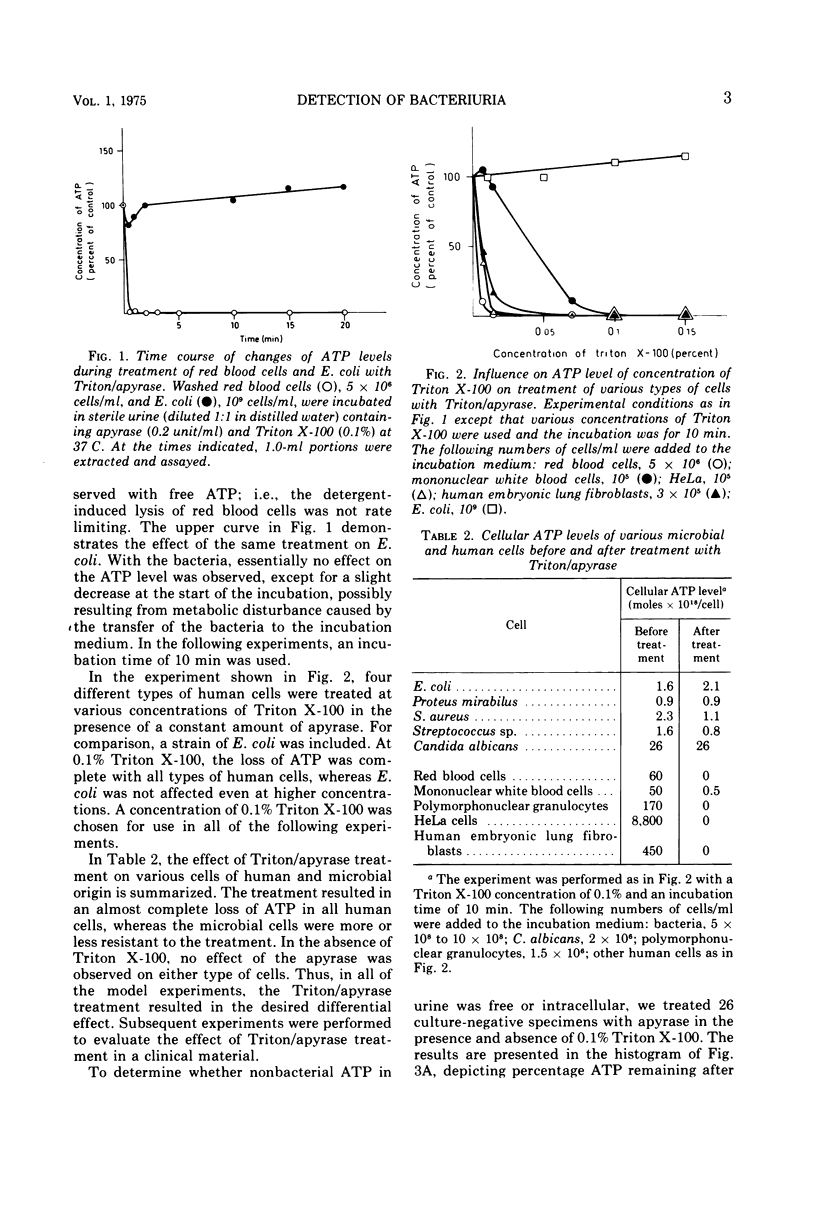
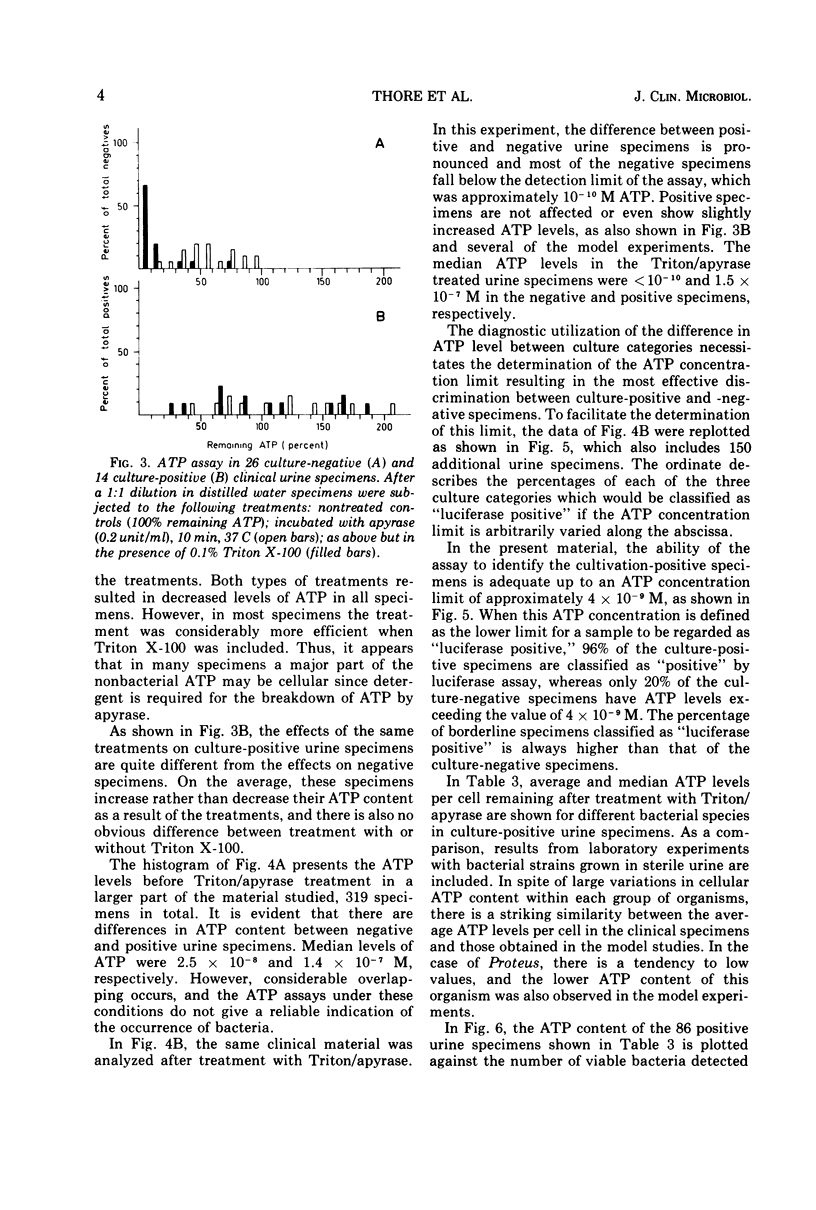
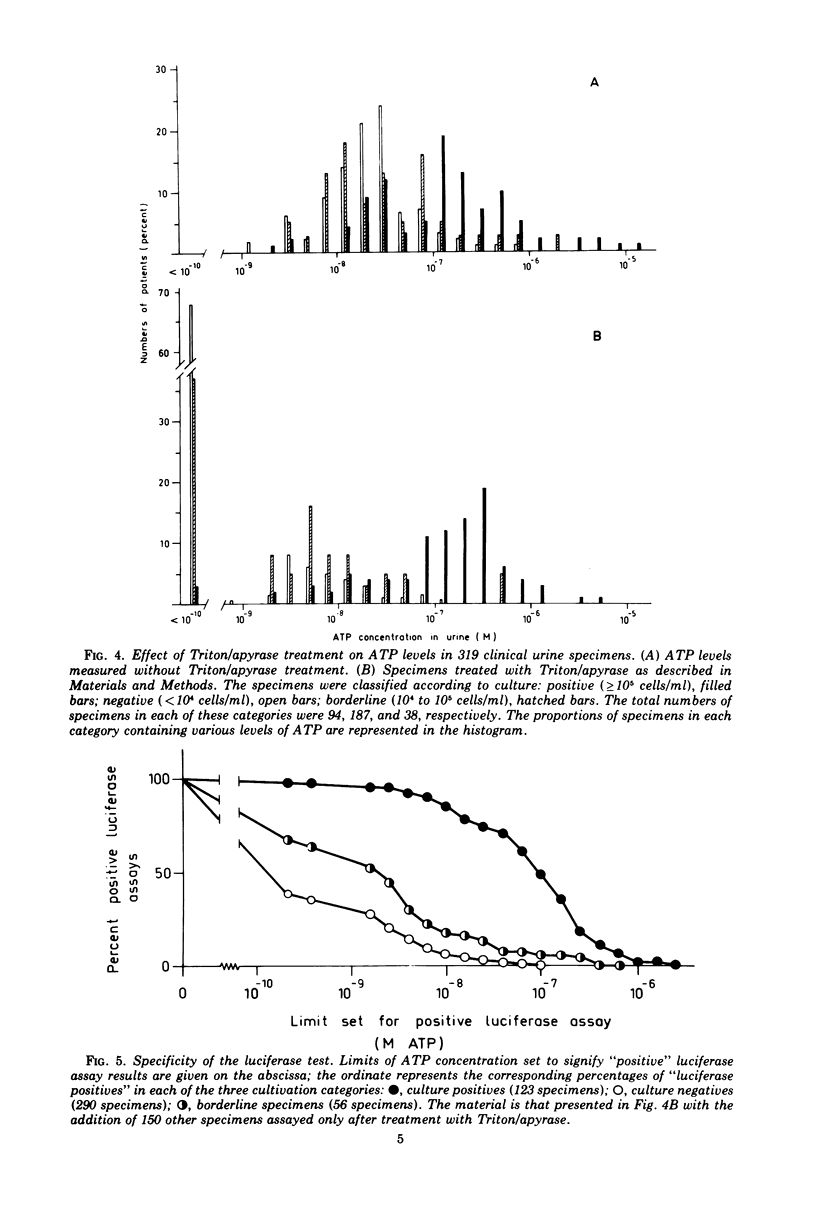
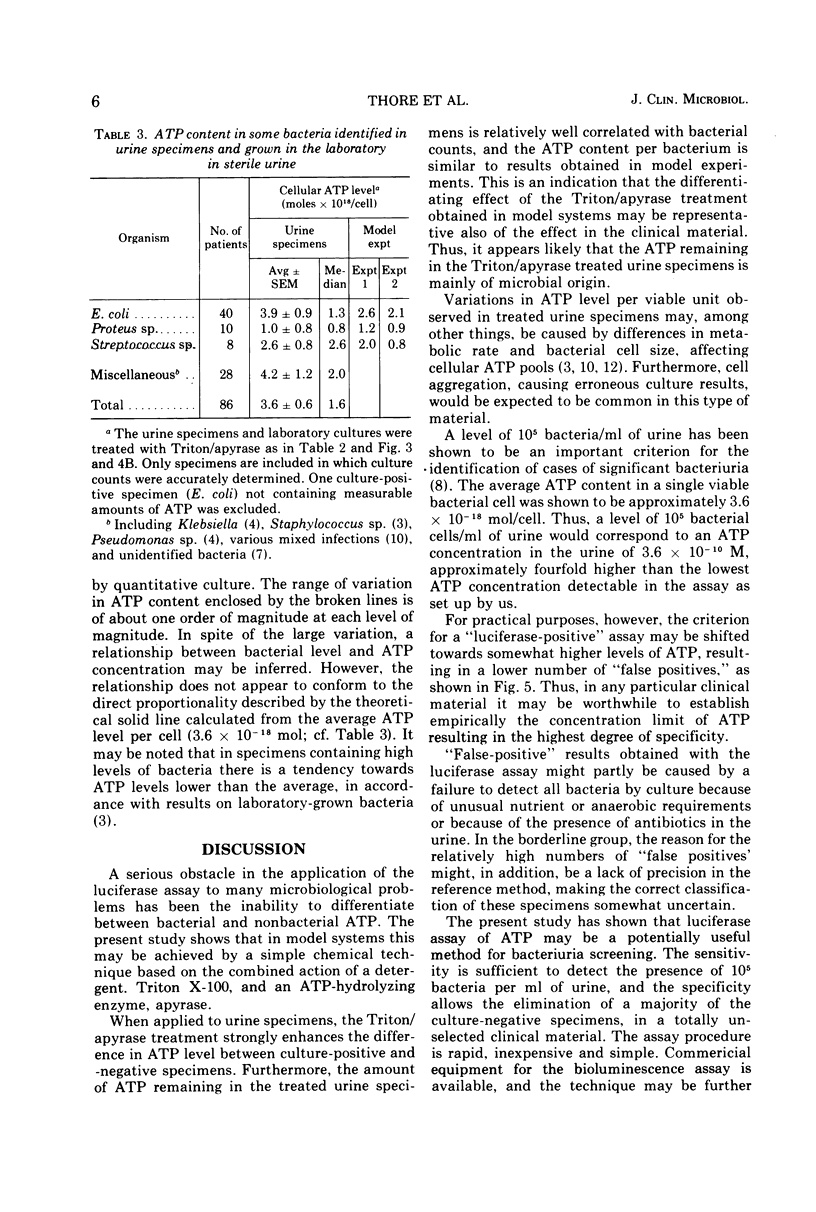
Abstract
A selective method for distinguishing bacterial and nonbacterial adenosine triphosphate (ATP) in clinical bacteriological specimens was studied. The method involved incubation of samples with the detergent Triton X-100 and the ATP-hydrolyzing enzyme apyrase. The incubation selectively destroyed ATP in suspensions of various human cells while not affecting the ATP content in microbial cells. ATP remaining in the sample after incubation was extracted in boiling buffer and assayed by the firefly luciferase assay. Application of the method to 469 clinical urine specimens showed that the ATP level after treatment with Triton/apyrase was correlated to bacterial counts and that the sensitivity of the assay was sufficient for the detection of 10(5) bacteria/ml. The ATP levels per bacterial cell remaining in the urine specimen after treatment with Triton/apyrase were close to values observed in laboratory-grown cultures. The specificity and sensitivity of the luciferase assay for the detection of urinary bacteria and its possible use as a bacteriuria screening method are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forrest W. W. Adenosine triphosphate pool during the growth cycle in Streptococcus faecalis. J Bacteriol. 1965 Oct;90(4):1013–1018. doi: 10.1128/jb.90.4.1013-1018.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Huzyk L., Clark D. J. Nucleoside triphosphate pools in synchronous cultures of Escherichia coli. J Bacteriol. 1971 Oct;108(1):74–81. doi: 10.1128/jb.108.1.74-81.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASS E. H. Bacteriuria and the diagnosis of infections of the urinary tract; with observations on the use of methionine as a urinary antiseptic. AMA Arch Intern Med. 1957 Nov;100(5):709–714. doi: 10.1001/archinte.1957.00260110025004. [DOI] [PubMed] [Google Scholar]
- Levin G. V., Usdin E., Slonim A. R. Rapid detection of microorganisms in aerospace water systems. Aerosp Med. 1968 Jan;39(1):14–16. [PubMed] [Google Scholar]
- STRANGE R. E., WADE H. E., DARK F. A. EFFECT OF STARVATION ON ADENOSINE TRIPHOSPHATE CONCENTRATION IN AEROBACTER AEROGENES. Nature. 1963 Jul 6;199:55–57. doi: 10.1038/199055a0. [DOI] [PubMed] [Google Scholar]
- Sharpe A. N., Woodrow M. N., Jackson A. K. Adenosinetriphosphate (ATP) levels in foods contaminated by bacteria. J Appl Bacteriol. 1970 Dec;33(4):758–767. doi: 10.1111/j.1365-2672.1970.tb02260.x. [DOI] [PubMed] [Google Scholar]
- Strange R. E. Rapid detection and assessment of sparse microbial populations. Adv Microb Physiol. 1972;8:105–141. doi: 10.1016/s0065-2911(08)60189-7. [DOI] [PubMed] [Google Scholar]
- Tärnvik A. Isolation of lymphocytes from blood. A procedure combining nylon fibre filtration and differential centrifugation. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(3):311–316. [PubMed] [Google Scholar]
- Van Dyke K., Stitzel R., McClellan T., Szustkiewicz C. An automated procedure for the sensitive and specific determination of ATP. Clin Chem. 1969 Jan;15(1):3–14. [PubMed] [Google Scholar]


