SUMMARY
IFN-γ is a pleiotropic cytokine that plays a key role in host resistance yet when not properly regulated can become host-detrimental. The interferon-inducible Immunity Related GTPase family M member 1 (Irgm1), previously characterized as an effector molecule required for macrophage microbicidal activity, has been shown recently to play a previously unrecognized role in regulating IFN-γ-dependent cell survival and host resistance. Irgm1 regulates the expansion / survival of mature effector CD4+ T lymphocytes by protecting them from IFN-γ induced autophagic cell death. Importantly, mice deficient in both IFN-γ and Irgm1 were rescued from the lymphocyte depletion and increased mortality that occurs in single Irgm1−/− animals following pathogen exposure. We propose that Irgm1 plays a major role in maintaining T lymphocyte homeostasis during host IFN-γ responses by protecting these cells from autophagy-dependent cell death.
Keywords: autophagy, cell death, IFN-γ, Th1, lymphocyte homeostasis, GTPase
While IFN-γ is required for host control of a wide range of intracellular pathogens by inducing NOS2 and other effector molecules in infected macrophages, unregulated production of or signaling by the cytokine can be host detrimental. Interestingly, the regulatory mechanisms that determine the beneficial versus detrimental outcomes of the IFN-γ response are poorly understood. A set of these genes strongly induced by interferons encodes the p47 kDa Immunity-Related GTPase (IRG) family 1, 2. We and others have shown that mice deficient in one member of the family, Irgm1 (also known as LRG47) 3are highly susceptible to a wide variety of different intracellular bacterial and protozoan infections 2, 4. Because Irgm1 has been shown to enhance intracellular pathogen killing by promoting phagolysosomal maturation and autophagy in vitro 5, 6, it is characterized as an effector molecule required for microbicidal activity in IFN-γ-activated macrophages. Unexpectedly, we observed that Mycobacterium avium 7 and Trypanosoma cruzi 8 infected Irgm1−/− mice develop a profound pancytopenia, a defect that we have recently shown to be associated with impaired expansion of Irgm1−/− hematopoietic stem and progenitor cell populations 9. These observations suggest that Irgm1 plays a broader and more fundamental regulatory role in host responses to infections beyond its proposed function in pathogen killing.
Focusing on mature CD4+ T lymphocytes we recently demonstrated that IFN-γ induced Irgm1 is critical for the survival of activated mature lymphocytes since in the absence of Irgm1, IFN-γ triggered the death of these cells. Importantly, the lymphopenia and mortality previously observed in M. avium-infected Irgm1−/− animals was completely abrogated in Irgm1−/−Ifng−/− double-deficient mice 10. These findings identified a previous unknown regulatory function for Irgm1 in IFN-γ-dependent host defense and lymphocyte survival in vivo. They are consistent with the conclusions from a recent in vitro study demonstrating that Irgm proteins negatively regulate the activation status of other GTPases within the same family and such that in the absence of this pathway some IRG members form aggregates and fail to reach pathogen-containing vacuoles 11. Together, these two studies argue that in the absence of Irgm1, dysregulated cellular responses, rather than defective effector cell function, account for the impaired resistance of Irgm1−/− mice to certain infections. This is explained for Mycobacterium avium infection in Figure 1.
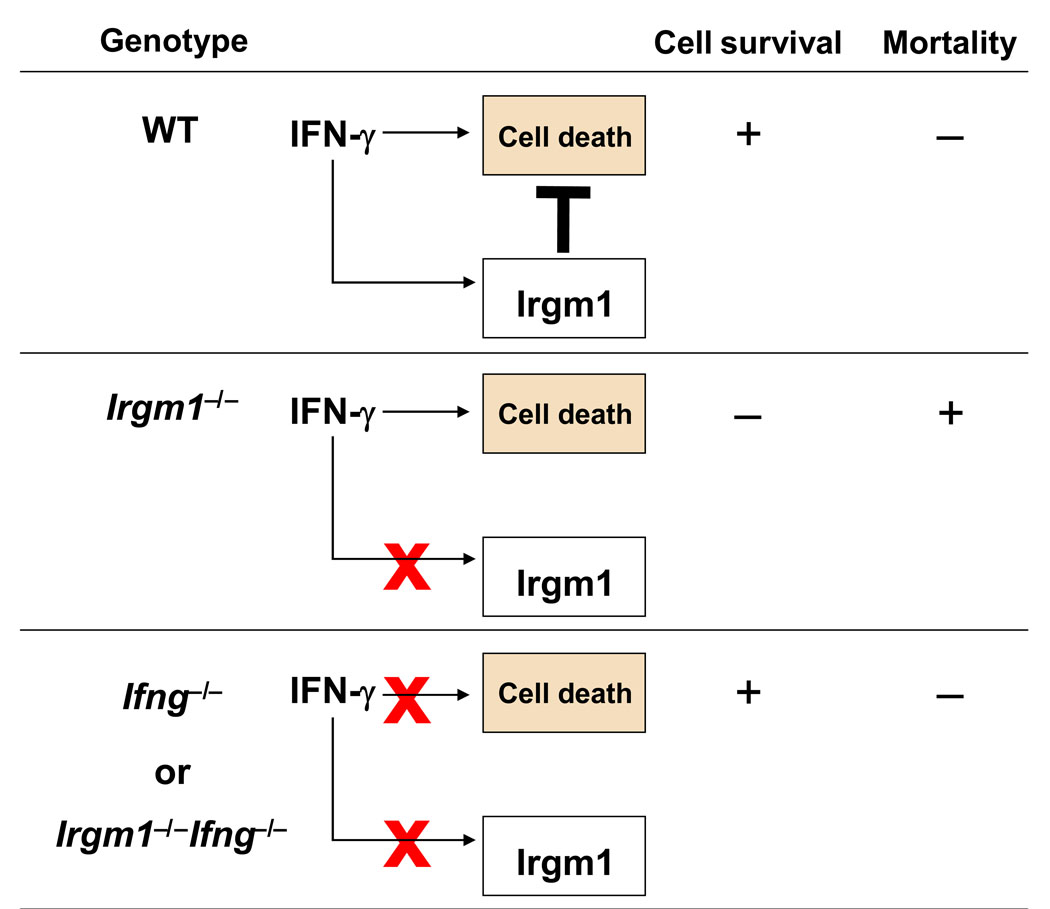
Figure 1.
Irgm1 regulates the outcome of IFN-γ response to persistent intracellular pathogens. In this model, interferon-inducible Irgm1 negatively regulates IFN-γ-dependent death program ensuring expansion of effector lymphocyte populations and survival of infected hosts. In the absence of such protective mechanism as observed in Irgm1−/− mice, exposure of IFN-γ leads to the death of activated CD4+ T cells and increased mortality to mycobacterial infection. This regulatory mechanism is revealed only in the presence of IFN-γ, as single IFN-γ or double Irgm1 and IFN-γ deficiency does not result in cell death or increased mortality to the infection.
We also investigated the mechanism of IFNγ-induced death of Irgm1−/− T cells and found that the Irgm1-dependent death is distinct from previously described T cell receptor triggered T cell death mechnaims and is independent of Fas ligand-Fas interactions. Moreover, both morphological analysis as well as studies with pharmacological inhibitors failed to reveal a significant role for caspase-dependent apoptosis in the IFN-γ-induced death of Irgm1−/− cells. Instead, we observed that following IFN-γ stimulation many living Irgm1−/− cells appeared to have significantly increased numbers of membrane-bound vacuoles closely resembling autophagosomes and autolysosomes in their morphology. Importantly, IFN-γ-induced death of Irgm1−/− T cells could be reduced by treatment with inhibitors of phosphoinositide 3-kinase or by silencing the expression of beclin-1 thus directly implicating the involvement of an autophagic pathway. Based on these observations, we suggested that Irgm1 controls a no-apoptotic cell death program that depends on autophagy.
The Irgm1-dependent autophagic death program in lymphocytes is clearly distinct from the autophagic cell death mechanism previously described in macrophages which requires caspase inhibition and receptor interacting protein (RIP) kinase induction 12, 13. Interestingly, the proposed functions for Irgm1 in autophagy also appear to be cell type-specific. Thus, in IFN-γ-stimulated macrophages Irgm1 has been shown to promote 6, 14rather than inhibit autophagy as observed in T cells. Although the mechanisms underlying this distinction are presently unclear, it is possible that Irgm1 plays a role in fine-tuning the extent of autophagy depending on the specific functions of the cell type. Macrophages are terminally differentiated non-dividing cells in which high levels of autophagy have been proposed to contribute both to their anti-microbial effector activity and enhanced capacity to degrade house keeping proteins. In contrast, effector T lymphocyte function requires extensive cycling activity. While basal autophagy is beneficial for normal cellular homeostasis and survival, excessive catabolic activity during mitosis would inhibit generation of essential organelles / proteins and lead to cell death. Indeed, we found that IFN-γ triggers death of proliferating but not resting Irgm1−/− CD4 T cells, suggesting that molecular events associated with increased nutrient uptake and protein turnover in cycling Irgm1−/− lymphocytes may contributed totheir death. This observation also raises the question of whether in addition to direct regulating autophagy pathway, Irgm1 may be critical in balancing IFN-γ-dependent cellular events upstream of Atg (autophagy related genes) that, if not properly regulated, could trigger autophagy related protein degradation cascades. Our current works focus on the mechanisms by which Irgm1 regulates the autophagic process in lymphocytes. Autophagy has now been shown to play a generalized role in maintaining cellular homeostasis through its involvement in both cell survival as well as cell death 15–18. Interestingly, the autophagic cell death pathway utilizes almost the same machinery that promotes cell survival. In this regard, our findings emphasize the pivotal role played by non-Atg specific factors, such as extracellular signal, level of autophagy, cell type and rate of metabolism, in determining the threshold of autophagy-dependent death.
In conclusion, our findings demonstrate that a single GTPase, Irgm1, can dictate the beneficial outcome of the IFN-γ response by providing a feedback mechanism essential for protecting activated CD4+ lymphocytes from IFN-γ-mediated death while enhancing the anti-microbial functions of the cytokine (Figure 2). We propose that this activity of Irgm1 is of special importance in the establishment and maintenance of Th1 responses to persistent intracellular infections. It is however also likely that the GTPase serves a similar role in the maintenance of cellular homeostasis in other non- T lymphocyte IFN-γ responsive lineages.
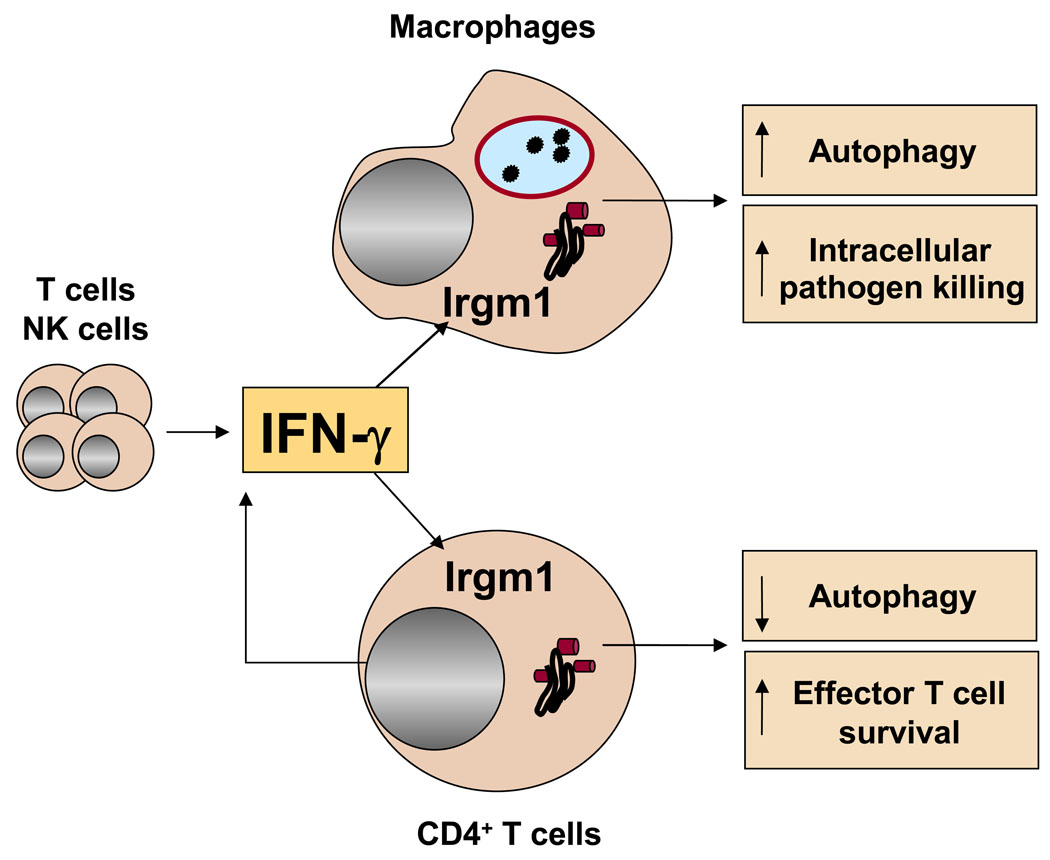
Figure 2.
Distinct role for Irgm1 in the regulation of autophagy in macrophages and CD4+ T cells. During Th1 response, IFN-γ produced by activated NK and T cells induces Irgm1 expression to maximize host control of intracellular pathogens. While promoting IFN-γ-dependent autophagy in macrophages to enhance clearance of intracellular pathogens, Irgm1 inhibits autophagic death of activated CD4+ T cells to amplify Th1 effector populations.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Addendum to:
The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing IFN-γ-induced cell death
Feng CG, Zheng L, Jankovic D, Báfica A, Cannons JL, Watford WT, Chaussabel D, Hieny S, Caspar P, Schwartzberg PL, Lenardo MJ, Sher A
Nat Immunol. 2008 Nov;9(11):1279-87
References
- 1.Boehm U, Guethlein L, Klamp T, Ozbek K, Schaub A, Futterer A, et al. Two families of GTPases dominate the complex cellular response to IFN-γ. J Immunol. 1998;161:6715–6723. [PubMed] [Google Scholar]
- 2.Taylor GA, Feng CG, Sher A. p47 GTPases: regulators of immunity to intracellular pathogens. Nat Rev Immunol. 2004;4:100–109. doi: 10.1038/nri1270. [DOI] [PubMed] [Google Scholar]
- 3.Sorace JM, Johnson RJ, Howard DL, Drysdale BE. Identification of an endotoxin and IFN-inducible cDNA: possible identification of a novel protein family. J Leukoc Biol. 1995;58:477–484. doi: 10.1002/jlb.58.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Taylor GA, Feng CG, Sher A. Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 5.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 6.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- 7.Feng CG, Collazo-Custodio CM, Eckhaus M, Hieny S, Belkaid Y, Elkins K, et al. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J Immunol. 2004;172:1163–1168. doi: 10.4049/jimmunol.172.2.1163. [DOI] [PubMed] [Google Scholar]
- 8.Santiago HC, Feng CG, Bafica A, Roffe E, Arantes RM, Cheever A, et al. Mice deficient in LRG-47 display enhanced susceptibility to Trypanosoma cruzi infection associated with defective hemopoiesis and intracellular control of parasite growth. J Immunol. 2005;175:8165–8172. doi: 10.4049/jimmunol.175.12.8165. [DOI] [PubMed] [Google Scholar]
- 9.Feng CG, Weksberg DC, Taylor GA, Sher A, Goodell MA. The p47 GTPase Lrg-47 (Irgm1) Links Host Defense and Hematopoietic Stem Cell Proliferation. Cell stem cell. 2008;2:83–89. doi: 10.1016/j.stem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng CG, Zheng L, Jankovic D, Bafica A, Cannons JL, Watford WT, et al. The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon-γ-induced cell death. Nat Immunol. 2008;9:1279–1287. doi: 10.1038/ni.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunn JP, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, et al. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. Embo J. 2008;27:2495–2509. doi: 10.1038/emboj.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006;281:19179–19187. doi: 10.1074/jbc.M513377200. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]