Abstract
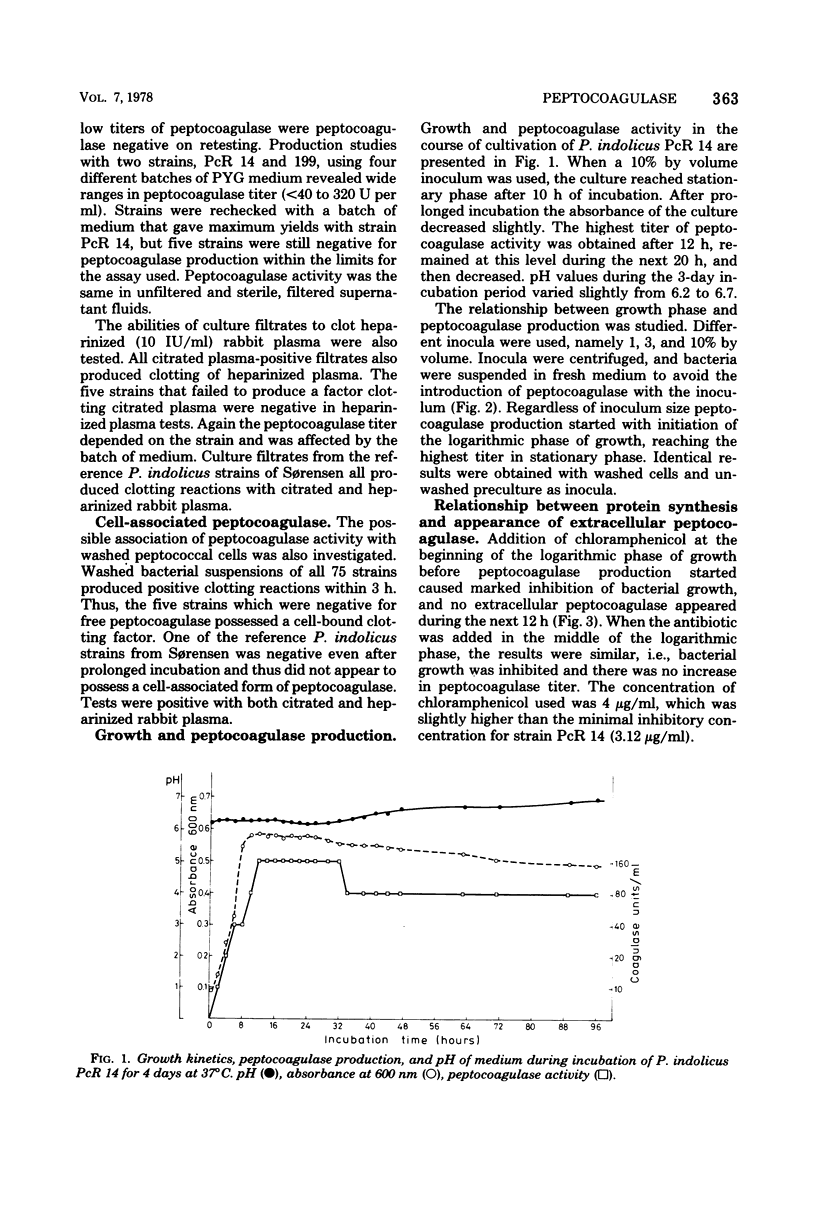
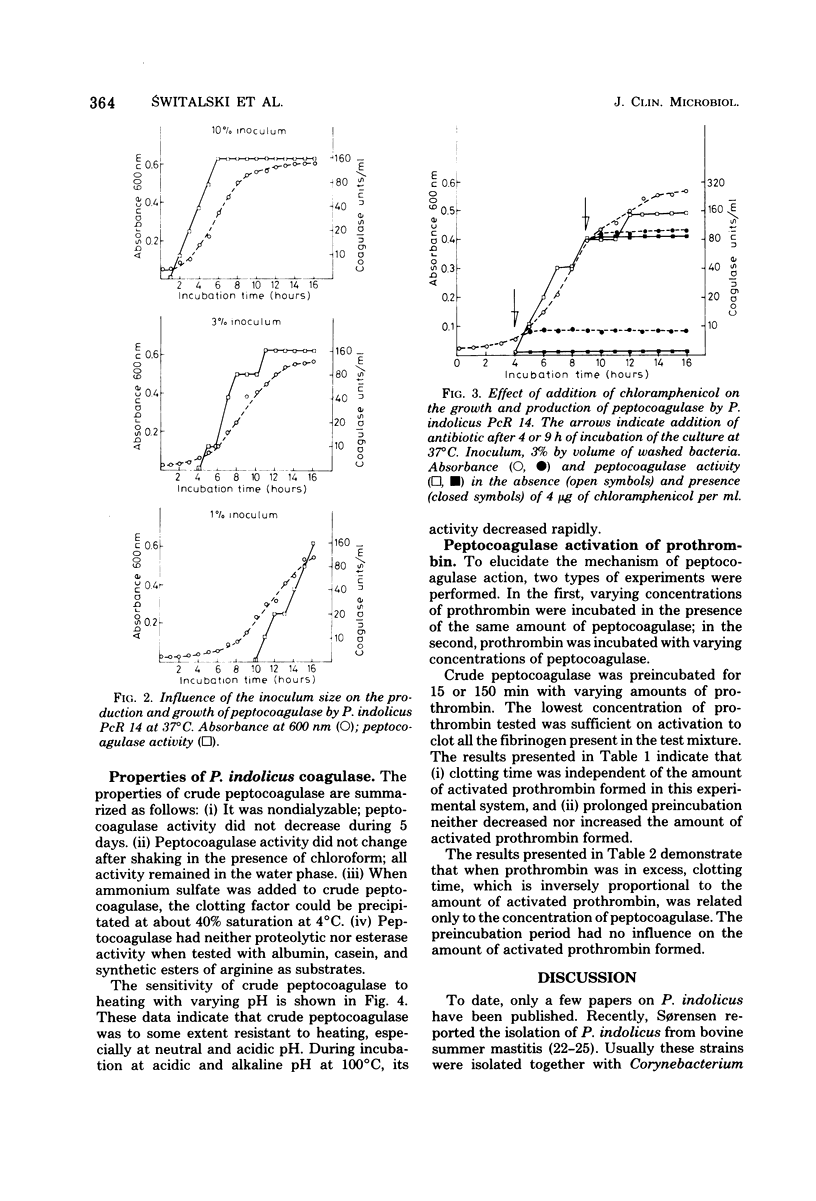
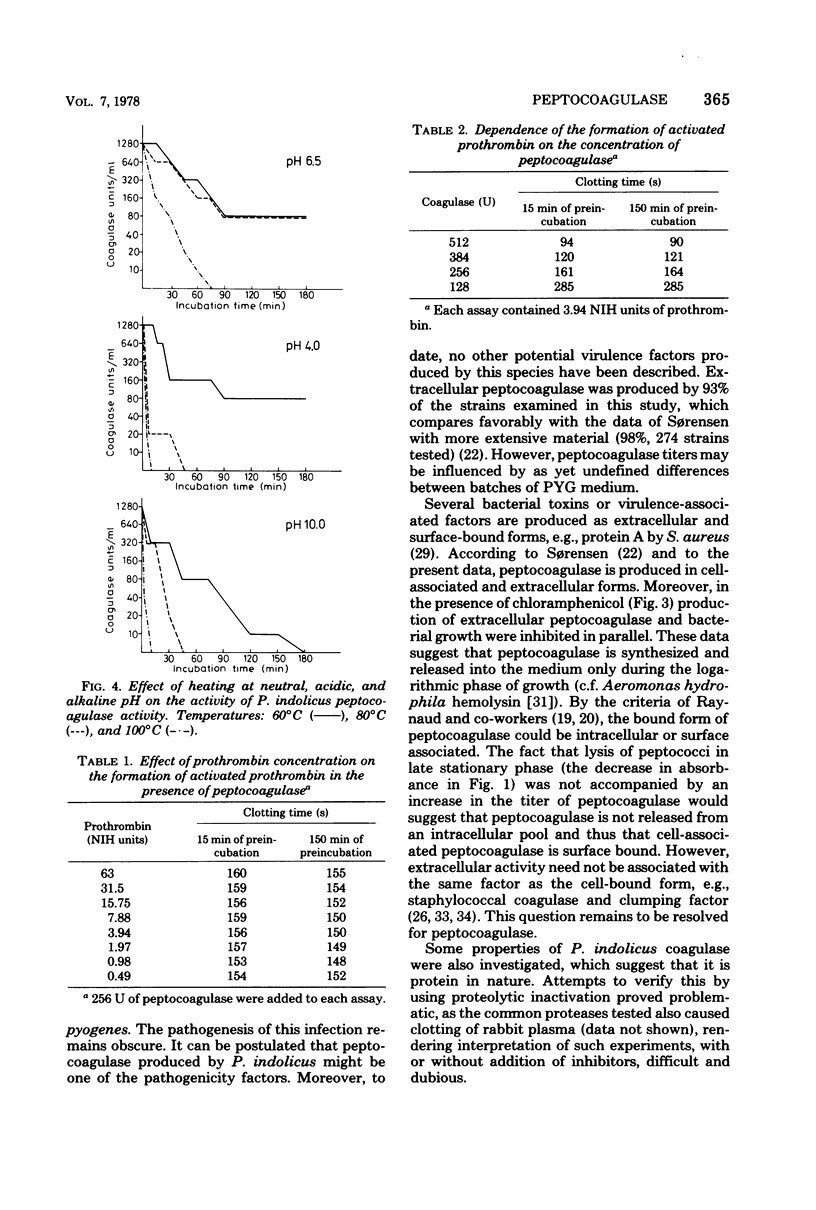
The production of a clotting factor (peptocoagulase) by bovine clinical isolates of Peptococcus indolicus and its nature were investigated. Extracellular peptocoagulase was demonstrated in culture filtrates of 93% and cell associated with washed cell suspensions of 100% of the 75 isolates tested. Both citrated and heparinized plasma were clotted. Crude peptocoagulase was nondialyzable, precipitated with (NH4)2SO4 at 40% saturation, somewhat resistant to heating at both neutral and acid pH, and chloroform insoluble. Culture filtrate did not contain proteolytic activity with albumin and casein, as substrates and no esterase activity was detected with tosylarginine and benzoylarginine methyl esters as substrates. The clotting reaction required peptocoagulase, prothrombin, and fibrinogen. The activation of prothrombin appeared to involve a stoichiometric reaction with peptocoagulase, possibly by formation of a stable complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbern R. A. On the nature of albumin-promoted coagulase release by Staphylococcus aureus. J Infect Dis. 1966 Dec;116(5):593–600. doi: 10.1093/infdis/116.5.593. [DOI] [PubMed] [Google Scholar]
- DREYER W. J., NEURATH H. The activation of chymotrypsinogen; isolation and identification of a peptide liberated during activation. J Biol Chem. 1955 Dec;217(2):527–539. [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K. Basic mechanisms in blood coagulation. Annu Rev Biochem. 1975;44:799–829. doi: 10.1146/annurev.bi.44.070175.004055. [DOI] [PubMed] [Google Scholar]
- EISLER D. M. Coagulation of human plasma by Pasteurella pestis. J Bacteriol. 1961 Feb;81:241–245. doi: 10.1128/jb.81.2.241-245.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS J. B., BUETTNER L. G., NIVEN C. F., Jr Occurrence of streptococci that give a false-positive coagulase test. J Bacteriol. 1952 Sep;64(3):433–434. doi: 10.1128/jb.64.3.433-434.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- KEKWICK R. A., MACKAY M. E., NANCE M. H., RECORD B. R. The purification of human fibrinogen. Biochem J. 1955 Aug;60(4):671–683. doi: 10.1042/bj0600671b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGNUSSON S. N-Terminal glycine analysis for the determination of the proteolytic activity of thrombin. Thromb Diath Haemorrh. 1960 Mar 1;4:167–177. [PubMed] [Google Scholar]
- MUSHIN R., KERR V. J. Clotting of citrated plasma and citrate utilization by intestinal gram-negative bacilli. J Gen Microbiol. 1954 Jun;10(3):445–451. doi: 10.1099/00221287-10-3-445. [DOI] [PubMed] [Google Scholar]
- Malhotra O. P., Carter J. R. Modified method for the preparation of purified bovine prothrombin of high specific activity. Thromb Diath Haemorrh. 1968 Mar 31;19(1):178–185. [PubMed] [Google Scholar]
- Moore H. B., Sutter V. L., Finegold S. M. Comparison of three procedures for biochemical testing of anaerobic bacteria. J Clin Microbiol. 1975 Jan;1(1):15–24. doi: 10.1128/jcm.1.1.15-24.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYNAUD M., TURPIN A., MANGALO R., BIZZINI B. Croissance et toxinogenèse. Ann Inst Pasteur (Paris) 1954 Dec;87(6):599–616. [PubMed] [Google Scholar]
- ROGERS H. J. The rate of formation of hyaluronidase, coagulase and total extracellular protein by strains of Staphylococcus aureus. J Gen Microbiol. 1954 Apr;10(2):209–220. doi: 10.1099/00221287-10-2-209. [DOI] [PubMed] [Google Scholar]
- Sorensen G. H. Micrococcus indolicus. Some biochemical properties, and the demonstration of sex antigenically different types. Acta Vet Scand. 1973;14(2):301–326. doi: 10.1186/BF03547448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G. H. Peptococcus (S. Micrococcus) indolicus. The demonstration of two varieties of hemolysin forming strains. Acta Vet Scand. 1975;16(2):218–225. doi: 10.1186/BF03546676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G. H. Studies on the occurrence of Peptococcus indolicus and Corynebacterium pyogenes in apparently healthy cattle. Acta Vet Scand. 1976;17(1):15–24. doi: 10.1186/BF03547939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Ohsaka A. Purification and characterization of a proteinase in the venom of Trimeresurus flavoviridis. Complete separation of the enzyme from hemorrhagic activity. Biochim Biophys Acta. 1970 Feb 11;198(2):293–307. doi: 10.1016/0005-2744(70)90062-8. [DOI] [PubMed] [Google Scholar]
- WOOD M. The clotting of rabbit plasma by group D Streptococci. J Gen Microbiol. 1959 Oct;21:385–388. doi: 10.1099/00221287-21-2-385. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Hedén L., Wadström T. Formation of extracellular haemolysin by Aeromonas hydrophila in relation to protease and staphylolytic enzyme. J Gen Microbiol. 1973 Sep;78(1):57–65. doi: 10.1099/00221287-78-1-57. [DOI] [PubMed] [Google Scholar]
- YOSHIDA A. Staphylococcal delta-hemolysin. I. Purification and chemical properties. Biochim Biophys Acta. 1963 Jun 4;71:544–553. doi: 10.1016/0006-3002(63)91126-0. [DOI] [PubMed] [Google Scholar]
- Zajdel M., Wegrzynowicz Z., Jeljaszewicz J., Pulverer G. Mechanism of action of staphylocoagulase and clumping factor. Contrib Microbiol Immunol. 1973;1:364–375. [PubMed] [Google Scholar]


