement with (BuN4)3HOPP (CH3CN, molecular sieves, room temperature, 5 days) followed by ion exchange, cellulose chromatography, lyophilization, and preparative high pressure liquid chromatography afforded 15-aza-14,15-dihydrogeranylgeranyl diphosphate (15-aza-GGPP; 7a) (15). Conversion of 15-aza-GGOH (6a) to the corresponding methanesulfonate (6c; CH3SO2Cl and EtsN in CH3CN at −30 °C, 30 min) followed by reaction with (Bu4N)3SPP (15) and molecular sieves (0 °C, 1 h) modeled after a procedure for preparation of GGSPP by Phan and Poulter (16) gave 15-aza-14,15-dihydrogeranylgeranyl thiolodiphosphate (15-aza-GGSPP; 7b; 46 mg, 85%) following ion exchange, centrifugation/extractions with MeOH, and flash chromatography on cellulose. Assays with these inhibitors were carried out with a 5-min preincubation of the inhibitor with 50 nm MBP-MtHPS prior to the addition of GGPP (to 5 μm) to initiate 3-min reactions. The syntheses of 7a and 7b are summarized in Fig. 2; for further experimental details, see supplemental material.
RESULTS
HPS Expression Construct
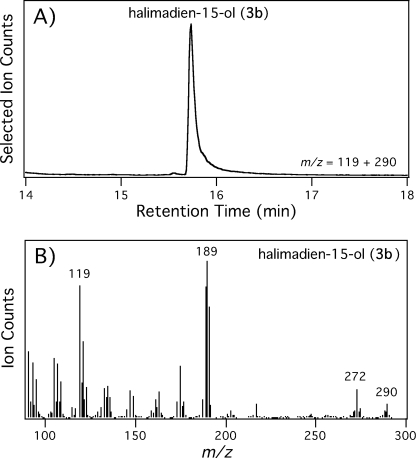
In the initial functional characterization report, it was noted that HPS was quite difficult to express recombinantly, requiring coexpression of the GroEL protein folding chaperone (7). Consistent with this, upon cloning the corresponding gene (Rv3377c/MtHPS) from M. tuberculosis (strain H37Rv), we found that recombinant expression resulted in activity only when MtHPS was fused to MBP, although several other fusion proteins were also assessed. The resulting MBP-MtHPS protein was able to convert GGPP to HPP (1a → 3a) completely, as determined by NMR structural analysis of the hydrolytically dephosphorylated alcohol 3b and comparison with that reported previously (7), wherein this compound (3b) was termed tuberculosinol (halimadien-15-ol). Notably, although initial assays were carried out with cell-free extracts, the presence of the MBP tag enabled rapid purification, with the purified MBP-MtHPS protein similarly able to convert GGPP (1a) to HPP (3a) completely (Fig. 3).
FIGURE 3.
MBP-MtHPS-mediated conversion of GGPP to HPP. A, selected ion chromatogram from gas chromatography-mass spectrometry analysis of halimadien-15-ol (3b) resulting from phosphatase-mediated hydrolysis of the HPP produced by purified MBP-MtHPS from GGPP. B, mass spectra of halimadien-15-ol (3b).
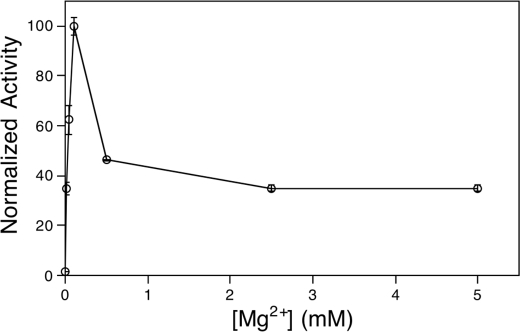
Magnesium Cofactor Dependence
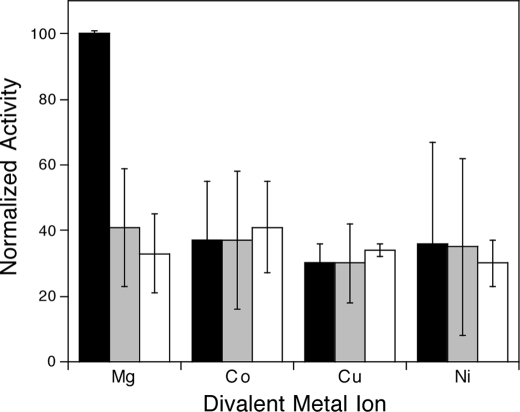
As with other characterized class II diterpene cyclases (14, 17–19), it was found in the initial characterization report that MtHPS requires Mg2+ as an enzymatic cofactor (7). After optimization of the assay for kinetic measurements, the ability of a variety of divalent cations to support MtHPS activity at various concentrations (0.1, 1, and 10 mm) was investigated. This included Mg2+, Co2+, Cu2+, Fe2+, Mn2+, Ca2+, and Zn2+, although because of interference with the secondary enzyme in our coupled assay (phosphatase), we were unable to measure kinetic rates with Mn2+ and Zn2+. Of the remaining divalent cations, MtHPS reacted most efficiently in the presence of low levels of Mg2+ (Fig. 4).
FIGURE 4.
Relative MBP-MtHPS activity with various divalent metal ion cofactors. Black bars, 0.1 mm; gray bars, 1 mm; white bars, 10 mm.
The decrease in activity observed with higher levels of divalent cations indicates that MtHPS undergoes substrate-like cofactor inhibition, much as been reported with plant class II diterpene cyclases (14). This was more closely investigated with a detailed analysis of Mg2+ dependence (Fig. 5), demonstrating that MtHPS is most active in the presence of 0.1 mm Mg2+ and displays a rapid loss of activity as the concentration is raised above this point (e.g. >50% loss at 0.5 mm), although the observed data did not fit well to the standard substrate inhibition equation. This presumably is a result of complex effects of Mg2+, which is expected to bind to the pyrophosphate moiety of GGPP (1a) and the enzyme itself, potentially to both catalytic and inhibitory sites.
FIGURE 5.
Mg2+ dependence of MBP-MtHPS activity.
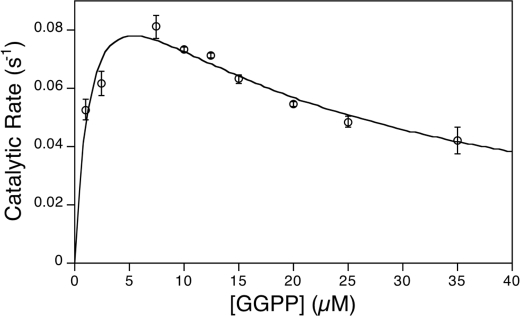
Kinetic Analysis
Substrate inhibition effects with GGPP (1a) have also been reported previously for some, although not all, class II diterpene cyclases (14, 18–22). Upon kinetic analysis of the GGPP (1a) concentration dependence of MtHPS activity, a clear substrate inhibition effect was observed. These data were readily fit to the standard substrate inhibition equation, with an apparent Km of 1.6 ± 0.6 μm, a Ki of 18 ± 6 μm, and a kcat of 0.12 ± 0.2 s−1 (Fig. 6).
FIGURE 6.
Kinetic analysis of MBP-HPS activity.
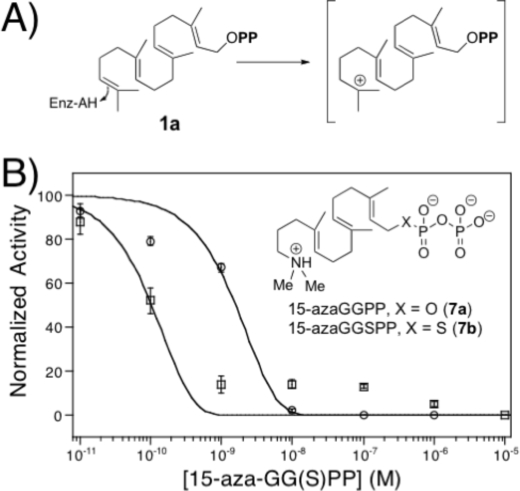
Inhibition of HPS
It has been shown previously that 15-aza-GGPP (7a), an analog of the high energy intermediate formed by protonation of the terminal carbon=carbon double bond of GGPP (the initial step in class II cyclization) (Fig. 7A), is a potent inhibitor of the class II diterpene cyclase activity of a bifunctional plant diterpene synthase, abietadiene synthase (23). Thus, the ability of 15-aza-GGPP to inhibit MtHPS activity was investigated here, with an observed IC50 of 2 nm (Fig. 7B), similar to that reported with abietadiene synthase (23). In addition, the potentially more stable thiolo analog, 15-aza-GGSPP (7b), proved to be an even more potent inhibitor, nominally exhibiting an IC50 of 0.1 nm, despite the larger size of the sulfur atom, the longer C–S and S–P bonds, and the presumably diminished affinity for the Mg2+ cofactor. However, small amounts of residual activity were evident with 7b at concentrations at which 7a was able to completely inhibit MBP-MtHPS. Notably, given that these assays were performed with 50 nm enzyme, the resulting relatively very low IC50 values indicate that only a small fraction of MBP-MtHPS is correctly folded (i.e. active and able to bind these inhibitors). Unfortunately, this uncertainty in amount of active enzyme precludes more precise analysis of binding affinity (i.e. Ki). Nevertheless, these findings suggest that 15-aza-GGSPP, as well as other thiophosphates (16), is likely to be useful as an inert, tight-binding active-site probe for diterpene cyclases.
FIGURE 7.
Inhibition of MBP-MtHPS. A, initial step and corresponding geranylgeran-15-yl+ diphosphate transition state of class II cyclization reaction; B, effect of 15-aza-GGPP (7a; ○) and 15-aza-GGSPP (7b; □), with structures shown (inset), on MBP-MtHPS activity.
Characterization of HPS from M. bovis
It has been noted previously that M. bovis (although not other mycobacteria) contains an operon homologous to that now associated with edaxadiene biosynthesis in M. tuberculosis (6, 7). Interestingly, in examining the M. bovis HPS homolog, we noticed that this gene (Mb3411c/MbHPS) is annotated as having a 3-bp for 4-bp frameshifting substitution (CAAT → AAC) toward its 3′-end (nucleotide ∼1220 of the open reading frame) in the reported genome sequences for M. bovis, both strain AF2122/97 (4) and the vaccination strain bacillus Calmette-Guérin Pasteur 1173P2 (24). Upon cloning MbHPS from M. bovis strain 95-1315, we confirmed this frameshift mutation, which leads to a change in C-terminal sequence relative to the last 95 amino acid residues found in MtHPS, including early termination after 483 instead of 501 residues. To determine whether this was sufficient to abrogate enzymatic activity, MbHPS was expressed as an MBP fusion protein using the same protocols used with MtHPS. This led to readily apparent expression of MBP-MbHPS, albeit with the expected smaller apparent size relative to MBP-MtHPS upon SDS-PAGE analysis. However, the MBP-MbHPS construct did not exhibit activity, with no conversion of GGPP (1a) to HPP (3a) found even upon extended incubations (up to 72 h) in the optimized assay conditions using either recombinant cell-free extracts or large amounts of purified enzymatic preparations.
DISCUSSION
M. tuberculosis is a highly efficient human pathogen that has infected over one-third of the global population (2). Establishment of an infection requires M. tuberculosis to halt endocytic maturation of the phagosome compartment created upon engulfment of the bacterium by macrophage cells of the human host immune system. There are multiple factors that contribute to the ability of M. tuberculosis to initiate and maintain arrest of these phagosome compartments at an early endocytic stage. One such factor appears to be the diterpene edaxadiene (4) (8), whose biosynthesis is initiated by the bicyclization and rearrangement of GGPP (1a → 3a) catalyzed by HPS (Fig. 1).
Arguably the most striking finding from the kinetic analysis of MtHPS reported here was its pronounced biphasic dependence on the Mg2+ level (Fig. 5). Previous results have established that class II diterpene cyclases can exhibit much less susceptibility to Mg2+ cofactor inhibition (14, 21), so this is not an intrinsic feature of such enzymes. Intriguingly, the phagosome appears to be a nutrient-deprived environment, with experiments demonstrating reduced concentrations of various elements in M. tuberculosis-containing vacuoles (25), which have a hypothesized resting Mg2+ concentration of 10–50 μm (26), close to the optimal 100 μm concentration for MtHPS activity. Given that bacterial intracellular concentrations of Mg2+ have been demonstrated to reflect that of their external environment (27), upon endocytic uptake, M. tuberculosis intracellular Mg2+ levels would be drastically reduced, leading to increased HPS activity. This should increase flux toward edaxadiene (4) production, which then acts to prevent phagosomal maturation. Thus, we hypothesize that the Mg2+ dependence of MtHPS activity represents a physiologically relevant biochemical mechanism that triggers (or at least increases) edaxadiene (4) biosynthesis upon phagosomal engulfment.
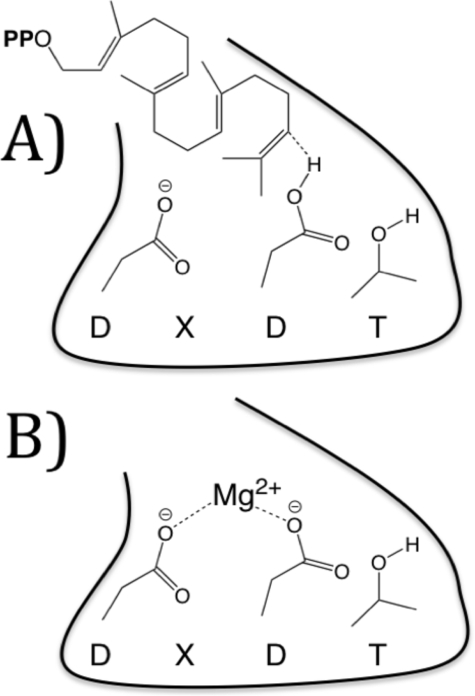
As suggested previously for plant class II diterpene cyclases (14), it seems likely that the site of inhibitory Mg2+ binding is the aspartate-rich motif found in such enzymes, whose productive role is to act synergistically as the catalytic acid that protonates the terminal carbon=carbon double bond of GGPP (1a) (9), which would provide an example of an intrasteric (i.e. within the active site) inhibitory mechanism. Although typically conserved as a DXDD sequence motif, the corresponding sequence was found to be DXDT in MtHPS (7). Nevertheless, this motif should also be capable of binding Mg2+ (Fig. 8), either alone or in complex with GGPP in a catalytically non-productive mode.
FIGURE 8.
Schematic model of the DXDT motif in the MtHPS active site. A, as an acid in productive catalysis; B, as a Mg2+-binding site, leading to intrasteric inhibition.
Regardless of such speculation on potential regulation and mechanism, it is particularly notable that HPS, and hence edaxadiene (4) biosynthetic capacity, appears to be conserved in M. tuberculosis but not in other mycobacteria. This now includes M. bovis, which had been noted previously as containing a corresponding (i.e. homologous) biosynthetic operon (6, 7). However, although HPS is completely conserved in all four M. tuberculosis strains with corresponding sequence information available (H37Rv, Haarlem, F11, and C), in the sequenced M. bovis strains AF2122/97 and bacillus Calmette-Guérin Pasteur 1173P2, as well as in the 95-1315 strain used here, the MbHPS homolog carries a frameshifting mutation, which our analysis indicates abolishes its enzymatic activity. Thus, M. bovis presumably does not produce edaxadiene (4). Notably, despite sharing >99.9% genome sequence identity (4), M. bovis appears to be less infectious in humans than M. tuberculosis (3) and is a significantly less common causative agent of tuberculosis (1). We hypothesize that the loss of edaxadiene (4) production indicated by our studies is a factor contributing to the reduced infectivity and/or virulence of M. bovis in humans. A role in infectivity would be consistent with the design of the initial genetic screen that identified the edaxadiene operon (6), which selected for factors playing a role in very early stages of infection, as well as the activity of edaxadiene (4) itself, as indicated by both the mutant phenotype (6) and its rapid (within 20 min) effect on phagosomal maturation in isolation (8). However, our data do not allow us to distinguish what role(s) edaxadiene (4) plays in the ability of M. tuberculosis to efficiently infect humans and cause tuberculosis.
Drug-resistant strains of M. tuberculosis have been steadily increasing in frequency, posing a serious threat to public health (28). The relevant biological activity of edaxadiene (4) indicates that the associated biosynthetic pathway may present a target of potential pharmaceutical interest. Here, we have demonstrated that the transition state analog 15-aza-GGPP (7a), demonstrated previously to be a tight-binding inhibitor of plant class II diterpene cyclases, is a very tight binding inhibitor of MtHPS as well. However, 15-aza-GGPP (7a) contains an easily hydrolyzed diphosphate ester bond. In an initial attempt to modify this inhibitor to increase stability while retaining strong affinity, we substituted the diphosphate ester with a more stable thiolo linkage. The resulting 15-aza-GGSPP analog (7b) also proved to be a potent inhibitor, indicating that at least such moderate modification to increase stability does not lead to loss of affinity. Thus, MtHPS and, hence, edaxadiene (4) production appears to be a viable drug target.
In conclusion, the results reported here, in combination with those reported previously (6, 8), suggest that edaxadiene (4) plays an early role in M. tuberculosis infection (i.e. immediately following phagosomal uptake). The loss of such biosynthetic capacity in M. bovis indicated by the studies reported here leads us to hypothesize that this contributes to the reduced infectivity and/or virulence of M. bovis relative to M. tuberculosis in humans. Accordingly, the evidence reported here suggesting that this biosynthetic pathway may be amendable to pharmaceutical intervention provides a potential new drug target against this devastating human pathogen.
Supplementary Material
Acknowledgments
We thank Dr. John Bannantine (United States Department of Agriculture National Animal Disease Center) for M. tuberculosis genomic DNA, Professor Chris Minion (Iowa State University) for M. bovis genomic DNA, and Dr. Matthew Hillwig for NMR structural analysis of halimadien-15-ol (3b).
This work was supported, in whole or in part, by National Institutes of Health Grants GM076324 (to R. J. P.) and GM13956 (to R. M. C.).
 The on-line version of this article (available at http://www.jbc.org) contains supplemental information concerning the preparation and characterization of 15-aza-GGSPP (7b) and NMR structural analysis of halimadien-15-ol (3b).
The on-line version of this article (available at http://www.jbc.org) contains supplemental information concerning the preparation and characterization of 15-aza-GGSPP (7b) and NMR structural analysis of halimadien-15-ol (3b).
- GGPP (1a)
- (E,E,E)-geranylgeranyl diphosphate
- HPP (3a)
- halimadienyl diphosphate
- HPS
- halimadienyl-diphosphate synthase
- Mt
- M. tuberculosis
- MBP
- maltose-binding protein
- GGOH
- geranylgeraniol
- 15-aza-GGPP (7a)
- 15-aza-14,15-dihydrogeranylgeranyl diphosphate
- 15-aza-GGSPP (7b)
- 15-aza-14,15-dihydrogeranylgeranyl thiolodiphosphate
- Mb
- M. bovis.
REFERENCES
- 1.Kumar V., Abbas A., Fausto N., Mitchell R. (2007) Robbins Basic Pathology, 8th Ed., pp. 516–517, Elsevier Science Publishing Co., Inc., New York [Google Scholar]
- 2.Russell D. G. (2007) Nat. Rev. Microbiol. 5,39–47 [DOI] [PubMed] [Google Scholar]
- 3.Grange J. M., Yates M. D. (1994) Vet. Microbiol. 40,137–151 [DOI] [PubMed] [Google Scholar]
- 4.Garnier T., Eiglmeier K., Camus J. C., Medina N., Mansoor H., Pryor M., Duthoy S., Grondin S., Lacroix C., Monsempe C., Simon S., Harris B., Atkin R., Doggett J., Mayes R., Keating L., Wheeler P. R., Parkhill J., Barrell B. G., Cole S. T., Gordon S. V., Hewinson R. G. (2003) Proc. Natl. Acad. Sci. U.S.A. 100,7877–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deretic V., Singh S., Master S., Harris J., Roberts E., Kyei G., Davis A., de Haro S., Naylor J., Lee H. H., Vergne I. (2006) Cell. Microbiol. 8,719–727 [DOI] [PubMed] [Google Scholar]
- 6.Pethe K., Swenson D. L., Alonso S., Anderson J., Wang C., Russell D. G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101,13642–13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano C., Okamura T., Sato T., Dairi T., Hoshino T. (2005) Chem. Commun. 2005,1016–1018 [DOI] [PubMed] [Google Scholar]
- 8.Mann F. M., Xu M., Chen X., Fulton D. B., Russell D. G., Peters R. J. (2009) J. Am. Chem. Soc., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prisic S., Xu J., Coates R. M., Peters R. J. (2007) ChemBioChem 8,869–874 [DOI] [PubMed] [Google Scholar]
- 10.Morrone D., Chambers J., Lowry L., Kim G., Anterola A., Bender K., Peters R. J. (2009) FEBS Lett. 583,475–480 [DOI] [PubMed] [Google Scholar]
- 11.Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S., Barry C. E., 3rd, Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., Connor R., Davies R., Devlin K., Feltwell T., Gentles S., Hamlin N., Holroyd S., Hornsby T., Jagels K., Krogh A., McLean J., Moule S., Murphy L., Oliver K., Osborne J., Quail M. A., Rajandream M. A., Rogers J., Rutter S., Seeger K., Skelton J., Squares R., Squares S., Sulston J. E., Taylor K., Whitehead S., Barrell B. G. (1998) Nature 393,537–544 [DOI] [PubMed] [Google Scholar]
- 12.Schmitt S. M., Fitzgerald S. D., Cooley T. M., Bruning-Fann C. S., Sullivan L., Berry D., Carlson T., Minnis R. B., Payeur J. B., Sikarskie J. (1997) J. Wildl. Dis. 33,749–758 [DOI] [PubMed] [Google Scholar]
- 13.Xu M., Hillwig M. L., Prisic S., Coates R. M., Peters R. J. (2004) Plant J. 39,309–318 [DOI] [PubMed] [Google Scholar]
- 14.Prisic S., Peters R. J. (2007) Plant Physiol. 144,445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravn M. M., Jin Q., Coates R. M. (2000) Eur. J. Org. Chem. 2000,1401–1410 [Google Scholar]
- 16.Phan R. M., Poulter C. D. (2001) J. Org. Chem. 66,6705–6710 [DOI] [PubMed] [Google Scholar]
- 17.Peters R. J., Croteau R. B. (2002) Biochemistry 41,1836–1842 [DOI] [PubMed] [Google Scholar]
- 18.Hamano Y., Kuzuyama T., Itoh N., Furihata K., Seto H., Dairi T. (2002) J. Biol. Chem. 277,37098–37104 [DOI] [PubMed] [Google Scholar]
- 19.Ikeda C., Hayashi Y., Itoh N., Seto H., Dairi T. (2007) J. Biochem. 141,37–45 [DOI] [PubMed] [Google Scholar]
- 20.Peters R. J., Flory J. E., Jetter R., Ravn M. M., Lee H. J., Coates R. M., Croteau R. B. (2000) Biochemistry 39,15592–15602 [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y., Toyomasu T., Hirose Y., Onodera Y., Mitsuhashi W., Yamane H., Sassa T., Dairi T. (2008) Biosci. Biotechnol. Biochem. 72,523–530 [DOI] [PubMed] [Google Scholar]
- 22.Kawaide H., Sassa T., Kamiya Y. (2000) J. Biol. Chem. 275,2276–2280 [DOI] [PubMed] [Google Scholar]
- 23.Peters R. J., Ravn M. M., Coates R. M., Croteau R. B. (2001) J. Am. Chem. Soc. 123,8974–8978 [DOI] [PubMed] [Google Scholar]
- 24.Brosch R., Gordon S. V., Garnier T., Eiglmeier K., Frigui W., Valenti P., Dos Santos S., Duthoy S., Lacroix C., Garcia-Pelayo C., Inwald J. K., Golby P., Garcia J. N., Hewinson R. G., Behr M. A., Quail M. A., Churcher C., Barrell B. G., Parkhill J., Cole S. T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104,5596–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner D., Maser J., Lai B., Cai Z., Barry C. E., 3rd, Höner Zu, Bentrup K., Russell D. G., Bermudez L. E. (2005) J. Immunol. 174,1491–1500 [DOI] [PubMed] [Google Scholar]
- 26.Garcia-del Portillo F., Foster J. W., Maguire M. E., Finlay B. B. (1992) Mol. Microbiol. 6,3289–3297 [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz C., Rosano C. L. (1967) J. Biol. Chem. 242,3719–3722 [PubMed] [Google Scholar]
- 28.Zignol M., Hosseini M. S., Wright A., Weezenbeek C. L., Nunn P., Watt C. J., Williams B. G., Dye C. (2006) J. Infect. Dis. 194,479–485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.