Abstract
The differentiation of embryonic stem cells (ESCs) into neurons and glial cells represents a promising cell-based therapy for neurodegenerative diseases. Because the rhesus macaque is physiologically and phylogenetically similar to humans, it is a clinically relevant animal model for ESC research. In this study, the pluripotency and neural differentiation potential of a rhesus monkey ESC line (ORMES6) was investigated. ORMES6 was derived from an in vitro produced blastocyst, which is the same way human ESCs have been derived. ORMES6 stably expressed the embryonic transcription factors POU5F1 (Oct4), Sox2 and NANOG. Stage-specific embryonic antigen 4 (SSEA 4) and the glycoproteins TRA-1-60 and TRA-1-81 were also expressed. The embryoid bodies (EBs) formed from ORMES6 ESCs spontaneously gave rise to cells of three germ layers. After exposure to basic fibroblast growth factor (bFGF) for 14–16 days, columnar rosette cells formed in the EB outgrowths. Sox2, microtubule-associated protein (MAP2), β-tublinIII and glial fibrillary acidic protein (GFAP) genes and Nestin, FoxD3, Pax6 and β-tublinIII antigens were expressed in the rosette cells. Oct4 and NANOG expression were remarkably down-regulated in these cells. After removal of bFGF from the medium, the rosette cells differentiated along neural lineages. The differentiated cells expressed MAP2, β-tublinIII, Neuro D and GFAP genes. Most differentiated cells expressed early neuron-specific antigen β-tublinIII (73±4.7%) and some expressed intermediate neuron antigen MAP2 (18±7.2%). However, some differentiated cells expressed the glial cell antigens A2B5 (7.17%±1.2%), GFAP (4.93±1.9%), S100 (7±3.5%) and O4 (0.2±70.2%). The rosette cells were transplanted into the striatum of immune-deficient NIHIII mice. The cells persisted for approximately 2 weeks and expressed Ki67, NeuN, MAP2 and GFAP. These results demonstrate that the rhesus monkey ESC line ORMES6 retains the pluripotent characteristics of ESCs and can be efficiently induced to differentiate along neural lineages.
Keywords: Embryonic stem cell, Rhesus monkey, Neurogenesis, Neural lineage differentiation
1. Introduction
Embryonic stem cells (ESCs) have the potential to generate cells of all three embryonic germ layers (Evans and Kaufman, 1981; Martin, 1981; Kuai et al., 2003; Copi et al., 2005; Li et al., 2005; Benzing et al., 2006; Kamochi et al., 2006; Takeshita et al., 2006; Kang et al., 2007). Because of their self-renewal capacity and pluripotency, ESC-based therapy holds great potential for the treatment of human diseases. However, before clinical applications are realized, the safety, efficacy and feasibility of this therapeutic approach must be established in animal models. Promising achievements of ESC transplantation have been made in mouse disease models (Kolossov et al., 2006; Hara et al., 2006; Soto-Gutiérrez et al., 2006; Lee et al., 2007; Riess et al., 2007; Rodríguez-Gómez et al., 2007). Differences between human and mouse ESCs are significant (Ginis et al., 2004), and the safety of ESC-derived cell transplantation in humans is still unclear.
The rhesus macaque has more than 90% DNA homology to humans and has long been used as a model to study various human diseases (Porrino et al., 1987; Cornblath et al., 1989; Rokkas et al., 1993; Baskin et al., 1998). Human and rhesus monkey ESCs share many characteristics including morphology, surface marker expression and developmental potential (Pau and Wolf, 2004). Rhesus monkey ESCs may thus play an intermediate translational role as therapeutic strategies evolve from rodent systems to human clinical applications. Rhesus monkey animal models for Krabbe disease, Parkinson’s disease, diabetes and spinal cord injury have been demonstrated (Porrino et al., 1987; Cornblath et al., 1989; Rokkas et al., 1993; Baskin et al., 1998). Transplantation of cells derived from rhesus monkey ESCs into these models will be perfect strategies to evaluate ESCs for human disease therapy.
ESC lines have been derived from both in vitro produced blastocysts and in vivo flushed blastocysts (Pau and Wolf, 2004). At present, 26 rhesus monkey ESC lines have been produced (Byrne et al., 2006). Eighteen of these cell lines (ORMES) were derived from in vitro fertilized embryos at the Oregon National Primate Research Center (Byrne et al., 2006). The remaining eight cell lines (R series) were made from in vivo produced blastocysts by Dr. James Thomson at the Wisconsin National Primate Research Center. The majority of available information on rhesus monkey ESC neural lineage differentiation has been obtained from studies on the cells derived from in vivo flushed blastocysts (Thomson et al., 1998; Kuo et al., 2003; Salli et al., 2004; Wolf et al., 2004), although no human ESC lines have been derived in that way. The unanswered question is whether or not the neural differentiation process of rhesus monkey ESCs derived from in vitro fertilized blastocysts is similar to that of human ESCs. To answer this question, the pluripotency and neural differentiation potential of ORMES6, an in vitro derived rhesus monkey ESC line (Pau and Wolf, 2004; Byrne et al., 2006), was investigated in this study.
Based on neural system development, two methods have been described for directing ESCs into neural lineage cells. One involves an embryoid body (EB) intermediate and complex media (Thomson et al., 1998; Kuo et al., 2003; Salli et al., 2004; Li et al., 2005); the other applies co-culture with cells such as PA6 mouse stromal cells, to expose the ESCs to stromal cell-derived inducing activity, and then culture as neurospheres (Morizane et al., 2006). Here, a procedure for efficient and reproducible differentiation of neural lineage cells from rhesus monkey ESCs with chemically complex media is described. The data demonstrate that the rhesus monkey ESCs retain the characteristics of pluripotent ESCs and can be efficiently induced to differentiate along neural lineages.
2. Materials and methods
2.1. ESC culture
Rhesus monkey ESC line ORMES6 was obtained from Dr. Don P. Wolf at the Oregon National Primate Research Center. This ESC line was derived from an in vitro produced blastocyst and has been previously characterized for ESC markers and the ability to differentiate into three germ layer cells (Pau and Wolf, 2004). The ORMES6 ESC line, starting at passage 26, was cultured in our lab according to Dr. Wolf’s instructions. Cells between passages 28 and 35 were used for neural differentiation.
Briefly, ESCs were co-cultured with mitotically inactivated mouse embryonic fibroblasts (MEFs) in ESC culture medium consisting of DMEM/F12 (Invitrogen, Carlsbad, CA), supplemented with 20% fetal bovine serum (Hyclone, Logan, UT), 100 mM MEM nonessential amino acids (Invitrogen), 2 mM L-glutamine (Invitrogen) and 0.1 mM β-mercaptoethanol (Sigma, St. Louis, MO). MEFs were derived from E13 mouse embryos and expanded in DMEM with 10% fetal bovine serum, 2 mM L-glutamine and antibiotics. Subconfluent MEF cultures were treated with 10 μg/ml Mitomycin C (Sigma) for 2 h, washed 3 times with PBS, trypsinized and plated at 4 × 104/cm2 onto 6-well plates (Fisher/Nunc, Hampton, NH) overnight. MEFs were then washed once with PBS and incubated in ESC medium for 1 day before plating ESCs. MEFs from passages 2–4 were used as feeder cells. ESC colonies were observed and the medium was changed daily. The ESCs were passaged every 5–6 days, or when the cell confluence reached about 80–90%. For passaging, cultures were incubated with dispase (1 mg/ml, Invitrogen) at 37 °C for 5–8 min. The dispase was aspirated, then the culture plates were rinsed with ESC culture medium and colonies were triturated into small clumps. One 6-well plate of ESCs was replated into four to five 6-well plates with freshly inactivated MEFs.
2.2. Formation of embryoid bodies (EBs)
Entire ESC colonies were detached from MEF feeder cells by exposure to dispase (1 mg/ml, 37 °C for 5–8 min). The dispase was aspirated, then the cell layer was rinsed with ESC culture medium and colonies were triturated into small clumps. Two 6-well plates of ESCs (about 80–90% confluence) were transferred into one low-attachment 6-well cell culture plate (Fisher, Hampton, NH) with ESC culture medium for EB formation. The EBs were cultured in suspension for 4 days.
2.3. Neural progenitor cell induction
EBs from one low-attachment 6-well plate were transferred onto one 0.1% gelatin-coated 10 cm diameter cell culture dish (Fisher/Nunc). The EBs were cultured in ESC culture medium for 48 h to permit attachment of the EBs. Then the culture medium was replaced with neural induction medium composed of DMEM/F12 (1:1) supplemented with N2 (Invitrogen), 100 mM MEM nonessential amino acids and basic fibroblast growth factor (bFGF) (10 ng/ml, Invitrogen). The EB outgrowths were maintained in neural induction medium and fed every 2 days. Within 8–10 days, neural tube-like columnar rosette cells formed in the EBs outgrowths.
With continued exposure to bFGF, the neural rosette cells expanded and formed multiple layers. They frequently composed the bulk of the EBs and were clearly demarcated from the surrounding flat cells. The EB outgrowth cells were incubated with dispase (0.2 mg/ml, 37 °C for 10–15 min) to separate the clusters of neural rosette cells from the surrounding flat cells. The neural rosette cell clumps retracted upon exposure to dispase, whereas the surrounding flat cells remained adherent. At this point, the neural rosette cells were dislodged by gently tilting the culture dish. The cell clumps were pelleted, gently triturated with a transfer pipette and plated into a new 10 cm diameter cell culture dish for 1 h to allow the contaminating flat cells to adhere. The floating neural rosette cell clumps were then transferred to another dish and cultured in neural induction medium. In order to obtain uncontaminated putative neural cells, this separation procedure was repeated 2–3 times, and the culture period of this step was over 21 days. Large neural rosette cell clumps were dissociated with a P100 pipette.
For neuronal and glial subtype differentiation, the neural rosette cell clumps were cultured on polyornithine and laminin coated 8-well plates (BD, Franklin Lakes, NJ) with NeuroCult® NS-A Differentiation Kit (Human) (Stem Cell Technologies, Vancouver, Canada) instead of neural induction medium. Morphological analysis, gene expression analysis and immunostaining were performed during the course of the differentiation.
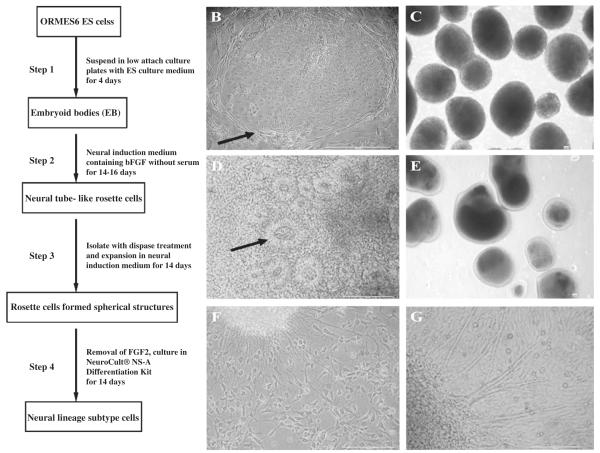
In summary, the whole differentiation procedure was divided into four stages: EB formation, neural rosette cell induction and isolation, expansion of neurosphere-like structures and neural subtype differentiation (Fig. 1A).
Fig. 1.
The protocol for ORMES6 ESC differentiation into neural lineage cells. (A) Sequential culture procedures for ORMES6 ESC differentiation into neural lineage subtype cells. (B) ORMES6 ESC colony (arrow) in an undifferentiated state on an MEF feeder layer; (C) EBs derived from ORMES6 ESCs; (D) columnar rosette cells (arrow) resembling the early neural tube in the EB outgrowth cells after exposure to bFGF for 14–16 days; (E) isolated rosette cells formed free-floating cell aggregates similar to neurospheres; (F) highly branched cells, similar in morphology to neural lineage cells, grew out from attached rosette cell aggregates. Neurite-like processes appeared after removing bFGF from the cell culture medium; (G) fibroblastoid and epithelia-like cells that result from the spontaneous differentiation of ORMES6 cells in the absence of cytokines and chemical inducers. Scale bar: 40 μm.
2.4. RT-PCR
The expression of mRNAs for markers of pluripotentiality and specific neural lineage genes was assessed on undifferentiated ORMES6 ESCs, neural rosette cells, neural rosette cell derived neural subtype cells and spontaneously differentiated ORMES6 cells. Table 1 lists the sequences of all the PCR primers and the sizes of PCR products. Total cellular RNA was isolated from the differentiating ESCs at each step identified in the protocol. Using the same induction steps, ORMES6 ESCs cultured in the ESC culture medium instead of in the chemically complex media were set as control samples. Total cellular RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) and treated with DNA-free® kit (Ambion, Austin, TX) to remove potential contamination of genomic DNA. A total of 500 ng of RNA was used as a template for reverse transcription with Reverse Transcription System (Promega, Madison, WI). Following the RT step, the concentration of the cDNA was adjusted to the same level in all samples and 100 ng cDNA was used for a standard PCR reaction for the described primer sets. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase, was used as a control for the efficiency of the PCR reaction. The PCR step was performed using the PCR Master Mix kit (Promega), and the PCR products were detected and analyzed by 2% agarose gel electrophoresis.
Table 1.
Primers used for RT-PCR
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| GAPDH | F: 5′ ATC ACC ATG GAG AAG GCT GG 3′ | 524 |
| R: 5′ CTT AGC GTA GCC CAG GAT GC 3′ | ||
| Oct4 | F: 5′ CTC CTG GAG GGC CAG GAA TC 3′ | 381 |
| R: 5′ CCA CAT CGG CCT GTG TAT AT 3′ | ||
| Sox2 | F: 5′ GGC AGC TAC AGC ATG ATG CAG GAC 3′ | 131 |
| R: 5′ CTG GTC ATG GAG TTG TAC TGC AGT 3′ | ||
| NANOG | F: 5′ CCT ATG CCT GTG ATT TGT GG 3′ | 208 |
| R: 5′ CCG GGA CCT TGT CTT CCT TT 3′ | ||
| Neuro-D | F: 5′ CCT CGA AGC CAT GAA CGC AG 3′ | 583 |
| R: 5′ GCT GTC CAT GGT ACC GTA AG 3′ | ||
| GFAP | F: 5′ ATC GAG AAG GTT CGC TTC CT 3′ | 194 |
| R: 5′ AGG TCC TGT GCC AGA TTG TC 3′ | ||
| Tublin-beta III | F: 5′ CGCATCATGAAC ACC TTC AG 3′ | 207 |
| R: 5′ CGA TAC CAG GTG GTT GAG GT 3′ | ||
| MAP 2 | F: 5′ GGA GTA ACC AAG AGC CCA GA 3′ | 205 |
| R: 5′ CAG GGG TAG TGG GTG TTG AG 3′ |
PCR primers: 245×182mm2 (96×96 DPI).
2.5. Immunocytochemistry
Cells for immunocytochemistry were cultured on chamber slides and fixed in 4% paraformaldehyde in PBS for 15 min, permeabilized with 0.1% Triton X-100 for 10 min and then blocked for 1 h at room temperature in PBS containing 4% goat serum (Invitrogen). Samples were then incubated in blocking buffer containing primary antibody for 1 h at room temperature and washed 3 times with PBS for 10 min each wash. The cells were then incubated with secondary antibodies conjugated with Texas Red or Alexa 488 (1:1000, Molecular Probes, Eugene, OR) for 1 h at room temperature. The samples were washed as above and mounted with 6-diamidino-2-phenylindole (DAPI; DAKO, Carpinteria, CA) containing mounting solution.
The following primary antibodies were used: anti-neuronal nuclear antigen (NeuN) (IgG, 1:100), anti-tubulin betaIII (IgG, 1:50), anti-nestin (rabbit polyclonal, 1:100), anti-FoxD3 (rabbit polyclonal, 1:50), anti-Pax6 (rabbit polyclonal, 1:100, Covance Research Products, Inc, Denver, PA), anti-microtubule-associated protein (MAP2) (IgG, 1:50), anti-A2B5 (IgM, 1:100), anti-glial fibrillary acidic protein (GFAP) (IgG, 1:100), anti-O4 (IgG, 1:100), anti-galactocerebroside (GalCer) (IgG, 1:100), anti-S100 (rabbit polyclonal, 1:100), anti-SSEA 4 (IgG, 1:66), anti-TRA-1-60 (IgM, 1:66), anti-TRA-1-81(IgM, 1:66), anti-Oct4 (rabbit polyclonal, 1:13.3, Santa Cruz Biotechnology, Santa Cruz, CA), anti-human Ki67 (IgG1, 1:100, DAKO, Carpinteria, CA) and anti-Brachyury (goat polyclonal, 1:13.3, Santa Cruz Biotechnology, Santa Cruz, CA). All primary antibodies were obtained from Chemicon (Billerica, MA), unless noted. Fluorescent samples were examined with a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan).
At least three independent experiments and at least 1000 cells per marker were analyzed. The percentage of the markers expressed by differentiated ORMES6 ESCs were determined and calculated as values±standard error.
2.6. Cell transplantation and in vivo experiments
All animal procedures conformed to requirements of the Animal Welfare Act, and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Tulane University. The neural rosette cell aggregates were dissociated into small cell clumps by pipetting gently. The cell suspensions were sampled and treated with trypsin (0.05% in 0.1% EDTA) for cell counting. The neural rosette cells were suspended in PBS at a concentration of about 105 cells/μl and placed on ice until transplantation. Immune-deficient mice (6–8 weeks old, NIHIII Immune-deficient Mouse, Charles River Laboratories, Inc., Wilmington, MA) were deeply anaesthetized and placed into a stereotactic apparatus. For transplantation, the scalp was opened, a hole was drilled and 2 μl of cell suspension (2×105 total cells) was injected into the striatum over a period of 5 min, the injection coordinates were as follows: anteroposterior (AP): 0.5 mm, mediolateral (ML): 1.25 mm, dorsoventral (DV): -3 mm. The transplantation of the cells was performed with a micropipette connected to a Hamilton syringe. Mice were sacrificed at 1, 2, 4 weeks post-transplantation and perfusion fixed with 4% paraformaldehyde. Brains were collected and postfixed for 4 h in 4 °C, then cryoprotected in 30% sucrose and sectioned into 10 μm thick sections from injection point. The tissue sections were blocked with 10% normal goat serum for 1 h at room temperature, and then incubated with primary antibodies 1 h at room temperature. The sections were washed 3 times before being incubated with secondary antibodies for 1 h at room temperature. The transplanted cells were identified with an antibody to a human-specific nuclear antigen (HNA) (Chemicon, Temecula, CA, 1:50). The HNA immunoreactive cells were later double stained with antibodies to GFAP (Sigma, 1:200), MAP2 (Chemicon, 1:100), NeuN (Chemicon, 1:50) and Ki67 (DAKO, 1:100). The sections were mounted with DAPI (DAKO, Carpinteria, CA) containing mounting solution. The slides were examined with a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan).
3. Results
3.1. ORMES6 ESCs
Rhesus monkey ESC line ORMES6, derived from an in vitro produced blastocyst, was propagated on mitotically inactivated MEFs. ORMES6 ESCs formed colonies with individual cells containing large nuclei and distinctive cell boundaries (Fig. 1B) in the undifferentiated state.
The quality of ORMES6 ESC cultures in our laboratory was confirmed by evaluating several markers that are known to be expressed by undifferentiated rhesus monkey ESCs (Pau and Wolf, 2004; Wolf et al., 2004; Byrne et al., 2006). Undifferentiated ORMES6 ESCs expressed stage-specific embryonic antigen 4 (SSEA 4) (Fig. 2A), transcription factor Oct4 (Fig. 2B) and glycoproteins TRA-1-60 (Fig. 2C) and TRA-1-81 (Fig. 2D). The undifferentiated ORMES6 ESCs expressed the Oct4, NANOG and Sox2 genes, which are essential embryonic transcription factors (Byrne et al., 2006) (Fig. 3, Lane 1). The expression of MAP2 and β-tublinIII mRNAs (Fig. 3, Lane 1) in undifferentiated ORMES6 ESCs indicated that some of the cells may have undergone spontaneous differentiation.
Fig. 2.

Undifferentiated ORMES6 ESCs express embryonic cell markers. (A) SSEA 4 (green); (B) Oct4 (red); (C) glycoprotein TRA-1-60 (green)/ Oct4 (red) and (D) glycoprotein TRA-1-81 (green)/Oct4 (red). The nuclei were stained with DAPI (blue). The undifferentiated ORMES6 ESCs expressed SSEA 4, Oct4, TRA-1-60 and TRA-1-81 antigens. Cells only stained with DAPI were MEF feeder cells. Scale bar: 40 μm.
Fig. 3.
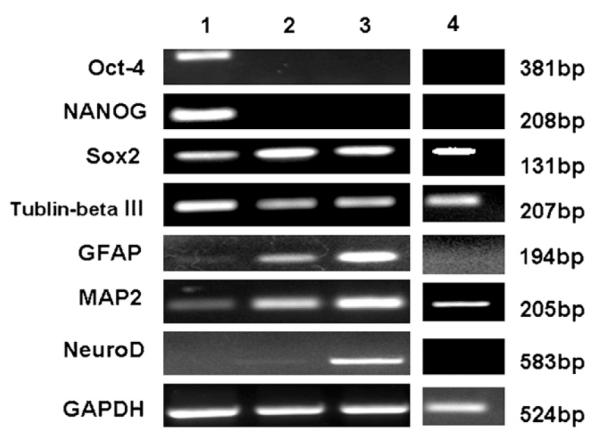
RT-PCR analysis of gene expression at select stages of differentiation. Oct4, Sox2 and NANOG are markers of pluripotent stem cells. MAP2, β-tublinIII, NeuroD are neuronal markers, and GFAP is glial cell marker. Undifferentiated ORMES6 ESCs expressed Oct4, NANOG, Sox2 stemness genes and MAP2, β-tublinIII neuronal genes. Oct4 and NANOG transcription factors were markedly down-regulated in the rosette cells. Sox2, MAP2, β-tublinIII neuronal genes and GFAP glial cell genes were expressed in the rosette cells. The neural subtype cells derived from rosette cells expressed Sox2, MAP2, β-tublinIII, GFAP and NeuroD genes. However, spontaneously differentiated cells only expressed β-tublinIII, Sox2 and MAP2 genes.
Lane 1: undifferentiated ORMES6 ESCs; Lane 2: neural progenitor cells (neural tube like-rosette cells) induced by neural induction medium (Step 2 of protocol); Lane 3: Neural subtype cells derived from rosette cells (Step 4); Lane 4: Spontaneously differentiated ORMES6 ESCs cultured in ESC medium without MEF cells.
EBs derived from ORMES6 ESCs were composed of all three germ layers. The EBs expressed markers of three germ layers (Fig. 4), namely β-tublinIII (Fig. 4A) as an ectodermal marker, alpha-fetoprotein (Fig. 4B) as an endodermal marker and both cardiac troponin I (Fig. 4C) and Brachyury (Fig. 4D) as mesodermal markers. Moreover, at 30 days some EBs spontaneously formed clusters of contracting cardiac cells (data not shown).
Fig. 4.

EBs derived from ORMES6 ESCs express markers of three germ layers. EBs derived from ORMES6 ES cell line, cultured with ES cell culture medium in low-attachment 6-well plates for 1 month, expressed three germ layer markers. (A) β-tublinIII as an ectodermal-derived neuronal marker (red label); (B) alpha-fetoprotein as an endodermal marker (green label); (C) cardiac troponin I as a mesodermal marker (green label) and (D) brachyury as a mesodermal marker (green label). The nuclei were counter-stained with DAPI (blue). Scale bar: 40 μm.
3.2. ORMES6 ESC-derived neural tube-like rosette cells
Undifferentiated ORMES6 ESCs were propagated on mitotically inactivated MEFs. To induce EBs, ESC colonies were detached from MEF feeder cells by exposure to dispase. The colonies were triturated into small cell clumps, which were plated onto a low-attachment surface and spontaneously formed spherical aggregates. When disassociated into single cells, the ESCs failed to aggregate. The ESC aggregates were cultured in suspension as EBs for 4 days (Fig. 1C). Then the EBs were plated onto gelatin-coated dishes with neural induction medium and allowed to attach. Neural induction medium does not include serum but does include bFGF.
A large number of small, elongated cells were noted in the EB outgrowths by 8–10 days. These small tightly packed epithelial-like cells developed in the center of EB outgrowth and fibroblast-like cells grew at the periphery. With continued exposure to bFGF for 14–17 days, the small, elongated cells formed a radial arrangement (Fig. 1D) which was organized similarly to that of the neural tube. These columnar rosette cells were isolated with dispase treatment and expanded in neural induction medium as free-floating cell aggregates (Fig. 1E), similar to neurosphere cultures derived from fetal brain tissues (Svendsen et al., 1996; Brüstle et al., 1997, 1998; Carpenter et al., 1999; Fricker et al., 1999; Vescovi et al., 1999; Reynolds and Rietze, 2005).
Transcription factor gene Sox2, neuron-specific genes MAP2 and β-tublinIII (Katsetos et al., 2003; Dehmelt and Halpain, 2005) and astrocyte gene GFAP (Baba et al., 1997) were expressed in neural rosette cells (Fig. 3, Lane 2). However, Oct4 and NANOG genes were markedly down-regulated in these cells.
Immunocytochemical analyses showed that more than 99% of neural rosette cells expressed nestin, which is specifically expressed in neuroepithelial stem cells (Fig. 5A) (Lendahl et al., 1990). Some of these cells also expressed Pax6 (15.60±6.3% of DAPI), FoxD3 (37.97±9.40% of DAPI) and Sox10 (23.40±2.83% of DAPI) antigens (Fig. 5B-D, F). During neural system development, Pax6, FoxD3 and Sox10 have been implicated in specification of the neural crest and neural tube (Mollaaghababa and Pavan, 2003; Barembaum and Bronner-Fraser, 2005; Lazzari et al., 2006). About 29.65±9.70% of neural rosette cells expressed the cell proliferation marker, Ki67 (Fig. 5E, F).
Fig. 5.
Expression of neuroepithelial markers by neural tube-like rosette cells. After exposure to bFGF for 14–16 days, neural tube-like rosette cells formed in the EBs outgrowth. The isolated rosette cells were positively stained for nestin, FoxD3, Pax6 and Sox10 that are considered as markers for neuroepithelial stem cells. The neural rosette cells were also positive for Ki67, which is a cell proliferation marker. (A) Nestin (green label); (B) FoxD3 (red label); (C) Pax6 (green label); (D) Sox10 (green label); (E) Ki67 (red label) and (F) percentage of each marker. The nuclei were counter-stained with DAPI (blue). Scale bar: 40 μm.
3.3. Neural subtype differentiation
Differentiation of isolated neurosphere-like rosette cell aggregates along neuronal and glial cell lineages was initiated by substituting the neural induction medium with the NeuroCult® NS-A Differentiation Kit. The cells were plated onto polyornithine and laminin coated cell culture dishes. About 4 days later, neurite-like processes appeared. Approximately 14 days after plating, extensive branches radiated out from the cell clumps (Fig. 1F).
ORMES6 ESCs treated to the same induction process, but cultured solely in the ESC culture medium instead of in the chemically complex media, were set as control samples. These spontaneously differentiated ESC cultures grew cells with morphologies that were not unique, consisting of fibroblast-like cells, epithelial-like cells and neural lineages-like cells (Fig. 1G).
The neural subtype cells expressed neural lineage genes Sox2, MAP2, β-tublinIII, GFAP and NeuroD (Fig. 3, Lane 3) (Katsetos et al., 2003; Dehmelt and Halpain, 2005; Wegner and Stolt, 2005; Hevner et al., 2006; Miyagi et al., 2006). However, the spontaneously differentiated cells only expressed Sox2, MAP2 and β-tublinIII genes (Fig. 3, Lane 4).
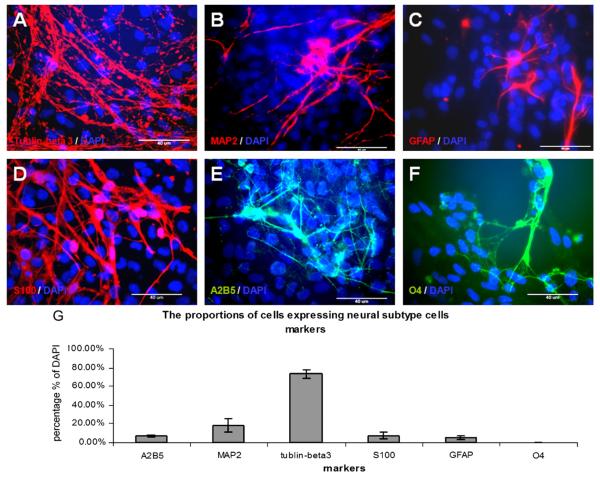
The majority of neural subtype cells derived from neurosphere-like rosette cell aggregates positively stained for β-tublinIII (73±4.7% of DAPI) and MAP2 (18±7.2% of DAPI) (Fig. 6A, B). NeuN is a mature neuronal marker (Mullen et al., 1992; Sarnat et al., 1998), and was negative in this study (data not shown). The mature oligodendrocyte marker, GalCer, was also negative (data not shown). However, some cells expressed the glial cell markers A2B5 (7.17±1.2% of DAPI), GFAP (4.93±1.9% of DAPI), S100 (7±3.5% of DAPI) and O4 (0.27±0.2% of DAPI) (Ingraham and Rising, 2000; Yasuda et al., 2004; Raponi et al., 2007)(Fig. 6C-G), suggesting that a subpopulation of cells retained the characteristics of the glial lineage.
Fig. 6.
Neural subtype cells derived from the rosette cells. The neural subtype cells differentiation was initiated by removal of bFGF from the culture medium. Most differentiated cells expressed neuronal cell markers, a small subpopulation of the differentiated cells expressed glial lineage cells markers. (A) Neuronal marker β-tublinIII (red label); (B) neuronal-specific protein MAP2 (red label); (C) glial cell marker GFAP (red label); (D) glial cell marker S100 (red label); (E) glial cell marker A2B5 (green label) and (F) oligodendrocyte marker O4 (green label). The nuclei are counter-stained with DAPI (blue). Scale bar: 40 μm. (G) The percentages of markers expressed by the differentiated neural subtype cells.
3.4. Transplantation of neurosphere-like rosette cells into SCID mice
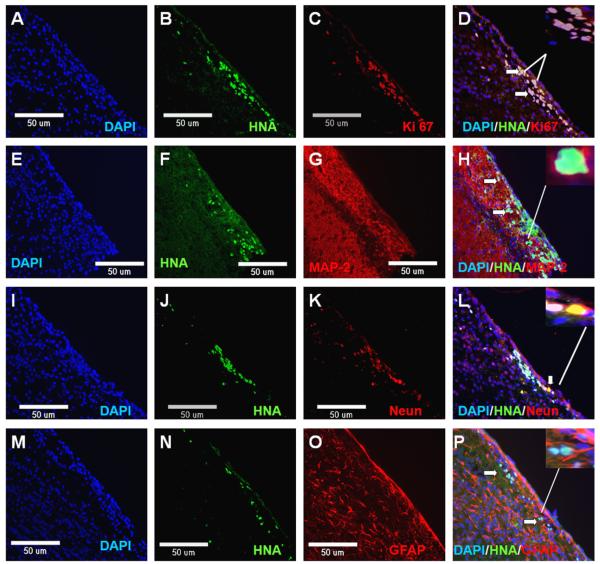
To assess the cell survival, proliferation, differentiation and tumorigenesis potential of ORMES6 ESC-derived rosette cells in vivo, the rosette cells were transplanted into the striatum of immune-deficient NIHIII mice. The cells could be found along the needle tract at 1 week (n = 3) and 2 weeks (n = 3) post-transplantation. And the number of surviving cells is very small. By 4 weeks, cells were not detected (n = 3). In brain tissue sections, the transplanted cells were distinguished from the host cells by HNA staining (Fig. 7B, F, J, N). Some of the donor cells were double stained with cell proliferation marker Ki67 (Fig. 7D), or neural lineage cell markers GFAP, MAP2 and NeuN (Fig. 7H, L, P).
Fig. 7.
Neural rosette cells derived from ORMES6 gave rise to neuron and glial cells in vivo. The rosette cells were transplanted into the striatum of NIHIII mice. At 1, 2, 4 weeks after transplantation, the mice were sacrificed and the brains collected for analysis. The cells could be found at 1 week (n = 3) and 2 weeks (n = 3) post-transplantation. And the number of surviving cells is very small. By 4 weeks, cells were not detected (n = 3). The transplanted cells were distinguished from the host cells by human-specific nuclear antigen (HNA) staining. The transplanted neural rosette cells expressed cell proliferation marker Ki67, astrocyte marker GFAP and neuron markers MAP2 and NeuN. (A) DAPI (blue label); (B) HNA (green label); (C) cell proliferation marker Ki67 (red label); (D) Merge; (E) DAPI (blue label); (F) HNA (green label); (G) neuronal-specific protein MAP (red label); (H) Merge; (I) DAPI (blue label); (J) HNA (green label); (K) neuron marker NeuN (red label); (L) Merge; (M) DAPI (blue label); (N) HNA (green label); (O) astrocyte marker GFAP (red label); and (P) Merge. The nuclei are counter-stained with DAPI (blue). Scale bar: 50 μm.
4. Discussion
The present study indicates that ORMES6, a rhesus monkey ESC line derived from an in vitro produced blastocyst, maintains the typical characteristics of ESCs (Pau and Wolf, 2004; Byrne et al., 2006) and can be efficiently induced to differentiate along neural lineages. Further, the neural differentiation process is similar to that for human ESCs (Zhang et al., 2001; Li et al., 2005; Roy et al., 2006). Rhesus monkey ESCs may therefore be a transitional model for human ESC research.
Under the ESC culture conditions used in this study, undifferentiated ORMES6 cells showed positive immunostaining for SSEA4 and glycoproteins TRA-1-60 and TRA-1-81, which are unique ESC markers (Pau and Wolf, 2004; Wolf et al., 2004; Byrne et al., 2006). The undifferentiated cells also expressed pluripotent transcription factor genes, Oct4, Sox2 and NANOG (Pau and Wolf, 2004; Byrne et al., 2006). When cultured in low-attachment plates with ESC culture medium, ORMES6-derived EBs spontaneously differentiated into cells representing three embryonic germ layers. These results demonstrate that the ORMES6 ESC line, from an in vitro produced blastocyst, has maintained the pluripotent characteristics of ESCs (Pau and Wolf, 2004; Byrne et al., 2006).
bFGF has numerous effects in the nervous system, ranging from regulation of stem cell proliferation and differentiation to neuronal survival (Gensburger et al., 1987; Ghosh and Greenberg, 1995; Tao et al., 1997). In this study, after exposure to bFGF for 8–10 days, columnar rosette cells formed in the EB outgrowths. The rosette cells formed radial arrangements, similar to neural tube structures. The rosette cells were isolated with dispase. The isolated rosette cells were expanded as free-floating cell aggregates in a suspension culture, similar to neurosphere cultures derived from fetal brains (Svendsen et al., 1996; Brüstle et al., 1997, 1998; Carpenter et al., 1999; Fricker et al., 1999; Vescovi et al., 1999) in a medium with bFGF. More than 99% of these rosette cells positively stained with nestin, a neuroepithelial stem cell marker (Lendahl et al., 1990). Neural crest stem cell markers FoxD3, Pax6 and Sox10 (Mollaaghababa and Pavan, 2003; Barembaum and Bronner-Fraser, 2005; Lazzari et al., 2006), and the cell proliferation marker Ki67 were also expressed in the neural rosette cells. These data suggest that bFGF has induced neural differentiation and neural crest stem cell proliferation.
Oct4, Sox2 and NANOG transcription factors work together to maintain the pluripotency of ESCs (Gensburger et al., 1987). In this study, Oct4 and NANOG genes were down-regulated after differentiation; however, Sox2 was continuously expressed. Other studies have shown that Sox2 is required for not only ESCs but also neural stem cell maintenance (Episkopou, 2005; Bani-Yaghoub et al., 2006; Pan and Thomson, 2007). The neural crest stem cell markers nestin, FoxD3, Pax6 and Sox10 antigens were expressed in the rosette cells, as were neuron-specific genes MAP2 and β-tublinIII (Katsetos et al., 2003; Dehmelt and Halpain, 2005) and the astrocyte gene GFAP (Baba et al., 1997). These data indicate that these columnar rosette cells have some characteristics in common with neural crest stem cells and the neural tube in early neurogenesis.
Some studies have demonstrated that removal of bFGF results in substantial neural differentiation (Namaka et al., 2001; Ito et al., 2003; Ding et al., 2006). In this study, neurite-like processes appeared after removal of bFGF from the culture medium. Cells with extensive branches radiated out from the rosette cell aggregates. RT-PCR analysis revealed that the differentiated neural subtype cells expressed intermediate neuronal progenitor differentiation markers MAP2 and β-tublinIII, and Neuro D which is a basic helix—loop—helix transcription factor capable of converting embryonic epidermal cells into fully differentiated neurons (Chae et al., 2004). Other researchers have also confirmed that removal of bFGF from culture medium increases NeuroD mRNAs, resulting in substantial neuronal differentiation (Ito et al., 2003). In all, 73±4.7% of the differentiated cells were positively stained for β-tublinIII and 18±7.2% expressed MAP2. However, there was no detectable expression of the mature neuron marker NeuN (Mullen et al., 1992; Sarnat et al., 1998). The NeuN protein first appears at the initiation of terminal differentiation of the neuron (Mullen et al., 1992). These data suggest that the neuronal cells derived from ORMES6 ESCs are not fully differentiated. The majority of differentiated cells were of neuronal lineage; however, a small fraction was of glial lineage. About 7±3.5% of differentiated cells expressed glial cell marker S100; 4.93±1.9% expressed astrocyte marker GFAP; 0.27±0.2% expressed oligodendrocyte marker O4 (Wegner and Stolt, 2005; Hevner et al., 2006; Miyagi et al., 2006). This suggests that a small subpopulation of the differentiated cells retains the characteristics of the glial lineage.
The definitive goal of this research is the use of the ESC-derived cells for disease therapy, but it is not known which stage in the differentiation protocol represents the optimal cell type for transplantation. The final step in the neural differentiation procedure is the removal of bFGF from the cell culture medium. The cell population present at that point cannot survive harvesting or replating in vitro, suggesting that they are not a viable source for transplantation studies. Neural rosette cells, present in the penultimate step and cultured with bFGF in the medium, can be harvested and replated but may have tumorigenic potential in vivo, as evidenced by the expression of Ki67, a cell proliferation marker. In order to assess survival, proliferation, differentiation and tumor formation of these cells in vivo, the neural rosette cells were transplanted into the striatum of SCID mice. In 2 weeks after transplantation, the cells proliferated and differentiated along neural lineage cells. At 4 weeks, no donor cells or tumor were observed. Some studies showed the ES cell derived neural lineage cell survived more than 8 weeks in chemical injured brains (Zhang et al., 2001; Li et al., 2005; Roy et al., 2006). The host microenvironment critically influences the eventual survival, proliferation and differentiation of grafted cells. In this study, the cells were transplanted into adult uninjured brain, the lack of space, cell—cell contacts and absence of damage signals may limit donor cell development. Many studies demonstrated that extensive and prolonged pre-differentiation of ES cells substantially reduced the incidence of tumor formation after grafting (Brederlau et al., 2006; Li et al., 2008). Brederlau et al. indicated no tumor formation in the brain transplanted with cells differentiated for 23 days, and 25% and 81.8% tumor formation with cells differentiated for 20 and 16 days, respectively. Brederlau et al. also revealed that cells differentiation for 23 days did not survive the grafting procedure as well as the cells cultured for 16 days. In our study, the total differentiation was over 28 days; the cell may undergo excessive differentiation, which may be another reason that the neural progenitor cells derived from ORMES6 ESC do not survive long time in the mouse brain.
To date, most research has explored neural lineage differentiation of primate ESCs with the R366.4 rhesus monkey line, which was derived from an in vivo flushed blastocyst (Thomson et al., 1998; Kuo et al., 2003; Salli et al., 2004; Wolf et al., 2004). However, there is no human ESC line derived from in vivo produced blastocysts. This study reports for the first time that neural precursor cells can be obtained with high efficiency and in large quantities from rhesus monkey ESCs derived from in vitro produced blastocysts and the neural differentiation process is similar to that for human ESCs (Zhang et al., 2001; Li et al., 2005; Roy et al., 2006). The differentiation of ORMES6 ESCs along the neural lineage also recapitulated the early stages of nervous system development.
Human ESC research is restricted by debates about ethics of their generation. Most, or all, of the NIH-approved human ESC lines are derived from oocytes of older and/or infertile women, and for that reason may not be ideal research materials. Human ESC research requires new cell lines. Human and nonhuman primate ESCs share many characteristics including morphology, surface marker expression and developmental potential (Pau and Wolf, 2004; Byrne et al., 2006). The rhesus macaque has more than 90% DNA homology to humans and has long been used as a model for study on various human diseases (Porrino et al., 1987; Cornblath et al., 1989; Rokkas et al., 1993; Baskin et al., 1998). Rhesus monkey ESCs can be used as a transitional model to accelerate ESC research. In the future, transplantation of rhesus monkey ESC-derived neural cells into a monkey neurological disease model will provide important preclinical safety and toxicity data for human ESC transplantation.
Acknowledgements
We thank Dr. Don Wolf for the generous gift of rhesus monkey ESC lines. The research was supported by Grant number RR00164 from National Center for Research Resources, National Institutes of Health and a grant from the state of Louisiana Millennium Health Excellence Fund and the Louisiana Gene Therapy Research Consortium.
References
- Baba H, Nakahira K, Morita N, Tanaka F, Akita H, Ikenaka K. GFAP gene expression during development of astrocyte. Dev. Neurosci. 1997;19:49–57. doi: 10.1159/000111185. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin. Cell Dev. Biol. 2005;16:642–646. doi: 10.1016/j.semcdb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Baskin GB, Ratterree M, Davison BB, Falkenstein KP, Clarke MR, England JD, Vanier MT, Luzi P, Rafi MA, Wenger DA. Genetic galactocerebrosidase deficiency (globoid cell leukodystrophy, Krabbe disease) in rhesus monkeys (Macaca mulatta) Lab. Anim. Sci. 1998;48:476–482. [PubMed] [Google Scholar]
- Benzing C, Segschneider M, Leinhaas A, Itskovitz-Eldor J, Brustle O. Neural conversion of human embryonic stem cell colonies in the presence of fibroblast growth factor-2. Neuroreport. 2006;17:1675–1681. doi: 10.1097/01.wnr.0000236861.01210.72. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24(6):1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Brüstle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RD. In vitro-generated neural precursors participate in mammalian brain development. Proc. Natl. Acad. Sci. USA. 1997;94:14809–14814. doi: 10.1073/pnas.94.26.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüstle O, Choudhary K, Karram K, Hüttner A, Murray K, Dubois-Dalcq M, McKay RD. Chimeric brains generated by intraventricular transplantation of fetal human brain cells into embryonic rats. Nat. Biotechnol. 1998;16:1040–1044. doi: 10.1038/3481. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Mitalipov SM, Clepper L, Wolf DP. Transcriptional profiling of rhesus monkey embryonic stem cells. Biol. Reprod. 2006;75:908–915. doi: 10.1095/biolreprod.106.053868. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, Wahlberg LU. In vitro expansion of a multipotent population of human neural progenitor cells. Exp. Neurol. 1999;158:265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- Chae JH, Stein GH, Lee JE. NeuroD: the predicted and the surprising. Mol. Cells. 2004;18:271–288. [PubMed] [Google Scholar]
- Copi A, Jungling K, Gottmann K. Activity- and BDNF-induced plasticity of miniature synaptic currents in ES cell-derived neurons integrated in a neocortical network. J. Neurophysiol. 2005;94:4538–4543. doi: 10.1152/jn.00155.2005. [DOI] [PubMed] [Google Scholar]
- Cornblath DR, Dellon AL, MacKinnon SE. Spontaneous diabetes mellitus in a rhesus monkey: neurophysiological studies. Muscle Nerve. 1989;12:233–235. doi: 10.1002/mus.880120312. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Messam CA, Li P, Selzer ME, Dichter MA, Haydon PG. Murine brain progenitor cells have the ability to differentiate into functional neurons and integrate into the CNS. Cell Transplant. 2006;15:699–710. doi: 10.3727/000000006783981468. [DOI] [PubMed] [Google Scholar]
- Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotent cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fricker RA, Carpenter MK, Winkler C, Greco C, Gates MA, Björklund A. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J. Neurosci. 1999;19:5990–6005. doi: 10.1523/JNEUROSCI.19-14-05990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensburger C, Labourdette G, Sensenbrenner M. Brain basic fibroblast growth factor stimulates the proliferation of rat neuronal precursor cells in vitro. FEBS Lett. 1987;217:1–5. doi: 10.1016/0014-5793(87)81230-9. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev. Biol. 2004;269:360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Hara A, Niwa M, Kumada M, Aoki H, Kunisada T, Oyama T, Yamamoto T, Kozawa O, Mori H. Intraocular injection of folate antagonist methotrexate induces neuronal differentiation of embryonic stem cells transplanted in the adult mouse retina. Brain Res. 2006;1085:33–42. doi: 10.1016/j.brainres.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Ingraham CA, Rising LJ. NBN defined medium supports the development of O4+/O1— immunopanned pro-oligodendroglia. Brain Res. Dev. Brain Res. 2000;125:1–8. doi: 10.1016/s0165-3806(00)00122-x. [DOI] [PubMed] [Google Scholar]
- Ito H, Nakajima A, Nomoto H, Furukawa S. Neurotrophins facilitate neuronal differentiation of cultured neural stem cells via induction of mRNA expression of basic helix-loop-helix transcription factors Mash1 and Math1. J. Neurosci. Res. 2003;71:648–658. doi: 10.1002/jnr.10532. [DOI] [PubMed] [Google Scholar]
- Kamochi H, Kurokawa MS, Yoshikawa H, Ueda Y, Masuda C, Takada E, Watanabe K, Sakakibara M, Natuki Y, Kimura K, Beppu M, Aoki H, Suzuki N. Transplantation of myocyte precursors derived from embryonic stem cells transfected with IGFII gene in a mouse model of muscle injury. Transplantation. 2006;82:516–526. doi: 10.1097/01.tp.0000229388.97549.55. [DOI] [PubMed] [Google Scholar]
- Kang X, Xie Y, Powell HM, Lee L. James, Belury MA, Lannutti JJ, Kniss DA. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials. 2007;28:450–458. doi: 10.1016/j.biomaterials.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Legido A, Perentes E, Mörk SJ. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J. Child Neurol. 2003;18:851–866. doi: 10.1177/088307380301801205. [DOI] [PubMed] [Google Scholar]
- Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE, Fleischmann BK. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J. Exp. Med. 2006;203:2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai XL, Cong XQ, Li XL, Xiao SD. Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transplant. 2003;9:1094–1099. doi: 10.1053/jlts.2003.50207. [DOI] [PubMed] [Google Scholar]
- Kuo HC, Pau KY, Yeoman RR, Mitalipov SM, Okano H, Wolf DP. Differentiation of monkey embryonic stem cells into neural lineages. Biol. Reprod. 2003;68:1727–1735. doi: 10.1095/biolreprod.102.012195. [DOI] [PubMed] [Google Scholar]
- Lazzari G, Colleoni S, Giannelli SG, Brunetti D, Colombo E, Lagutina I, Galli C, Broccoli V. Direct derivation of neural rosettes from cloned bovine blastocysts: a model of early neurulation events and neural crest specification in vitro. Stem Cells. 2006;24:2514–2521. doi: 10.1634/stemcells.2006-0149. [DOI] [PubMed] [Google Scholar]
- Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, McKercher S, Meerloo J, Muller FJ, Park KI, Butters TD, Dwek RA, Schwartz P, Tong G, Wenger D, Lipton SA, Seyfried TN, Platt FM, Snyder EY. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat. Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31(3):146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi S, Nishimoto M, Saito T, Ninomiya M, Sawamoto K, Okano H, Muramatsu M, Oguro H, Iwama A, Okuda A. The Sox2 regulatory region 2 functions as a neural stem cell-specific enhancer in the telencephalon. J. Biol. Chem. 2006;281:13374–13381. doi: 10.1074/jbc.M512669200. [DOI] [PubMed] [Google Scholar]
- Mollaaghababa R, Pavan WJ. The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia. Oncogene. 2003;22:3024–3034. doi: 10.1038/sj.onc.1206442. [DOI] [PubMed] [Google Scholar]
- Morizane A, Takahashi J, Shinoyama M, Ideguchi M, Takagi Y, Fukuda H, Koyanagi M, Sasai Y, Hashimoto N. Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J. Neurosci. Res. 2006;83:1015–1027. doi: 10.1002/jnr.20799. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Namaka MP, Sawchuk M, MacDonald SC, Jordan LM, Hochman S. Neurogenesis in postnatal mouse dorsal root ganglia. Exp. Neurol. 2001;172:606–609. doi: 10.1006/exnr.2001.7761. [DOI] [PubMed] [Google Scholar]
- Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- Pau KY, Wolf DP. Derivation and characterization of monkey embryonic stem cells. Reprod. Biol. Endocrinol. 2004;2:41. doi: 10.1186/1477-7827-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Burns RS, Crane AM, Palombo E, Kopin IJ, Sokoloff L. Changes in local cerebral glucose utilization associated with Parkinson’s syndrome induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the primate. Life Sci. 1987;40:1657–1664. doi: 10.1016/0024-3205(87)90014-2. [DOI] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Riess P, Molcanyi M, Bentz K, Maegele M, Simanski C, Carlitscheck C, Schneider A, Hescheler J, Bouillon B, Schäfer U, Neugebauer E. Embryonic stem cell transplantation after experimental traumatic brain injury dramatically improves neurological outcome, but may cause tumors. J. Neurotrauma. 2007;24:216–225. doi: 10.1089/neu.2006.0141. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Gómez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, Green MV, Thanos PK, Ichise M, Pike VW, Innis RB, McKay RD. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokkas CK, Sundaresan S, Shuman TA, Palazzo RS, Nitta T, Despotis GJ, Burns TC, Wareing TH, Kouchoukos NT. Profound systemic hypothermia protects the spinal cord in a primate model of spinal cord ischemia. J. Thorac. Cardiovasc. Surg. 1993;106:1024–1035. [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Salli U, Reddy AP, Salli N, Lu NZ, Kuo HC, Pau FK, Wolf DP, Bethea CL. Serotonin neurons derived from rhesus monkey embryonic stem cells: similarities to CNS serotonin neurons. Exp. Neurol. 2004;188:351–364. doi: 10.1016/j.expneurol.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev. 1998;20:88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- Soto-Gutiérrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y, Tanaka K, Narushima M, Miki A, Ueda T, Jun HS, Yoon JW, Lebkowski J, Tanaka N, Fox IJ. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat. Biotechnol. 2006;24:1412–1419. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Clarke DJ, Rosser AE, Dunnett SB. Survival and differentiation of rat and human epidermal growth factor-responsive precursor cells following grafting into the lesioned adult central nervous system. Exp. Neurol. 1996;137:376–388. doi: 10.1006/exnr.1996.0039. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Kodama M, Yamamoto H, Ikarashi Y, Ueda S, Teratani T, Yamamoto Y, Tamatani T, Kanegasaki S, Ochiya T, Quinn G. Streptozotocin-induced partial beta cell depletion in nude mice without hyperglycaemia induces pancreatic morphogenesis in transplanted embryonic stem cells. Diabetologia. 2006;49:2948–2958. doi: 10.1007/s00125-006-0432-z. [DOI] [PubMed] [Google Scholar]
- Tao Y, Black IB, DiCicco-Bloom E. In vivo neurogenesis is inhibited by neutralizing antibodies to basic fibroblast growth factor. J. Neurobiol. 1997;33:289–296. [PubMed] [Google Scholar]
- Thomson JA, Marshall VS, Trojanowski JQ. Neural differentiation of rhesus embryonic stem cells. APMIS. 1998;106:149–156. doi: 10.1111/j.1699-0463.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Gritti A, Galli R, Parati EA. Isolation and intracerebral grafting of nontransformed multipotential embryonic human CNS stem cells. J. Neurotrauma. 1999;16:689–693. doi: 10.1089/neu.1999.16.689. [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Kuo HC, Pau KY, Lester L. Progress with nonhuman primate embryonic stem cell. Biol. Reprod. 2004;71:1766–1771. doi: 10.1095/biolreprod.104.029413. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Tateishi N, Shimoda T, Satoh S, Ogitani E, Fujita S. Relationship between S100beta and GFAP expression in astrocytes during infarction and glial scar formation after mild transient ischemia. Brain Res. 2004;1021:20–31. doi: 10.1016/j.brainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]